Reproductive Health and Assisted Conception in Celiac Disease and Non-Celiac Gluten Sensitivity: A Narrative Review
Abstract
1. Introduction
2. Methodology
2.1. Literature Search Strategy
2.2. Study Selection and Data Synthesis
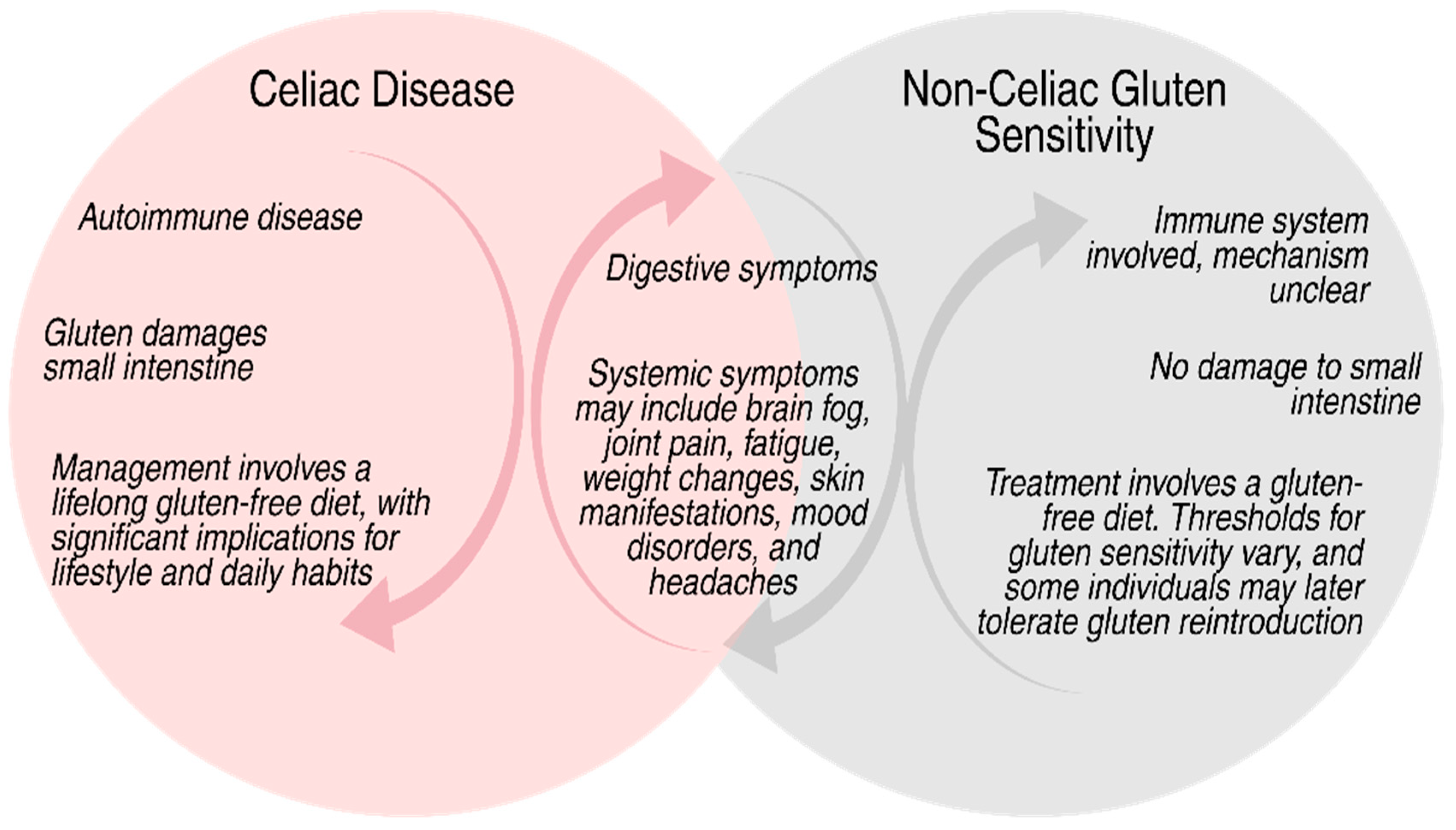
3. Gluten-Related Disorders and Their Systematic Effects
4. Impact of Gluten-Related Disorders on Female Reproductive Health
5. Impact of Gluten-Related Disorders on Male Reproductive Health
6. Gluten-Related Disorders and Assisted Conception Outcomes
7. Biological Mechanisms in Gluten-Related Reproductive Dysfunction
7.1. Immune Activation and Autoimmunity in Affected Individuals
7.2. Chronic Inflammation and Cytokine Dysregulation in Gluten-Related Conditions
7.3. Increased Intestinal Permeability “Leaky Gut” in Gluten-Sensitive Patients
7.4. Nutrient Malabsorption in Individuals with Gluten-Related Disorders
7.5. Hormonal Imbalances Related to Gluten-Related Conditions
7.6. Oxidative Stress in Gluten-Affected Individuals
7.7. Emerging Mechanisms: Epigenetics and Microbiota in Gluten-Related
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feuer, S.K.; Camarano, L.; Rinaudo, P.F. ART and health: Clinical outcomes and insights on molecular mechanisms from rodent studies. Mol. Hum. Reprod. 2013, 19, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Evans, K.E.; Hopper, A.D.; Smillie, D.M.; Sanders, D.S. A prospective study into the aetiology of lymphocytic duodenosis. Aliment. Pharmacol. Ther. 2010, 32, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Santolaria, S.; Sanders, D.S.; Fernandez-Banares, F. Systematic review: Noncoeliac gluten sensitivity. Aliment. Pharmacol. Ther. 2015, 41, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Gujral, N.; Freeman, H.J.; Thomson, A.B. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef]
- Lerner, A.; Benzvi, C.; Vojdani, A. Gluten is a Proinflammatory Inducer of Autoimmunity. J. Transl. Gastroenterol. 2024, 2, 109–124. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Iven, J. Non-coeliac gluten sensitivity: Piecing the puzzle together. United Eur. Gastroenterol. J. 2015, 3, 160–165. [Google Scholar] [CrossRef]
- Ferrari, R. Writing narrative style literature reviews. Med. Writ. 2015, 24, 230–235. [Google Scholar] [CrossRef]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef]
- Vats, V.; Makineni, P.; Hemaida, S.; Haider, A.; Subramani, S.; Kaur, N.; Butt, A.N.; Scott-Emuakpor, R.; Zahir, M.; Mathew, M.; et al. Gluten Intolerance and Its Association With Skin Disorders: A Narrative Review. Cureus 2023, 15, e44549. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef]
- Shukla, S.; Shrivastava, D. Nutritional Deficiencies and Subfertility: A Comprehensive Review of Current Evidence. Cureus 2024, 16, e66477. [Google Scholar] [CrossRef] [PubMed]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Obel, C.; Hammer Bech, B.; Olsen, J.; Basso, O. Infertility, infertility treatment, and fetal growth restriction. Obstet. Gynecol. 2007, 110, 1326–1334. [Google Scholar] [CrossRef]
- Ghunaim, M.; Seedi, A.; Alnuman, D.; Aljohani, S.; Aljuhani, N.; Almourai, M.; Alsuhaymi, S. Impact of a Gluten-Free Diet in Adults With Celiac Disease: Nutritional Deficiencies and Challenges. Cureus 2024, 16, e74983. [Google Scholar] [CrossRef] [PubMed]
- Alecsandru, D.; Lopez-Palacios, N.; Castano, M.; Aparicio, P.; Garcia-Velasco, J.A.; Nunez, C. Exploring undiagnosed celiac disease in women with recurrent reproductive failure: The gluten-free diet could improve reproductive outcomes. Am. J. Reprod. Immunol. 2020, 83, e13209. [Google Scholar] [CrossRef]
- Arvanitakis, K.; Siargkas, A.; Germanidis, G.; Dagklis, T.; Tsakiridis, I. Adverse pregnancy outcomes in women with celiac disease: A systematic review and meta-analysis. Ann. Gastroenterol. 2023, 36, 12–24. [Google Scholar] [CrossRef]
- Cardenas-Torres, F.I.; Cabrera-Chavez, F.; Figueroa-Salcido, O.G.; Ontiveros, N. Non-Celiac Gluten Sensitivity: An Update. Medicina 2021, 57, 526. [Google Scholar] [CrossRef]
- Herrera-Quintana, L.; Navajas-Porras, B.; Vazquez-Lorente, H.; Hinojosa-Nogueira, D.; Corrales-Borrego, F.J.; Lopez-Garzon, M.; Plaza-Diaz, J. Celiac Disease: Beyond Diet and Food Awareness. Foods 2025, 14, 377. [Google Scholar] [CrossRef]
- Glimberg, I.; Haggard, L.; Lebwohl, B.; Green, P.H.R.; Ludvigsson, J.F. The prevalence of celiac disease in women with infertility-A systematic review with meta-analysis. Reprod. Med. Biol. 2021, 20, 224–233. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Krawczyk, A.; Kretek, A.; Pluta, D.; Kowalczyk, K.; Czech, I.; Radosz, P.; Madej, P. Gluten-free diet—Remedy for infertility or dangerous trend? Ginekol. Pol. 2022, 93, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Peshevska-Sekulovska, M.; Gulinac, M.; Rangelov, R.; Docheva, D.; Velikova, T.; Sekulovski, M. Navigating the Challenges of Gluten Enteropathy and Infertility: The Role of Celiac-Related Antibodies and Dietary Changes. Antibodies 2023, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Mullerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Torroglosa, I.; Garcia-Velasco, J.A.; Alecsandru, D. The Impacts of Inflammatory and Autoimmune Conditions on the Endometrium and Reproductive Outcomes. J. Clin. Med. 2024, 13, 3724. [Google Scholar] [CrossRef]
- Saccone, G.; Berghella, V.; Sarno, L.; Maruotti, G.M.; Cetin, I.; Greco, L.; Khashan, A.S.; McCarthy, F.; Martinelli, D.; Fortunato, F.; et al. Celiac disease and obstetric complications: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2016, 214, 225–234. [Google Scholar] [CrossRef]
- Freeman, H.J. Reproductive changes associated with celiac disease. World J. Gastroenterol. 2010, 16, 5810–5814. [Google Scholar] [CrossRef]
- Wieser, H.; Ciacci, C.; Soldaini, C.; Gizzi, C.; Pellegrini, L.; Santonicola, A. Fertility in Celiac Disease: The Impact of Gluten on Male and Female Reproductive Health. Nutrients 2025, 17, 1575. [Google Scholar] [CrossRef]
- Singh, P.; Arora, S.; Lal, S.; Strand, T.A.; Makharia, G.K. Celiac Disease in Women With Infertility: A Meta-Analysis. J. Clin. Gastroenterol. 2016, 50, 33–39. [Google Scholar] [CrossRef]
- de la Fuente-Munoz, E.; Fernandez-Arquero, M.; Subbhi-Issa, N.; Guevara-Hoyer, K.; Suarez, L.P.; Laborda, R.G.; Sanchez, M.; Ochoa-Grullon, J.; Guzman-Fulgencio, M.; Villegas, A.; et al. Recurrent reproductive failure and celiac genetic susceptibility, a leading role of gluten. Front. Immunol. 2024, 15, 1451552. [Google Scholar] [CrossRef]
- Casella, G.; Orfanotti, G.; Giacomantonio, L.; Bella, C.D.; Crisafulli, V.; Villanacci, V.; Baldini, V.; Bassotti, G. Celiac disease and obstetrical-gynecological contribution. Gastroenterol. Hepatol. Bed Bench 2016, 9, 241–249. [Google Scholar]
- Prasad, S.; Singh, P.; Singh, A.; Mehtab, W.; Rajput, S.; Dang, S.; Chauhan, A.; Rajput, M.S.; Kachhawa, G.; Jagannath, S.; et al. Reproductive functions and pregnancy outcome in female patients with celiac disease. J. Gastroenterol. Hepatol. 2024, 39, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J. Androgen Physiology, Pharmacology, Use and Misuse. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Vanderhout, S.M.; Rastegar Panah, M.; Garcia-Bailo, B.; Grace-Farfaglia, P.; Samsel, K.; Dockray, J.; Jarvi, K.; El-Sohemy, A. Nutrition, genetic variation and male fertility. Transl. Androl. Urol. 2021, 10, 1410–1431. [Google Scholar] [CrossRef]
- Martinelli, S.; Nannini, G.; Cianchi, F.; Coratti, F.; Amedei, A. The Impact of Microbiota-Immunity-Hormone Interactions on Autoimmune Diseases and Infection. Biomedicines 2024, 12, 616. [Google Scholar] [CrossRef] [PubMed]
- Delcroix-Gomez, C.; Delcroix, M.H.; Jamee, A.; Gauthier, T.; Marquet, P.; Aubard, Y. Fetal growth restriction, low birth weight, and preterm birth: Effects of active or passive smoking evaluated by maternal expired CO at delivery, impacts of cessation at different trimesters. Tob. Induc. Dis. 2022, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Emokpae, M.A.; Brown, S.I. Effects of lifestyle factors on fertility: Practical recommendations for modification. Reprod. Fertil. 2021, 2, R13–R26. [Google Scholar] [CrossRef]
- Pieczynska, J. Do celiac disease and non-celiac gluten sensitivity have the same effects on reproductive disorders? Nutrition 2018, 48, 18–23. [Google Scholar] [CrossRef]
- Manza, F.; Lungaro, L.; Costanzini, A.; Caputo, F.; Carroccio, A.; Mansueto, P.; Seidita, A.; Raju, S.A.; Volta, U.; De Giorgio, R.; et al. Non-Celiac Gluten/Wheat Sensitivity-State of the Art: A Five-Year Narrative Review. Nutrients 2025, 17, 220. [Google Scholar] [CrossRef]
- Zugna, D.; Richiardi, L.; Akre, O.; Stephansson, O.; Ludvigsson, J.F. Celiac disease is not a risk factor for infertility in men. Fertil. Steril. 2011, 95, 1709–1713.e3. [Google Scholar] [CrossRef]
- Costa, S.T.B.; Sanmarful, I.S. Primary amenorrhoea as a manifestation of coeliac disease. BMJ Case Rep. 2021, 14, e239260. [Google Scholar] [CrossRef]
- Bianchi, P.I.; Aronico, N.; Santacroce, G.; Broglio, G.; Lenti, M.V.; Di Sabatino, A. Nutritional Consequences of Celiac Disease and Gluten-Free Diet. Gastroenterol. Insights 2024, 15, 878–894. [Google Scholar] [CrossRef]
- Andrade, D.L.; Viana, M.C.; Esteves, S.C. Differential Diagnosis of Azoospermia in Men with Infertility. J. Clin. Med. 2021, 10, 3144. [Google Scholar] [CrossRef]
- Lauret, E.; Rodrigo, L. Celiac disease and autoimmune-associated conditions. Biomed. Res. Int. 2013, 2013, 127589. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Botella, A.; Velasco, I.; Acien, M.; Saez-Espinosa, P.; Todoli-Torro, J.L.; Sanchez-Romero, R.; Gomez-Torres, M.J. Impact of Heavy Metals on Human Male Fertility-An Overview. Antioxidants 2021, 10, 1473. [Google Scholar] [CrossRef]
- Bold, J.; Rostami, K. Non-coeliac gluten sensitivity and reproductive disorders. Gastroenterol. Hepatol. Bed Bench 2015, 8, 294–297. [Google Scholar] [PubMed]
- Jain, M.; Singh, M. Assisted Reproductive Technology (ART) Techniques. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Boczar, A.; Dryja, P.; Jarmołowicz, J.; Stawicka, I.; Orzołek, I.; Solisch, S. A Comparison of the Effectiveness of Different Assisted Reproductive Techniques (ART) in Couples with Unexplained Infertility. J. Educ. Health Sport 2024, 71, 56073. [Google Scholar] [CrossRef]
- Sciorio, R.; Tramontano, L.; Greco, P.F.; Greco, E. Morphological assessment of oocyte quality during assisted reproductive technology cycle. JBRA Assist. Reprod. 2024, 28, 511–520. [Google Scholar] [CrossRef]
- Inversetti, A.; Zambella, E.; Guarano, A.; Dell’Avanzo, M.; Di Simone, N. Endometrial Microbiota and Immune Tolerance in Pregnancy. Int. J. Mol. Sci. 2023, 24, 2995. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Aguililla, S.; Farrais, S.; Lopez-Palacios, N.; Arau, B.; Senosiain, C.; Corzo, M.; Fernandez-Jimenez, N.; Ruiz-Carnicer, A.; Fernandez-Banares, F.; Gonzalez-Garcia, B.P.; et al. Diagnosis of celiac disease on a gluten-free diet: A multicenter prospective quasi-experimental clinical study. BMC Med. 2025, 23, 182. [Google Scholar] [CrossRef]
- Tersigni, C.; Castellani, R.; de Waure, C.; Fattorossi, A.; De Spirito, M.; Gasbarrini, A.; Scambia, G.; Di Simone, N. Celiac disease and reproductive disorders: Meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum. Reprod. Update 2014, 20, 582–593. [Google Scholar] [CrossRef]
- Patt, Y.S.; Lahat, A.; David, P.; Patt, C.; Eyade, R.; Sharif, K. Unraveling the Immunopathological Landscape of Celiac Disease: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 15482. [Google Scholar] [CrossRef]
- Zuvarox, T.; Belletieri, C. Malabsorption Syndromes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Dmytriv, T.R.; Storey, K.B.; Lushchak, V.I. Intestinal barrier permeability: The influence of gut microbiota, nutrition, and exercise. Front. Physiol. 2024, 15, 1380713. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, J.S.; Hernandez, L.; Shah, J.G.; Konikkara, J.; Naiyer, A.J.; Lee, A.R.; Ciaccio, E.; Minaya, M.T.; Green, P.H.; Bhagat, G. Serum cytokine elevations in celiac disease: Association with disease presentation. Hum. Immunol. 2010, 71, 50–57. [Google Scholar] [CrossRef]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of Inflammation on Male Reproductive Tract. J. Reprod. Infertil. 2015, 16, 123–129. [Google Scholar] [PubMed]
- Barbaro, M.R.; Cremon, C.; Morselli-Labate, A.M.; Di Sabatino, A.; Giuffrida, P.; Corazza, G.R.; Di Stefano, M.; Caio, G.; Latella, G.; Ciacci, C.; et al. Serum zonulin and its diagnostic performance in non-coeliac gluten sensitivity. Gut 2020, 69, 1966–1974. [Google Scholar] [CrossRef]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.A.; et al. Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef]
- Scarampi, M.; Mengoli, C.; Miceli, E.; Di Stefano, M. Vitamins and Celiac Disease: Beyond Vitamin D. Metabolites 2025, 15, 78. [Google Scholar] [CrossRef]
- Abu-Ouf, N.M.; Jan, M.M. The impact of maternal iron deficiency and iron deficiency anemia on child's health. Saudi Med. J. 2015, 36, 146–149. [Google Scholar] [CrossRef]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef]
- Freeman, H.J. Endocrine manifestations in celiac disease. World J. Gastroenterol. 2016, 22, 8472–8479. [Google Scholar] [CrossRef]
- Leonard, M.M.; Sapone, A.; Catassi, C.; Fasano, A. Celiac Disease and Nonceliac Gluten Sensitivity: A Review. JAMA 2017, 318, 647–656. [Google Scholar] [CrossRef]
- Potiris, A.; Moustakli, E.; Trismpioti, E.; Drakaki, E.; Mavrogianni, D.; Matsas, A.; Zikopoulos, A.; Sfakianakis, A.; Tsakiridis, I.; Dagklis, T.; et al. From Inflammation to Infertility: How Oxidative Stress and Infections Disrupt Male Reproductive Health. Metabolites 2025, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Al Theyab, A.; Almutairi, T.; Al-Suwaidi, A.M.; Bendriss, G.; McVeigh, C.; Chaari, A. Epigenetic Effects of Gut Metabolites: Exploring the Path of Dietary Prevention of Type 1 Diabetes. Front. Nutr. 2020, 7, 563605. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Stavros, S.; Katopodis, P.; Potiris, A.; Drakakis, P.; Dafopoulos, S.; Zachariou, A.; Dafopoulos, K.; Zikopoulos, K.; Zikopoulos, A. Gut Microbiome Dysbiosis and Its Impact on Reproductive Health: Mechanisms and Clinical Applications. Metabolites 2025, 15, 390. [Google Scholar] [CrossRef]
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.N.; Jia, Y.; Dong, P.J.; Liu, Z.Z.; He, D.D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Papakonstantinou, A.; Moustakli, E.; Potiris, A.; Zikopoulos, A.; Tsarna, E.; Christodoulaki, C.; Tsakiridis, I.; Dagklis, T.; Panagopoulos, P.; Drakakis, P.; et al. Behind-the-Scenes Actors in Fertility: A Comprehensive Review of the Female Reproductive Tract Microbiome and Its Clinical Relevance. Life 2025, 15, 916. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmed, S.H.; Choucair, F.; Chouliaras, S.; Awwad, J.; Terranegra, A. A disturbed communication between hypothalamic-pituitary-ovary axis and gut microbiota in female infertility: Is diet to blame? J. Transl. Med. 2025, 23, 92. [Google Scholar] [CrossRef]
- Pecora, F.; Persico, F.; Gismondi, P.; Fornaroli, F.; Iuliano, S.; de’Angelis, G.L.; Esposito, S. Gut Microbiota in Celiac Disease: Is There Any Role for Probiotics? Front. Immunol. 2020, 11, 957. [Google Scholar] [CrossRef]
- Infertility Workup for the Women’s Health Specialist: ACOG Committee Opinion Summary, Number 781. Obstet. Gynecol 2019, 133, 1294–1295. [CrossRef]
- Serin, Y.; Manini, C.; Amato, P.; Verma, A.K. The Impact of a Gluten-Free Diet on Pregnant Women with Celiac Disease: Do We Need a Guideline to Manage Their Health? Gastrointest. Disord. 2024, 6, 675–691. [Google Scholar] [CrossRef]
- Rodrigo, L. (Ed.) . Celiac Disease and Gluten-Free Diet; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Palanski, B.A.; Weng, N.; Zhang, L.; Hilmer, A.J.; Fall, L.A.; Swaminathan, K.; Jabri, B.; Sousa, C.; Fernandez-Becker, N.Q.; Khosla, C.; et al. An efficient urine peptidomics workflow identifies chemically defined dietary gluten peptides from patients with celiac disease. Nat. Commun. 2022, 13, 888. [Google Scholar] [CrossRef]
- Syage, J.A.; Kelly, C.P.; Dickason, M.A.; Ramirez, A.C.; Leon, F.; Dominguez, R.; Sealey-Voyksner, J.A. Determination of gluten consumption in celiac disease patients on a gluten-free diet. Am. J. Clin. Nutr. 2018, 107, 201–207. [Google Scholar] [CrossRef]
- Monachesi, C.; Verma, A.K.; Catassi, G.N.; Franceschini, E.; Gatti, S.; Gesuita, R.; Lionetti, E.; Catassi, C. Determination of Urinary Gluten Immunogenic Peptides to Assess Adherence to the Gluten-Free Diet: A Randomized, Double-Blind, Controlled Study. Clin. Transl. Gastroenterol. 2021, 12, e00411. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, A.M.; Zammarchi, I.; Valerii, M.C.; Spisni, E.; Saracino, I.M.; Lanzarotto, F.; Ricci, C. Gluten-Free Diet and Other Celiac Disease Therapies: Current Understanding and Emerging Strategies. Nutrients 2024, 16, 1006. [Google Scholar] [CrossRef] [PubMed]
- Juneau, C.R.; Franasiak, J.M.; Goodman, L.R.; Marin, D.; Scott, K.; Morin, S.J.; Neal, S.A.; Juneau, J.E.; Scott, R.T. Celiac disease is not more prevalent in patients undergoing in vitro fertilization and does not affect reproductive outcomes with or without treatment: A large prospective cohort study. Fertil. Steril. 2018, 110, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Arcieri, M.; Abrami, C.; Graziano, A.; Restaino, S.; Barbui, E.; Rizzante, E.; D’Ippolito, S.; Vizzielli, G.; Driul, L. The influence of celiac disease on fertility and pregnancy: An Italian survey. Arch. Gynecol. Obstet. 2024, 310, 2907–2914. [Google Scholar] [CrossRef]
- Discepolo, V.; Kelly, C.P.; Koning, F.; Schuppan, D. How Future Pharmacologic Therapies for Celiac Disease Will Complement the Gluten-Free Diet. Gastroenterology 2024, 167, 90–103. [Google Scholar] [CrossRef]
- Smolinska, S.; Jurkiewicz, K.; Majsiak, E. Recent Advances in Gluten-Related Disorders. Int. J. Mol. Sci. 2025, 26, 1749. [Google Scholar] [CrossRef]
- Stanciu, D.; Staykov, H.; Dragomanova, S.; Tancheva, L.; Pop, R.S.; Ielciu, I.; Crisan, G. Gluten Unraveled: Latest Insights on Terminology, Diagnosis, Pathophysiology, Dietary Strategies, and Intestinal Microbiota Modulations-A Decade in Review. Nutrients 2024, 16, 3636. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Aguililla, S.; Farrais, S.; Senosiain, C.; Lopez-Palacios, N.; Arau, B.; Ruiz-Carnicer, A.; Sanchez-Dominguez, R.; Corzo, M.; Casado, I.; Pujals, M.; et al. Elucidating Immune Cell Changes in Celiac Disease: Revealing New Insights from Spectral Flow Cytometry. Int. J. Mol. Sci. 2025, 26, 2877. [Google Scholar] [CrossRef] [PubMed]
- Popp, A.; Laurikka, P.; Czika, D.; Kurppa, K. The role of gluten challenge in the diagnosis of celiac disease: A review. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 691–700. [Google Scholar] [CrossRef]
- Di Simone, N.; De Spirito, M.; Di Nicuolo, F.; Tersigni, C.; Castellani, R.; Silano, M.; Maulucci, G.; Papi, M.; Marana, R.; Scambia, G.; et al. Potential new mechanisms of placental damage in celiac disease: Anti-transglutaminase antibodies impair human endometrial angiogenesis. Biol. Reprod. 2013, 89, 88. [Google Scholar] [CrossRef] [PubMed]
| Aspect | Female Reproductive Health | Male Reproductive Health |
|---|---|---|
| Prevalence of CD in Infertile Individuals | Higher prevalence in women with unexplained infertility (2–8%) [31] | Limited data; underexplored but possible underdiagnosis [22] |
| Menstrual and Hormonal Effects | Delayed menarche, amenorrhea, early menopause; disrupted ovarian hormone production [21] | Hypogonadism, low testosterone; elevated LH and FSH suggestive of testicular dysfunction [32] |
| Nutritional Deficiency Impact | Iron, folate, vitamin D, calcium—affects ovulation, endometrial receptivity, and fetal development | Zinc, selenium, folate, B12—impairs spermatogenesis and sperm motility [33] |
| Endometrial/Testicular Effects | Impaired endometrial receptivity; inflammation and possible autoantibody interference with implantation [16] | Potential autoimmune impact on testicular function; possible anti-sperm antibodies (hypothetical) [34] |
| Pregnancy/Fertility Outcomes | Increased risk of miscarriage, IUGR, low birth weight, preterm birth [35] | Decreased sperm count, motility, morphology; subfertility [33] |
| Response to GFD | Restoration of ovulation and menstrual regularity; improved ART outcomes and natural conception [36] | Improved sperm parameters and hormonal balance after GFD adherence [22] |
| Role of NCGS | Suspected contributor to reproductive symptoms in some women (e.g., infertility, endometriosis-like symptoms) [37] | Largely unknown; minimal data currently available [38] |
| Mechanism | Description | Effect on Reproductive Health |
|---|---|---|
| Immune Dysregulation and Inflammation [52] | Gluten causes immune activation and inflammation that can spread beyond the gut. | Affects oocyte quality, fertilization, implantation, and placenta development. |
| Nutrient Malabsorption [53] | Damage to the intestine reduces absorption of important vitamins and minerals like iron and folate. | Leads to hormone problems, poor oocyte quality, miscarriage, and birth defects. |
| Autoimmunity and Molecular Mimicry [5] | Autoantibodies from gluten reaction may attack reproductive tissues by mistake. | Can damage ovaries or placenta and cause fertility issues. |
| Increased Intestinal Permeability [54] | Gluten can make the gut leak, letting harmful substances enter the bloodstream. | Causes inflammation that may harm reproductive organs. |
| Gut Microbiota Imbalance [55] | Gluten and gluten-free diets change gut bacteria, affecting immune and hormone balance. | Can disrupt fertility through immune and hormonal changes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustakli, E.; Christopoulos, P.; Potiris, A.; Zikopoulos, A.; Drakaki, E.; Karampitsakos, T.; Anagnostaki, I.; Kathopoulis, N.; Katopodis, P.; Galani, A.; et al. Reproductive Health and Assisted Conception in Celiac Disease and Non-Celiac Gluten Sensitivity: A Narrative Review. Nutrients 2025, 17, 2215. https://doi.org/10.3390/nu17132215
Moustakli E, Christopoulos P, Potiris A, Zikopoulos A, Drakaki E, Karampitsakos T, Anagnostaki I, Kathopoulis N, Katopodis P, Galani A, et al. Reproductive Health and Assisted Conception in Celiac Disease and Non-Celiac Gluten Sensitivity: A Narrative Review. Nutrients. 2025; 17(13):2215. https://doi.org/10.3390/nu17132215
Chicago/Turabian StyleMoustakli, Efthalia, Panagiotis Christopoulos, Anastasios Potiris, Athanasios Zikopoulos, Eirini Drakaki, Theodoros Karampitsakos, Ismini Anagnostaki, Nikolaos Kathopoulis, Periklis Katopodis, Apostolia Galani, and et al. 2025. "Reproductive Health and Assisted Conception in Celiac Disease and Non-Celiac Gluten Sensitivity: A Narrative Review" Nutrients 17, no. 13: 2215. https://doi.org/10.3390/nu17132215
APA StyleMoustakli, E., Christopoulos, P., Potiris, A., Zikopoulos, A., Drakaki, E., Karampitsakos, T., Anagnostaki, I., Kathopoulis, N., Katopodis, P., Galani, A., Christodoulaki, C., Zachariou, A., Drakakis, P., & Stavros, S. (2025). Reproductive Health and Assisted Conception in Celiac Disease and Non-Celiac Gluten Sensitivity: A Narrative Review. Nutrients, 17(13), 2215. https://doi.org/10.3390/nu17132215









