Vitamin D Status Determines the Effect of Cabergoline on Sexual Function and Depressive Symptoms in Hyperprolactinemic Women
Abstract
1. Introduction
2. Materials and Methods
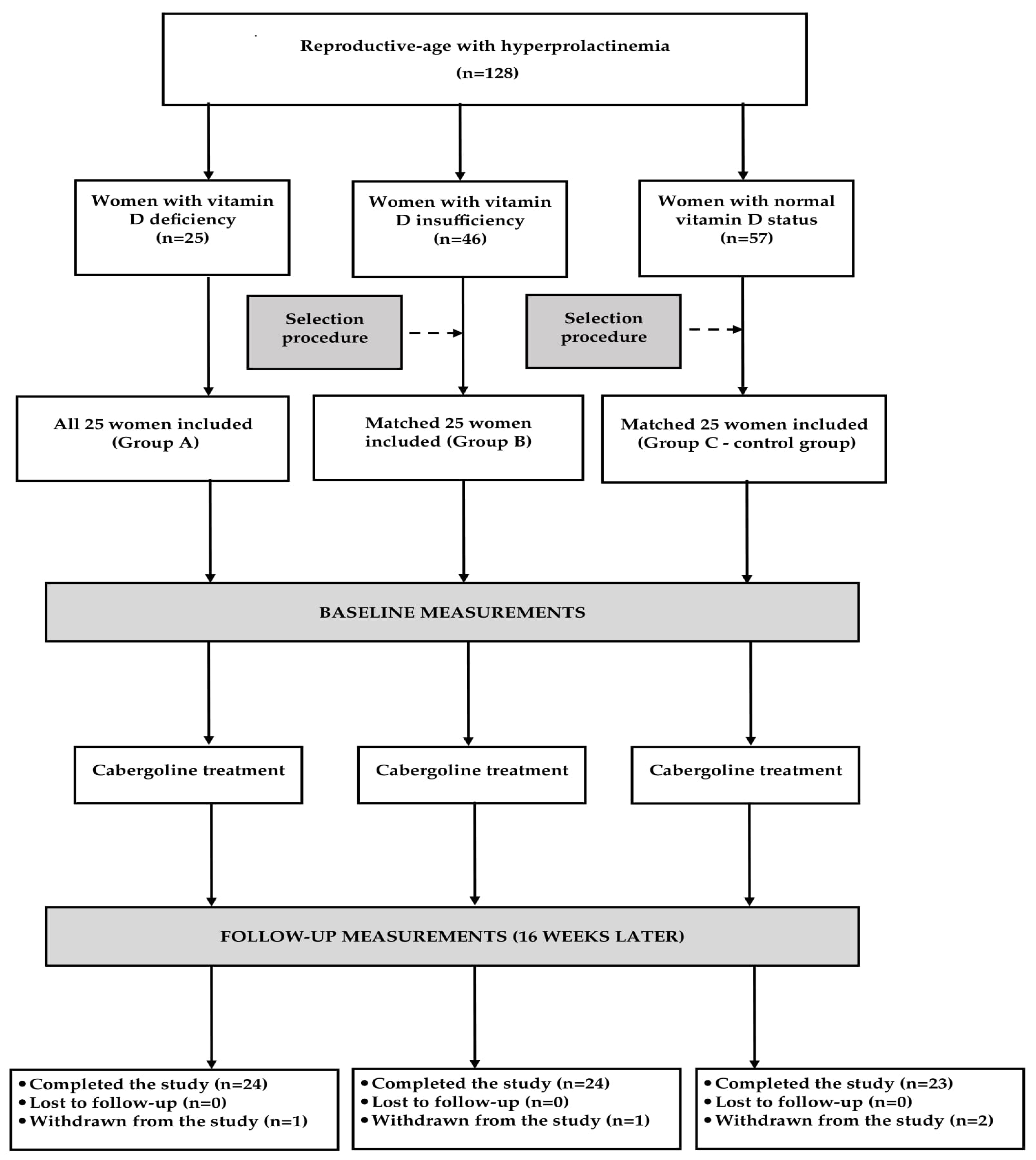
2.1. Study Population
2.2. Study Design
2.3. Laboratory Assays
2.4. Questionnaires
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Groups
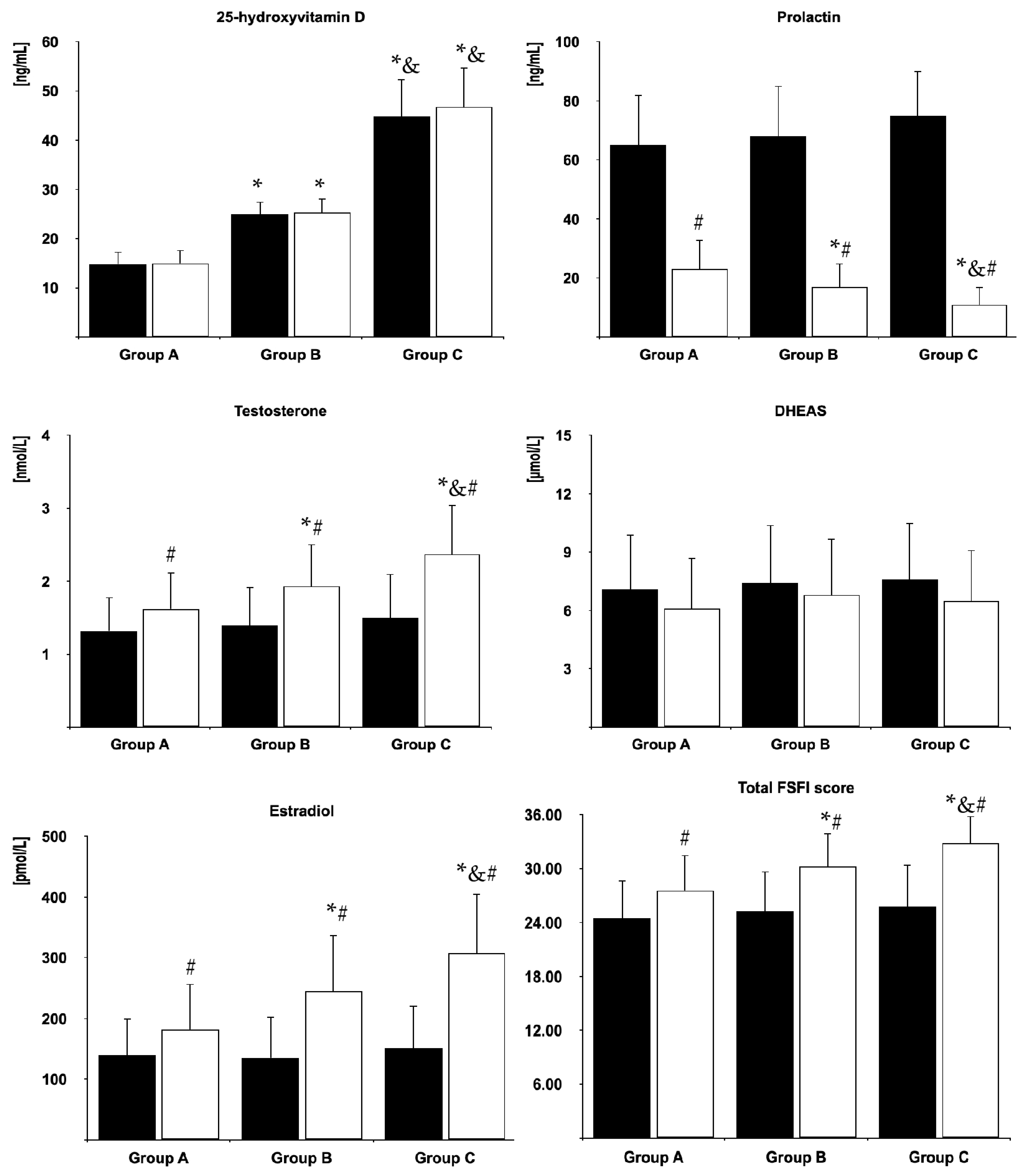
3.2. Biochemical Variables
3.3. Sexual Functioning
3.4. Depressive Symptoms
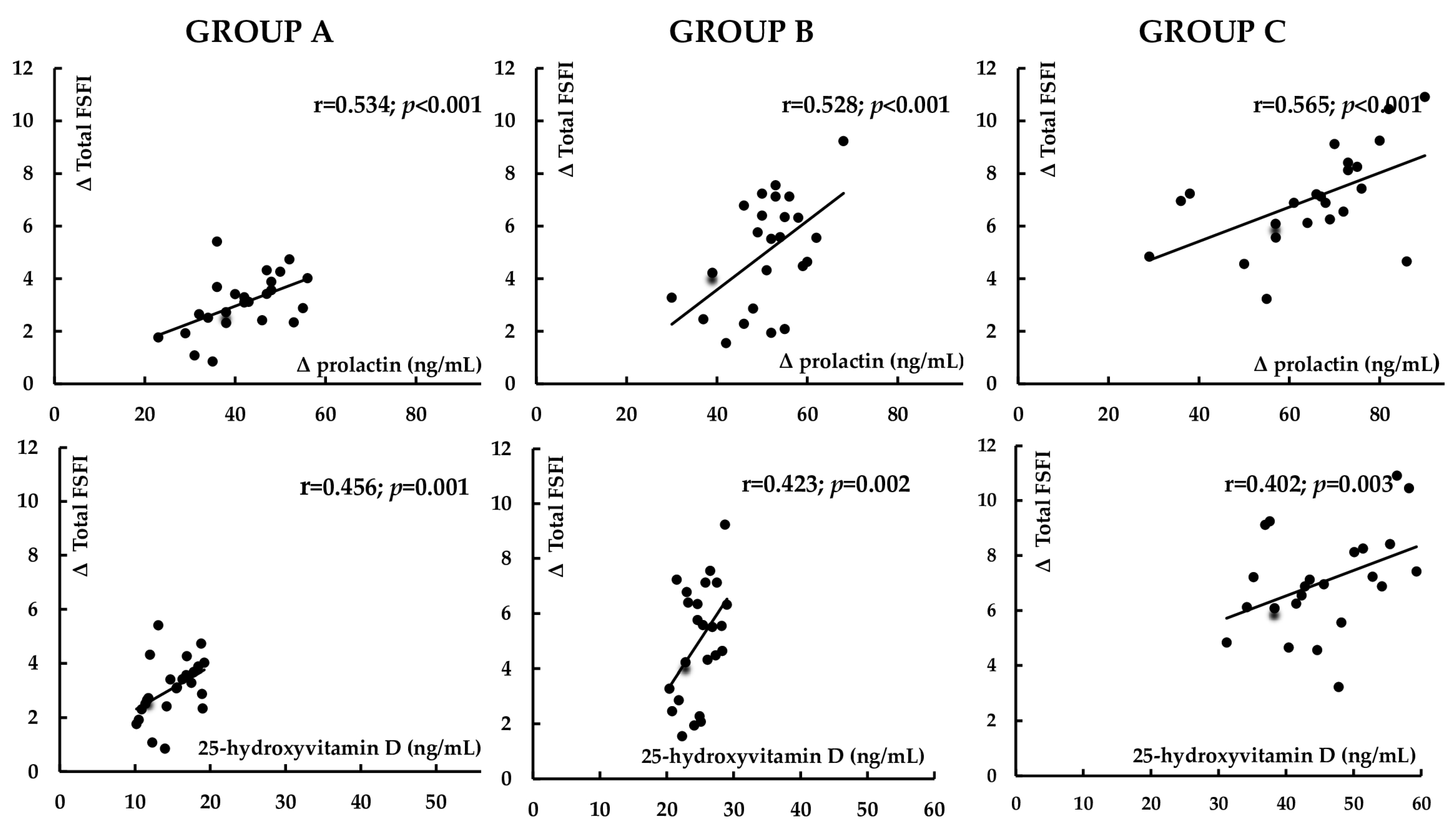
3.5. Correlations
3.6. Multivariate Regression Analysis
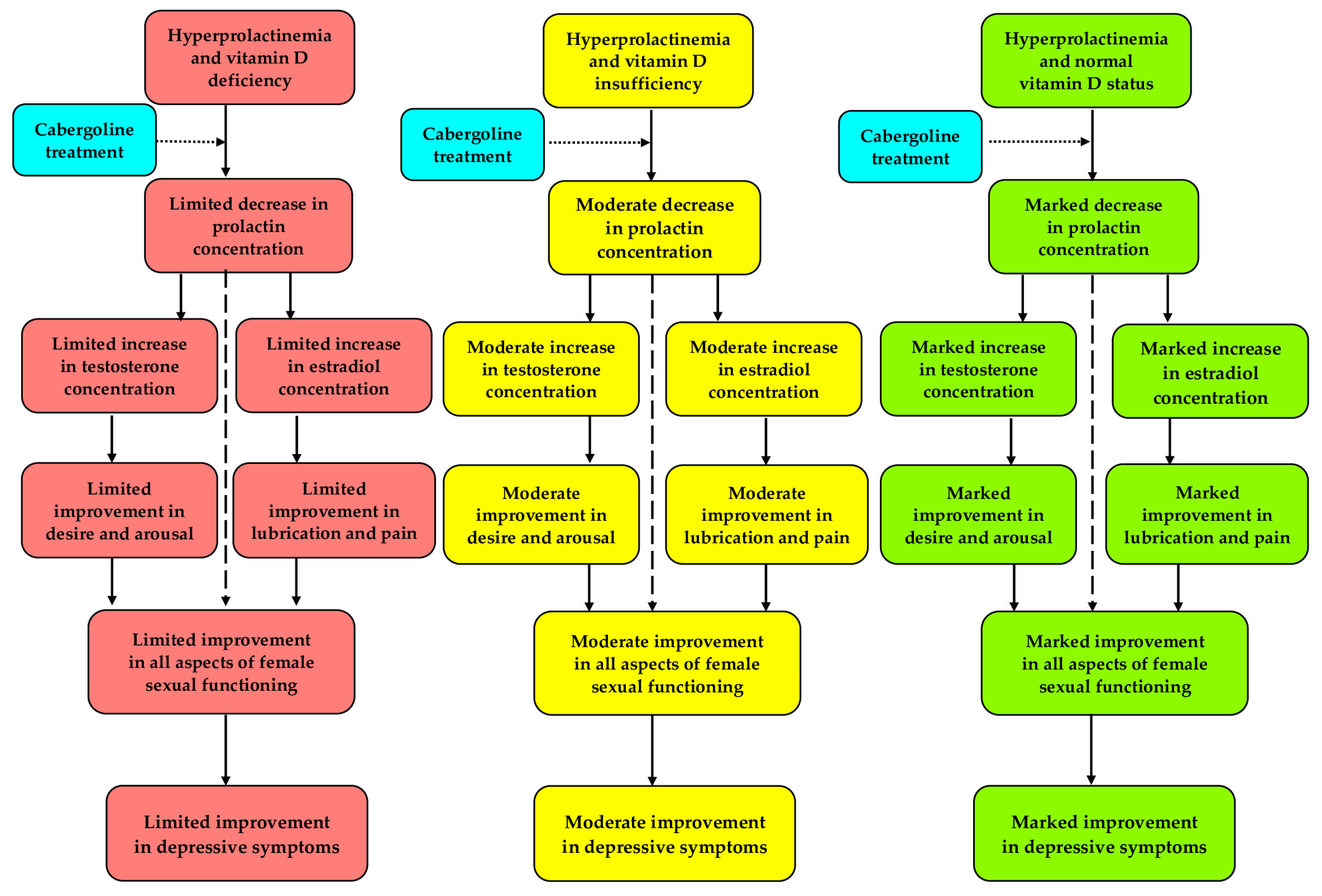
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilar, L.; Vilar, C.F.; Lyra, R.; Freitas, M.D. Pitfalls in the diagnostic evaluation of hyperprolactinemia. Neuroendocrinology 2019, 109, 7–19. [Google Scholar] [CrossRef]
- Romijn, J.A. Hyperprolactinemia and prolactinoma. Handb. Clin. Neurol. 2014, 124, 185–195. [Google Scholar]
- Lundberg, P.O.; Hulter, B. Sexual dysfunction in patients with hypothalamo-pituitary disorders. Exp. Clin. Endocrinol. 1991, 98, 81–88. [Google Scholar] [CrossRef]
- Kadioglu, P.; Yalin, A.S.; Tiryakioglu, O.; Gazioglu, N.; Oral, G.; Sanli, O.; Onem, K.; Kadioglu, A. Sexual dysfunction in women with hyperprolactinemia: A pilot study report. J. Urol. 2005, 174, 1921–1925. [Google Scholar] [CrossRef]
- Krysiak, R.; Drosdzol-Cop, A.; Skrzypulec-Plinta, V.; Okopień, B. Sexual function and depressive symptoms in young women with elevated macroprolactin content: A pilot study. Endocrine 2016, 53, 291–298. [Google Scholar] [CrossRef]
- Noroozzadeh, M.; Tehrani, F.R.; Mobarakabadi, S.S.; Farahmand, M.; Dovom, M.R. Sexual function and hormonal profiles in women with and without polycystic ovary syndrome: A population-based study. Int. J. Impot. Res. 2017, 29, 1–6. [Google Scholar] [CrossRef]
- Benetti-Pinto, C.L.; Nácul, A.P.; Rosa, E.; Silva, A.C.; Maciel, G.A.; Dos Santos Nunes Nogueira, V.; Elias, P.C.; Martins, M.; Kasuki, L.; Garmes, H.M.; et al. Hyperprolactinemia in women: Treatment. Rev. Bras. Ginecol. Obstet. 2024, 46, e-FPS05. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The effect of bromocriptine treatment on sexual functioning and depresssive symptoms in women with mild hyperprolactinemia. Pharmacol. Rep. 2018, 70, 227–232. [Google Scholar] [CrossRef]
- Krysiak, R.; Okopień, B. Sexual functioning in hyperprolactinemic patients treated with cabergoline or bromocriptine. Am. J. Ther. 2019, 26, e430–e440. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Sexual function and depressive symptoms in young women with hypoprolactinaemia. Clin. Endocrinol. 2020, 93, 482–488. [Google Scholar] [CrossRef]
- Krysiak, R.; Gilowska, M.; Okopień, B. Sexual function and depressive symptoms in young women with low vitamin D status: A pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 204, 108–112. [Google Scholar] [CrossRef]
- Inal, Z.O.; Inal, H.A.; Gorkem, U. Sexual function and depressive symptoms in primary infertile women with vitamin D deficiency undergoing IVF treatment. Taiwan J. Obstet. Gynecol. 2020, 59, 91–98. [Google Scholar] [CrossRef]
- Canat, M.; Canat, L.; Öztürk, F.Y.; Eroğlu, H.; Atalay, H.A.; Altuntaş, Y. Vitamin D3 deficiency is associated with female sexual dysfunction in premenopausal women. Int. Urol. Nephrol. 2016, 48, 1789–1795. [Google Scholar] [CrossRef]
- Eickman, K.; Maxwell, R.; McGinnis, L.K.; Stanczyk, F.; Legro, R.; Lindheim, S.R. Total and bioavailable 25-hydroxyvitamin D is not associated with improved sexual dysfunction following vitamin D supplementation in women with polycystic ovarian syndrome: A pilot study. J. Sex. Med. 2024, 21, 240–247. [Google Scholar] [CrossRef]
- Rafati, M.; Bazrafshan, E.; Shaki, F.; Ghalini-Moghaddam, T.; Moghimi, M. The relationship between serum vitamin D, testosterone, and oxidative stress levels in women with sexual dysfunction: A case-controlled study. Taiwan J. Obstet. Gynecol. 2024, 63, 673–678. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. Sexual function and depressive symptoms in young women with euthyroid Hashimoto’s thyroiditis receiving vitamin D, selenomethionine and myo-inositol: A pilot study. Nutrients 2023, 15, 2815. [Google Scholar] [CrossRef]
- Vitale, S.G.; Caruso, S.; Rapisarda, A.M.; Cianci, S.; Cianci, A. Isoflavones, calcium, vitamin D and inulin improve quality of life, sexual function, body composition and metabolic parameters in menopausal women: Result from a prospective, randomized, placebo-controlled, parallel-group study. Prz. Menopauzalny 2018, 17, 32–38. [Google Scholar] [CrossRef]
- Sarebani, Z.; Chegini, V.; Chen, H.; Aali, E.; Mirzadeh, M.; Abbaspour, M.; Griffiths, M.D.; Alimoradi, Z. Effect of vitamin D vaginal suppository on sexual functioning among postmenopausal women: A three-arm randomized controlled clinical trial. Obstet. Gynecol. Sci. 2023, 66, 208–220. [Google Scholar] [CrossRef]
- Youssef, E.; Badie, M.S.; Ismail, D.; Gamal, A.; Eldamanhoury, H.M. The effectiveness of vitamin D as an alternative to FDA-approved treatment and other therapies for managing vulvovaginal atrophy and sexual inactivity in postmenopausal women. A systematic review and meta-analysis. Int. J. Gynaecol. Obstet. 2025, 56, 219–233. [Google Scholar] [CrossRef]
- Eskildsen, P.C.; Lund, B.; Sørensen, O.H.; Lund, B.; Bishop, J.E.; Norman, A.W. Acromegaly and vitamin D metabolism: Effect of bromocriptine treatment. J. Clin. Endocrinol. Metab. 1979, 49, 484–486. [Google Scholar] [CrossRef]
- Krysiak, R.; Basiak, M.; Machnik, G.; Szkróbka, W.; Okopień, B. Vitamin D status determines cardiometabolic effects of cabergoline in women with elevated prolactin levels: A pilot study. Nutrients 2023, 15, 2303. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Parkes, A.S. Seasonal variation in human sexual activity. Eugen. Soc. Symp. 1968, 4, 128–145. [Google Scholar]
- Lukmanji, A.; Williams, J.V.; Bulloch, A.G.; Bhattarai, A.; Patten, S.B. Seasonal variation in symptoms of depression: A Canadian population-based study. J. Affect. Disord. 2019, 255, 142–149. [Google Scholar] [CrossRef]
- Benucci, A.; Tommasi, M.; Fantappié, B.; Scardigli, S.; Ottanelli, S.; Pratesi, E.; Romano, S. Serum 25-hydroxyvitamin D levels in normal subjects: Seasonal variations and relationships with parathyroid hormone and osteocalcin. J. Nucl. Biol. Med. 1993, 37, 77–82. [Google Scholar]
- Kivelä, A.; Kauppila, A.; Ylöstalo, P.; Vakkuri, O.; Leppäluoto, J. Seasonal, menstrual and circadian secretions of melatonin, gonadotropins and prolactin in women. Acta Physiol. Scand. 1988, 132, 321–327. [Google Scholar] [CrossRef]
- Kauppila, A.; Pakarinen, A.; Kirkinen, P.; Mäkilä, U. The effect of season on the circulating concentrations of anterior pituitary, ovarian and adrenal cortex hormones and hormone binding proteins in the subarctic area; evidence of increased activity of the pituitary-ovarian axis in spring. Gynecol. Endocrinol. 1987, 1, 137–150. [Google Scholar] [CrossRef]
- Wilkinson, T.; Li, B.; Soule, S.; Hunt, P. The utility of rested prolactin sampling in the evaluation of hyperprolactinaemia. Intern. Med. J. 2024, 54, 307–311. [Google Scholar] [CrossRef]
- Rosen, R.; Brown, C.; Heiman, J.; Leiblum, S.; Meston, C.; Shabsigh, R.; Ferguson, D.; D’Agostino, R., Jr. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital. Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef]
- Wiegel, M.; Meston, C.; Rosen, R. The Female Sexual Function Index (FSFI): Cross-validation and development of clinical cut-off scores. J. Sex Marital. Ther. 2005, 31, 1–20. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI-II: Beck Depression Inventory Manual, 2nd ed.; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders—DSM-IV-TR, 4th ed.; American Psychiatric Publishing: Washington, DC, USA, 1994. [Google Scholar]
- Cea García, J.; Márquez Maraver, F.; Rubio Rodríguez, M.C. Cross-sectional study on the impact of age, menopause and quality of life on female sexual function. J. Obstet. Gynaecol. 2022, 42, 1225–1232. [Google Scholar] [CrossRef]
- Ferrández Infante, A.; Novella Arribas, B.; Khan, K.S.; Zamora, J.; Jurado López, A.R.; Fragoso Pasero, M.; Suárez Fernández, C. Obesity and female sexual dysfunctions: A systematic review of prevalence with meta-analysis. Semergen 2023, 49, 102022. [Google Scholar] [CrossRef]
- Doumas, M.; Tsiodras, S.; Tsakiris, A.; Douma, S.; Chounta, A.; Papadopoulos, A.; Kanellakopoulou, K.; Giamarellou, H. Female sexual dysfunction in essential hypertension: A common problem being uncovered. J. Hypertens. 2006, 24, 2387–2392. [Google Scholar] [CrossRef]
- Rains, C.P.; Bryson, H.M.; Fitton, A. Cabergoline. A review of its pharmacological properties and therapeutic potential in the treatment of hyperprolactinaemia and inhibition of lactation. Drugs 1995, 49, 255–279. [Google Scholar] [CrossRef]
- Bancos, I.; Nannenga, M.R.; Bostwick, J.M.; Silber, M.H.; Erickson, D.; Nippoldt, T.B. Impulse control disorders in patients with dopamine agonist-treated prolactinomas and nonfunctioning pituitary adenomas: A case-control study. Clin. Endocrinol. 2014, 80, 863–868. [Google Scholar] [CrossRef]
- Davis, S.R.; Worsley, R.; Miller, K.K.; Parish, S.J.; Santoro, N. Androgens and female sexual function and dysfunction—Findings from the Fourth International Consultation of Sexual Medicine. J. Sex. Med. 2016, 13, 168–178. [Google Scholar] [CrossRef]
- Davison, S.L.; Davis, S.R. Androgens in women. J. Steroid Biochem. Mol. Biol. 2003, 85, 363–366. [Google Scholar] [CrossRef]
- Pilz, S.; Frisch, S.; Koertke, H.; Kuhn, J.; Dreier, J.; Obermayer-Pietsch, B.; Wehr, E.; Zittermann, A. Effect of vitamin D supplementation on testosterone levels in men. Horm. Metab. Res. 2011, 43, 223–225. [Google Scholar] [CrossRef]
- Chang, E.M.; Kim, Y.S.; Won, H.J.; Yoon, T.K.; Lee, W.S. Association between sex steroids, ovarian reserve, and vitamin D levels in healthy nonobese women. J. Clin. Endocrinol. Metab. 2014, 99, 2526–2532. [Google Scholar] [CrossRef]
- Ahonen, M.H.; Zhuang, Y.H.; Aine, R.; Ylikomi, T.; Tuohimaa, P. Androgen receptor and vitamin D receptor in human ovarian cancer: Growth stimulation and inhibition by ligands. Int. J. Cancer 2000, 86, 40–46. [Google Scholar] [CrossRef]
- Gracia, C.R.; Freeman, E.W.; Sammel, M.D.; Lin, H.; Mogul, M. Hormones and sexuality during transition to menopause. Obstet. Gynecol. 2007, 109, 831–840. [Google Scholar] [CrossRef]
- Koppelman, M.C.; Parry, B.L.; Hamilton, J.A.; Alagna, S.W.; Loriaux, D.L. Effect of bromocriptine on affect and libido in hyperprolactinemia. Am. J. Psychiatry 1987, 144, 1037–1041. [Google Scholar]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Yoon, H.S.; Kunugi, H. Cabergoline, a dopamine receptor agonist, has an antidepressant-like property and enhances brain-derived neurotrophic factor signaling. Psychopharmacology 2010, 211, 291–301. [Google Scholar] [CrossRef]
- Płudowski, P.; Ducki, C.; Konstantynowicz, J.; Jaworski, M. Vitamin D status in Poland. Pol. Arch. Med. Wewn. 2016, 126, 530–539. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor, and 1alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Papenberg, G.; Jonasson, L.; Karalija, N.; Johansson, J.; Köhncke, Y.; Salami, A.; Andersson, M.; Axelsson, J.; Wåhlin, A.; Riklund, K.; et al. Mapping the landscape of human dopamine D2/3 receptors with [(11)C]raclopride. Brain Struct. Funct. 2019, 224, 2871–2882. [Google Scholar] [CrossRef]

| Variable | Group A | Group B | Group C |
|---|---|---|---|
| Number of patients | 24 | 24 | 23 |
| Age (years) | 32 ± 6 | 31 ± 7 | 32 ± 6 |
| Body mass index (kg/m2) | 23.5 ± 4.9 | 23.2 ± 5.1 | 22.8 ± 4.7 |
| Smokers (%)/Number of cigarettes a day (n)/Duration of smoking (months) | 38/9 ± 7/95 ± 40 | 42/9 ± 6/89 ± 38 | 43/10 ± 7/85 ± 35 |
| Reasons for prolactin excess: drug-induced hyperprolactinemia/microprolactinoma/brain injury/empty sella syndrome/idiopathic hyperprolactinemia | 38/25/17/17/4 | 38/25/17/12/8 | 35/26/17/13/9 |
| Physical activity: total/several times a week/once a week/once a month (%) | 100/46/46/8 | 96/42/46/8 | 95/43/43/9 |
| Primary or vocational/secondary/university education (%) | 12/38/50 | 12/42/46 | 9/39/52 |
| Occupational activity/Blue-collar/white-collar/pink-collar workers (%) | 88/21/42/25 | 88/17/42/29 | 87/22/43/22 |
| Number of sexual partners (n) | 2.7 ± 1.0 | 2.5 ± 1.0 | 2.4 ±0.9 |
| Number of marriages (n)/duration of marriages (months) | 1.1 ±0.5/42 ± 15 | 1.2± 0.5/40 ± 15 | 1.1 ± 0.6/38 ± 12 |
| Number of deliveries (n)/Number of miscarriages (n) | 1.3 ± 0.6/0.5 ± 0.5 | 1.2 ± 0.6/0.5 ± 0.5 | 1.2 ± 0.5/0.7 ± 0.5 |
| Stress exposure (%) | 79 | 83 | 78 |
| Systolic blood pressure (mm Hg) | 125 ± 16 | 123 ± 17 | 119 ± 15 |
| Diastolic blood pressure (mm Hg) | 78 ± 6 | 77 ± 5 | 76 ± 6 |
| Total daily vitamin D intake (µg) | 8.0 ± 2.8 | 12.7 ± 3.8 & | 26.8 ± 8.6 |
| Users of vitamin D3 supplements (%) 1 | 4 *& | 38 & | 57 |
| Variable | Group A | Group B | Group C |
|---|---|---|---|
| BDI-II score (mean ± standard deviation) | |||
| Before treatment | 13.7 ± 3.2 | 13.0 ± 2.8 | 12.6 ± 2.9 |
| At the end of the study | 12.9 ± 3.1 | 12.1 ± 3.2 | 10.2 ± 3.0 *&# |
| Depression symptoms (n [%]) | |||
| Before treatment | 13 [54] | 12 [50] | 10 [43] |
| At the end of the study | 11 [46] | 10 [42] | 5 [21] *&# |
| Mild symptoms (n [%]) | |||
| Before treatment | 12 [50] | 12 [50] | 10 [43] |
| At the end of the study | 11 [46] | 10 [42] | 5 [21] *&# |
| Moderate symptoms (n [%]) | |||
| Before treatment | 1 [4] | 0 [0] | 0 [0] |
| At the end of the study | 0 [0] | 0 [0] | 0 [0] |
| Severe symptoms (n [%]) | |||
| Before treatment | 0 [0] | 0 [0] | 0 [0] |
| At the end of the study | 0 [0] | 0 [0] | 0 [0] |
| Correlated Variables | Group A | Group B | Group C | |
|---|---|---|---|---|
| Δ Prolactin | Prolactin | 0.563 *** | 0.588 *** | 0.655 *** |
| Δ Prolactin | 25OHD | 0.487 *** | 0.447 ** | 0.418 ** |
| Δ Prolactin | Δ Testosterone | 0.315 * | 0.325 * | 0.341 * |
| Δ Prolactin | Δ Estradiol | 0.542 *** | 0.567 *** | 0.602 *** |
| Δ Testosterone | 25OHD | 0.422 ** | 0.448 ** | 0.456 ** |
| Δ Sexual desire | Δ Prolactin | 0.412 ** | 0.395 ** | 0.408 ** |
| Δ Sexual arousal | Δ Prolactin | 0.388 ** | 0.411 ** | 0.425 ** |
| Δ Lubrication | Δ Prolactin | 0.312 * | 0.385 ** | 0.342 * |
| Δ Orgasm | Δ Prolactin | 0.515 *** | 0.532 *** | 0.608 *** |
| Δ Sexual satisfaction | Δ Prolactin | 0.568 *** | 0.594 *** | 0.572 *** |
| Δ Pain | Δ Prolactin | 0.353 * | 0.324 * | 0.305 * |
| Δ Sexual desire | 25OHD | 0.168 | 0.155 | 0.175 |
| Δ Sexual arousal | 25OHD | 0.184 | 0.122 | 0.103 |
| Δ Lubrication | 25OHD | 0.455 ** | 0.421 ** | 0.397 ** |
| Δ Orgasm | 25OHD | 0.462 ** | 0.442 ** | 0.411 ** |
| Δ Sexual satisfaction | 25OHD | 0.432 ** | 0.421 ** | 0.388 ** |
| Δ Pain | 25OHD | 0.412 ** | 0.376 ** | 0.354 * |
| Δ Sexual desire | Δ Testosterone | 0.483 *** | 0.523 *** | 0.551 *** |
| Δ Sexual arousal | Δ Testosterone | 0.462 ** | 0.487 *** | 0.514 *** |
| Δ Lubrication | Δ Estradiol | 0.415 ** | 0.420 ** | 0.437 ** |
| Δ Pain | Δ Estradiol | 0.429 ** | 0.452 ** | 0.429 ** |
| Δ BDI-II | Δ Total FSFI score | 0.345 * | 0.364 * | 0.359 * |
| Δ BDI-II | Δ Sexual desire | 0.384 * | 0.392 ** | 0.408 ** |
| Δ BDI-II | Δ Sexual arousal | 0.402 ** | 0.428 ** | 0.342 ** |
| Δ BDI-II | Δ Lubrication | 0.646 *** | 0.594 *** | 0.602 *** |
| Δ BDI-II | Δ Orgasm | 0.423 ** | 0.405 ** | 0.317 * |
| Δ BDI-II | Δ Sexual satisfaction | 0.385 ** | 0.427 * | 0.406 * |
| Δ BDI-II | Δ Pain | 0.622 *** | 0.586 *** | 0.614 *** |
| Δ BDI-II | 25OHD | 0.478 *** | 0.457 ** | 0.443 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysiak, R.; Kowalcze, K.; Ott, J.; Deledda, A.; Okopień, B. Vitamin D Status Determines the Effect of Cabergoline on Sexual Function and Depressive Symptoms in Hyperprolactinemic Women. Nutrients 2025, 17, 1813. https://doi.org/10.3390/nu17111813
Krysiak R, Kowalcze K, Ott J, Deledda A, Okopień B. Vitamin D Status Determines the Effect of Cabergoline on Sexual Function and Depressive Symptoms in Hyperprolactinemic Women. Nutrients. 2025; 17(11):1813. https://doi.org/10.3390/nu17111813
Chicago/Turabian StyleKrysiak, Robert, Karolina Kowalcze, Johannes Ott, Andrea Deledda, and Bogusław Okopień. 2025. "Vitamin D Status Determines the Effect of Cabergoline on Sexual Function and Depressive Symptoms in Hyperprolactinemic Women" Nutrients 17, no. 11: 1813. https://doi.org/10.3390/nu17111813
APA StyleKrysiak, R., Kowalcze, K., Ott, J., Deledda, A., & Okopień, B. (2025). Vitamin D Status Determines the Effect of Cabergoline on Sexual Function and Depressive Symptoms in Hyperprolactinemic Women. Nutrients, 17(11), 1813. https://doi.org/10.3390/nu17111813







