The Role of Magnesium in Depression, Migraine, Alzheimer’s Disease, and Cognitive Health: A Comprehensive Review
Abstract
1. Introduction
2. Methods
- AND was used to link related concepts (e.g., magnesium AND depression AND neurotransmitters);
- OR connected synonyms (e.g., depression OR major depressive disorder);
- NOT was used to exclude irrelevant studies.
2.1. Application of the PICO Model
2.2. The Inclusion and Exclusion Criteria
2.3. The Data Extraction and Analysis Methods
- Study type (e.g., RCT, longitudinal study);
- Sample size and demographic characteristics;
- Magnesium dosage and administration route;
- Measurement methods and primary outcome variables.
3. The Role of Magnesium Supplementation in the Treatment of Depression
4. The Role of Magnesium in the Pathophysiology of Migraine
5. The Role of Magnesium in Dementia Prevention and Slowing the Progression of Alzheimer’s Disease
6. Epidemiological and Clinical Evidence
6.1. Serum Magnesium Levels and Dementia Risk
6.2. Magnesium Intake and Supplementation and Cognitive Outcomes
7. Prevention, Therapy, and Neurological Disorders: A Scientific Discussion on the Role of Magnesium
8. Recommendations and Future Directions
9. Limitations
10. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Danciu, C.; Antal, D.; Avram, S.; Buda, V.; Pavel, I.Z.; Minda, D.; Ardelean, F.; Nicolov, M.; Dehelean, C. Essential mineral elements: Macronutrients and micronutrients from herbs in human health. In Plant Sources Potential Health Benefits; Nova Science Publishers, Inc.: New York, NY, USA, 2019; p. 1. [Google Scholar]
- Kumar, A.; Mehan, S.; Tiwari, A.; Khan, Z.; Das Gupta, G.; Narula, A.S.; Samant, R. Magnesium (Mg2+): Essential mineral for neuronal health: From cellular biochemistry to cognitive health and behavior regulation. Curr. Pharm. Des. 2024, 30, 3074–3107. [Google Scholar] [CrossRef] [PubMed]
- Schutten, J.C.; Joris, P.J.; Minović, I.; Post, A.; van Beek, A.P.; de Borst, M.H.; Mensink, R.P.; Bakker, S.J.L. Long-term magnesium supplementation improves glucocorticoid metabolism: A post-hoc analysis of an intervention trial. Clin. Endocrinol. 2021, 94, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.A.; Panonnummal, R. A mini review on the various facets effecting brain delivery of magnesium and its role in neurological disorders. Biol. Trace Elem. Res. 2023, 201, 4238–4253. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, magnesium, selenium and depression: A review of the evidence, potential mechanisms and implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The role of magnesium in neurological disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef]
- Kochlik, B.; Herpich, C.; Moreno-Villanueva, M.; Klaus, S.; Muller-Werdan, U.; Weinberger, B.; Fiegl, S.; Toussaint, O.; Debacq-Chainiaux, F.; Schon, C.; et al. Associations of circulating GDF15 with combined cognitive frailty and depression in older adults of the MARK-AGE study. Geroscience 2024, 46, 1657–1669. [Google Scholar] [CrossRef]
- Dibello, V.; Custodero, C.; Cavalcanti, R.; Lafornara, D.; Dibello, A.; Lozupone, M.; Daniele, A.; Pilotto, A.; Panza, F.; Solfrizzi, V. Impact of periodontal disease on cognitive disorders, dementia, and depression: A systematic review and meta-analysis. Geroscience 2024, 46, 5133–5169. [Google Scholar] [CrossRef]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model-are we there yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef]
- Desai, P.; Krueger, K.R.; Mendes de Leon, C.; Wilson, R.S.; Evans, D.A.; Rajan, K.B. Depressive Symptoms, Glial Fibrillary Acid Protein Concentrations, and Cognitive Decline in a Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad129. [Google Scholar] [CrossRef]
- Lu, K.; Wang, W.; Wang, J.; Du, Q.; Li, C.; Wei, Y.; Yao, M.; Zhang, T.; Yin, F.; Ma, Y. Depressive intensity, duration, and their associations with cognitive decline: A population-based study in Korea. Geroscience 2025, 47, 1–19. [Google Scholar] [CrossRef]
- Karakose, S.; Luchetti, M.; Stephan, Y.; Sutin, A.R.; Terracciano, A. Life satisfaction and risk of dementia over 18 years: An analysis of the National Alzheimer’s Coordinating Center dataset. Geroscience 2024, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.Y.; Wood Alexander, M.; Cogo-Moreira, H.; Wu, C.Y.; Eid, M.; Herrmann, N.; Gallagher, D.; Edwards, J.D.; Lanctot, K.L.; Marzolini, S.; et al. Longitudinal relationships between depressive symptoms, functional impairment, and physical activity in later late life. Geroscience 2024, 47, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Salwierz, P.; Thapa, S.; Taghdiri, F.; Vasilevskaya, A.; Anastassiadis, C.; Tang-Wai, D.F.; Golas, A.C.; Tartaglia, M.C. Investigating the association between a history of depression and biomarkers of Alzheimer’s disease, cerebrovascular disease, and neurodegeneration in patients with dementia. Geroscience 2024, 46, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Saggu, S.; Bai, A.; Aida, M.; Rehman, H.; Pless, A.; Ware, D.; Deak, F.; Jiao, K.; Wang, Q. Monoamine alterations in Alzheimer’s disease and their implications in comorbid neuropsychiatric symptoms. Geroscience 2024, 47, 457–482. [Google Scholar] [CrossRef]
- Pieruccini-Faria, F.; Hachinski, V.; Son, S.; Montero-Odasso, M. Apathy, gait slowness, and executive dysfunction (AGED) triad: Opportunities to predict and delay dementia onset. Geroscience 2024, 47, 1–13. [Google Scholar] [CrossRef]
- Valsdottir, V.; Magnusdottir, B.B.; Chang, M.; Sigurdsson, S.; Gudnason, V.; Launer, L.J.; Jonsdottir, M.K. Cognition and brain health among older adults in Iceland: The AGES-Reykjavik study. Geroscience 2022, 44, 2785–2800. [Google Scholar] [CrossRef]
- Jung, S.J.; Lee, G.B.; Nishimi, K.; Chibnik, L.; Koenen, K.C.; Kim, H.C. Association between psychological resilience and cognitive function in older adults: Effect modification by inflammatory status. Geroscience 2021, 43, 2749–2760. [Google Scholar] [CrossRef]
- Barak, Y.; Barson, D.; Davie, G.; Glue, P.; Paleacu, D. Internalize at your peril: Internalizing disorders as risk factors for dementia-cohort study. Geroscience 2021, 43, 253–261. [Google Scholar] [CrossRef]
- Serefko, A.; Szopa, A.; Poleszak, E. Magnesium and depression. Magnes. Res. 2016, 3, 29. [Google Scholar] [CrossRef]
- Derom, M.-L.; Sayón-Orea, C.; Martínez-Ortega, J.M.; Martínez-González, M.A. Magnesium and depression: A systematic review. Nutr. Neurosci. 2013, 16, 191–206. [Google Scholar] [CrossRef]
- Ryszewska-Pokraśniewicz, B.; Mach, A.; Skalski, M.; Januszko, P.; Wawrzyniak, Z.M.; Poleszak, E.; Nowak, G.; Pilc, A.; Radziwoń-Zaleska, M. Effects of Magnesium Supplementation on Unipolar Depression: A Placebo-Controlled Study and Review of the Importance of Dosing and Magnesium Status in the Therapeutic Response. Nutrients 2018, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, E.K.; Littenberg, B.; MacLean, C.D.; Kennedy, A.G.; Daley, C. Role of magnesium supplementation in the treatment of depression: A randomized clinical trial. PLoS ONE 2017, 12, e0180067. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Li, H.; Quan, Z.; Qing, H. Appropriate macronutrients or mineral elements are beneficial to improve depression and reduce the risk of depression. Int. J. Mol. Sci. 2023, 24, 7098. [Google Scholar] [CrossRef] [PubMed]
- Eby III, G.A.; Eby, K.L. Magnesium for treatment-resistant depression: A review and hypothesis. Med. Hypotheses 2010, 74, 649–660. [Google Scholar] [CrossRef]
- Firth, J.; Teasdale, S.B.; Allott, K.; Siskind, D.; Marx, W.; Cotter, J.; Veronese, N.; Schuch, F.; Smith, L.; Solmi, M. The efficacy and safety of nutrient supplements in the treatment of mental disorders: A meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019, 18, 308–324. [Google Scholar] [CrossRef]
- Silberstein, S. Migraine pathophysiology and its clinical implications. Cephalalgia 2004, 24, 2–7. [Google Scholar] [CrossRef]
- Dolati, S.; Rikhtegar, R.; Mehdizadeh, A.; Yousefi, M. The role of magnesium in pathophysiology and migraine treatment. Biol. Trace Elem. Res. 2020, 196, 375–383. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Sabico, S.; Al-Daghri, N.M.; Barbagallo, M. Magnesium and Migraine. Nutrients 2025, 17, 725. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Yeh, T.-H.; Yin-Cheng, H.; Pin-Yuan, C. Effects of intravenous and oral magnesium on reducing migraine: A meta-analysis of randomized controlled trials. Pain. Physician 2016, 19, E97. [Google Scholar]
- Freedman, V.A.; Cornman, J.C. Dementia Prevalence, Incidence, and Mortality Trends Among U.S. Adults Ages 72 and Older, 2011–2021. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, S22–S31. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Castrogiovanni, P.; Vinciguerra, M.; Imbesi, R.; Ulivieri, M.; Fazio, F.; Blennow, K.; Zetterberg, H.; Di Rosa, M. A sex-stratified analysis of neuroimmune gene expression signatures in Alzheimer’s disease brains. Geroscience 2023, 45, 523–541. [Google Scholar] [CrossRef] [PubMed]
- van Setten, A.; Uleman, J.F.; Melis, R.J.F.; Lawlor, B.; Riksen, N.P.; Claassen, J.; de Heus, R.A.A.; Group, N.S. No association between markers of systemic inflammation and endothelial dysfunction with Alzheimer’s disease progression: A longitudinal study. Geroscience 2024, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- DeVries, S.A.; Conner, B.; Dimovasili, C.; Moore, T.L.; Medalla, M.; Mortazavi, F.; Rosene, D.L. Immune proteins C1q and CD47 may contribute to aberrant microglia-mediated synapse loss in the aging monkey brain that is associated with cognitive impairment. Geroscience 2024, 46, 2503–2519. [Google Scholar] [CrossRef]
- Andonian, B.J.; Hippensteel, J.A.; Abuabara, K.; Boyle, E.M.; Colbert, J.F.; Devinney, M.J.; Faye, A.S.; Kochar, B.; Lee, J.; Litke, R.; et al. Inflammation and aging-related disease: A transdisciplinary inflammaging framework. Geroscience 2024, 47, 515–542. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, X.; Zhao, S.; Zhong, S.; Li, Z.; Yan, Y.; Zhang, B.; Chen, Y. Interferon Regulatory Factor 5 Regulates the Phagocytosis of Microglia and Alleviate Alzheimer’s Pathology. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae031. [Google Scholar] [CrossRef]
- Giudici, K.V.; de Souto Barreto, P.; Guyonnet, S.; Morley, J.E.; Nguyen, A.D.; Aggarwal, G.; Parini, A.; Li, Y.; Bateman, R.J.; Vellas, B.; et al. TNFR-1 and GDF-15 Are Associated With Plasma Neurofilament Light Chain and Progranulin Among Community-Dwelling Older Adults: A Secondary Analysis of the MAPT Study. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 569–578. [Google Scholar] [CrossRef]
- Ayyanar, M.P.; Vijayan, M. A review on gut microbiota and miRNA crosstalk: Implications for Alzheimer’s disease. Geroscience 2024, 47, 1–47. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Shah, R.; Alford, N.; Mishra, S.P.; Jain, S.; Hansen, B.; Sanberg, P.; Molina, A.J.A.; Yadav, H. The Triple Alliance: Microbiome, Mitochondria, and Metabolites in the Context of Age-Related Cognitive Decline and Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2187–2202. [Google Scholar] [CrossRef]
- Luo, H.; Wu, B.; Gonzalez, H.M.; Stickel, A.; Kaste, L.M.; Tarraf, W.; Daviglus, M.L.; Sanders, A.E.; Cai, J. Tooth Loss, Periodontal Disease, and Mild Cognitive Impairment Among Hispanic/Latino Immigrants: The Moderating Effects of Age at Immigration. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 949–957. [Google Scholar] [CrossRef]
- Pszczolowska, M.; Walczak, K.; Miskow, W.; Mroziak, M.; Chojdak-Lukasiewicz, J.; Leszek, J. Mitochondrial disorders leading to Alzheimer’s disease-perspectives of diagnosis and treatment. Geroscience 2024, 46, 2977–2988. [Google Scholar] [CrossRef]
- Kugler, B.A.; Lysaker, C.R.; Franczak, E.; Hauger, B.M.; Csikos, V.; Stopperan, J.A.; Allen, J.A.; Stanford, J.A.; Koch, L.G.; Britton, S.L.; et al. Intrinsic aerobic capacity modulates Alzheimer’s disease pathological hallmarks, brain mitochondrial function and proteome during aging. Geroscience 2024, 46, 4955–4967. [Google Scholar] [CrossRef] [PubMed]
- Seman, A.; Chandra, P.K.; Byrum, S.D.; Mackintosh, S.G.; Gies, A.J.; Busija, D.W.; Rutkai, I. Targeting mitochondria in the aged cerebral vasculature with SS-31, a proteomic study of brain microvessels. Geroscience 2023, 45, 2951–2965. [Google Scholar] [CrossRef] [PubMed]
- Waigi, E.W.; Pernomian, L.; Crockett, A.M.; Costa, T.J.; Townsend, P., Jr.; Webb, R.C.; McQuail, J.A.; McCarthy, C.G.; Hollis, F.; Wenceslau, C.F. Vascular dysfunction occurs prior to the onset of amyloid pathology and Abeta plaque deposits colocalize with endothelial cells in the hippocampus of female APPswe/PSEN1dE9 mice. Geroscience 2024, 46, 5517–5536. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Tang, C.; Zhang, H.; Border, J.J.; Liu, Y.; Shin, S.M.; Yu, H.; Roman, R.J.; Fan, F. Longitudinal characterization of cerebral hemodynamics in the TgF344-AD rat model of Alzheimer’s disease. Geroscience 2023, 45, 1471–1490. [Google Scholar] [CrossRef]
- Fang, X.; Border, J.J.; Rivers, P.L.; Zhang, H.; Williams, J.M.; Fan, F.; Roman, R.J. Amyloid beta accumulation in TgF344-AD rats is associated with reduced cerebral capillary endothelial Kir2.1 expression and neurovascular uncoupling. Geroscience 2023, 45, 2909–2926. [Google Scholar] [CrossRef]
- Csiszar, A.; Ungvari, A.; Patai, R.; Gulej, R.; Yabluchanskiy, A.; Benyo, Z.; Kovacs, I.; Sotonyi, P.; Kirkpartrick, A.C.; Prodan, C.I.; et al. Atherosclerotic burden and cerebral small vessel disease: Exploring the link through microvascular aging and cerebral microhemorrhages. Geroscience 2024, 46, 5103–5132. [Google Scholar] [CrossRef]
- Ting, K.K.; Coleman, P.; Kim, H.J.; Zhao, Y.; Mulangala, J.; Cheng, N.C.; Li, W.; Gunatilake, D.; Johnstone, D.M.; Loo, L.; et al. Vascular senescence and leak are features of the early breakdown of the blood-brain barrier in Alzheimer’s disease models. Geroscience 2023, 45, 3307–3331. [Google Scholar] [CrossRef]
- Nyul-Toth, A.; Patai, R.; Csiszar, A.; Ungvari, A.; Gulej, R.; Mukli, P.; Yabluchanskiy, A.; Benyo, Z.; Sotonyi, P.; Prodan, C.I.; et al. Linking peripheral atherosclerosis to blood-brain barrier disruption: Elucidating its role as a manifestation of cerebral small vessel disease in vascular cognitive impairment. Geroscience 2024, 46, 6511–6536. [Google Scholar] [CrossRef]
- Waigi, E.W.; Webb, R.C.; Moss, M.A.; Uline, M.J.; McCarthy, C.G.; Wenceslau, C.F. Soluble and insoluble protein aggregates, endoplasmic reticulum stress, and vascular dysfunction in Alzheimer’s disease and cardiovascular diseases. Geroscience 2023, 45, 1411–1438. [Google Scholar] [CrossRef]
- Bakhtiari, A.; Benedek, K.; Law, I.; Fagerlund, B.; Mortensen, E.L.; Osler, M.; Lauritzen, M.; Larsson, H.B.W.; Vestergaard, M.B. Early cerebral amyloid-beta accumulation and hypermetabolism are associated with subtle cognitive deficits before accelerated cerebral atrophy. Geroscience 2024, 46, 769–782. [Google Scholar] [CrossRef]
- van Gennip, A.C.E.; Satizabal, C.L.; Tracy, R.P.; Sigurdsson, S.; Gudnason, V.; Launer, L.J.; van Sloten, T.T. Associations of plasma NfL, GFAP, and t-tau with cerebral small vessel disease and incident dementia: Longitudinal data of the AGES-Reykjavik Study. Geroscience 2024, 46, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, F.; Alzheimer’s disease Neuroimaging Initiative. Local molecular and connectomic contributions of tau-related neurodegeneration. Geroscience 2024, 47, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Billaud, C.H.A.; Yu, J.; Alzheimer’s Disease Neuroimaging Initiative. Fixel-based and tensor-derived white matter abnormalities in relation to memory impairment and neurocognitive disorders. Geroscience 2024, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, P.; Mak, H.K.F.; Hui, E.S. The structural-functional-connectivity coupling of the aging brain. Geroscience 2024, 46, 3875–3887. [Google Scholar] [CrossRef]
- Yan, Y.; Gao, M.; Geng, Z.; Wu, Y.; Xiao, G.; Wang, L.; Pang, X.; Yang, C.; Zhou, S.; Li, H.; et al. Abnormal EEG microstates in Alzheimer’s disease: Predictors of beta-amyloid deposition degree and disease classification. Geroscience 2024, 46, 4779–4792. [Google Scholar] [CrossRef]
- Wojtecki, L.; Cont, C.; Stute, N.; Galli, A.; Schulte, C.; Trenado, C. Electrical brain networks before and after transcranial pulsed shockwave stimulation in Alzheimer’s patients. Geroscience 2024, 47, 953–964. [Google Scholar] [CrossRef]
- Williamson, J.N.; James, S.A.; Mullen, S.P.; Sutton, B.P.; Wszalek, T.; Mulyana, B.; Mukli, P.; Yabluchanskiy, A.; Alzheimer’s Disease Neuroimaging Initiative Consortium; Yang, Y. Sex differences in interacting genetic and functional connectivity biomarkers in Alzheimer’s disease. Geroscience 2024, 46, 6071–6084. [Google Scholar] [CrossRef]
- Williamson, J.; James, S.A.; Mukli, P.; Yabluchanskiy, A.; Wu, D.H.; Sonntag, W.; Alzheimer’s Disease Neuroimaging Initiative, C.; Yang, Y. Sex difference in brain functional connectivity of hippocampus in Alzheimer’s disease. Geroscience 2024, 46, 563–572. [Google Scholar] [CrossRef]
- Garcia-Colomo, A.; Nebreda, A.; Carrasco-Gomez, M.; de Frutos-Lucas, J.; Ramirez-Torano, F.; Spuch, C.; Comis-Tuche, M.; Bruna, R.; Alfonsin, S.; Maestu, F. Longitudinal changes in the functional connectivity of individuals at risk of Alzheimer’s disease. Geroscience 2024, 46, 2989–3003. [Google Scholar] [CrossRef]
- Morrison, C.; Dadar, M.; Kamal, F.; Collins, D.L.; Alzheimer’s Disease Neuroimaging Initiative. Differences in Alzheimer’s Disease-Related Pathology Profiles Across Apolipoprotein Groups. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad254. [Google Scholar] [CrossRef]
- Rasmussen, L.T.; de Labio, R.W.; Dos Santos, M.P.; Fredi, B.M.; Baisi Chagas, E.F.; Chen, E.S.; Turecki, G.; Smith, M.A.C.; Payao, S.L.M. Changes in Expression of Key Genes in Alzheimer’s Disease: A Specific Brain Tissue Change. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae023. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, Z.; Zhuang, Z.; Zhao, Y.; Zhang, L.; Wang, W.; Song, Z.; Dong, X.; Xiao, W.; Huang, N.; et al. Polysocial and Polygenic Risk Scores and All-Cause Dementia, Alzheimer’s Disease, and Vascular Dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad262. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Zheng, Y.; Jiang, T.; Hou, J.; Cao, R.; Cai, J.; Ma, E.; Wang, W.; Song, W.; Xie, C. A Risk Variant rs6922617 in TREM Is Discrepantly Associated With Defining Neuropathological Hallmarks in the Alzheimer’s Continuum. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Smith, J.A.; Wang, Y.Z.; Chintalapati, M.; Ammous, F.; Yu, M.; Moorjani, P.; Ganna, A.; Gross, A.; Dey, S.; et al. Polygenic Risk Scores for Alzheimer’s Disease and General Cognitive Function Are Associated With Measures of Cognition in Older South Asians. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 743–752. [Google Scholar] [CrossRef]
- Zhou, S.; Ma, G.; Luo, H.; Shan, S.; Xiong, J.; Cheng, G. Identification of 5 Potential Predictive Biomarkers for Alzheimer’s Disease by Integrating the Unified Test for Molecular Signatures and Weighted Gene Coexpression Network Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 653–658. [Google Scholar] [CrossRef]
- Kim, Y.; Wang, M.; Sharp, S.J.; Au Yeung, S.L.; Luo, S.; Jang, H.; Jiesisibieke, Z.L.; Shi, Q.; Chen, Z.; Brage, S. Incidence of Dementia and Alzheimer’s Disease, Genetic Susceptibility, and Grip Strength Among Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad224. [Google Scholar] [CrossRef]
- Zapater-Fajari, M.; Diaz-Galvan, P.; Cedres, N.; Rydberg Sterner, T.; Ryden, L.; Sacuiu, S.; Waern, M.; Zettergren, A.; Zetterberg, H.; Blennow, K.; et al. Biomarkers of Alzheimer’s Disease and Cerebrovascular Disease in Relation to Depressive Symptomatology in Individuals With Subjective Cognitive Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad216. [Google Scholar] [CrossRef]
- Guo, J.; Marseglia, A.; Shang, Y.; Dove, A.; Grande, G.; Fratiglioni, L.; Xu, W. Association Between Late-Life Weight Change and Dementia: A Population-based Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 143–150. [Google Scholar] [CrossRef]
- Ha, J.; Kwak, S.; Kim, K.Y.; Kim, H.; Cho, S.Y.; Kim, M.; Lee, J.Y.; Kim, E. Relationship Between Adipokines, Cognition, and Brain Structures in Old Age Depending on Obesity. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 120–128. [Google Scholar] [CrossRef]
- Lerfald, M.; Allore, H.; Nilsen, T.I.L.; Eldholm, R.S.; Martinez-Velilla, N.; Selbaek, G.; Ernstsen, L. Longitudinal Patterns of Systolic Blood Pressure, Diastolic Blood Pressure, Cardiorespiratory Fitness, and Their Association With Dementia Risk: The HUNT Study. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae161. [Google Scholar] [CrossRef]
- Beydoun, H.A.; Szymkowiak, D.; Kinney, R.; Beydoun, M.A.; Zonderman, A.B.; Tsai, J. Is the Risk of Alzheimer’s Disease and Related Dementias Among U.S. Veterans Influenced by the Intersectionality of Housing Status, HIV/AIDS, Hepatitis C, and Psychiatric Disorders? J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae153. [Google Scholar] [CrossRef] [PubMed]
- Aravena, J.M.; Lee, J.; Schwartz, A.E.; Nyhan, K.; Wang, S.Y.; Levy, B.R. Beneficial Effect of Societal Factors on APOE-epsilon2 and epsilon4 Carriers’ Brain Health: A Systematic Review. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad237. [Google Scholar] [CrossRef] [PubMed]
- Aiken-Morgan, A.T.; Capuano, A.W.; Wilson, R.S.; Barnes, L.L. Changes in Body Mass Index and Incident Mild Cognitive Impairment Among African American Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad263. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Capuano, A.W.; VanderHorst, V.; Wilson, R.S.; Oveisgharan, S.; Schneider, J.A.; Bennett, D.A. Brain beta-Amyloid Links the Association of Change in Body Mass Index With Cognitive Decline in Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 277–285. [Google Scholar] [CrossRef]
- Campbell, E.B.; Delgadillo, M.; Lazzeroni, L.C.; Louras, P.N.; Myers, J.; Yesavage, J.; Fairchild, J.K. Cognitive Improvement Following Physical Exercise and Cognitive Training Intervention for Older Adults With MCI. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 554–560. [Google Scholar] [CrossRef]
- Chang, Y.K.; Etnier, J.L.; Li, R.H.; Ren, F.F.; Ai, J.Y.; Chu, C.H. Acute Exercise Effect on Neurocognitive Function Among Cognitively Normal Late-Middle-Aged Adults With/Without Genetic Risk of AD: The Moderating Role of Exercise Volume and APOE Genotype. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad179. [Google Scholar] [CrossRef]
- Grande de Franca, N.A.; Diaz, G.; Lengele, L.; Soriano, G.; Caspar-Bauguil, S.; Saint-Aubert, L.; Payoux, P.; Rouch, L.; Vellas, B.; de Souto Barreto, P.; et al. Associations Between Blood Nutritional Biomarkers and Cerebral Amyloid-beta: Insights From the COGFRAIL Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad248. [Google Scholar] [CrossRef]
- Kallianpur, K.J.; Masaki, K.H.; Chen, R.; Willcox, B.J.; Allsopp, R.C.; Davy, P.; Dodge, H.H. Weak Social Networks in Late Life Predict Incident Alzheimer’s Disease: The Kuakini Honolulu-Asia Aging Study. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 663–672. [Google Scholar] [CrossRef]
- Lange-Maia, B.S.; Wagner, M.; Rogers, C.A.; Mehta, R.I.; Bennett, D.A.; Tangney, C.; Schoeny, M.E.; Halloway, S.; Arvanitakis, Z. Profiles of Lifestyle Health Behaviors and Postmortem Dementia-Related Neuropathology. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae100. [Google Scholar] [CrossRef]
- Marino, F.R.; Deal, J.A.; Dougherty, R.J.; Bilgel, M.; Tian, Q.; An, Y.; Simonsick, E.M.; Resnick, S.M.; Ferrucci, L.; Spira, A.P.; et al. Differences in Daily Physical Activity by Alzheimer’s Risk Markers Among Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae119. [Google Scholar] [CrossRef]
- Morita, A.; Fujiwara, T.; Murayama, H.; Machida, M.; Inoue, S.; Shobugawa, Y. Association Between Trajectory of Socioeconomic Position and Regional Brain Volumes Related to Dementia: Results From the NEIGE Study. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad269. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Nyarko Hukportie, D.; Wan, Z.; Li, F.R.; Wu, X.B. The Association Between Exposure to Air Pollution and Dementia Incidence: The Modifying Effect of Smoking. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, N.; Pouget, A.M.; Grivaud, A.; Nogueira, L.; Larvor, F.; Marchand, P.; Schmidt, E.; Le Bizec, B. First Observations of a Potential Association Between Accumulation of Per- and Polyfluoroalkyl Substances in the Central Nervous System and Markers of Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad208. [Google Scholar] [CrossRef]
- Finch, C.E.; Thorwald, M.A. Inhaled Pollutants of the Gero-Exposome and Later-Life Health. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae107. [Google Scholar] [CrossRef]
- Ścibior, A.; Llopis, J.; Dobrakowski, P.P.; Męcik-Kronenberg, T. Magnesium (Mg) and Neurodegeneration: A Comprehensive Overview of Studies on Mg Levels in Biological Specimens in Humans Affected Some Neurodegenerative Disorders with an Update on Therapy and Clinical Trials Supplemented with Selected Animal Studies. Int. J. Mol. Sci. 2024, 25, 12595. [Google Scholar] [CrossRef]
- Tyczyńska, M.; Gędek, M.; Brachet, A.; Stręk, W.; Flieger, J.; Teresiński, G.; Baj, J. Trace elements in Alzheimer’s disease and dementia: The current state of knowledge. J. Clin. Med. 2024, 13, 2381. [Google Scholar] [CrossRef]
- Maier, J.A.; Locatelli, L.; Fedele, G.; Cazzaniga, A.; Mazur, A. Magnesium and the brain: A focus on neuroinflammation and neurodegeneration. Int. J. Mol. Sci. 2022, 24, 223. [Google Scholar] [CrossRef]
- Huang, J.-H.; Lu, Y.-F.; Cheng, F.-C.; Lee, J.N.-Y.; Tsai, L.-C. Correlation of magnesium intake with metabolic parameters, depression and physical activity in elderly type 2 diabetes patients: A cross-sectional study. Nutr. J. 2012, 11, 1–10. [Google Scholar] [CrossRef]
- Al-Dujaili, A.H.; Al-Hakeim, H.K.; Twayej, A.J.; Maes, M. Total and ionized calcium and magnesium are significantly lowered in drug-naïve depressed patients: Effects of antidepressants and associations with immune activation. Metab. Brain Dis. 2019, 34, 1493–1503. [Google Scholar] [CrossRef]
- Woodward, G.; CM Wan, J.; Viswanath, K.; Zaman, R. Serum Vitamin D and Magnesium levels in a psychiatric cohort. Psychiatr. Danub. 2019, 31, 221–226. [Google Scholar]
- Samad, N.; Yasmin, F.; Manzoor, N. Biomarkers in drug free subjects with depression: Correlation with tryptophan. Psychiatry Investig. 2019, 16, 948. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Islam, M.R.; Shalahuddin Qusar, M.; Islam, M.S.; Kabir, M.H.; Mustafizur Rahman, G.; Islam, M.S.; Hasnat, A. Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: A case-control study. BMC Psychiatry 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Pourmehr, H.; Dolatkhah, N.; Gassab-Abdollahi, N.; Farrin, N.; Mojtahedi, M.; Farshbaf-Khalili, A. Screening of depression in overweight and obese pregnant women and its predictors. J. Obstet. Gynaecol. Res. 2019, 45, 2169–2177. [Google Scholar] [CrossRef]
- Szkup, M.; Jurczak, A.; Brodowska, A.; Brodowska, A.; Noceń, I.; Chlubek, D.; Laszczyńska, M.; Karakiewicz, B.; Grochans, E. Analysis of relations between the level of Mg, Zn, Ca, Cu, and Fe and depressiveness in postmenopausal women. Biol. Trace Elem. Res. 2017, 176, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Afsharfar, M.; Shahraki, M.; Shakiba, M.; Asbaghi, O.; Dashipour, A. The effects of magnesium supplementation on serum level of brain derived neurotrophic factor (BDNF) and depression status in patients with depression. Clin. Nutr. ESPEN 2021, 42, 381–386. [Google Scholar] [CrossRef]
- Barragán-Rodríguez, L.; Rodríguez-Morán, M.; Guerrero-Romero, F. Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: A randomized, equivalent trial. Magnes. Res. 2008, 21, 218–223. [Google Scholar]
- Rodríguez-Morán, M.; Guerrero-Romero, F.; Barragán-Zuñiga, J.; Gamboa-Gómez, C.I.; Weyman-Vela, Y.; Arce-Quiñones, M.; Simental-Mendía, L.E.; Martínez-Aguilar, G. Combined oral supplementation with magnesium plus vitamin D alleviates mild to moderate depressive symptoms related to long-COVID: An open-label randomized, controlled clinical trial. Magnes. Res. 2024, 37, 49–57. [Google Scholar]
- Rajizadeh, A.; Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Dehghani, A. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double-blind, placebo-controlled trial. Nutrition 2017, 35, 56–60. [Google Scholar] [CrossRef]
- Abiri, B.; Sarbakhsh, P.; Vafa, M. Randomized study of the effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammation, and SIRT1 in obese women with mild to moderate depressive symptoms. Nutr. Neurosci. 2022, 25, 2123–2135. [Google Scholar] [CrossRef]
- Shakya, P.R.; Melaku, Y.A.; Page, A.; Gill, T.K. Association between dietary patterns and adult depression symptoms based on principal component analysis, reduced-rank regression and partial least-squares. Clin. Nutr. 2020, 39, 2811–2823. [Google Scholar] [CrossRef]
- Fard, F.E.; Mirghafourvand, M.; Mohammad-Alizadeh Charandabi, S.; Farshbaf-Khalili, A.; Javadzadeh, Y.; Asgharian, H. Effects of zinc and magnesium supplements on postpartum depression and anxiety: A randomized controlled clinical trial. Women Health 2017, 57, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.M.; Atlas, S.E.; Qadir, S.; Musselman, D.; Goldberg, S.; Woolger, J.M.; Corredor, R.; Abbas, M.H.; Arosemena, L.; Caccamo, S.; et al. Double-blind, randomized crossover study of intravenous infusion of magnesium sulfate versus 5% dextrose on depressive symptoms in adults with treatment-resistant depression. Psychiatry Clin. Neurosci. 2017, 71, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, E.; Kabir-Ahmadi, M.; Noah, L.; Mazur, A.; Dye, L.; Hellhammer, J.; Pickering, G.; Dubray, C. Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: A randomized, single-blind clinical trial. PLoS ONE 2018, 13, e0208454. [Google Scholar] [CrossRef] [PubMed]
- Derom, M.L.; Martínez-González, M.A.; Sayón-Orea Mdel, C.; Bes-Rastrollo, M.; Beunza, J.J.; Sánchez-Villegas, A. Magnesium intake is not related to depression risk in Spanish university graduates. J. Nutr. 2012, 142, 1053–1059. [Google Scholar] [CrossRef]
- Nazarinasab, M.; Behrouzian, F.; Abdi, L.; Sadegh Moghaddam, A.A.; Sadeghi, S. Investigating the effect of magnesium supplement in patients with major depressive disorder under selective serotonin reuptake inhibitor treatment. J. Fam. Med. Prim. Care 2022, 11, 7800–7805. [Google Scholar] [CrossRef]
- Yablon, L.A.; Mauskop, A. Magnesium in headache. In Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar] [PubMed]
- Mauskop, A.; Varughese, J. Why all migraine patients should be treated with magnesium. J. Neural Transm. 2012, 119, 575–579. [Google Scholar] [CrossRef]
- Mauskop, A.; Altura, B.M. Role of magnesium in the pathogenesis and treatment of migraines. Clin. Neurosci. 1998, 5, 24–27. [Google Scholar]
- Peikert, A.; Wilimzig, C.; Köhne-Volland, R. Prophylaxis of migraine with oral magnesium: Results from a prospective, multi-center, placebo-controlled and double-blind randomized study. Cephalalgia 1996, 16, 257–263. [Google Scholar] [CrossRef]
- Miller, A.C.; Pfeffer, B.K.; Lawson, M.R.; Sewell, K.A.; King, A.R.; Zehtabchi, S. Intravenous Magnesium Sulfate to Treat Acute Headaches in the Emergency Department: A Systematic Review. Headache 2019, 59, 1674–1686. [Google Scholar] [CrossRef]
- Choi, H.; Parmar, N. The use of intravenous magnesium sulphate for acute migraine: Meta-analysis of randomized controlled trials. Eur. J. Emerg. Med. 2014, 21, 2–9. [Google Scholar] [CrossRef]
- Cete, Y.; Dora, B.; Ertan, C.; Ozdemir, C.; Oktay, C. A randomized prospective placebo-controlled study of intravenous magnesium sulphate vs. metoclopramide in the management of acute migraine attacks in the Emergency Department. Cephalalgia 2005, 25, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Corbo, J.; Esses, D.; Bijur, P.E.; Iannaccone, R.; Gallagher, E.J. Randomized clinical trial of intravenous magnesium sulfate as an adjunctive medication for emergency department treatment of migraine headache. Ann. Emerg. Med. 2001, 38, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Demirkaya, S.; Vural, O.; Dora, B.; Topçuoğlu, M.A. Efficacy of intravenous magnesium sulfate in the treatment of acute migraine attacks. Headache 2001, 41, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Bordini, C.A.; Tepper, S.J.; Speciali, J.G. Intravenous magnesium sulphate in the acute treatment of migraine without aura and migraine with aura. A randomized, double-blind, placebo-controlled study. Cephalalgia 2002, 22, 345–353. [Google Scholar] [CrossRef]
- Shahrami, A.; Assarzadegan, F.; Hatamabadi, H.R.; Asgarzadeh, M.; Sarehbandi, B.; Asgarzadeh, S. Comparison of therapeutic effects of magnesium sulfate vs. dexamethasone/metoclopramide on alleviating acute migraine headache. J. Emerg. Med. 2015, 48, 69–76. [Google Scholar] [CrossRef]
- Matin, H.; Taghian, F.; Chitsaz, A. Artificial intelligence analysis to explore synchronize exercise, cobalamin, and magnesium as new actors to therapeutic of migraine symptoms: A randomized, placebo-controlled trial. Neurol. Sci. 2022, 43, 4413–4424. [Google Scholar] [CrossRef]
- Kandil, M.; Jaber, S.; Desai, D.; Nuñez Cruz, S.; Lomotan, N.; Ahmad, U.; Cirone, M.; Burkins, J.; McDowell, M. MAGraine: Magnesium compared to conventional therapy for treatment of migraines. Am. J. Emerg. Med. 2021, 39, 28–33. [Google Scholar] [CrossRef]
- Gaul, C.; Diener, H.C.; Danesch, U. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo-controlled, double-blind, multicenter trial. J. Headache Pain 2015, 16, 516. [Google Scholar] [CrossRef]
- Rahimdel, A.; Eslami, M.H.; Zeinali, A. A Randomized Controlled Study Ofmagnesium Sulfate Versusdihydroergotamine in The Management Ofacute Migraine Attacks. Pak. J. Neurol. Sci. 2007, 2, 92–95. [Google Scholar]
- Ginder, S.; Oatman, B.; Pollack, M. A prospective study of i.v. magnesium and i.v. prochlorperazine in the treatment of headaches. J. Emerg. Med. 2000, 18, 311–315. [Google Scholar] [CrossRef]
- Khani, S.; Hejazi, S.A.; Yaghoubi, M.; Sharifipour, E. Comparative study of magnesium, sodium valproate, and concurrent magnesium-sodium valproate therapy in the prevention of migraine headaches: A randomized controlled double-blind trial. J. Headache Pain 2021, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Razian, A.; Heidari, M. The efficacy of magnesium oxide and sodium valproate in prevention of migraine headache: A randomized, controlled, double-blind, crossover study. Acta Neurol. Belg. 2021, 121, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Tarighat Esfanjani, A.; Mahdavi, R.; Ebrahimi Mameghani, M.; Talebi, M.; Nikniaz, Z.; Safaiyan, A. The effects of magnesium, L-carnitine, and concurrent magnesium-L-carnitine supplementation in migraine prophylaxis. Biol. Trace Elem. Res. 2012, 150, 42–48. [Google Scholar] [CrossRef]
- Köseoglu, E.; Talaslioglu, A.; Gönül, A.S.; Kula, M. The effects of magnesium prophylaxis in migraine without aura. Magnes. Res. 2008, 21, 101–108. [Google Scholar]
- Mattiuzzi, C.; Lippi, G. Worldwide disease epidemiology in the older persons. Eur. Geriatr. Med. 2020, 11, 147–153. [Google Scholar] [CrossRef]
- Pedroza, P.; Miller-Petrie, M.K.; Chen, C.; Chakrabarti, S.; Chapin, A.; Hay, S.; Tsakalos, G.; Wimo, A.; Dieleman, J.L. Global and regional spending on dementia care from 2000–2019 and expected future health spending scenarios from 2020–2050: An economic modelling exercise. EClinicalMedicine 2022, 45, 101337. [Google Scholar] [CrossRef]
- Ghalibaf, A. The Role of Nurses in Improving the Quality of Life of Alzheimer’s Disease. Bachelor’s Thesis, JAMK University of Applied Sciences, Jyväskylä, Finland, 2024. [Google Scholar]
- Mangiaterra, S. The Human Rights Perspective on Dementia: From Prevention to Social Care Priority in National Agenda. Master’s Thesis, Università Degli Studi di Padova, Padova, Italy, 2023. [Google Scholar]
- Memudu, A.E.; Olukade, B.A.; Alex, G.S. Neurodegenerative Diseases: Alzheimer’s Disease. In Integrating Neuroimaging, Computational Neuroscience, and Artificial Intelligence; CRC Press: Boca Raton, FL, USA, 2024; pp. 128–147. [Google Scholar]
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Solomon, A.; Mangialasche, F.; Richard, E.; Andrieu, S.; Bennett, D.A.; Breteler, M.; Fratiglioni, L.; Hooshmand, B.; Khachaturian, A.S.; Schneider, L.S. Advances in the prevention of Alzheimer’s disease and dementia. J. Intern. Med. 2014, 275, 229–250. [Google Scholar] [CrossRef]
- Kurowska, A.; Ziemichód, W.; Herbet, M.; Piątkowska-Chmiel, I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients 2023, 15, 1436. [Google Scholar] [CrossRef]
- Patel, V.; Akimbekov, N.S.; Grant, W.B.; Dean, C.; Fang, X.; Razzaque, M.S. Neuroprotective effects of magnesium: Implications for neuroinflammation and cognitive decline. Front. Endocrinol. 2024, 15, 1406455. [Google Scholar] [CrossRef]
- Zhou, X.; Hollern, D.; Liao, J.; Andrechek, E.; Wang, H. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell Death Dis. 2013, 4, e560. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Addae, J.I. The pharmacological manipulation of glutamate receptors and neuroprotection. Eur. J. Pharmacol. 2002, 447, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, M.; Kim, S.-J. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J. Neural Transm. 2012, 119, 891–910. [Google Scholar] [CrossRef] [PubMed]
- Paula Monteiro, C.; Nunes Matias, C.; Bicho, M.; Santa-Clara, H.; José Laires, M. Coordination between antioxidant defences might be partially modulated by magnesium status. Magnes. Res. 2016, 29, 161–168. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated tau in Alzheimer’s disease and other tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Zhu, D.; Su, Y.; Fu, B.; Xu, H. Magnesium reduces blood-brain barrier permeability and regulates amyloid-β transcytosis. Mol. Neurobiol. 2018, 55, 7118–7131. [Google Scholar] [CrossRef]
- Toffa, D.H.; Magnerou, M.A.; Kassab, A.; Djibo, F.H.; Sow, A.D. Can magnesium reduce central neurodegeneration in Alzheimer’s disease? Basic evidences and research needs. Neurochem. Int. 2019, 126, 195–202. [Google Scholar] [CrossRef]
- Fatima, G.; Dzupina, A.; Alhmadi, H.B.; Magomedova, A.; Siddiqui, Z.; Mehdi, A.; Hadi, N.; MEHDI, A. Magnesium matters: A comprehensive review of its vital role in health and diseases. Cureus 2024, 16, e71392. [Google Scholar] [CrossRef]
- Eckert, A.; Keil, U.; Marques, C.A.; Bonert, A.; Frey, C.; Schüssel, K.; Müller, W.E. Mitochondrial dysfunction, apoptotic cell death, and Alzheimer’s disease. Biochem. Pharmacol. 2003, 66, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium is a key player in neuronal maturation and neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef] [PubMed]
- Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in aging, health and diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Cheng, Y.; Li, R.; Wang, Y.; Chen, Y.; Scott, T.; Tucker, K.L. Magnesium and cognitive health in adults: A systematic review and meta-analysis. Adv. Nutr. 2024, 15, 100272. [Google Scholar] [CrossRef]
- Zhu, X.; Borenstein, A.R.; Zheng, Y.; Zhang, W.; Seidner, D.L.; Ness, R.; Murff, H.J.; Li, B.; Shrubsole, M.J.; Yu, C.; et al. Ca:Mg Ratio, APOE Cytosine Modifications, and Cognitive Function: Results from a Randomized Trial. J. Alzheimers Dis. 2020, 75, 85–98. [Google Scholar] [CrossRef]
- Du, K.; Zheng, X.; Ma, Z.T.; Lv, J.Y.; Jiang, W.J.; Liu, M.Y. Association of Circulating Magnesium Levels in Patients With Alzheimer’s Disease From 1991 to 2021: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 13, 799824. [Google Scholar] [CrossRef]
- Chen, C.; Xun, P.; Unverzagt, F.; McClure, L.A.; Irvin, M.R.; Judd, S.; Cushman, M.; He, K. Serum magnesium concentration and incident cognitive impairment: The reasons for geographic and racial differences in stroke study. Eur. J. Nutr. 2021, 60, 1511–1520. [Google Scholar] [CrossRef]
- Thomassen, J.Q.; Tolstrup, J.S.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Plasma concentrations of magnesium and risk of dementia: A general population study of 102 648 individuals. Clin. Chem. 2021, 67, 899–911. [Google Scholar] [CrossRef]
- Alam, A.B.; Lutsey, P.L.; Gottesman, R.F.; Tin, A.; Alonso, A. Low Serum Magnesium is Associated with Incident Dementia in the ARIC-NCS Cohort. Nutrients 2020, 12, 3074. [Google Scholar] [CrossRef]
- Kieboom, B.C.; Licher, S.; Wolters, F.J.; Ikram, M.K.; Hoorn, E.J.; Zietse, R.; Stricker, B.H.; Ikram, M.A. Serum magnesium is associated with the risk of dementia. Neurology 2017, 89, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Alateeq, K.; Walsh, E.I.; Ambikairajah, A.; Cherbuin, N. Association between dietary magnesium intake, inflammation, and neurodegeneration. Eur. J. Nutr. 2024, 63, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Etgen, T.; Sander, D.; Bickel, H.; Förstl, H. Mild cognitive impairment and dementia: The importance of modifiable risk factors. Dtsch. Ärzteblatt Int. 2011, 108, 743. [Google Scholar]
- Ni, Y.; Deng, F.; Yu, S.; Zhang, J.; Zhang, X.; Huang, D.; Zhou, H. A randomized, double-blind, placebo-controlled trial to evaluate the therapeutic effect of magnesium-L-threonate supplementation for persistent pain after breast cancer surgery. Breast Cancer Targets Ther. 2023, 15, 495–504. [Google Scholar] [CrossRef]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y. Self-Reported Dietary Intake of Potassium, Calcium, and Magnesium and Risk of Dementia in the J apanese: The H isayama Study. J. Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef]
- Cherbuin, N.; Kumar, R.; Sachdev, P.S.; Anstey, K.J. Dietary mineral intake and risk of mild cognitive impairment: The PATH through life project. Front. Aging Neurosci. 2014, 6, 4. [Google Scholar] [CrossRef]
- Lo, K.; Liu, Q.; Madsen, T.; Rapp, S.; Chen, J.-C.; Neuhouser, M.; Shadyab, A.; Pal, L.; Lin, X.; Shumaker, S. Relations of magnesium intake to cognitive impairment and dementia among participants in the Women’s Health Initiative Memory Study: A prospective cohort study. BMJ Open 2019, 9, e030052. [Google Scholar] [CrossRef]
- Kimura, Y.; Yoshida, D.; Ohara, T.; Hata, J.; Honda, T.; Hirakawa, Y.; Shibata, M.; Oishi, E.; Sakata, S.; Furuta, Y. Long-term association of vegetable and fruit intake with risk of dementia in Japanese older adults: The Hisayama study. BMC Geriatr. 2022, 22, 257. [Google Scholar] [CrossRef]
- Tao, M.H.; Liu, J.; Cervantes, D. Association between magnesium intake and cognition in US older adults: National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Alzheimers Dement. 2022, 8, e12250. [Google Scholar] [CrossRef]
- Tao, M.H.; Chuang, S.C.; Wu, I.C.; Chan, H.T.; Cheng, C.W.; Chen, H.L.; Lee, M.M.; Chang, H.Y.; Hsiung, C.A.; Hsu, C.C. Cross-sectional and longitudinal associations of magnesium intake and cognition in the Healthy Aging Longitudinal Study in Taiwan. Eur. J. Nutr. 2024, 63, 3061–3073. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, C.; Zhao, Q.; Wu, W.; Liang, X.; Xiao, Z.; Mortimer, J.A.; Borenstein, A.R.; Dai, Q.; Ding, D. Dietary calcium and magnesium intake and risk for incident dementia: The Shanghai Aging Study. Alzheimers Dement. 2022, 8, e12362. [Google Scholar] [CrossRef]
- Tzeng, N.-S.; Chung, C.-H.; Lin, F.-H.; Huang, C.-F.; Yeh, C.-B.; Huang, S.-Y.; Lu, R.-B.; Chang, H.-A.; Kao, Y.-C.; Yeh, H.-W. Magnesium oxide use and reduced risk of dementia: A retrospective, nationwide cohort study in Taiwan. Curr. Med. Res. Opin. 2018, 34, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, Q.; Li, S.; Dai, F.; Qian, W.; Hewlings, S.; Yan, T.; Wang, Y. A Magtein(®), Magnesium L-Threonate, -Based Formula Improves Brain Cognitive Functions in Healthy Chinese Adults. Nutrients 2022, 14, 5235. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kawashima, R. Nutrients and dementia: Prospective study. Nutrients 2023, 15, 842. [Google Scholar] [CrossRef]
- Cohen-Hagai, K.; Feldman, D.; Turani-Feldman, T.; Hadary, R.; Lotan, S.; Kitay-Cohen, Y. Magnesium deficiency and minimal hepatic encephalopathy among patients with compensated liver cirrhosis. Isr. Med. Assoc. J. 2018, 20, 533–538. [Google Scholar]
- Alateeq, K.; Walsh, E.I.; Cherbuin, N. Dietary magnesium intake is related to larger brain volumes and lower white matter lesions with notable sex differences. Eur. J. Nutr. 2023, 62, 1–13. [Google Scholar] [CrossRef]
- Eby, G.A.; Eby, K.L.; Murk, H. Magnesium and major depression. In Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar] [PubMed]
- Papri, S.S.; Sonia, S.B.A. Role of magnesium in major depressive disorder. Bangladesh J. Pharmacol. 2025, 20, 41–50. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, Y.; Ruan, Y.; Ou, W.; Hu, Z.; Li, W.; Xiao, N.; Liao, W.; Liu, J.; Liu, Z. Magnesium-L-threonate Ameliorates Cognitive Deficit by Attenuating Adult Hippocampal Neurogenesis Impairment in a Mouse Model of Alzheimer’s Disease. Exp. Neurobiol. 2025, 34, 53. [Google Scholar] [CrossRef]
- Shahi, A.; Aslani, S.; Ataollahi, M.; Mahmoudi, M. The role of magnesium in different inflammatory diseases. Inflammopharmacology 2019, 27, 649–661. [Google Scholar] [CrossRef]
- McConeghy, K.W.; Hatton, J.; Hughes, L.; Cook, A.M. A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs 2012, 26, 613–636. [Google Scholar] [CrossRef]
- Denniss, R.J. Micronutrient Intervention Effects on Cognitive Outcomes in Post-Acute Traumatic Brain Injury; Sheffield Hallam University (United Kingdom): Sheffield, UK, 2020. [Google Scholar]
- Tardy, A.-L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and minerals for energy, fatigue and cognition: A narrative review of the biochemical and clinical evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed]
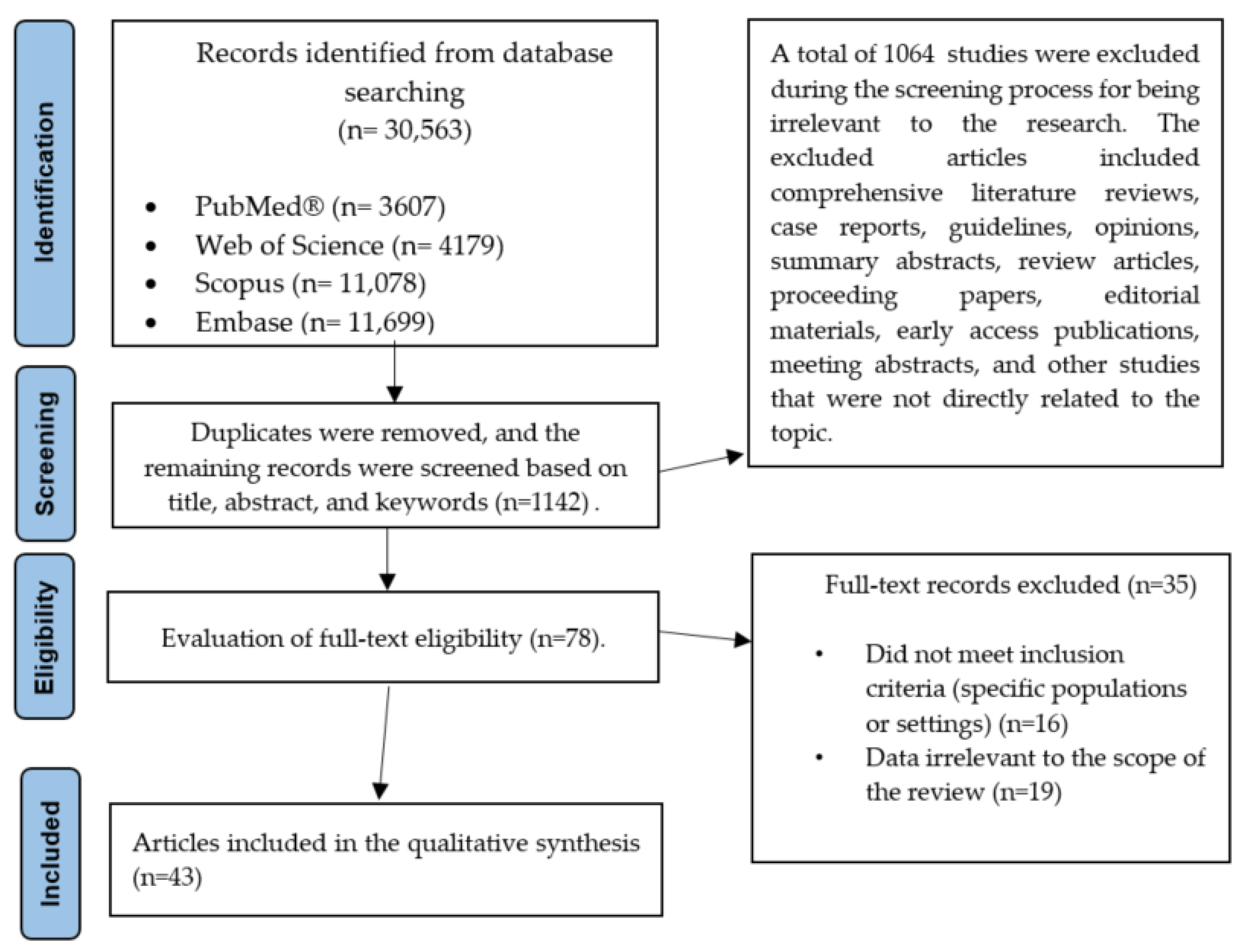
| Category | Description |
|---|---|
| Population (P) | Adults and elderly individuals (≥18 years), patients with depression, migraine, or Alzheimer’s disease |
| Intervention (I) | Magnesium supplementation in any form (oral, intravenous), the effects of a magnesium-rich diet |
| Comparison (C) | Placebo or other standard treatments, comparison between individuals with low and normal magnesium levels |
| Outcome (O) | Improvement in mood and cognitive function, reduction in depressive symptoms (e.g., Beck Depression Inventory scores), changes in migraine attack frequency and intensity, slowing of Alzheimer’s disease progression (e.g., Mini-Mental State Examination (MMSE) score changes) |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Randomized controlled/clinical trials | Animal studies and in vitro research |
| Longitudinal and prospective cohort studies | Non-peer-reviewed articles, conference abstracts, opinion papers |
| Full-text, peer-reviewed scientific articles | Case reports |
| Studies conducted on human participants | Studies published in languages other than English or Hungarian |
| Study (Ref.) | Intervention | Duration | Participants | Outcomes Measured | Key Findings |
|---|---|---|---|---|---|
| Afsharfar et al. [96] | Oral magnesium oxide | 500 mg/day for 8 weeks | 46 depressed patients (randomized into Mg and placebo groups) | Beck Depression Inventory scores, serum BDNF, serum Mg | Significant improvement in BDI scores (p = 0.01) and serum Mg levels (p = 0.001); no change in BDNF (p = 0.507) |
| Barragán-Rodríguez et al. [97] | Oral magnesium chloride (MgCl2) | 450 mg of elemental Mg/day for 12 weeks | 23 elderly patients with type 2 diabetes and hypomagnesemia | Yasavage and Brink depression scores, serum Mg levels | No difference in depression (p = 0.27); higher serum Mg in the MgCl2 group (p < 0.0005) |
| Rodríguez-Morán et al. [98] | Oral magnesium chloride + vitamin D | 1300 mg of MgCl2 + 4000 IU of vitamin D daily for 4 months | 60 long COVID patients with mild to moderate depression | Beck Depression Inventory (BDI) scores | A significant reduction in BDI scores (p < 0.01 intervention; p < 0.05 control) |
| Rajizadeh et al. [99] | Oral magnesium oxide (MgO) | 500 mg/day for 8 weeks | 60 depressed patients (Mg vs. placebo groups) | Beck Depression Inventory-II scores, serum Mg levels | Significant reductions in BDI scores (p = 0.02) and Mg (p = 0.002); 15.65 points (Mg) vs. 10.40 (placebo) |
| Abiri et al. [100] | Oral magnesium + vitamin D | 50,000 IU of vitamin D weekly + 250 mg of Mg daily for 8 weeks | 108 obese women with mild to moderate depression | BDI-II scores, inflammatory markers, 25(OH)-D, serum Mg | Significant improvements in markers; no significant difference in BDI-II scores |
| Shakya et al. [101] | Dietary patterns (PCA, RRR, PLS) | N/A | 1743 adults from the North West Adelaide Health Study (NWAHS) | CES-D scores, dietary patterns | ‘Prudent’ diet inversely and ‘Western’ diet positively associated with depression (PCA: OR = 0.57, 2.04) |
| Fard et al. [102] | Oral magnesium sulfate, zinc sulfate | 27 mg of zinc sulfate or 320 mg of magnesium sulfate for 8 weeks | 99 women postpartum (randomized into groups) | Edinburgh Postnatal Depression Scale scores, Anxiety Inventory scores | No significant difference in depression (p = 0.553) or anxiety |
| Mehdi et al. [103] | IV magnesium sulfate | 4 g of magnesium sulfate in 5% dextrose for 8 days (5-day washout) | 12 subjects with mild to moderate treatment-resistant depression | Serum Mg, PHQ-9 scores | A significant increase in serum Mg (p = 0.02); a decrease in PHQ-9 scores (p = 0.02) |
| Tarleton et al. [23] | Oral magnesium chloride | 248 mg/day for 6 weeks | 126 adults with mild to moderate depression | PHQ-9 scores, GAD-7 scores, adherence, adverse effects | Significant improvements in PHQ-9 (−6.0 points, p < 0.001) and GAD-7 (−4.5 points, p < 0.001) scores |
| Ryszewska-Pokraśniewicz et al. [22] | Oral magnesium aspartate | 120 mg/day for 8 weeks with fluoxetine | 37 patients with recurrent depression disorder | HDRS scores, serum Mg levels, pharmaco-EEG | No significant changes in HDRS scores but increased effectiveness with magnesium augmentation |
| Pouteau et al. [104] | Oral magnesium + vitamin B6 | 300 mg of Mg + 30 mg of vitamin B6 for 8 weeks | 264 healthy adults with stress (DASS-42 > 18) | DASS-42 stress subscale scores | A 24% greater improvement in severe stress with Mg + B6 (p = 0.0203) |
| Derom et al. [105] | Dietary magnesium intake | Median follow-up of 6.3 years | 12,939 Spanish university graduates | Depression incidence (self-reported, antidepressant use) | No association between Mg intake and depression risk (p-trend = 0.59) |
| Nazarinasab et al. [106] | Oral magnesium supplement, 250 mg/day | 6 weeks of Mg vs. placebo + SSRI treatment | 60 patients with major depressive disorder (MDD) | Beck Depression Inventory-II scores | A significant improvement in BDI scores at 4 and 6 weeks (p = 0.02, p = 0.001) |
| Study (Ref.) | Intervention | Duration | Participants | Outcomes Measured | Key Findings |
|---|---|---|---|---|---|
| Magnesium supplementation only | |||||
| Demirkaya et al. [115] | IV MgSO4 (1 g) | Single 15 min infusion | 30 patients (15 Mg, 15 placebo) with migraine | Pain, symptoms, side effects at 0 and 30 min and 2 h | 87% pain-free with Mg vs. 0% with the placebo; total symptom relief: 100% vs. 20%; mild side effects |
| Bigal et al. [116] | IV MgSO4 (1 g) | Single 10 mL infusion | 120 patients (60 with aura, 60 without aura) | Pain, nausea, photophobia, phonophobia, aura | With aura: significant relief (NNT = 2.7); without aura: no pain/nausea relief (NNT = 5.98); ↓ photo/phonophobia |
| Combined supplementation | |||||
| Cete et al. [113] | IV MgSO4 (2 g) + metoclopramide (10 mg) | Single 10 min infusion | 113 adults with migraine (IHS criteria), three groups | VAS scores at 0, 15, and 30 min; rescue meds; recurrence at 24 h | All groups improved by >25 mm; no VAS differences; the placebo needed more rescue meds |
| Corbo et al. [114] | IV MgSO4 (2 g) + metoclopramide (20 mg) vs. placebo | Max of three doses at 15 min intervals | 44 adults with acute migraine | VAS scores at 0–45 min; function; side effects | The Mg group was less effective (–16 mm); NNH = 4; worse functional outcomes |
| Shahrami et al. [117] | IV MgSO4 (1 g) vs. 8 mg dexamethasone + 10 mg metoclopramide | Single IV dose | 70 adults, randomized into two equal groups | NRS scores at the baseline, 20 min, 1 h, and 2 h | MgSO4 led to faster and greater pain reductions (2 h NRS scores: 1.3 vs. 2.5); p < 0.0001 |
| Matin et al. [118] | Oral magnesium (250 mg) + vitamin B12 (1 mg) ± HIIT | 2 months | 60 women, four randomized groups | CGRP levels, MIDAS scores, frequency, intensity, duration | HIIT + Mg reduced CGRP levels and migraine indicators the most; supported by in silico anti-inflammatory findings |
| Kandil et al. [119] | IV Mg (2 g), metoclopramide (10 mg), or prochlorperazine (10 mg) | Single IV dose | 157 adult ED migraine patients, randomized into three groups | NRS scores at 30, 60, and 120 min; ED stays; rescue meds; adverse events | No significant difference at 30 or 60 min (e.g., ΔNRS score at 60 min: Mg: –4, p = 0.27); similar side effect rates |
| Gaul et al. [120] | Oral Mg (1100 mg) + riboflavin (400 mg) + CoQ10 (150 mg) (combined supplement) | 3 months (after the 4-week baseline) | 130 adults with ≥3 migraines/month | Migraine days, pain intensity, HIT-6 scores, subjective benefit | Days: –1.8 vs. –1.0 (NS); pain (p = 0.03), HIT-6 scores (p = 0.01), subjective efficacy (p = 0.01) |
| Rahimdel et al. [121] | IV MgSO4 (1 g in 100 mL saline) vs. DHE | Single IV dose | 120 severe migraine patients in the ER | VAS scores at 30, 60, and 90 min | Mg group significantly better at 60 and 90 min (VAS scores: 2.48 vs. 3.48 at 90 min; p < 0.05) |
| Ginder et al. [122] | IV MgSO4 (1 g) vs. prochlorperazine | Single IV dose | 36 ED patients with acute headache | VAS score before and 30 min post-infusion | Pain relief: 90% (prochlorperazine) vs. 56% (Mg), significant; Mg’s effect not related to serum Mg levels |
| Khani et al. [123] | Oral magnesium (500 mg/day), sodium valproate (400 mg/day), and their combination for 3 months | A: VPA 200 mg BID + P; B: VPA 200 mg BID + Mg 250 mg BID; C: Mg 250 mg BID + P | 222 patients (18–65 years), ≥4 migraines/month; three randomized groups | Frequency, severity, duration, painkillers/month, MIDAS scores, HIT-6 scores | All groups improved (p < 0.001); combo > valproate > Mg alone; greater MIDAS/HIT-6 score reduction in combo and valproate groups (p < 0.001) |
| Karimi et al. [124] | Oral magnesium oxide (500 mg BID) vs. sodium valproate (400 mg BID) | 8 weeks, crossover | 70 migraine patients; 63 completed | Monthly attack frequency, headache days, headache hours | No significant difference between treatments; both effective and safe |
| Tarighat Esfanjani et al. [125] | Oral magnesium oxide (500 mg/day) and L-carnitine (500 mg/day) | 12 weeks; Mg 500 mg/day, L-carnitine 500 mg/day, combo = same doses | 133 migraine patients, randomized into three intervention groups and one control group | Attacks/month, days/month, severity, and serum Mg and L-carnitine levels | All interventions reduced migraine indicators (p < 0.05); ANOVA: significant frequency reduction (p = 0.008); Mg had an independent significant effect |
| Köseoglu et al. [126] | Oral Mg citrate (600 mg/day) | 3 months | 40 patients with migraine without aura (30 Mg, 10 placebo), aged 20–55 | Attack frequency, severity, P1 amplitude (VEP), cortical perfusion (SPECT) | Mg group: ↓ frequency (p = 0.005), ↓ severity (p < 0.001), ↓ P1 (p < 0.05); ↑ cortical perfusion (p = 0.001–0.01); all vs. placebo significant |
| Study (Ref.) | Intervention | Duration | Participants | Outcomes Measured | Key Findings | Cognitive Outcome Measures |
|---|---|---|---|---|---|---|
| Alam et al. [155] | Baseline serum magnesium levels | 24 years | 12,040 dementia-free adults | Dementia incidence; cognitive function | Lowest serum magnesium quintile linked to a 24% higher dementia risk (HR = 1.24); no link with cognitive decline rates | Dementia incidence, DWRT, DSST, WFT |
| Zhu et al. [151] | Oral personalized magnesium supplementation vs. a placebo | 12 weeks | 250 (subgroup >65 years, high Ca:Mg ratios) | Cognition; APOE gene methylation | Reducing Ca:Mg to ~2.3 improved cognition by 9.1% (p = 0.03), mediated partly by epigenetic changes | MoCA |
| Ni et al. [159] | Oral Mg-L-threonate (1.2 g/day) vs. a placebo | 12 weeks; 3- and 6-month follow-up | 109 post-breast cancer surgery patients | Pain, mood, sleep, cognition | No significant benefits in terms of pain, mood, sleep, or cognition; combination therapies suggested | TICS |
| Ozawa et al. [160] | Dietary intake of K, Ca, and Mg (FFQ) | 17 years of follow-up | 1081 Japanese adults ≥60, dementia-free | Incidence of all-cause dementia, VaD, AD | Higher intake of K, Ca, and Mg linked to a lower risk of all-cause dementia and vascular dementia; no link with AD | Incidence of dementia, AD, and VaD |
| Cherbuin et al. [161] | Dietary intake of Mg, K, Fe (questionnaire) | 8 years of follow-up | 1406 cognitively healthy adults (mean age: 62.5) | Risk of MCI and mild cognitive disorders | Higher Mg intake associated with reduced MCI/MCD risk; higher K and Fe intake linked to increased risk | MMSE |
| Lo et al. [162] | Dietary and supplemental Mg intake (FFQ) | >20 years of follow-up | 6473 postmenopausal women (65–79 years) | Physician-adjudicated MCI and probable dementia (PD) | Moderate Mg intake (Q2–Q5) associated with a lower risk of MCI and PD; non-linear relationship, no significant effect at extremes | Modified MMSE |
| Kimura et al. [163] | Dietary intake of vegetables, fruits, and nutrients (FFQ) | 24 years | 1071 Japanese adults ≥60, dementia-free | Incident dementia, AD, VaD | Higher vegetable intake linked to a 27% lower dementia risk and a 31% lower AD risk; no association with VaD; higher Mg, Ca, K, vitamin A and C, and riboflavin intake also protective; fruit intake not significant | Incidence of dementia, AD, and VaD |
| Tao et al. [164] | Total magnesium intake (diet + supplements) | NHANES 2011–2014 | 2508 adults ≥60 years | Global cognitive z-scores; serum vitamin D levels | Higher magnesium intake linked to better cognition, especially in women, non-Hispanic Whites, and those with sufficient vitamin D levels | CERAD, AF, DSST |
| Tao et al. [165] | Dietary magnesium intake (FFQ) | 6 years | 5663 adults ≥55 years | Cognitive tests (MMSE, DSST, CDT); impairment risk | Higher intake linked to a lower cognitive impairment risk in men (MMSE, DSST); in women, only MMSE results were significant. This effect was independent of vitamin D in men. | MMSE, DSST, CDT |
| Luo et al. [166] | Dietary Ca, Mg, and Ca:Mg ratio (FFQ) | 5 years | 1565 dementia-free urban older adults | Incident dementia (DSM-IV) | Lowest tertile for Ca (<339.1 mg/day) and Mg (<202.1 mg/day) intake linked to the highest dementia risk; in the subgroup with Ca:Mg ≤1.69, a Mg intake >267.5 mg/day increased dementia risk (HR: 3.97)—highlights the importance of Ca:Mg balance | MMSE, Conflicting Instructions Task, Stick Design Test, modified Common Objects Sorting Test, Auditory Verbal Learning Test, modified Fuld Object Memory Evaluation, and Trail Making Test Parts A and B |
| Tzeng et al. [167] | Oral magnesium oxide (MgO) use vs. no use | 10 years | 1547 MgO users vs. 4641 matched controls (≥50 years) | Incidence of dementia (Cox regression) | MgO users showed a significantly lower dementia risk (adjusted HR: 0.517, p = 0.001) | Incidence of AD, VaD, and non-VaD |
| Zhang et al. [168] | Oral magnesium L-threonate (Magtein® PS: 400 mg Mg L-threonate + phosphatidylserine + vitamins C, D, B6) | 30 days | 109 healthy Chinese adults (18–65 years) | Clinical Memory Test (5 subtests + memory quotient | The Magtein®PS group showed significant improvements in all memory domains; older adults benefited most. | The Clinical Memory Test |
| Takeuchi et al. [169] | Web-based 24 h dietary assessment of macronutrients and minerals (alcohol, sugars, fats, magnesium, protein) | 12 years | 161,376 middle-aged and older UK adults (the UK Biobank cohort) | Incidence of all-cause dementia (hospital records, death registry) | Higher dementia risk associated with no alcohol intake and high sugar/carbohydrate, very low/high fat, very low/high Mg, and the highest protein intake; moderate intake linked to lower risk | Dementia incidence |
| Cohen-Hagai et al. [170] | Oral magnesium oxide (520 mg) vs. a placebo | 8 weeks | 29 outpatients with liver cirrhosis | Serum/intracellular Mg; cognition | 83% had cognitive impairments; cognitive scores correlated with Mg levels | The MoCA, the CCT, digit span examinations, and the Lowenstein Occupational Cognitive Assessment |
| Alateeq et al. [171] | Dietary magnesium intake (24 h recall); latent trajectory analysis | 17 years | 6001 individuals aged 40–73 | Brain volumes (GM, WM, hippocampus), white matter lesions (WMLs), blood pressure | Higher Mg intake associated with larger brain volumes and fewer WMLs, especially in post-menopausal women; BP did not mediate outcomes | Brain volumes (GM, WM, LHC, RHC, WMLs) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, P.; Lehoczki, A.; Fekete, M.; Jarecsny, T.; Kryczyk-Poprawa, A.; Zábó, V.; Major, D.; Fazekas-Pongor, V.; Csípő, T.; Varga, J.T. The Role of Magnesium in Depression, Migraine, Alzheimer’s Disease, and Cognitive Health: A Comprehensive Review. Nutrients 2025, 17, 2216. https://doi.org/10.3390/nu17132216
Varga P, Lehoczki A, Fekete M, Jarecsny T, Kryczyk-Poprawa A, Zábó V, Major D, Fazekas-Pongor V, Csípő T, Varga JT. The Role of Magnesium in Depression, Migraine, Alzheimer’s Disease, and Cognitive Health: A Comprehensive Review. Nutrients. 2025; 17(13):2216. https://doi.org/10.3390/nu17132216
Chicago/Turabian StyleVarga, Péter, Andrea Lehoczki, Mónika Fekete, Tamás Jarecsny, Agata Kryczyk-Poprawa, Virág Zábó, Dávid Major, Vince Fazekas-Pongor, Tamás Csípő, and János Tamás Varga. 2025. "The Role of Magnesium in Depression, Migraine, Alzheimer’s Disease, and Cognitive Health: A Comprehensive Review" Nutrients 17, no. 13: 2216. https://doi.org/10.3390/nu17132216
APA StyleVarga, P., Lehoczki, A., Fekete, M., Jarecsny, T., Kryczyk-Poprawa, A., Zábó, V., Major, D., Fazekas-Pongor, V., Csípő, T., & Varga, J. T. (2025). The Role of Magnesium in Depression, Migraine, Alzheimer’s Disease, and Cognitive Health: A Comprehensive Review. Nutrients, 17(13), 2216. https://doi.org/10.3390/nu17132216





