Microbiota-Modulating Strategies in Neonates Undergoing Surgery for Congenital Gastrointestinal Conditions: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection Process
2.4. Data Synthesis
3. Results
3.1. Dynamics of the Microbiota in a Healthy Term Newborn
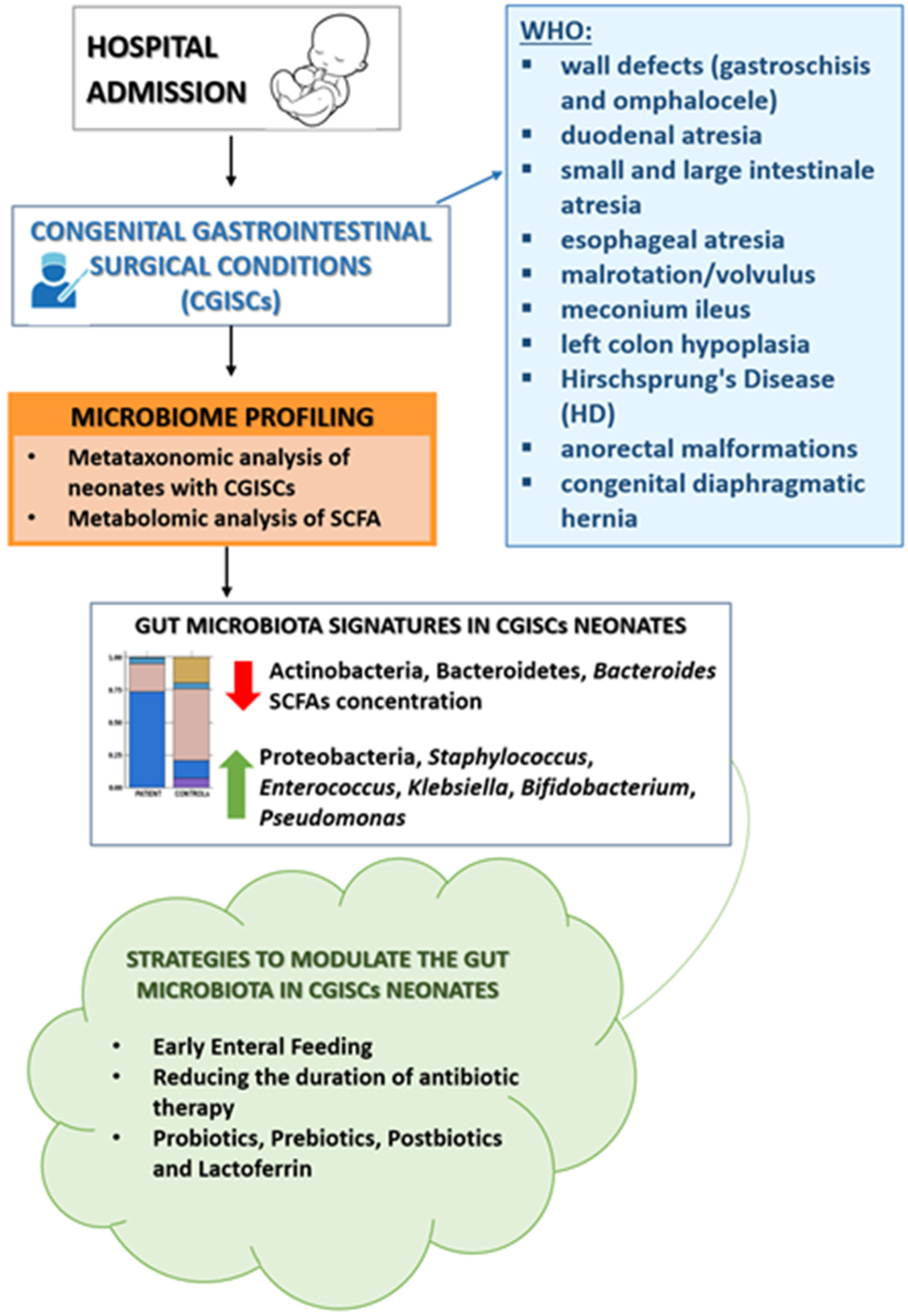
3.2. Dynamics of the Microbiota in the Surgical Newborn
3.3. Strategies to Modulate the Intestinal Microbiota in Newborns Undergoing Gastrointestinal Surgery
3.3.1. Early Enteral Feeding
| N. | Author, Year | Type of Study | Level of Evidence | Age | Number of Patients | Main Topic | Key Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Rao SC et al., 2020 | Prospective Cohort Study | Level II | Newborns (≥36 weeks) | 73 | Gut microbiota composition in neonates with CGISCs vs. healthy infants | By the second week of life, neonates with congenital gastrointestinal surgical conditions (CGISCs) developed gut dysbiosis, characterized by a lower abundance of Bifidobacterium and Bacteroides and higher levels of Pseudomonas and Escherichia–Shigella compared to healthy infants. Stool SCFA levels were significantly lower in CGISCs. Findings suggest the potential for probiotic interventions. | [41] |
| 2 | Issac A et al., 2023 | Systematic Review and Meta-analysis | Level I | 10 days–6.5 years | 488 | Early enteral nutrition (EEN) vs. late enteral nutrition (LEN) | Early enteral nutrition (EEN) after gastrointestinal surgery showed shorten hospital stays, faster recovery by promoting earlier fecal movement, and reduced postoperative wound infections. No significant impact on anastomosis leakage, vomiting, or abdominal distension. | [51] |
| 3 | Behera BK et al., 2022 | Systematic Review and Meta-analysis | Level I | 0–18 years | 286 | EEN vs. LEN in children following bowel anastomosis surgery | Early enteral nutrition after bowel anastomosis surgery showed a statistically significant lower incidence of surgical site infections, septic complications, and pooled overall complications compared to LEN group. The time to the passage of first feces and the length of hospitalization were significantly lower in the EEN group. No significant difference in anastomotic leaks, abdominal distension, wound dehiscence, or vomiting between EEN and LEN. | [52] |
| 4 | Kohler JA et al., 2013 | Retrospective Study | Level III | Newborns | 90 | Exclusive HM vs. formula feeding after gastroschisis repair | Exclusive HM feeding led to significantly shorter time to full enteral feeds and hospital discharge compared to formula-fed infants. Findings suggest HM should be prioritized in post-gastroschisis feeding strategies. | [55] |
| 5 | Brindle ME et al., 2013 | Consensus Based Guidelines | Level I | Newborns (≥37 weeks) | Not Applicable | ERAS recommendations for perioperative care in neonatal intestinal surgery | Seventeen evidence-based recommendations for Enhanced Recovery After Surgery (ERAS) in neonatal intestinal surgery. Key points include early enteral feeding within 24–48 h, prioritization of h milk, perioperative fluid management, prevention of hypothermia, structured perioperative communication, and parental involvement in care. | [58] |
| 6 | Rao SC et al., 2018 | Systematic Review | Level I | Newborns | 32 | Probiotic use, gut microbiota composition, clinical outcomes in infants with gastrointestinal surgical conditions | Limited evidence on probiotics in neonates with gastrointestinal surgical conditions. Two RCTs (N = 32) evaluated probiotic supplementation in neonates with GI surgical conditions. One RCT (N = 24) found no significant differences in overall microbial composition, though probiotics increased Bifidobacteriaceae and reduced Clostridiaceae, Enterobacteriaceae, Enterococcaceae, Staphylococcaceae, and Streptococcaceae. Another RCT (N = 8) showed increased Streptococcaceae in the probiotic group but unexpectedly higher Bifidobacteriaceae in the control group. No significant differences in TPN duration, antibiotic therapy, or length of hospital stay were observed. Surgical stress appears to significantly affect gut microbiota. | [59] |
| 7 | Powell WT et al., 2016 | Randomized Controlled Trial (RCT) | Level I | Newborns (>34 weeks) | 24 | Probiotic use, fecal microbiota composition, hospital stay | Bifidobacterium infantis probiotic altered gut microbiota composition but had no significant impact on length of hospital stay. | [60] |
| 8 | Murakami et al., 2016 | Randomized Controlled Trial (RCT) | Level I | Newborns (≥37 weeks) | 13 | Probiotic use, fecal microbiota composition | The authors randomized 8 CGISC patients; 4 received Bifidobacterium animalis subsp. lactis LKM512 (LKM) and 4 received placebo; 3 healthy newborns served as controls. Stool analysis revealed similar results for both groups; particularly, there were more Streptcoccaceae in stools from those who received probiotics and, unexpectedly, there were more Bifidobacteriaceae in samples from those who did not receive probiotics. The authors concluded that stress after surgery has an important impact on GM. | [61] |
| 9 | Rao SC et al., 2022 | Randomized Controlled Trial (RCT) | Level I | Newborns (>35 weeks) | 61 | Gut microbiota, SCFA levels, clinical outcomes | Probiotic supplementation in neonates with CGISC significantly reduced the relative abundance of pathogenic bacterial families (p = 0.044) and increased Bifidobacteriaceae levels (p < 0.001) at two weeks. SCFA levels were higher in the probiotic group (p = 0.008), with no significant differences in hospital stay, infections, or antibiotic use. Postnatal head circumference restriction was less severe in the probiotic group (p = 0.013). No probiotic-related infections were reported, supporting safety and potential benefits for gut microbiota modulation. | [62] |
| 10 | Trivedi A et al., 2024 | Systematic Review | Level I | Newborns (>35 weeks) | 61 | Use of probiotics after gastrointestinal surgery for postoperative management | Probiotics showed little to no effect on the incidence of proven sepsis (OR 0.64, 95% CI 0.16–2.55) or time to full enteral feeds (MD 0.63 days, 95% CI −4.02 to 5.28). No deaths were reported before hospital discharge. After two weeks of supplementation, infants receiving probiotics had a significantly higher abundance of beneficial intestinal microflora (Bifidobacteriaceae) compared to the placebo group. | [63] |
| 11 | Trivedi A et al., 2023 | Systematic review | Level I | Newborns (>37 weeks) | Not applicable | Lactoferrin for postoperative management after gastrointestinal surgery | No RCTs were identified evaluating the efficacy of lactoferrin in postoperative term neonates after gastrointestinal surgery. There is no current evidence to support or refute its use in reducing sepsis, mortality, or improving enteral feeding. | [64] |
3.3.2. Reducing the Duration of Antibiotic Therapy
3.3.3. Probiotics
3.3.4. Prebiotics
3.3.5. Postbiotics
3.3.6. Lactoferrin
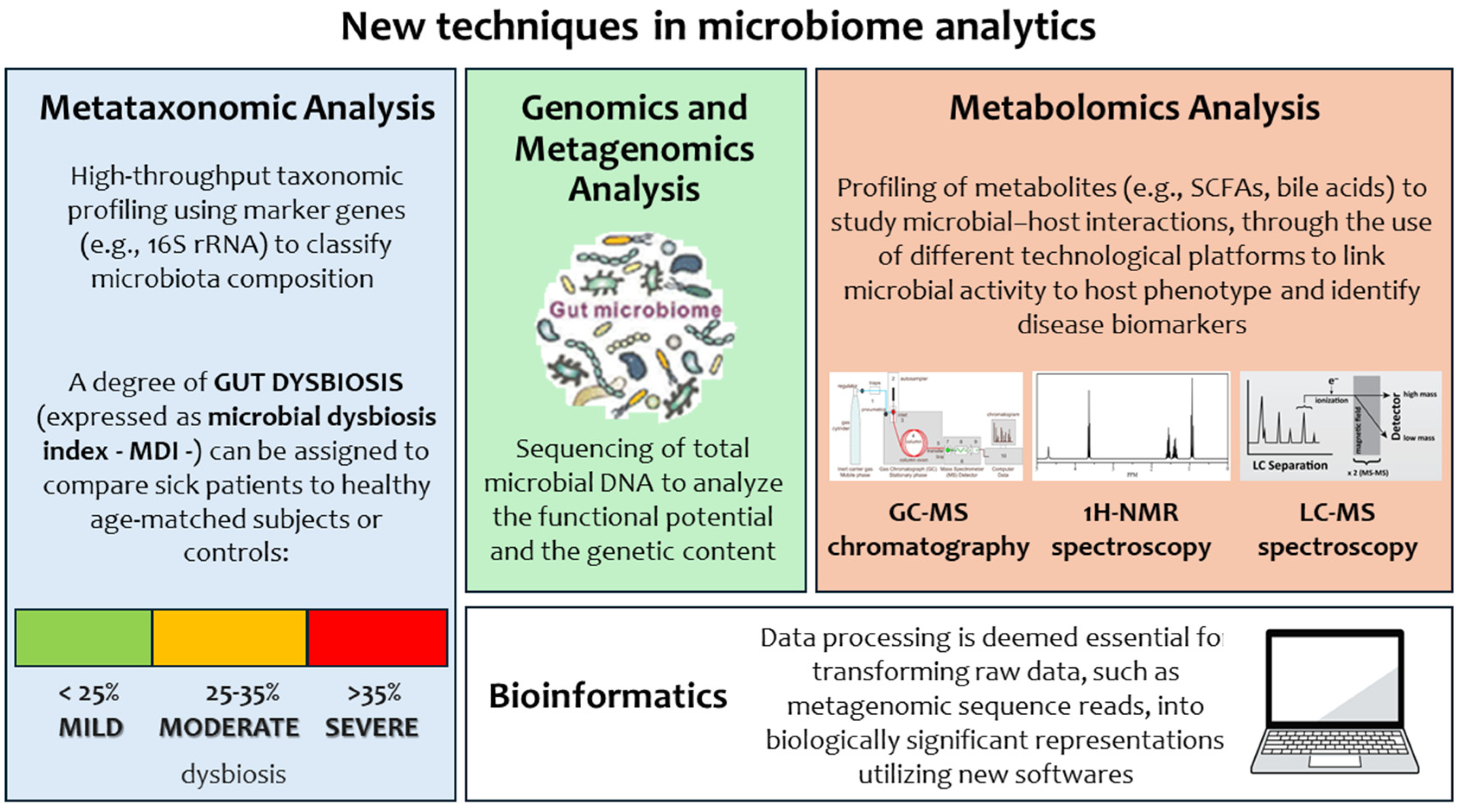
3.4. The Impact of Microbiome Analytics: A New Approach in Clinical Microbiology to Decipher Neonatal Pathological Conditions from the Laboratory Point of View
4. Discussion
4.1. Research Gaps
4.2. Clinical Implications
4.3. Limitations of the Review
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NICU | Neonatal Intensive Care Unit |
| SCFAs | Short-Chain Fatty Acids |
| CGISCs | Congenital Gastrointestinal Surgical Conditions |
| TPN | Total Parenteral Nutrition |
| HD | Hirshsprung’s Disease |
| CDH | Congenital Diaphragmatic Hernia |
| NEC | Necrotizing Enterocolitis |
| PPIs | Proton Pump Inhibitors |
| H2RAs | H2-Receptor Antagonists |
| SBS | Short Bowel Syndrome |
| EOS | Early-Onset Sepsis |
| LOS | Late-Onset Sepsis |
| HM | Human Milk |
| DHM | Donor Human Milk |
| RCT | Randomized Controlled Trial |
| MDI | Microbial Dysbiosis Index |
| GM | Gut Microbiota |
| HMOs | Human Milk Oligosaccharides |
| GOSs | Galacto-Oligosaccharides |
| FOSs | Fructo-Oligosaccharides |
| bLf | Bovine Lactoferrin |
| Lf | Lactoferrin |
| ERAS | Enhanced Recovery After Surgery |
| GA | Gestational Age |
| PNALD | Parenteral Nutrition-Associated Liver Disease |
| BSI | Bloodstream Infections |
| LEN | Late Enteral Nutrition |
| EEN | Early Enteral Nutrition |
| SSI | Surgical Site Infection |
| TLR | Toll-Like Receptor |
| LPS | Lipopolysaccharides |
| MDRO | Multidrug-Resistant Organism |
| UTI | Urinary Tract Infection |
| FM | Formula Milk |
References
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Kelly, J.R.; Minuto, C.; Cryan, J.F.; Clarke, G.; Dinan, T.G. Cross Talk: The Microbiota and Neurodevelopmental Disorders. Front. Neurosci. 2017, 11, 490. [Google Scholar] [CrossRef]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef]
- Ficara, M.; Pietrella, E.; Spada, C.; Della Casa Muttini, E.; Lucaccioni, L.; Iughetti, L.; Berardi, A. Changes of Intestinal Microbiota in Early Life. J. Matern. Fetal Neonatal Med. 2020, 33, 1036–1043. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.; Finlay, B. The Intestinal Microbiome in Early Life: Health and Disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef] [PubMed]
- Kaplina, A.; Kononova, S.; Zaikova, E.; Pervunina, T.; Petrova, N.; Sitkin, S. Necrotizing Enterocolitis: The Role of Hypoxia, Gut Microbiome, and Microbial Metabolites. Int. J. Mol. Sci. 2023, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Tarracchini, C.; Milani, C.; Longhi, G.; Fontana, F.; Mancabelli, L.; Pintus, R.; Lugli, G.A.; Alessandri, G.; Anzalone, R.; Viappiani, A.; et al. Unraveling the Microbiome of Necrotizing Enterocolitis: Insights in Novel Microbial and Metabolomic Biomarkers. Microbiol. Spectr. 2021, 9, e0117621. [Google Scholar] [CrossRef]
- McDonnell, L.; Gilkes, A.; Ashworth, M.; Rowland, V.; Harries, T.H.; Armstrong, D.; White, P. Association between Antibiotics and Gut Microbiome Dysbiosis in Children: Systematic Review and Meta-Analysis. Gut Microbes 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Piper, H.G.; Fan, D.; Coughlin, L.A.; Ho, E.X.; McDaniel, M.M.; Channabasappa, N.; Kim, J.; Kim, M.; Zhan, X.; Xie, Y.; et al. Severe Gut Microbiota Dysbiosis Is Associated with Poor Growth in Patients with Short Bowel Syndrome. J. Parenter. Enter. Nutr. 2017, 41, 1202–1212. [Google Scholar] [CrossRef]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal Dysbiosis in Preterm Infants Preceding Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 26, 5. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human Gut Colonisation May Be Initiated in Utero by Distinct Microbial Communities in the Placenta and Amniotic Fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef]
- Chong, C.Y.L.; Bloomfield, F.H.; O’Sullivan, J.M. Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients 2018, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; Van Den Brandt, P.A.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Browne, H.P.; Shao, Y.; Lawley, T.D. Mother–Infant Transmission of Human Microbiota. Curr. Opin. Microbiol. 2022, 69, 102173. [Google Scholar] [CrossRef]
- Beller, L.; Deboutte, W.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Valles-Colomer, M.; Rymenans, L.; Jansen, D.; Van Espen, L.; Papadaki, M.I.; et al. Successional Stages in Infant Gut Microbiota Maturation. mBio 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The Mode of Delivery Affects the Diversity and Colonization Pattern of the Gut Microbiota during the First Year of Infants’ Life: A Systematic Review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Mueller, N.T.; Differding, M.K.; Østbye, T.; Hoyo, C.; Benjamin-Neelon, S.E. Association of Birth Mode of Delivery with Infant Faecal Microbiota, Potential Pathobionts, and Short Chain Fatty Acids: A Longitudinal Study over the First Year of Life. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1293–1303. [Google Scholar] [CrossRef]
- Cortes-Macías, E.; Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; González, S.; Martínez-Costa, C.; Collado, M.C. Maternal Diet Shapes the Breast Milk Microbiota Composition and Diversity: Impact of Mode of Delivery and Antibiotic Exposure. J. Nutr. 2021, 151, 330–340. [Google Scholar] [CrossRef]
- Arboleya, S.; Saturio, S.; Gueimonde, M. Impact of Intrapartum Antibiotics on the Developing Microbiota: A Review. Microbiome Res. Rep. 2022, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Catassi, G.; Aloi, M.; Giorgio, V.; Gasbarrini, A.; Cammarota, G.; Ianiro, G. The Role of Diet and Nutritional Interventions for the Infant Gut Microbiome. Nutrients 2024, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Boudry, G.; Charton, E.; Le Huerou-Luron, I.; Ferret-Bernard, S.; Le Gall, S.; Even, S.; Blat, S. The Relationship Between Breast Milk Components and the Infant Gut Microbiota. Front. Nutr. 2021, 8, 629740. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145.e5. [Google Scholar] [CrossRef]
- Notarbartolo, V.; Giuffre, M.; Montante, C.; Corsello, G.; Carta, M. Composition of Human Breast Milk Microbiota and Its Role in Children’s Health. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 194–210. [Google Scholar] [CrossRef]
- Rodríguez, J.M. The Origin of Human Milk Bacteria: Is There a Bacterial Entero-Mammary Pathway during Late Pregnancy and Lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef]
- Fehr, K.; Moossavi, S.; Sbihi, H.; Boutin, R.C.T.; Bode, L.; Robertson, B.; Yonemitsu, C.; Field, C.J.; Becker, A.B.; Mandhane, P.J.; et al. Breastmilk Feeding Practices Are Associated with the Co-Occurrence of Bacteria in Mothers’ Milk and the Infant Gut: The CHILD Cohort Study. Cell Host Microbe 2020, 28, 285–297.e4. [Google Scholar] [CrossRef]
- Bajic, D.; Wiens, F.; Wintergerst, E.; Deyaert, S.; Baudot, A.; Van den Abbeele, P. HMOs Exert Marked Bifidogenic Effects on Children’s Gut Microbiota Ex Vivo, Due to Age-Related Bifidobacterium Species Composition. Nutrients 2023, 15, 1701. [Google Scholar] [CrossRef]
- Hundshammer, C.; Minge, O. In Love with Shaping You—Influential Factors on the Breast Milk Content of Human Milk Oligosaccharides and Their Decisive Roles for Neonatal Development. Nutrients 2020, 12, 3568. [Google Scholar] [CrossRef]
- Mills, D.A.; German, J.B.; Lebrilla, C.B.; Underwood, M.A. Translating Neonatal Microbiome Science into Commercial Innovation: Metabolism of Human Milk Oligosaccharides as a Basis for Probiotic Efficacy in Breast-Fed Infants. Gut Microbes 2023, 15, 2192458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Collado, M.C.; Wopereis, H.; Salminen, S.; Knol, J.; Roeselers, G. The Bifidogenic Effect Revisited—Ecology and Health Perspectives of Bifidobacterial Colonization in Early Life. Microorganisms 2020, 8, 1855. [Google Scholar] [CrossRef] [PubMed]
- Saturio, S.; Nogacka, A.M.; Alvarado-jasso, G.M.; Salazar, N.; de los Reyes-Gavilán, C.G.; Gueimonde, M.; Arboleya, S. Role of Bifidobacteria on Infant Health. Microorganisms 2021, 9, 2415. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, V.; Indrio, F.; Verduci, E.; Calcaterra, V.; Pop, T.L.; Mari, A.; Zuccotti, G.V.; Cokugras, F.C.; Pettoello-Mantovani, M.; Goulet, O. Term Infant Formulas Influencing Gut Microbiota: An Overview. Nutrients 2021, 13, 4200. [Google Scholar] [CrossRef]
- Verkhnyatskaya, S.; Ferrari, M.; De Vos, P.; Walvoort, M.T.C. Shaping the Infant Microbiome with Non-Digestible Carbohydrates. Front. Microbiol. 2019, 10, 343. [Google Scholar] [CrossRef]
- Indrio, F.; Gutierrez Castrellon, P.; Vandenplas, Y.; Cagri Dinleyici, E.; Francavilla, R.; Mantovani, M.P.; Grillo, A.; Beghetti, I.; Corvaglia, L.; Aceti, A. Health Effects of Infant Formula Supplemented with Probiotics or Synbiotics in Infants and Toddlers: Systematic Review with Network Meta-Analysis. Nutrients 2022, 14, 5175. [Google Scholar] [CrossRef]
- De Rose, D.U.; Santisi, A.; Ronchetti, M.P.; Martini, L.; Serafini, L.; Betta, P.; Maino, M.; Cavigioli, F.; Cocchi, I.; Pugni, L.; et al. Invasive Candida Infections in Neonates after Major Surgery: Current Evidence and New Directions. Pathogens 2021, 10, 319. [Google Scholar] [CrossRef]
- Auriti, C.; De Rose, D.U.; Santisi, A.; Martini, L.; Ronchetti, M.P.; Ravà, L.; Antenucci, V.; Bernaschi, P.; Serafini, L.; Catarzi, S.; et al. Incidence and Risk Factors of Bacterial Sepsis and Invasive Fungal Infection in Neonates and Infants Requiring Major Surgery: An Italian Multicentre Prospective Study. J. Hosp. Infect. 2022, 130, 122–130. [Google Scholar] [CrossRef]
- Daskalakis, G.; Psarris, A.; Koutras, A.; Fasoulakis, Z.; Prokopakis, I.; Varthaliti, A.; Karasmani, C.; Ntounis, T.; Domali, E.; Theodora, M.; et al. Maternal Infection and Preterm Birth: From Molecular Basis to Clinical Implications. Children 2023, 10, 907. [Google Scholar] [CrossRef]
- Putignani, L.; Del Chierico, F.; Petrucca, A.; Vernocchi, P.; Dallapiccola, B. The Human Gut Microbiota: A Dynamic Interplay with the Host from Birth to Senescence Settled during Childhood. Pediatr. Res. 2014, 76, 2–10. [Google Scholar] [CrossRef]
- Rao, S.C.; Esvaran, M.; Patole, S.K.; Simmer, K.N.; Gollow, I.; Keil, A.; Wemheuer, B.; Chen, L.; Conway, P.L. Gut Microbiota in Neonates with Congenital Gastrointestinal Surgical Conditions: A Prospective Study. Pediatr. Res. 2020, 88, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, A.F.; Pan, A.; Lam, V.; Gouthro, K.C.; Simpson, P.M.; Salzman, N.H.; Nghiem-Rao, T.H. Longitudinal Changes in the Gut Microbiome of Infants on Total Parenteral Nutrition. Pediatr. Res. 2019, 86, 107–114. [Google Scholar] [CrossRef]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia Muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front Immunol 2022, 13, 934695. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Quagliariello, A.; Schiavoni, M.; Petito, V.; Russo, A.; Reddel, S.; Del Chierico, F.; Ianiro, G.; Scaldaferri, F.; Neri, M.; et al. Towards a Disease-Associated Common Trait of Gut Microbiota Dysbiosis: The Pivotal Role of Akkermansia Muciniphila. Dig. Liver Dis. 2020, 52, 1002–1010. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Gorshkova, E.A.; Bondareva, M.A.; Podosokorskaya, O.A.; Sheynova, A.D.; Yakovleva, A.S.; Bonch-Osmolovskaya, E.A.; Nedospasov, S.A.; Kruglov, A.A.; Drutskaya, M.S. Akkermansia Muciniphila—Friend or Foe in Colorectal Cancer? Front. Immunol. 2023, 14, 1303795. [Google Scholar] [CrossRef]
- Korpela, K.; Mutanen, A.; Salonen, A.; Savilahti, E.; De Vos, W.M.; Pakarinen, M.P. Intestinal Microbiota Signatures Associated with Histological Liver Steatosis in Pediatric-Onset Intestinal Failure. J. Parenter. Enter. Nutr. 2017, 41, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Esaiassen, E.; Fjalstad, J.W.; Juvet, L.K.; van den Anker, J.N.; Klingenberg, C. Antibiotic Exposure in Neonates and Early Adverse Outcomes: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2017, 72, 1858–1870. [Google Scholar] [CrossRef]
- Thänert, R.; Thänert, A.; Ou, J.; Bajinting, A.; Burnham, C.A.D.; Engelstad, H.J.; Tecos, M.E.; Ndao, I.M.; Hall-Moore, C.; Rouggly-Nickless, C.; et al. Antibiotic-Driven Intestinal Dysbiosis in Pediatric Short Bowel Syndrome Is Associated with Persistently Altered Microbiome Functions and Gut-Derived Bloodstream Infections. Gut Microbes. 2021, 13, 1940792. [Google Scholar] [CrossRef]
- Santos, V.S.; Freire, M.S.; Santana, R.N.S.; Martins-Filho, P.R.S.; Cuevas, L.E.; Gurgel, R.Q. Association between Histamine-2 Receptor Antagonists and Adverse Outcomes in Neonates: A Systematic Review and Meta-Analysis. PLoS ONE 2019, 14, e0214135. [Google Scholar] [CrossRef]
- Terrin, G.; Canani, R.B.; Passariello, A.; Caoci, S.; De Curtis, M. Inhibitors of Gastric Acid Secretion Drugs Increase Neonatal Morbidity and Mortality. J. Matern. Fetal Neonatal. Med. 2012, 25, 77–79. [Google Scholar] [CrossRef][Green Version]
- Issac, A.; Dhiraaj, S.; Halemani, K.; Thimmappa, L.; Mishra, P.; Kumar, B.; Mavinatop, A. Efficacy of Early Enteral Nutrition on Gastrointestinal Surgery Outcomes: A Systematic Review and Meta-Analysis. Eur. J. Pediatr. Surg. 2023, 33, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.K.; Misra, S.; Tripathy, B.B. Systematic Review and Meta-Analysis of Safety and Efficacy of Early Enteral Nutrition as an Isolated Component of Enhanced Recovery After Surgery [ERAS] in Children after Bowel Anastomosis Surgery. J. Pediatr. Surg. 2022, 57, 1473–1479. [Google Scholar] [CrossRef]
- Savoie, K.B.; Bachier-Rodriguez, M.; Jones, T.L.; Jeffreys, K.; Papraniku, D.; Sevilla, W.M.A.; Tillman, E.; Huang, E.Y. Standardization of Feeding Advancement after Neonatal Gastrointestinal Surgery: Does It Improve Outcomes? Nutr. Clin. Pract. 2016, 31, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Hobson, D.; Spence, K.; Trivedi, A.; Thomas, G. Differences in Attitudes to Feeding Post Repair of Gastroschisis and Development of a Standardized Feeding Protocol. BMC Pediatr. 2019, 19, 475. [Google Scholar] [CrossRef]
- Kohler, J.A.; Perkins, A.M.; Bass, W.T. Human Milk versus Formula after Gastroschisis Repair: Effects on Time to Full Feeds and Time to Discharge. J. Perinatol. 2013, 33, 627–630. [Google Scholar] [CrossRef]
- Bertino, E.; Giuliani, F.; Baricco, M.; Di Nicola, P.; Peila, C.; Vassia, C.; Chiale, F.; Pirra, A.; Cresi, F.; Martano, C.; et al. Benefits of Donor Milk in the Feeding of Preterm Infants. Early Hum. Dev. 2013, 89, S3–S6. [Google Scholar] [CrossRef]
- Picaud, J.C. Review Highlights the Importance of Donor Human Milk Being Available for Very Low Birth Weight Infants. Acta Paediatr. Int. J. Paediatr. 2022, 111, 1127–1133. [Google Scholar] [CrossRef]
- Brindle, M.E.; McDiarmid, C.; Short, K.; Miller, K.; MacRobie, A.; Lam, J.Y.K.; Brockel, M.; Raval, M.V.; Howlett, A.; Lee, K.S.; et al. Consensus Guidelines for Perioperative Care in Neonatal Intestinal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2020, 44, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Simmer, K.; Patole, S. Probiotic Supplementation in Neonates with Major Gastrointestinal Surgical Conditions: A Systematic Review. J. Matern. Fetal Neonatal Med. 2018, 31, 1517–1523. [Google Scholar] [CrossRef]
- Powell, W.T.; Borghese, R.A.; Kalanetra, K.M.; Mirmiran, M.; Mills, D.A.; Underwood, M.A. Probiotic Administration in Infants with Gastroschisis: A Pilot Randomized Placebo-Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 852–857. [Google Scholar] [CrossRef]
- Murakami, H.; Shimomura, Y.; Matsumoto, M.; Lane, G.J.; Yamataka, A.; Okawada, M. Intestinal Microbiota in Neonates Requiring Urgent Surgery: Assessing the Role of Probiotics Using Fecal DNA Sequencing. Pediatr. Surg. Int. 2016, 32, 37–43. [Google Scholar] [CrossRef]
- Rao, S.; Esvaran, M.; Chen, L.; Keil, A.D.; Gollow, I.; Simmer, K.; Wemheuer, B.; Conway, P.; Patole, S. Probiotic Supplementation in Neonates with Congenital Gastrointestinal Surgical Conditions: A Pilot Randomised Controlled Trial. Pediatr. Res. 2022, 92, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Teo, E.; Walker, K.S. Probiotics for the Postoperative Management of Term Neonates after Gastrointestinal Surgery. Cochrane Database Syst. Rev. 2024, 2024, CD012265. [Google Scholar] [CrossRef]
- Trivedi, A.; Maheshwari, R.; Tarnow-Mordi, W.O.; Saxena, N. Lactoferrin for the Postoperative Management of Term Neonates after Gastrointestinal Surgery. Cochrane Database Syst. Rev. 2023, 2023, CD012218. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of Gut Microbiota of Healthy Adults Following Antibiotic Exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Riddle, S.; Agarwal, N.; Haberman, B.; Karpen, H.; Miquel-Verges, F.; Nayak, S.P.; Sullivan, K.; Williams, S.; Zaniletti, I.; Jacobson, E. Gastroschisis and Low Incidence of Early-Onset Infection: A Case for Antimicrobial Stewardship. J. Perinatol. 2022, 42, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Gargiullo, L.; Del Chierico, F.; D’Argenio, P.; Putignani, L. Gut Microbiota Modulation for Multidrug-Resistant Organism Decolonization: Present and Future Perspectives. Front Microbiol. 2019, 10, 1704. [Google Scholar] [CrossRef]
- Prasad, P.A.; Wong-McLoughlin, J.; Patel, S.; Coffin, S.E.; Zaoutis, T.E.; Perlman, J.; DeLaMora, P.; Alba, L.; Ferng, Y.H.; Saiman, L. Surgical Site Infections in a Longitudinal Cohort of Neonatal Intensive Care Unit Patients. J. Perinatol. 2016, 36, 300–305. [Google Scholar] [CrossRef]
- Duque-Estrada, E.O.; Duarte, M.R.; Rodrigues, D.M.; Raphael, M.D. Wound Infections in Pediatric Surgery: A Study of 575 Patients in a University Hospital. Pediatr. Surg. Int. 2003, 19, 436–438. [Google Scholar] [CrossRef]
- Gilje, E.A.; Hossain, M.J.; Vinocur, C.D.; Berman, L. Surgical Site Infections in Neonates Are Independently Associated with Longer Hospitalizations. J. Perinatol. 2017, 37, 1130–1134. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery. Am. J. Health Syst. Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef] [PubMed]
- Zwittink, R.D.; Renes, I.B.; van Lingen, R.A.; van Zoeren-Grobben, D.; Konstanti, P.; Norbruis, O.F.; Martin, R.; Groot Jebbink, L.J.M.; Knol, J.; Belzer, C. Association between Duration of Intravenous Antibiotic Administration and Early-Life Microbiota Development in Late-Preterm Infants. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Datta, A.; Massoumi, R.L.; Gross, E.R.; Uhing, M.; Arca, M.J. Antibiotic Stewardship in the Newborn Surgical Patient: A Quality Improvement Project in the Neonatal Intensive Care Unit. Surgery 2017, 162, 1295–1303. [Google Scholar] [CrossRef]
- Vu, L.T.; Vittinghoff, E.; Nobuhara, K.K.; Farmer, D.L.; Lee, H. Surgical Site Infections in Neonates and Infants: Is Antibiotic Prophylaxis Needed for Longer than 24 h? Pediatr. Surg. Int. 2014, 30, 587–592. [Google Scholar] [CrossRef]
- He, P.; Yu, L.; Tian, F.; Chen, W.; Zhang, H.; Zhai, Q. Effects of Probiotics on Preterm Infant Gut Microbiota Across Populations: A Systematic Review and Meta-Analysis. Adv. Nutr. 2024, 15, 100233. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cai, J.; Su, Q.; Li, Q.; Meng, X. Human Milk Oligosaccharides Combine with Bifidobacterium Longum to Form the “Golden Shield” of the Infant Intestine: Metabolic Strategies, Health Effects, and Mechanisms of Action. Gut Microbes 2024, 16, 2430418. [Google Scholar] [CrossRef]
- Dargenio, V.N.; Cristofori, F.; Brindicci, V.F.; Schettini, F.; Dargenio, C.; Castellaneta, S.P.; Iannone, A.; Francavilla, R. Impact of Bifidobacterium Longum Subspecies Infantis on Pediatric Gut Health and Nutrition: Current Evidence and Future Directions. Nutrients 2024, 16, 3510. [Google Scholar] [CrossRef]
- Ananthan, A.; Balasubramanian, H.; Rath, C.; Muthusamy, S.; Rao, S.; Patole, S. Lactobacillus Rhamnosus GG as a Probiotic for Preterm Infants: A Strain Specific Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2024, 78, 830–846. [Google Scholar] [CrossRef]
- Ang, J.L.; Athalye-Jape, G.; Rao, S.; Bulsara, M.; Patole, S. Limosilactobacillus Reuteri DSM 17938 as a Probiotic in Preterm Infants: An Updated Systematic Review with Meta-Analysis and Trial Sequential Analysis. J. Parenter. Enter. Nutr. 2023, 47, 963–981. [Google Scholar] [CrossRef]
- Van Rossum, T.; Haiß, A.; Knoll, R.L.; Marißen, J.; Podlesny, D.; Pagel, J.; Bleskina, M.; Vens, M.; Fortmann, I.; Siller, B.; et al. Bifidobacterium and Lactobacillus Probiotics and Gut Dysbiosis in Preterm Infants: The PRIMAL Randomized Clinical Trial. JAMA Pediatr. 2024, 178, 985–995. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The Pros, Cons, and Many Unknowns of Probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Navarro-Tapia, E.; Sebastiani, G.; Sailer, S.; Toledano, L.A.; Serra-Delgado, M.; García-Algar, Ó.; Andreu-Fernández, V. Probiotic Supplementation during the Perinatal and Infant Period: Effects on Gut Dysbiosis and Disease. Nutrients 2020, 12, 2243. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, F.; Siva, N.; Raghupathy, M.K.; Lewis, L.E.S.; Barche, A.; Purkayastha, J.; Nayak, B.S. Probiotic Effect in Preterm Neonates with Sepsis—A Systematic Review Protocol. F1000Res 2022, 11, 913. [Google Scholar] [CrossRef]
- Morreale, C.; Giaroni, C.; Baj, A.; Folgori, L.; Barcellini, L.; Dhami, A.; Agosti, M.; Bresesti, I. Effects of Perinatal Antibiotic Exposure and Neonatal Gut Microbiota. Antibiotics 2021, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Catassi, G.; Mateo, S.G.; Occhionero, A.S.; Esposito, C.; Giorgio, V.; Aloi, M.; Gasbarrini, A.; Cammarota, G.; Ianiro, G. The Importance of Gut Microbiome in the Perinatal Period. Eur. J. Pediatr. 2024, 183, 5085–5101. [Google Scholar] [CrossRef]
- Klerk, D.H.; van Avezaath, L.K.; Loeffen, E.A.H.; Hulscher, J.B.F.; Kooi, E.M.W. Fetal–Neonatal Exposure to Antibiotics and NEC Development: A Systematic Review and Meta-Analysis. Front Pediatr. 2023, 10, 1102884. [Google Scholar] [CrossRef] [PubMed]
- Wala, S.J.; Ragan, M.V.; Sajankila, N.; Volpe, S.G.; Purayil, N.; Dumbauld, Z.; Besner, G.E. Probiotics and Novel Probiotic Delivery Systems. Semin. Pediatr. Surg. 2023, 32, 151307. [Google Scholar] [CrossRef]
- Sowden, M.; van Weissenbruch, M.M.; Bulabula, A.N.H.; van Wyk, L.; Twisk, J.; van Niekerk, E. Effect of a Multi-Strain Probiotic on the Incidence and Severity of Necrotizing Enterocolitis and Feeding Intolerances in Preterm Neonates. Nutrients 2022, 14, 3305. [Google Scholar] [CrossRef]
- Athalye-Jape, G.; Esvaran, M.; Patole, S.; Simmer, K.; Nathan, E.; Doherty, D.; Keil, A.; Rao, S.; Chen, L.; Chandrasekaran, L.; et al. Effect of Single versus Multistrain Probiotic in Extremely Preterm Infants: A Randomised Trial. BMJ Open Gastroenterol. 2022, 9, e000811. [Google Scholar] [CrossRef]
- Kruth, S.S.; Willers, C.; Persad, E.; Sjöström, E.S.; Lagerström, S.R.; Rakow, A. Probiotic Supplementation and Risk of Necrotizing Enterocolitis and Mortality among Extremely Preterm Infants—The Probiotics in Extreme Prematurity in Scandinavia (PEPS) Trial: Study Protocol for a Multicenter, Double-Blinded, Placebo-Controlled, and Registry-Based Randomized Controlled Trial. Trials 2024, 25, 259. [Google Scholar] [CrossRef]
- Arumugam, S.; Lau, C.S.M.; Chamberlain, R.S. Probiotics and Synbiotics Decrease Postoperative Sepsis in Elective Gastrointestinal Surgical Patients: A Meta-Analysis. J. Gastrointest. Surg. 2016, 20, 1123–1131. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Q.; Liu, Y.; Fan, D. Effect of Perioperative Probiotics and Synbiotics on Postoperative Infections after Gastrointestinal Surgery: A Systematic Review with Meta-Analysis. J. Parenter. Enter. Nutr. 2017, 41, 1051–1062. [Google Scholar] [CrossRef]
- Lytvyn, L.; Quach, K.; Banfield, L.; Johnston, B.C.; Mertz, D. Probiotics and Synbiotics for the Prevention of Postoperative Infections Following Abdominal Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Hosp. Infect. 2016, 92, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.C.; Patole, S.K. Probiotic Research in Neonates with Congenital Gastrointestinal Surgical Conditions—Now Is the Time. Microb. Biotechnol. 2019, 12, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, T.; Majarikar, S.; Deshmukh, M.; Ananthan, A.; Balasubramanian, H.; Keil, A.; Patole, S. Probiotic Sepsis in Preterm Neonates—A Systematic Review. Eur. J. Pediatr. 2022, 181, 2249–2262. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, C.H.P.; Embleton, N.D.; Lapillonne, A.; Mihatsch, W.A.; Salvatore, S.; Canani, R.B.; Dinleyici, E.C.; Domellöf, M.; Guarino, A.; Gutiérrez-Castrellón, P.; et al. Reevaluating the FDA’s Warning against the Use of Probiotics in Preterm Neonates: A Societal Statement by ESPGHAN and EFCNI. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Esvaran, M.; Chen, L.; Kok, C.; Keil, A.D.; Gollow, I.; Simmer, K.; Wemheuer, B.; Conway, P.; Patole, S. Probiotic Supplementation for Neonates with Congenital Gastrointestinal Surgical Conditions: Guidelines for Future Research. Pediatr. Res. 2023, 93, 49–55. [Google Scholar] [CrossRef]
- Liu, Z.S.; Chen, P.W. Featured Prebiotic Agent: The Roles and Mechanisms of Direct and Indirect Prebiotic Activities of Lactoferrin and Its Application in Disease Control. Nutrients 2023, 15, 2759. [Google Scholar] [CrossRef]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Gabrielli, O. Prebiotics in Human Milk: A Review. Dig. Liver Dis. 2006, 38, S291–S294. [Google Scholar] [CrossRef]
- Miqdady, M.; Al Mistarihi, J.; Azaz, A.; Rawat, D. Prebiotics in the Infant Microbiome: The Past, Present, and Future. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 1–14. [Google Scholar] [CrossRef]
- Ferro, L.E.; Crowley, L.N.; Bittinger, K.; Friedman, E.S.; Decker, J.E.; Russel, K.; Katz, S.; Kim, J.K.; Trabulsi, J.C. Effects of Prebiotics, Probiotics, and Synbiotics on the Infant Gut Microbiota and Other Health Outcomes: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5620–5642. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Henry, K.C.; Abrahamsson, T.R.; Wu, R.Y.; Sherman, P.M. Probiotics, Prebiotics, and Synbiotics for the Prevention of Necrotizing Enterocolitis. Adv. Nutr. 2016, 7, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Ross, R.P.; Ryan, C.A.; Dempsey, E.M.; Stanton, C. Probiotics, Prebiotics, and Synbiotics for the Prevention of Necrotizing Enterocolitis. Front. Nutr. 2021, 8, 667188. [Google Scholar] [CrossRef] [PubMed]
- Srinivasjois, R.; Rao, S.; Patole, S. Prebiotic Supplementation in Preterm Neonates: Updated Systematic Review and Meta-Analysis of Randomised Controlled Trials. Clin. Nutr. 2013, 32, 958–965. [Google Scholar] [CrossRef]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Al Olqi, N.; Al Uraimi, T. Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Mahboobipour, A.A.; Bitaraf, A.; Mohammadi, P.; Khosravifar, M.; Babaei, H.; Shahidolahi, A. Effects of Synbiotics on Necrotizing Enterocolitis and Full Enteral Feeding in Very Low Birth Weight Infants: A Double-Blind, Randomized Controlled Trial. Medicine 2024, 103, e39647. [Google Scholar] [CrossRef]
- Dilli, D.; Aydin, B.; Fettah, N.D.; Özyazici, E.; Beken, S.; Zenciroğlu, A.; Okumuş, N.; Özyurt, B.M.; Ipek, M.Ş.; Akdağ, A.; et al. The Propre-Save Study: Effects of Probiotics and Prebiotics Alone or Combined on Necrotizing Enterocolitis in Very Low Birth Weight Infants. J. Pediatr. 2015, 166, 545–551.e1. [Google Scholar] [CrossRef]
- Morniroli, D.; Vizzari, G.; Consales, A.; Mosca, F.; Giannì, M.L. Postbiotic Supplementation for Children and Newborn’s Health. Nutrients 2021, 13, 781. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Ansari, A.; Bose, S.; You, Y.; Park, S.; Kim, Y. Molecular Mechanism of Microbiota Metabolites in Preterm Birth: Pathological and Therapeutic Insights. Int. J. Mol. Sci. 2021, 22, 8145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, J.; Wang, N.; Li, Y.; Sun, X.; Zhang, Y.; Zhang, H. Lactobacillus Casei Zhang Modulate Cytokine and Toll-like Receptor Expression and Beneficially Regulate Poly I:C-Induced Immune Responses in RAW264.7 Macrophages. Microbiol. Immunol. 2013, 57, 54–62. [Google Scholar] [CrossRef]
- Szajewska, H.; Salminen, S. Evidence on Postbiotics in Infants and Children. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Kołodziej, M.; Skórka, A.; Pieścik-Lech, M. Infant Formulas with Postbiotics: An Updated Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 823–829. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Zhao, Z.; Ding, R.; Sun, J.; Chi, C. Safety and Efficacy of Adding Postbiotics in Infant Formula: A Systematic Review and Meta-Analysis. Pediatr. Res. 2024, 95, 43–51. [Google Scholar] [CrossRef]
- De Bernardo, G.; D’Urso, G.; Spadarella, S.; Giordano, M.; Leone, G.; Casapullo, A. Analysis of the Fecal Metabolomic Profile in Breast vs. Different Formula Milk Feeding in Late Preterm Infants. Metabolites 2024, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Giannì, M.L.; Morniroli, D.; Mosca, F.; Rescigno, M. Can Postbiotics Represent a New Strategy for NEC? Adv. Exp. Med. Biol. 2024, 1449, 43–57. [Google Scholar] [CrossRef]
- Rascón-Cruz, Q.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Nakamura-Bencomo, S.I.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory Effects of Lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Farnaud, S.; Evans, R.W. Lactoferrin—A Multifunctional Protein with Antimicrobial Properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin\-a Natural Immunity Molecule. Molecules 2011, 16, 6992–7012. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Mostert, M.; Stronati, M. Lactoferrin for Prevention of Neonatal Infections. Curr. Opin. Infect. Dis. 2011, 24, 177–182. [Google Scholar] [CrossRef]
- Manzoni, P.; Decembrino, L.; Stolfi, I.; Pugni, L.; Rinaldi, M.; Cattani, S.; Romeo, M.G.; Messner, H.; Laforgia, N.; Vagnarelli, F.; et al. Lactoferrin and Prevention of Late-Onset Sepsis in the Pre-Term Neonates. Early Hum. Dev. 2010, 86, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Lista, G.; Gallo, E.; Marangione, P.; Priolo, C.; Fontana, P.; Guardione, R.; Farina, D. Routine Lactobacillus Rhamnosus GG Administration in VLBW Infants: A Retrospective, 6-Year Cohort Study. Early Hum. Dev. 2011, 87, S35–S38. [Google Scholar] [CrossRef]
- Razak, A.; Hussain, A. Lactoferrin Supplementation to Prevent Late-Onset Sepsis in Preterm Infants: A Meta-Analysis. Am. J. Perinatol. 2021, 38, 283–290. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral Lactoferrin Supplementation for Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants. Cochrane Database Syst. Rev. 2017, 6, CD007137. [Google Scholar] [CrossRef]
- Pammi, M.; Gautham, K.S. Enteral Lactoferrin Supplementation for Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants. Cochrane Database Syst. Rev. 2020, 3, CD007137. [Google Scholar] [CrossRef]
- Griffiths, J.; Jenkins, P.; Vargova, M.; Bowler, U.; Juszczak, E.; King, A.; Linsell, L.; Murray, D.; Partlett, C.; Patel, M.; et al. Enteral Lactoferrin to Prevent Infection for Very Preterm Infants: The ELFIN RCT. Health Technol. Assess. 2018, 22, I–60. [Google Scholar] [CrossRef]
- Tarnow-Mordi, W.O.; Abdel-Latif, M.E.; Martin, A.; Pammi, M.; Robledo, K.; Manzoni, P.; Osborn, D.; Lui, K.; Keech, A.; Hague, W.; et al. The Effect of Lactoferrin Supplementation on Death or Major Morbidity in Very Low Birthweight Infants (LIFT): A Multicentre, Double-Blind, Randomised Controlled Trial. Lancet Child Adolesc. Health 2020, 4, 444–454. [Google Scholar] [CrossRef]
- Ochoa, T. Is Lactoferrin Still a Treatment Option to Reduce Neonatal Sepsis? Lancet Child Adolesc. 2020, 4, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Militello, M.A.; Rizzollo, S.; Tavella, E.; Messina, A.; Pieretto, M.; Boano, E.; Carlino, M.; Tognato, E.; Spola, R.; et al. Is Lactoferrin More Effective in Reducing Late-Onset Sepsis in Preterm Neonates Fed Formula Than in Those Receiving Mother’s Own Milk? Secondary Analyses of Two Multicenter Randomized Controlled Trials. Am. J. Perinatol. 2019, 36, S120–S125. [Google Scholar] [CrossRef]
- D’Amico, F.; Decembrino, N.; Muratore, E.; Turroni, S.; Muggeo, P.; Mura, R.; Perruccio, K.; Vitale, V.; Zecca, M.; Prete, A.; et al. Oral Lactoferrin Supplementation during Induction Chemotherapy Promotes Gut Microbiome Eubiosis in Pediatric Patients with Hematologic Malignancies. Pharmaceutics 2022, 14, 1705. [Google Scholar] [CrossRef]
- Del Chierico, F.; Vernocchi, P.; Bonizzi, L.; Carsetti, R.; Castellazzi, A.M.; Dallapiccola, B.; de Vos, W.; Guerzoni, M.E.; Manco, M.; Marseglia, G.L.; et al. Early-Life Gut Microbiota under Physiological and Pathological Conditions: The Central Role of Combined Meta-Omics-Based Approaches. J. Proteom. 2012, 75, 4580–4587. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Vernocchi, P.; Petrucca, A.; Paci, P.; Fuentes, S.; Praticò, G.; Capuani, G.; Masotti, A.; Reddel, S.; Russo, A.; et al. Phylogenetic and Metabolic Tracking of Gut Microbiota during Perinatal Development. PLoS ONE 2015, 10, e0137347. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Xu, C.; Chen, J.; Ma, X.; Shi, L.; Shi, W.; Du, L.; Ni, Y. Alteration of the Gut Microbiota after Surgery in Preterm Infants with Necrotizing Enterocolitis. Front Pediatr. 2023, 11, 993759. [Google Scholar] [CrossRef]
- Stewart, C.J.; Fatemizadeh, R.; Parsons, P.; Lamb, C.A.; Shady, D.A.; Petrosino, J.F.; Hair, A.B. Using Formalin Fixed Paraffin Embedded Tissue to Characterize the Preterm Gut Microbiota in Necrotising Enterocolitis and Spontaneous Isolated Perforation Using Marginal and Diseased Tissue. BMC Microbiol. 2019, 19, 52. [Google Scholar] [CrossRef]
- Romano-Keeler, J.; Shilts, M.H.; Tovchigrechko, A.; Wang, C.; Brucker, R.M.; Moore, D.J.; Fonnesbeck, C.; Meng, S.; Correa, H.; Lovvorn, H.N.; et al. Distinct Mucosal Microbial Communities in Infants with Surgical Necrotizing Enterocolitis Correlate with Age and Antibiotic Exposure. PLoS ONE 2018, 13, e0206366. [Google Scholar] [CrossRef]
- Toto, F.; Marangelo, C.; Scanu, M.; De Angelis, P.; Isoldi, S.; Abreu, M.T.; Cucchiara, S.; Stronati, L.; Del Chierico, F.; Putignani, L. A Novel Microbial Dysbiosis Index and Intestinal Microbiota-Associated Markers as Tools of Precision Medicine in Inflammatory Bowel Disease Paediatric Patients. Int. J. Mol. Sci. 2024, 25, 9618. [Google Scholar] [CrossRef]
| Key Points or Limitations | Details |
|---|---|
| Sample size | Many studies have a limited number of participants, which can reduce the statistical power and the generalizability of the results. The sample size should be calculated based on the result we aim to demonstrate, with a larger sample if we want to demonstrate a clinical benefit (e.g., reduction in mortality, NEC, LOS, time to reach full enteral feeding). Only large-scale studies can determine the benefits and risks of probiotic administration in these patients. |
| Heterogeneity of protocols | Differences in the probiotic strains used, therapy combinations, and definitions of primary outcomes can complicate comparisons between studies. |
| Randomization | Using “treatment allocation by minimization” or “rank minimization” could balance confounders such as mode of delivery and severity of the surgical condition. |
| Product safety and Quality | Probiotics available as dietary supplements may not meet the stringent safety, efficacy, and manufacturing standards required for pharmaceuticals. |
| Risk of infection | There is a potential (although very low) risk of sepsis associated with probiotic administration, especially in highly vulnerable neonates. |
| Challenges in studying gut microbiota | In neonates undergoing major surgery, surgical stress, inflammation, intravenous nutrition, and antibiotics can affect the gut microbiota, making it difficult to assess the effectiveness of probiotic administration. Stool samples should be collected and adequately stored for microbiota analysis and SCFA level measurements. |
| From bench to bedside | While probiotics may increase the proportion of “good” bacteria in the gut, it is unclear whether this translates into significant clinical benefits. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decembrino, N.; Scuderi, M.G.; Betta, P.M.; Leonardi, R.; Bartolone, A.; Marsiglia, R.; Marangelo, C.; Pane, S.; De Rose, D.U.; Salvatori, G.; et al. Microbiota-Modulating Strategies in Neonates Undergoing Surgery for Congenital Gastrointestinal Conditions: A Narrative Review. Nutrients 2025, 17, 2234. https://doi.org/10.3390/nu17132234
Decembrino N, Scuderi MG, Betta PM, Leonardi R, Bartolone A, Marsiglia R, Marangelo C, Pane S, De Rose DU, Salvatori G, et al. Microbiota-Modulating Strategies in Neonates Undergoing Surgery for Congenital Gastrointestinal Conditions: A Narrative Review. Nutrients. 2025; 17(13):2234. https://doi.org/10.3390/nu17132234
Chicago/Turabian StyleDecembrino, Nunzia, Maria Grazia Scuderi, Pasqua Maria Betta, Roberta Leonardi, Agnese Bartolone, Riccardo Marsiglia, Chiara Marangelo, Stefania Pane, Domenico Umberto De Rose, Guglielmo Salvatori, and et al. 2025. "Microbiota-Modulating Strategies in Neonates Undergoing Surgery for Congenital Gastrointestinal Conditions: A Narrative Review" Nutrients 17, no. 13: 2234. https://doi.org/10.3390/nu17132234
APA StyleDecembrino, N., Scuderi, M. G., Betta, P. M., Leonardi, R., Bartolone, A., Marsiglia, R., Marangelo, C., Pane, S., De Rose, D. U., Salvatori, G., Grosso, G., Di Domenico, F. M., Dotta, A., Putignani, L., Capolupo, I., & Di Benedetto, V. (2025). Microbiota-Modulating Strategies in Neonates Undergoing Surgery for Congenital Gastrointestinal Conditions: A Narrative Review. Nutrients, 17(13), 2234. https://doi.org/10.3390/nu17132234









