Assessment of the Gut Microbiome in Patients with Coexisting Irritable Bowel Syndrome and Chronic Fatigue Syndrome
Abstract
1. Introduction
1.1. Chronic Fatigue Syndrome: Historical Perspective and Diagnostic Evolution
1.2. Gut Microbiome Alterations in Chronic Fatigue Syndrome
1.3. Tryptophan Metabolism and the Microbiota–Gut–Brain Axis
1.4. Study Rationale and Objectives
2. Materials and Methods
2.1. Participants and Study Design
- Group I (n = 40): patients with IBS-U and no chronic fatigue,
- Group II (n = 40): patients with IBS-U and coexisting chronic fatigue syndrome (IBS-CFS), meeting established diagnostic criteria.
2.2. Fatigue Assessment
2.3. Laboratory Tests
2.4. Breathing Test
2.5. Dysbiosis Test
2.6. Nutritional Recommendations
2.7. Ethical Issues
2.8. Data Analysis
3. Results
3.1. Participant Demographic Assessment
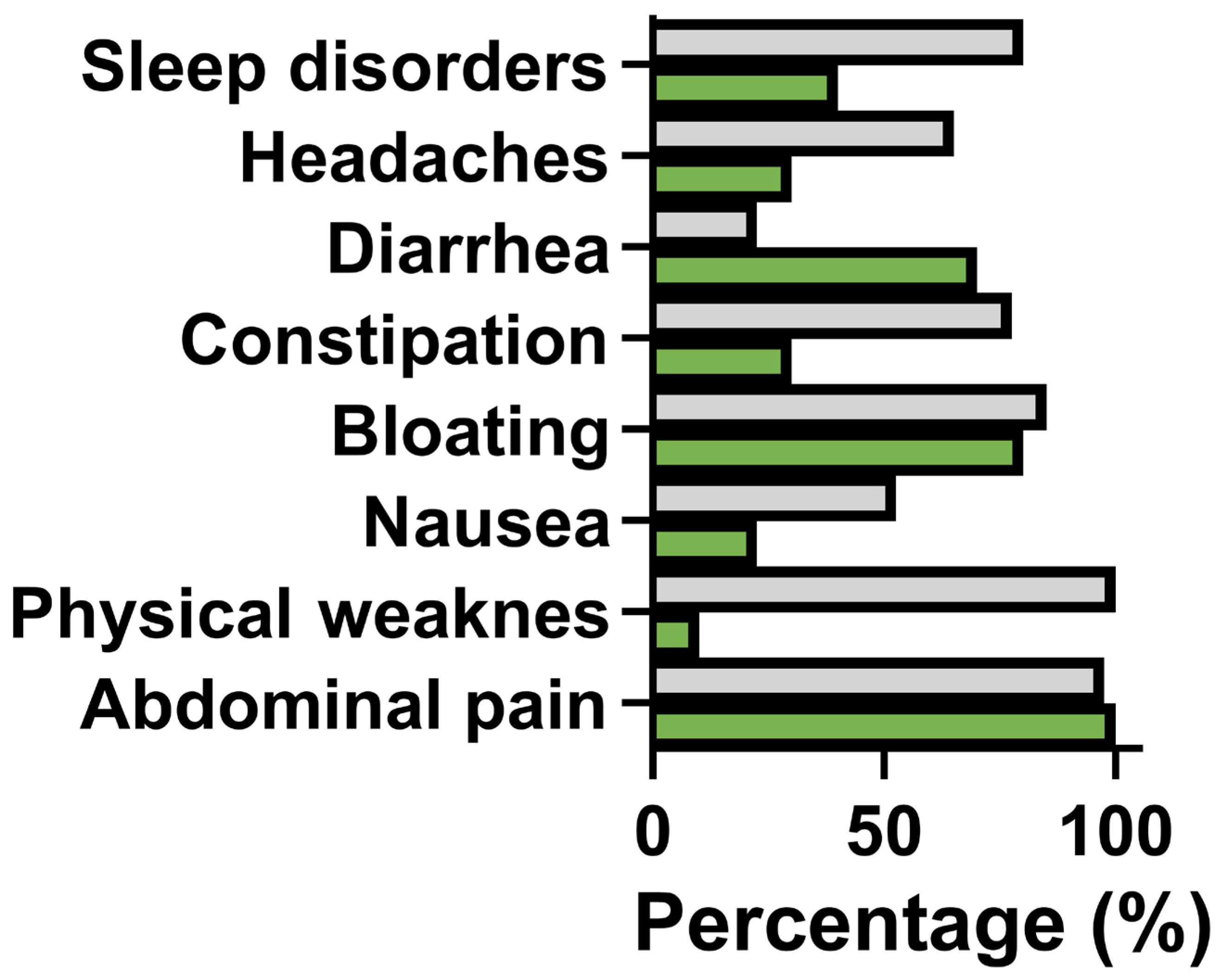
3.2. Assessment of the Most Common Ailments
3.3. Comparison of the Laboratory Data
3.4. Comparison of Breath Hydrogen-Methane Test Results
3.5. Gut Microbiome Profile Composition
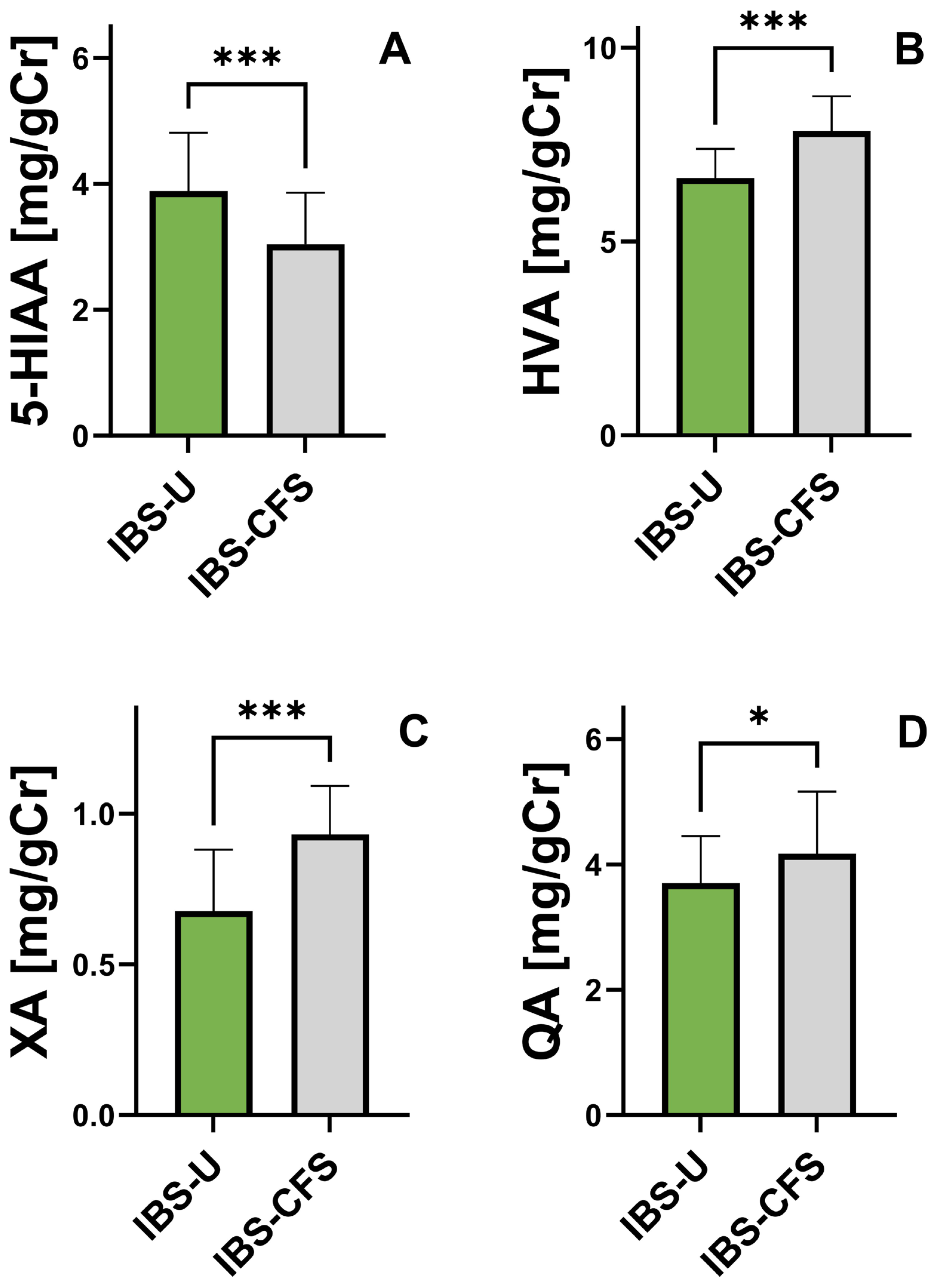
3.6. Comparison of Urinary Phenylalanine and Tryptophan Metabolite Concentrations
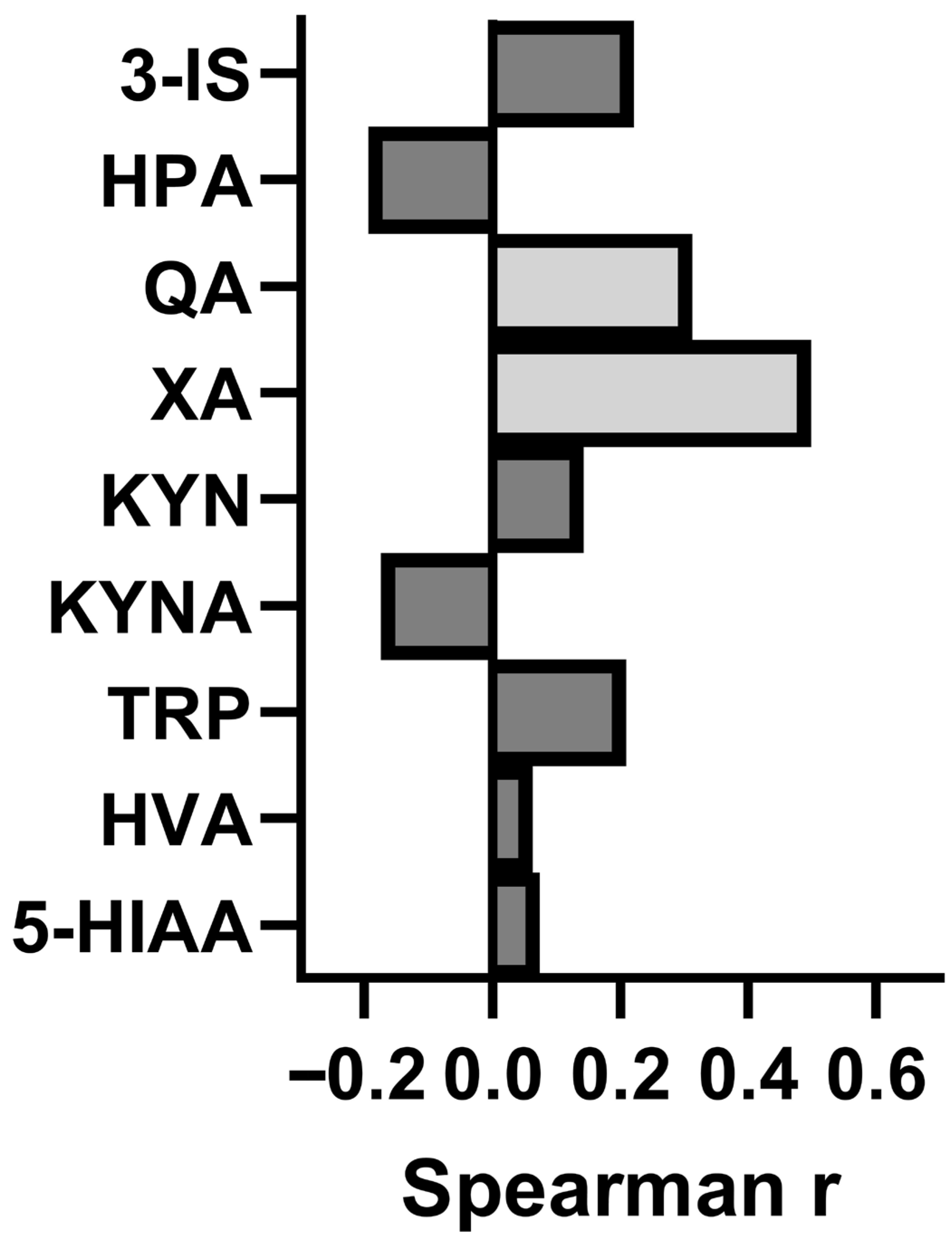
3.7. Assessment of the Relationship Between Concentration of the Studied Metabolites and Intensity of CFS
3.8. Post-Hoc Power Analysis
4. Discussion
4.1. Microbiome Composition in IBS-CFS: Comparison with Literature
4.2. Breath Test Findings and Bacterial Overgrowth Patterns
4.3. Tryptophan Metabolism Dysregulation and the Microbiota–Gut–Brain Axis
4.4. Interpretation Caveats: Biomarker Associations vs. Mechanistic Significance
4.5. Study Limitations and Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brurberg, K.G.; Fønhus, M.S.; Larun, L.; Flottorp, S.; Malterud, K. Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): A systematic review. BMJ Open 2014, 4, e003973. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Maes, M.; Berk, M.; Puri, B.K. Myalgic encephalomyelitis or chronic fatigue syndrome: How could the illness develop. Metabol. Brain Dis. 2019, 34, 385–415. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.P. Chronic Fatigue Syndrome: A Working Case Definition. Ann. Intern. Med. 1988, 108, 387. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations and Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Washington, DC, USA, 2015. Available online: http://www.ncbi.nlm.nih.gov/books/NBK274235 (accessed on 10 February 2015).
- Tate, W.; Walker, M.; Sweetan, E.; Heliweli, A.; Peppercorn, K.; Edgar, C.; Blair, A.; Chatterjee, A. Molecular Mechanism of Neuroinflommaton in ME/CFS snd Long COVID to Sustain Disease and Promote Relapses. Front. Neurol. 2022, 13, 877772. [Google Scholar] [CrossRef]
- Nagy-Szakal, D.; Williams, B.L.; Mishra, N.; Che, X.; Lee, B.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; Levine, S.; Montoya, J.G.; et al. Fecal Metagenomic Profiles in Subgroups of Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Microbiome 2017, 5, 44. [Google Scholar] [CrossRef]
- Fremont, M.; Coomans, D.; Massart, S.; De Meirleir, K. High-Throughput 16S rRNA Gene Sequencing Reveals Alterations of Intestinal Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Anaerobe 2013, 22, 50–56. [Google Scholar] [CrossRef]
- Armstrong, C.W.; McGregor, N.; Lewis, D.P.; Butt, H.L.; Gooley, P.R. The Association of Fecal Microbiota and Fecal, Blood Serum and Urine Metabolites in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Metabolomics 2017, 13, 8. [Google Scholar] [CrossRef]
- Navaneetharaja, N.; Griffiths, V.; Wileman, T.; Carding, S. A Role for the Intestinal Microbiota and Virome in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)? J. Clin. Med. 2016, 5, 55. [Google Scholar] [CrossRef]
- Maes, M.; Mihaylova, I.; Leunis, J.-C. Increased Serum IgA and IgM Against LPS of Enterobacteria in Chronic Fatigue Syndrome (CFS): Indication for the Involvement of Gram-Negative Enterobacteria in the Etiology of CFS and for the Presence of an Increased Gut–Intestinal Permeability. J. Affect. Disord. 2007, 99, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Lupo, G.F.D.; Rocchetti, G.; Lucini, L.; Lorusso, L.; Manara, E.; Bertelli, M.; Puglisi, E.; Capelli, E. Potential Role of Microbiome in Chronic Fatigue Syndrome/Myalgic Encephalomyelits (CFS/ME). Sci. Rep. 2021, 11, 7043. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.R.; Giloteaux, L. The Gut Microbiome in Myalgic Encephalomyelitis. Biochemist 2017, 39, 10–13. [Google Scholar] [CrossRef]
- Maes, M.; Twisk, F.N.; Kubera, M.; Ringel, K.; Leunis, J.-C.; Geffard, M. Increased IgA Responses to the LPS of Commensal Bacteria Is Associated With Inflammation and Activation of Cell-Mediated Immunity in Chronic Fatigue Syndrome. J. Affect. Disord. 2012, 136, 909–917. [Google Scholar] [CrossRef]
- Barker, E.; Fujimura, S.F.; Fadem, M.B.; Landay, A.L.; Levy, J.A. Immunologic Abnormalities Associated With Chronic Fatigue Syndrome. Clin. Infect. Dis. 1994, 18, S136–S141. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A. Gut Inflammation in Chronic Fatigue Syndrome. Nutr. Metab. 2010, 7, 79. [Google Scholar] [CrossRef]
- Berstad, A.; Hauso, O.; Berstad, K.; Berstad, J.E.R. From IBS to ME—The Dysbiotic March Hypothesis. Med. Hypotheses 2020, 140, 109648. [Google Scholar] [CrossRef]
- El-Salhy, M. Intestinal bacteria associated with irritable bowel syndrome and chronić fatigue syndrome. Neurogastroenterol. Motil. 2023, 35, e14621. [Google Scholar] [CrossRef]
- Galland, L. The Gut Microbiome and the Brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef]
- Raij, T.; Raij, K. Association between fatigue, peripheral serotonin, and L-carnitine in hypothyroidism and in chronic fatigue syndrome. Front. Endocrinol. 2024, 15, 1358404. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Kashi, A.A.; Davis, R.W.; Phair, R.D. The IDO Metabolic Trap Hypothesis for the Etiology of ME/CFS. Diagnostics 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Laurans, L.; Venteclef, N.; Haddad, Y.; Chajadine, M.; Alzaid, F.; Metghalchi, S.; Sovran, B.; Denis, R.G.P.; Dairou, J.; Cardellini, M.; et al. Genetic Deficiency of Indoleamine 2,3-Dioxygenase Promotes Gut Microbiota-Mediated Metabolic Health. Nat. Med. 2018, 24, 1113–1120. [Google Scholar] [CrossRef]
- Liu, W.-l.; Lin, Y.-H.; Xiao, H.; Xing, S.; Chen, H.; Chi, P.-D.; Zhang, G.; Longnecker, R.M. Epstein-Barr Virus Infection Induces Indoleamine 2,3-Dioxygenase Expression in Human Monocyte-Derived Macrophages Through P38/Mitogen-Activated Protein Kinase and NF- B Pathways: Impairment in T Cell Functions. J. Virol. 2014, 88, 6660–6671. [Google Scholar] [CrossRef]
- Magro, F.; Vieira-Coelho, M.A.; Fraga, S.; Serrao, M.M.; Veloso, F.T.; Robeiro, T.; Soares da Silva, P. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel diseases. Dig. Dis. Sci. 2002, 47, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitterr modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bactria of Gut Microbiota Regulate Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gran, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impact of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Wang, J.-K.; Yao, S.-K. Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation. Evid.-Based Complement. Altern. Med. 2021, 2021, 5560310. [Google Scholar] [CrossRef]
- Napolitano, M.; Fasulo, E.; Ungaro, F.; Massimino, L.; Sinagra, E.; Danese, S.; Mandarino, F.V. Gut Dysbiosis in Irritable Bowel Syndrome: A Narrative Review on Correlation with Disease Subtypes and Novel Therapeutic Implications. Microorganisms 2023, 11, 2369. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ruan, G.; Chen, L.; Ying, S.; Li, G.; Xu, F.; Xiao, Z.; Tian, Y.; Lv, L.; Ping, Y.; et al. Naurotransmitter and Intestinal Interactions: Focus on the Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. Front. Endocrinol. 2021, 13, 817100. [Google Scholar] [CrossRef]
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef]
- Palsson, O.S.; Baggish, J.S.; Turner, M.J.; Whithead, W.E. Patients Show Frequent Fluctuations between Loose/Watery andHard/Lumpy Stools: Implications for treatment. Am. J. Gastroenterol. 2012, 107, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steiberg, A.D. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erthematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Saad, R.; Long, M.D.; Rao, S.S. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020, 115, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Casén, C.; Vebo, H.C.; Sekelja, M.; Hegge, F.T.; Karlson, M.K.; Ciemniejewska, E.; Dzankovic, S.; Frøyland, C.; Nestestog, R.; Engstrand, L.; et al. Deviations in human gut microbiota: A novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015, 42, 71–83. [Google Scholar] [CrossRef]
- Jackson, M.L.; Butt, H.; Ball, M.; Lewis, D.P.; Bruck, D. Sleep Quality and the Treatment of Intestinal Microbiota Imbalance in Chronic Fatigue Syndrome: A Pilot Study. Sleep Sci. 2015, 8, 124–133. [Google Scholar] [CrossRef]
- Shukla, S.K.; Cook, D.; Meyer, J.; Vernon, S.D.; Le, T.; Clevidence, D.; Robertson, C.E.; Schrodi, S.J.; Yale, S.; Frank, D.N.; et al. Changes in Gut and Plasma Microbiome Following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). PLoS ONE 2015, 10, e0145453. [Google Scholar] [CrossRef]
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal Microbiota, Probiotics and Mental Health: From Metchnikoff to Modern Advances: Part II—Contemporary Contextual Research. Gut Pathog. 2013, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Wallis, A.; Ball, M.; McKechnie, S.; Butt, H.; Lewis, D.P.; Bruck, D. Examining Clinical Similarities Between Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and D-Lactic Acidosis: A Systematic Review. J Transl. Med. 2017, 15, 129. [Google Scholar] [CrossRef]
- Russell, A.; Hepgul, N.; Nikkheslat, N.; Borsini, A.; Zajkowska, Z.; Moll, N.; Forton, D.; Agarwal, K.; Chalder, T.; Mondelli, V.; et al. Persistent Fatigue Induced by Interferon-Alpha: A Novel, InflammationBased, Proxy Model of Chronic Fatigue Syndrome. Psychoneuroendocrinology 2019, 100, 276–285. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.N.; Coe, S.; Izadi, H. A Retrospective Outcome Study of 42 Patients With Chronic Fatigue Syndrome, 30 of Whom had Irritable Bowel Syndrome. Half Were Treated With Oral Approaches, and Half Were Treated with Faecal Microbiome Transplantation. Hum. Microbiome J. 2019, 13, 100061. [Google Scholar] [CrossRef]
- Corbitt, M.; Campagnolo, N.; Staines, D.; Marshall-Gradisnik, S. A Systematic Review of Probiotic Interventions for Gastrointestinal Symptoms and Irritable Bowel Syndrome in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME). Probiotics Antimicrob. Proteins 2018, 10, 466–477. [Google Scholar] [CrossRef]
- Chojnacki, J.; Konrad, P.; Mędrek-Socha, M.; Kaczka, A.; Błońska, A.; Zajdel, R.; Chojnacki, C.; Gąsiorowska, A. The Variability of Tryptophan Metabolism in Patients With Mixed Type of Irritable Bowel Syndrome. Int. J. Mol. Sci. 2023, 24, 2650. [Google Scholar] [CrossRef]
- Sheedy, J.R.; Wettenhall, R.E.H.; Scanlon, D.; Gooley, P.R.; Lewis, D.P.; McGregor, N.; I Stapleton, D.; Butt, H.L.; De Meirleir, K.L. Increased D-Lactic Acid Intestinal Bacteria in Patients With Chronic Fatigue Syndrome. In Vivo 2009, 23, 621–628. [Google Scholar] [PubMed]
- Wallis, A.; Ball, M.; Butt, H.; Lewis, D.P.; McKechnie, S.; Paull, P.; Jaa-Kwee, A.; Bruck, D. Open-Label Pilot for Treatment Targeting Gut Dysbiosis in Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome: Neuropsychological Symptoms and Sex Comparisons. J. Transl. Med. 2018, 16, 24. [Google Scholar] [CrossRef]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced Diversity and Altered Composition of the Gut Microbiome in Individuals With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Microbiome 2016, 4, 30. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Blomstrand, E. Branched-chain amino acids and central fatigue. J. Nutr. 2006, 136, 274S–276S. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Fernstrom, M.H. Exercise, serum free tryptophan, and central fatigue. J Nutr. 2006, 136, 553S–559S. [Google Scholar] [CrossRef]
- Yamashita, M.; Yamamoto, T. Tryptophan and kynurenic acid may produce an amplified effect in central fatigue induced by chronic sleep disorder. Int. J. Tryptophan Res. 2014, 7, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Azechi, H.; Board, M. Essential role of excessive tryptophan and its neurometabolites in fatigue. Can. J. Neurol. Sci. 2012, 39, 40–47. [Google Scholar] [CrossRef]
- Yamashita, M.; Yamamoto, T. Tryptophan circuit in fatigue: From blood to brainand cognition. Brain Res. 2017, 1675, 116–126. [Google Scholar] [CrossRef]
- Åkesson, K.; Pettersson, S.; Ståhl, S.; Surowiec, I.M. Kynurenine pathway is altered in patients with SLE and associated with severe fatigue. Lupus Sci. Med. 2018, 5, e000254. [Google Scholar] [CrossRef]
- Németh, H.; Toldi, J.; Vécsei, L. Role of kynurenines in the central and peripheral nervous systems. Curr. Neurovasc. Res. 2005, 2, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Błońska, A.; Konrad, P.; Chojnacki, M.; Podogrocki, M.; Popławski, M. Changes in Tryptophan Metabolism on serotonin and Kynurenine Pathways in Patients with Irritable Bowel Syndrome. Int. J. Mol. Sci. 2023, 15, 1262. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Muñiz, P.U.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de la Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple target. Oxid. Med. Cell Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef]
- Carpenedo, R.; Pittaluga, A.; Cozzi, A.; Attuci, S.; Galli, A.; Moroni, F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur. J. Neurosci. 2001, 13, 2141–2147. [Google Scholar] [CrossRef]
- Wu, H.Q.; Pereira, E.F.; Bruno, J.P.; Pellicciari, R.; Albuquerque, E.X.; Schwarcz, R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J. Mol. Neurosci. 2010, 40, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lu, L.; Hua, Y.; Huang, K.; Wang, I.; Huang, L. Vasculopathy in the setting of cardiorenal syndrome: Roles of protein-bound uremic toxins. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1–H13. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.S.; Bralley, J.A. Clinical applications of urinary organic acids. Part 2. Dysbiosis markers. Altern. Med. Rev. 2008, 13, 292–306. [Google Scholar] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome—A Review on the Pathology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Jovanovic, F.; Jovanovic, V.; Knezevic, N.N. Glucocoricoid Hormones as Modulators of the Kynurenine Pathway in Chronic Pain Conditions. Cells 2023, 12, 1178. [Google Scholar] [CrossRef]
- Tomczyk, T.; Urbańska, E.M. Experimental hypothyroidism raises brain kynurenic acid—Novel aspect of thyroid dysfunction. Eur. J. Pharmacol. 2020, 883, 173363. [Google Scholar] [CrossRef]
- Meier, T.B.; Drevets, W.C.; Teague, T.K.; Wurfl, B.E.; Muller, S.C.; Bodurka, J.; Dantzer, R.; Savitz, J. Kynurenic acid is reduced in females and contraceptive users: Implications for depression. Brain Behav. Immun. 2018, 67, 59–64. [Google Scholar] [CrossRef]
- Bakker, L.; Choe, K.; Eussen, S.J.P.M.; Ramarkes, I.H.G.B.; van den Hove, D.L.A.; Kenis, G.; Rutten, B.P.F.; Verhey, F.R.J.; Köhler, S. Relation of the kynurenine pathway with normal age: A systemic review. Mech. Ageing Dev. 2024, 217, 111890. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Panahi, H.K.S.; Kavyani, B.; Heng, B.; Tan, V.; Braidy, N.; Guillemin, G.J. The Role of Kynurenine Pathway and NAD+ Metabolsm in Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome. Aging Dis. 2022, 13, 698–711. [Google Scholar] [CrossRef]
- Sullivan, Å.; Nord, C.E.; Evengård, B. Effect of Supplement With Lactic-Acid Producing Bacteria on Fatigue and Physical Activity in Patients With Chronic Fatigue Syndrome. Nutr. J. 2009, 8, 4. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Perna, S.; Giacosa, A.; Peroni, G.; Castellazzi, A.M. Using Probiotics in Clinical Practice: Where Are We Now? A Review of Existing Meta-Analyses. Gut Microbes 2017, 8, 521–543. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. Gut Feelings: The Emerging Biology of Gut–Brain Communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yu, Z.; Liu, Y.; Li, C.; Hu, H.; Hu, J.-C.; Liu, M.; Yang, Q.; Gu, P.; Li, J.; et al. Gut-X axis. Imeta 2025, 4, e270. [Google Scholar] [CrossRef] [PubMed]
| Feature | Group I (n = 40) | IBS-CFS (n = 40) | p-Value |
|---|---|---|---|
| Hb (g/dL) | 38.1 ± 6.6 | 38.3 ± 7.1 | 0.869 |
| Fe (µg/dL) | 93.2 ± 0.9 | 23.0 ± 1.1 | 0.067 |
| GFR (mL/min) | 98.1 ± 14.9 | 88.6 ± 18.5 | 0.061 |
| ALT (µ/L) | 15.6 ± 3.5 | 14.9 ± 3.8 | 0.059 |
| AST (µ/L) | 13.9 ± 2.1 | 14.3 ± 1.8 | 0.092 |
| CRP (mg/L) | 2.3 ± 1.8 | 3.1 ± 2.3 | 0.188 |
| FC (µg/g) | 26.6 ± 15.7 | 30.9 ± 12.8 | 0.509 |
| TSH (µIU/mL) | 2.16 ± 0.6 | 3.21 ± 0.89 | 0.863 |
| Vit. D3 (ng/mL) | 40.8 ± 3.9 | 38.6 ± 4.7 | 0.912 |
| Vit. B12 (pg/L) | 389.0 ± 102.3 | 441 ± 121.2 | 0.822 |
| HbA1c (%) | 5.7 ± 0.4 | 5.2 ± 0.6 | 0.752 |
| Ions (Time, min) | Group I (ppm) | Group II (ppm) | p-Value |
|---|---|---|---|
| Hydrogen (0) | 10.9 ± 4.72 | 9.63 ± 5.31 | 0.064 |
| Hydrogen (90) | 47.2 ± 12.4 | 31.8 ± 6.76 * | 0.049 |
| Hydrogen (180) | 79.4 ± 18.2 | 69.1 ± 16.3 | 0.052 |
| Methane (0) | 3.73 ± 1.62 | 5.12 ± 2.63 | 0.058 |
| Methane (90) | 8.56 ± 1.19 | 13.2 ± 3.25 * | 0.036 |
| Methane (180) | 11.8 ± 2.03 | 16.4 ± 2.35 ** | 0.009 |
| Dysbiosis Indicators | Group I | Group II | p-Value |
|---|---|---|---|
| DI (points) | 3.69 ± 1.04 | 3.72 ± 1.28 | 0.746 |
| SDI (points) | 2.07 ± 0.59 | 2.23 ± 0.51 ** | 0.003 |
| Bacteria Species | Increase (%), Group I/II | p-Value | Decrease (%) Group I/II | p-Value |
|---|---|---|---|---|
| Bifidobacterium spp. | 42.5/30.0 | 0.352 | 22.5/30.0 | 0.611 |
| Bacteroidetes spp. | 40.0/15.0 | 0.024 * | 15.0/25.0 | 0.402 |
| Lactobacillus spp. | 25.0/10.0 | 0.141 | 17.5/35.0 | 0.127 |
| Streptococcus spp. | 10.0/10.0 | 0.709 | 10.0/15.0 | 0.735 |
| Faecalibacterium prausnitzii | 10.0/25.0 | 0.141 | 10.0/25.0 | 0.141 |
| Akkermansia muciniphila | 5.0/15.0 | 0.264 | 5.0/15.0 | 0.264 |
| Clostridium spp. | 15.0/10.0 | 0.735 | 10.0/22.5 | 0.365 |
| Escherichia spp. | 10.0/15.0 | 0.735 | 15.0/15.0 | 0.747 |
| Prevotella spp. | 20.0/22.5 | 0.990 | 10.0/15.0 | 0.735 |
| Ruminococcus v. | 10.0/15.0 | 0.735 | 27.5/25.0 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojnacki, M.; Błońska, A.; Kaczka, A.; Chojnacki, J.; Walecka-Kapica, E.; Romanowska, N.; Przybylowska-Sygut, K.; Popławski, T. Assessment of the Gut Microbiome in Patients with Coexisting Irritable Bowel Syndrome and Chronic Fatigue Syndrome. Nutrients 2025, 17, 2232. https://doi.org/10.3390/nu17132232
Chojnacki M, Błońska A, Kaczka A, Chojnacki J, Walecka-Kapica E, Romanowska N, Przybylowska-Sygut K, Popławski T. Assessment of the Gut Microbiome in Patients with Coexisting Irritable Bowel Syndrome and Chronic Fatigue Syndrome. Nutrients. 2025; 17(13):2232. https://doi.org/10.3390/nu17132232
Chicago/Turabian StyleChojnacki, Marcin, Aleksandra Błońska, Aleksandra Kaczka, Jan Chojnacki, Ewa Walecka-Kapica, Natalia Romanowska, Karolina Przybylowska-Sygut, and Tomasz Popławski. 2025. "Assessment of the Gut Microbiome in Patients with Coexisting Irritable Bowel Syndrome and Chronic Fatigue Syndrome" Nutrients 17, no. 13: 2232. https://doi.org/10.3390/nu17132232
APA StyleChojnacki, M., Błońska, A., Kaczka, A., Chojnacki, J., Walecka-Kapica, E., Romanowska, N., Przybylowska-Sygut, K., & Popławski, T. (2025). Assessment of the Gut Microbiome in Patients with Coexisting Irritable Bowel Syndrome and Chronic Fatigue Syndrome. Nutrients, 17(13), 2232. https://doi.org/10.3390/nu17132232





