Triglyceride–Glucose-Based Anthropometric Indices for Predicting Incident Cardiovascular Disease: Relative Fat Mass (RFM) as a Robust Indicator
Abstract
1. Introduction
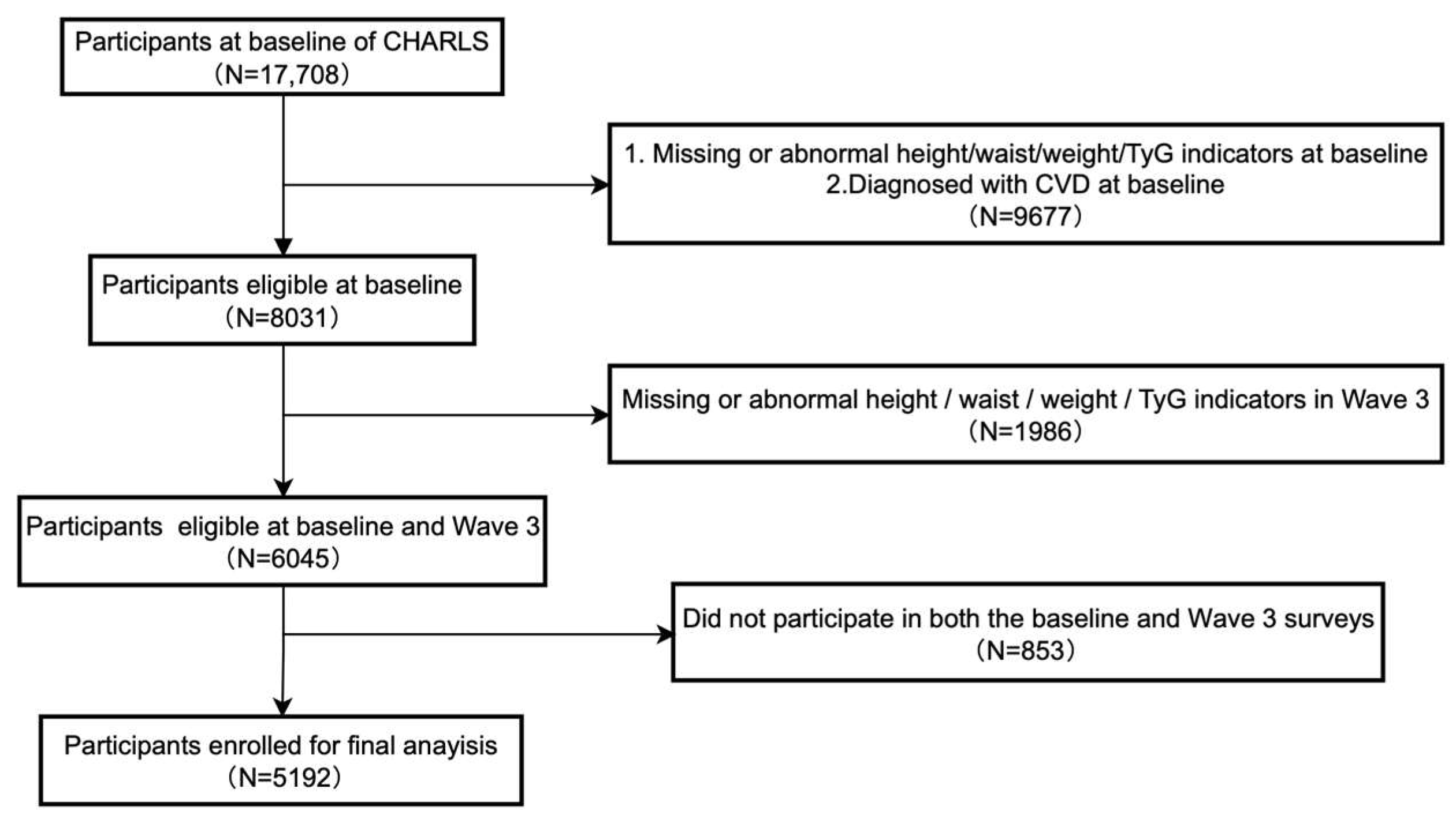
2. Materials and Methods
2.1. Study Design and Data Collection
2.1.1. Study Design
2.1.2. Data Collection
2.2. Data Assessment and Definitions
2.2.1. Exposure Assessment
2.2.2. Definition of CVD
2.2.3. Definition of Hypertension
2.2.4. Definition of Diabetes
2.2.5. Definition of Obesity
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics of the Participants
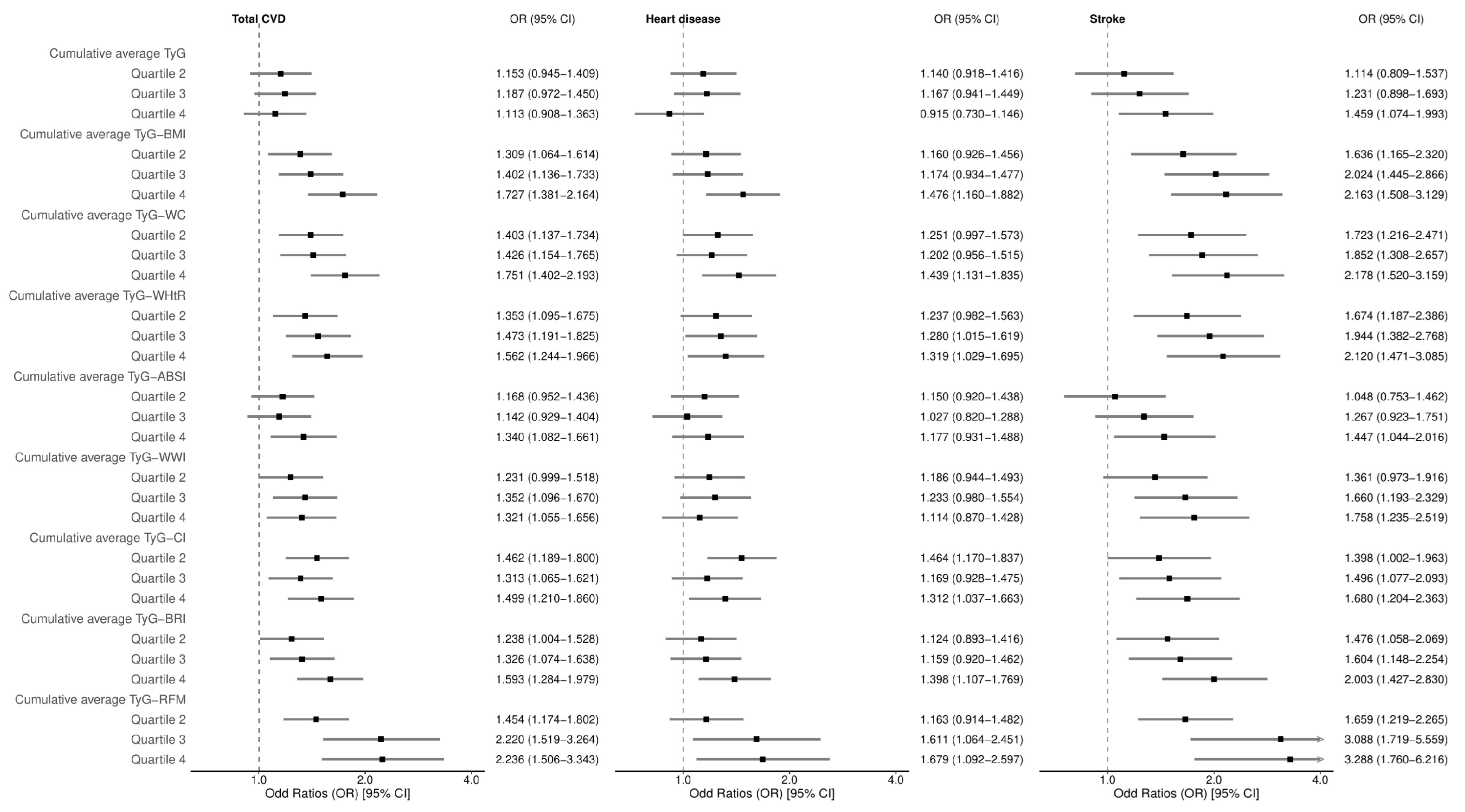
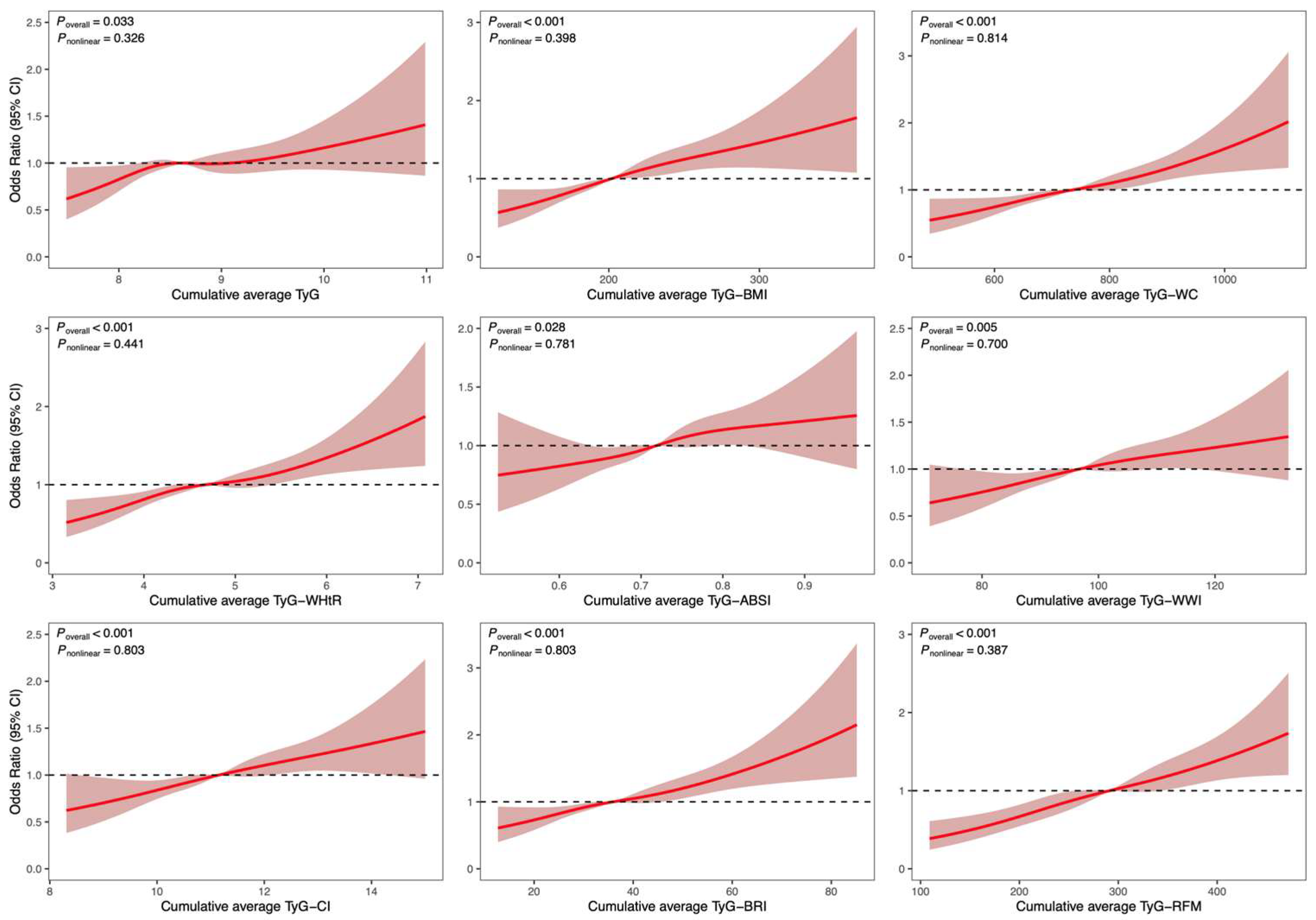
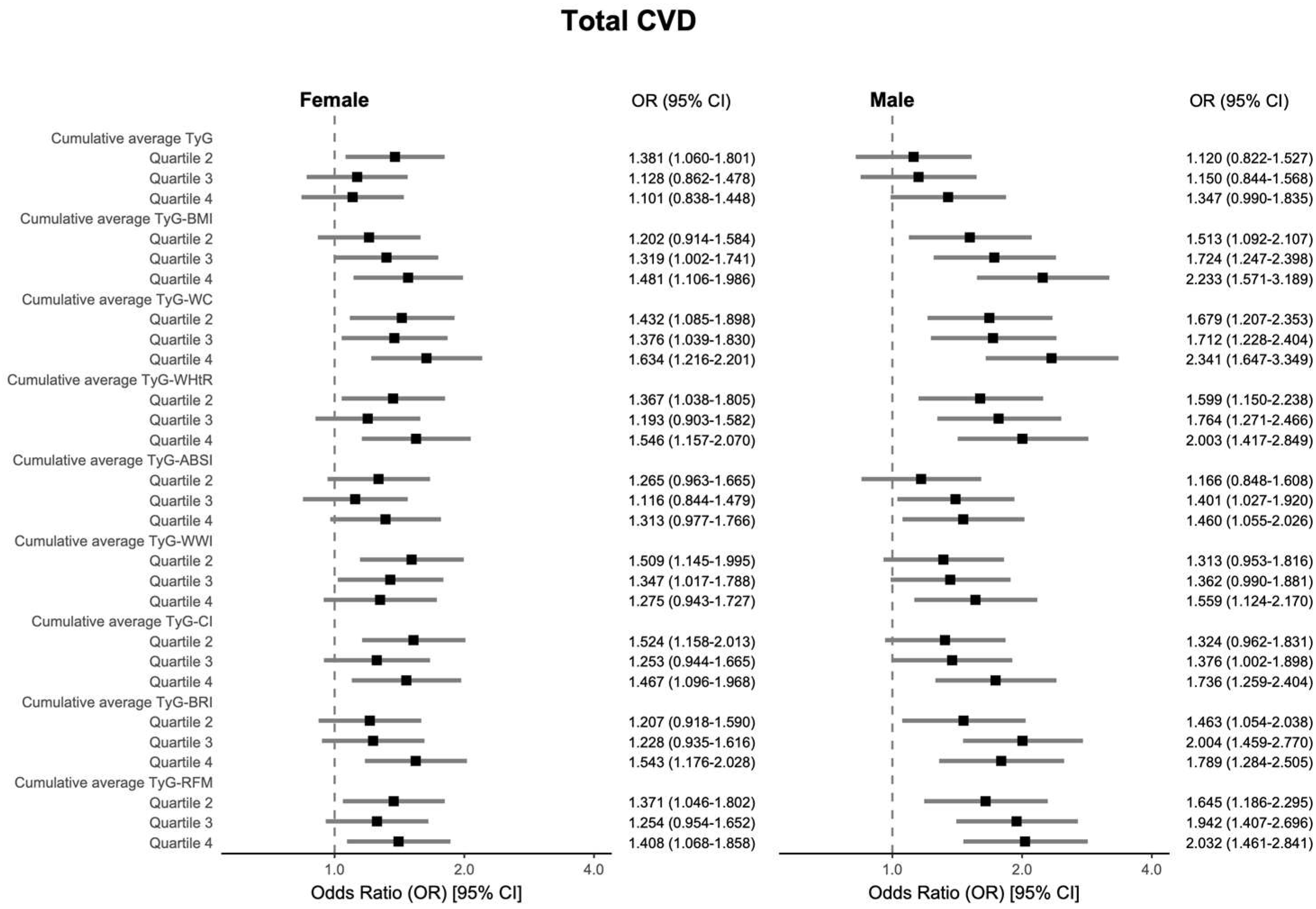
3.2. Analysis of the Association Between Cumulative Average TyG-AIs and CVD-Related Events
3.3. Receiver Operating Characteristic (ROC) Curve Analysis
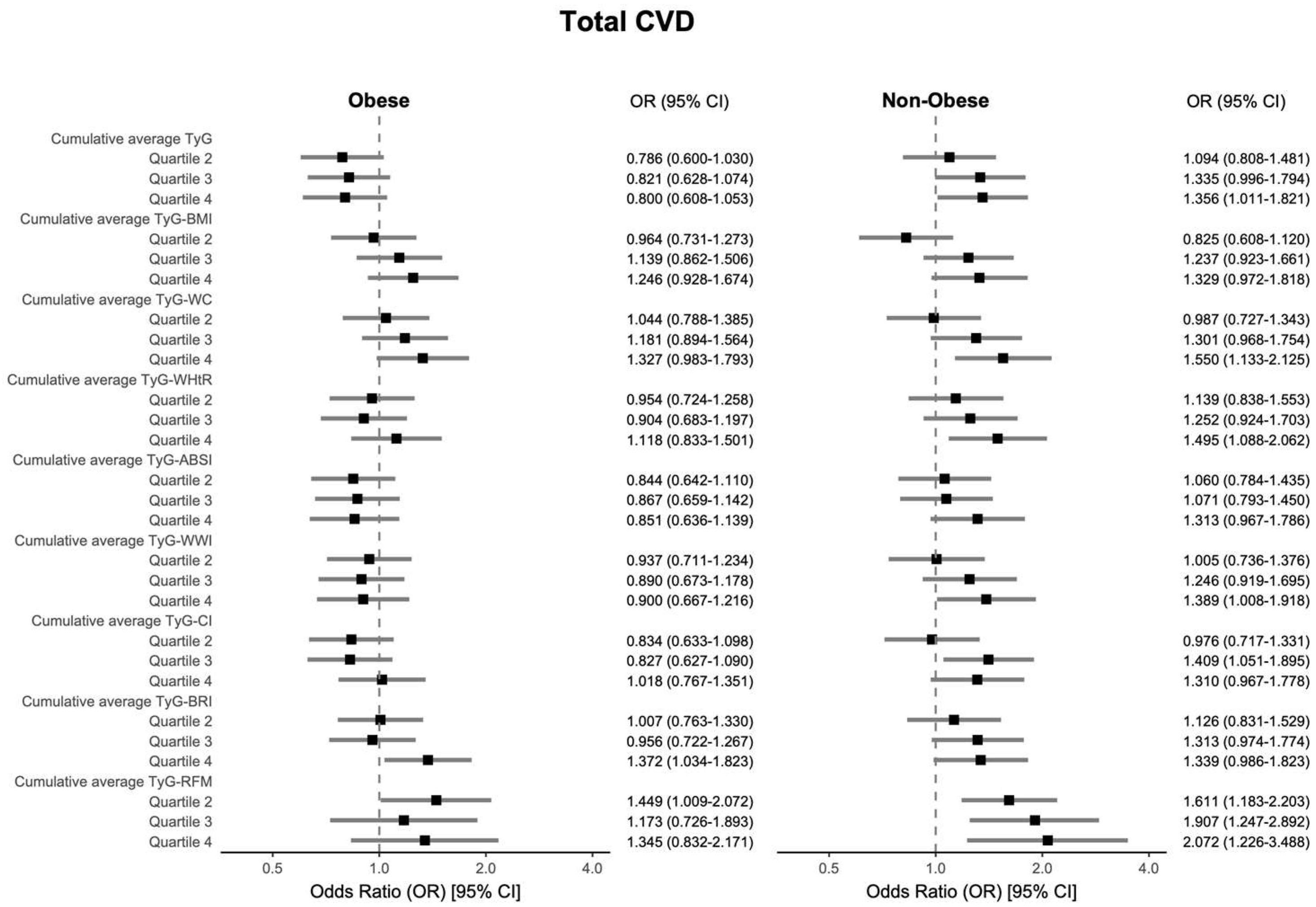
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular disease |
| IR | Insulin resistance |
| T2DM | Type 2 diabetes mellitus |
| NAFLD | Nonalcoholic fatty liver disease |
| TyG | Triglyceride–glucose |
| AIs | Anthropometric indicators |
| BMI | Body mass index |
| WC | Waist circumference |
| WHtR | Waist-to-height ratio |
| WWI | Weight-adjusted waist index |
| ABSI | A body shape index |
| BRI | Body roundness index |
| CI | Conicity index |
| RFM | Relative fat mass |
| TyG-AI | Triglyceride–glucose anthropometric indicator |
| CHARLS | China Health and Retirement Longitudinal Study |
| FBG | Fasting blood glucose |
| TG | Triglycerides |
| TC | Total cholesterol |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| HbA1c | Glycated hemoglobin |
| HDL-c | High-density lipoprotein cholesterol |
| LDL-c | Low-density lipoprotein cholesterol |
| SD | Standard deviation |
| IQR | Interquartile range |
| OR | Odds ratio |
| 95% CI | 95% confidence interval |
| GVIF | Generalized variance inflation factor |
| RCS | Restricted cubic splines |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| NO | Nitric oxide |
| SGK-1 | Serum and glucocorticoid-regulated kinase 1 |
| FFA | Free fatty acid |
References
- Wang, Y.; Wang, X.; Wang, C.; Zhou, J. Global, Regional, and National Burden of Cardiovascular Disease, 1990–2021: Results From the 2021 Global Burden of Disease Study. Cureus 2024, 16, e74333. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, G.-Y.; Jing, W.-Z.; Liu, J.; Liu, M. Global Trends and Regional Differences in Incidence and Mortality of Cardiovascular Disease, 1990–2019: Findings from 2019 Global Burden of Disease Study. Eur. J. Prev. Cardiol. 2023, 30, 276–286. [Google Scholar] [CrossRef]
- Fawzy, A.M.; Lip, G.Y.H. Cardiovascular Disease Prevention: Risk Factor Modification at the Heart of the Matter. Lancet Reg. Health West. Pac. 2021, 17, 100291. [Google Scholar] [CrossRef]
- Jung, I.; Koo, D.-J.; Lee, W.-Y. Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective. Diabetes Metab. J. 2024, 48, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ji, H.; Sun, W.; An, X.; Lian, F. Triglyceride Glucose (TyG) Index: A Promising Biomarker for Diagnosis and Treatment of Different Diseases. Eur. J. Intern. Med. 2025, 131, 3–14. [Google Scholar] [CrossRef]
- Selvi, N.M.K.; Nandhini, S.; Sakthivadivel, V.; Lokesh, S.; Srinivasan, A.R.; Sumathi, S. Association of Triglyceride–Glucose Index (TyG Index) with HbA1c and Insulin Resistance in Type 2 Diabetes Mellitus. Mædica 2021, 16, 375–381. [Google Scholar] [CrossRef]
- Mohajan, D.; Mohajan, H.K. Obesity and Its Related Diseases: A New Escalating Alarming in Global Health. J. Innov. Med. Res. 2023, 2, 12–23. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Dang, K.; Wang, X.; Hu, J.; Zhang, Y.; Cheng, L.; Qi, X.; Liu, L.; Ming, Z.; Tao, X.; Li, Y. The Association between Triglyceride-Glucose Index and Its Combination with Obesity Indicators and Cardiovascular Disease: NHANES 2003–2018. Cardiovasc. Diabetol. 2024, 23, 8. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Luo, X.; Xiao, Y.; Tu, T.; Liu, C.; Liu, Q.; Wang, C.; Dai, Y.; Zhang, Z.; et al. The Triglyceride–Glucose Index and Its Obesity-Related Derivatives as Predictors of All-Cause and Cardiovascular Mortality in Hypertensive Patients: Insights from NHANES Data with Machine Learning Analysis. Cardiovasc. Diabetol. 2025, 24, 47. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Shi, B.; Sun, S.; Wang, S.; Pang, S.; Wu, X. Association between the Cumulative Average Triglyceride Glucose-Body Mass Index and Cardiovascular Disease Incidence among the Middle-Aged and Older Population: A Prospective Nationwide Cohort Study in China. Cardiovasc. Diabetol. 2024, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yin, T.; Pan, M.; Qin, L.; Zhang, L.; Wang, X.; Zheng, W.; Liu, C.; Chen, R. The Mediating Effect of TyG-Related Indicators between Long-Term Exposure to Particulate Matter and Cardiovascular Disease: Evidence from a National Longitudinal Cohort Study. Lipids Health Dis. 2024, 23, 319. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xing, Q.; Wang, X.; Chen, Y.; Kong, J.; Li, J.; Yue, B. Exploring the Association between the TyG-WHtR Index and the Incidence of Stroke in the Obese Population: Based on NHANES Data from 1998 to 2018. J. Stroke Cerebrovasc. Dis. 2025, 34, 108209. [Google Scholar] [CrossRef]
- Wang, M.; Xu, K.; Yang, J.; Bennett, D.A.; Du, H.; Liu, X. Normal-Weight Obesity Subtypes and 10-Year Risks of Major Vascular Diseases in 0.3 Million Adults. Clin. Nutr. Edinb. Scotl. 2025, 45, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, N.H.; Kwon, T.Y.; Kim, S.G. A Novel Adiposity Index as an Integrated Predictor of Cardiometabolic Disease Morbidity and Mortality. Sci. Rep. 2018, 8, 16753. [Google Scholar] [CrossRef]
- Woolcott, O.O.; Bergman, R.N. Relative Fat Mass (RFM) as a New Estimator of Whole-Body Fat Percentage ─ A Cross-Sectional Study in American Adult Individuals. Sci. Rep. 2018, 8, 10980. [Google Scholar] [CrossRef]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between Body Roundness with Body Fat and Visceral Adipose Tissue Emerging from a New Geometrical Model. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef]
- Krakauer, N.Y.; Krakauer, J.C. A New Body Shape Index Predicts Mortality Hazard Independently of Body Mass Index. PLoS ONE 2012, 7, e39504. [Google Scholar] [CrossRef]
- Woolcott, O.O.; Samarasundera, E.; Heath, A.K. Association of Relative Fat Mass (RFM) Index with Diabetes-Related Mortality and Heart Disease Mortality. Sci. Rep. 2024, 14, 30823. [Google Scholar] [CrossRef]
- Suthahar, N.; Meems, L.M.G.; Withaar, C.; Gorter, T.M.; Kieneker, L.M.; Gansevoort, R.T.; Bakker, S.J.L.; van Veldhuisen, D.J.; de Boer, R.A. Relative Fat Mass, a New Index of Adiposity, Is Strongly Associated with Incident Heart Failure: Data from PREVEND. Sci. Rep. 2022, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Suthahar, N.; Wang, K.; Zwartkruis, V.W.; Bakker, S.J.L.; Inzucchi, S.E.; Meems, L.M.G.; Eijgenraam, T.R.; Ahmadizar, F.; Sijbrands, E.G.; Gansevoort, R.T.; et al. Associations of Relative Fat Mass, a New Index of Adiposity, with Type-2 Diabetes in the General Population. Eur. J. Intern. Med. 2023, 109, 73–78. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort Profile: The China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef]
- Shi, Z.; Tuomilehto, J.; Kronfeld-Schor, N.; Alberti, G.K.; Stern, N.; El-Osta, A.; Bilu, C.; Einat, H.; Zimmet, P. The Circadian Syndrome Predicts Cardiovascular Disease Better than Metabolic Syndrome in Chinese Adults. J. Intern. Med. 2021, 289, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Cao, L.-F.; Ma, W.-Z.; Gao, Y.-J.; Luo, M.-S.; Zhu, J.; Li, T.; Zhou, D. Association between Sarcopenia and Cardiovascular Disease among Middle-Aged and Older Adults: Findings from the China Health and Retirement Longitudinal Study. EClinicalMedicine 2022, 44, 101264. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, J.; Liu, S.; Zhuang, W. The Change of Triglyceride-Glucose Index May Predict Incidence of Stroke in the General Population over 45 Years Old. Cardiovasc. Diabetol. 2023, 22, 132. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Yu, J.; Yi, Q.; Chen, G.; Hou, L.; Liu, Q.; Xu, Y.; Qiu, Y.; Song, P. The Visceral Adiposity Index and Risk of Type 2 Diabetes Mellitus in China: A National Cohort Analysis. Diabetes Metab. Res. Rev. 2022, 38, e3507. [Google Scholar] [CrossRef]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and Diagnostic Criteria of Clinical Obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, K.; He, R. The Association of Triglyceride-Glucose and Triglyceride-Glucose Related Indices with the Risk of Heart Disease in a National. Cardiovasc. Diabetol. 2025, 24, 54. [Google Scholar] [CrossRef]
- Kim, J.H. Multicollinearity and Misleading Statistical Results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin Resistance, Cardiovascular Stiffening and Cardiovascular Disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin Resistance and Cardiovascular Disease. J. Int. Med. Res. 2023, 51, 03000605231164548. [Google Scholar] [CrossRef]
- Sasaki, N.; Maeda, R.; Ozono, R.; Nakano, Y.; Higashi, Y. Association of Obesity with Serum Free Fatty Acid Levels in Individuals at Different Stages of Prediabetes. Clin. Obes. 2022, 12, e12496. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Sironi, A.M.; Sicari, R.; Folli, F.; Gastaldelli, A. Ectopic Fat Storage, Insulin Resistance, and Hypertension. Curr. Pharm. Des. 2011, 17, 3074–3080. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, W.; Song, T.; Wang, X.; Wang, Q.; Li, J.; Liu, X.; Hao, B.; Chen, T.; Guo, J. Changes in the Combination of the Triglyceride-Glucose Index and Obesity Indicators Estimate the Risk of Cardiovascular Disease. Cardiovasc. Diabetol. 2024, 23, 192. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Wang, Y.; Wan, Q. Association between TyG Index with Obesity Indicators and Coronary Heart Disease: A Cohort Study. Sci. Rep. 2025, 15, 8920. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, Y.; Nakao, Y.M.; Masuda, I.; Higashiyama, A.; Takegami, M.; Nishimura, K.; Watanabe, M.; Ohkubo, T.; Okamura, T.; Miyamoto, Y. Risk for Metabolic Diseases in Normal Weight Individuals with Visceral Fat Accumulation: A Cross-Sectional Study in Japan. BMJ Open 2017, 7, e013831. [Google Scholar] [CrossRef]
- Nomura, K.; Eto, M.; Kojima, T.; Ogawa, S.; Iijima, K.; Nakamura, T.; Araki, A.; Akishita, M.; Ouchi, Y. Visceral Fat Accumulation and Metabolic Risk Factor Clustering in Older Adults. J. Am. Geriatr. Soc. 2010, 58, 1658–1663. [Google Scholar] [CrossRef]
- Patel, P.; Abate, N. Body Fat Distribution and Insulin Resistance. Nutrients 2013, 5, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.B.d.M.; Leão, L.M.C.S.M.; Tavares, A.B.W.; Lopes, K.G.; Kraemer-Aguiar, L.G. Fat Distribution and Its Correlation with Insulin Resistance, Androgen Markers, and Proinflammatory Cytokines in Polycystic Ovary Syndrome. Horm. Metab. Res. 2024, 57, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Christakoudi, S.; Tsilidis, K.K.; Muller, D.C.; Freisling, H.; Weiderpass, E.; Overvad, K.; Söderberg, S.; Häggström, C.; Pischon, T.; Dahm, C.C.; et al. A Body Shape Index (ABSI) Achieves Better Mortality Risk Stratification than Alternative Indices of Abdominal Obesity: Results from a Large European Cohort. Sci. Rep. 2020, 10, 14541. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, N.Y.; Krakauer, J.C. An Anthropometric Risk Index Based on Combining Height, Weight, Waist, and Hip Measurements. J. Obes. 2016, 2016, 8094275. [Google Scholar] [CrossRef]

| Characteristics | Non-CVD | CVD | ||||
|---|---|---|---|---|---|---|
| Non-Obese n = 2090 | Obese n = 1720 | p Value | Non-Obese n = 588 | Obese n = 794 | p Value | |
| Female, n (%) | 876 (41.91) | 1085 (63.08) | <0.001 | 281 (47.79) | 533 (67.13) | <0.001 |
| Age | 58 (52–65) | 56 (50–63) | <0.001 | 61 (54–67) | 60 (54–65) | 0.020 |
| Education, n (%) | 0.101 | 0.532 | ||||
| Primary or less | 991 (47.44) | 797 (46.36) | 311 (52.89) | 390 (49.12) | ||
| Junior high | 491 (23.50) | 366 (21.29) | 129 (21.94) | 180 (22.67) | ||
| Senior high | 435 (20.82) | 387 (22.51) | 93 (15.82) | 142 (17.88) | ||
| College or more | 172 (8.23) | 169 (9.83) | 55 (9.35) | 82 (10.33) | ||
| Married, n (%) | 1777 (85.02) | 1501 (87.27) | 0.052 | 480 (81.63) | 686 (86.40) | 0.019 |
| SBP, mmHg | 119.50 (109.50–133.00) | 128.00 (116.25–142.50) | <0.001 | 126.00 (113.50–141.00) | 134.50 (121.00–149.50) | <0.001 |
| DBP, mmHg | 71.00 (64.00–79.00) | 76.50 (69.00–84.50) | <0.001 | 73.00 (65.50–81.50) | 78.50 (71.00–86.75) | <0.001 |
| Ever smoked, n (%) | 992 (47.56) | 507 (29.48) | <0.001 | 269 (45.83) | 228 (28.72) | <0.001 |
| Ever drank, n (%) | 914 (43.82) | 584 (33.97) | <0.001 | 248 (42.32) | 272 (34.30) | 0.003 |
| Current smoker, n (%) | 835 (40.05) | 387 (22.55) | <0.001 | 207 (35.63) | 157 (19.80) | <0.001 |
| Current drinker, n (%) | 826 (39.58) | 501 (29.13) | <0.001 | 191 (32.54) | 210 (26.45) | 0.016 |
| Dyslipidemia, n (%) | 59 (2.88) | 171 (10.20) | <0.001 | 52 (9.01) | 145 (18.73) | <0.001 |
| Hypertension, n (%) | 249 (12.02) | 461 (26.94) | <0.001 | 132 (22.60) | 353 (44.68) | <0.001 |
| Diabetes, n (%) | 59 (2.85) | 109 (6.42) | <0.001 | 26 (4.45) | 84 (10.71) | <0.001 |
| Glucose, mg/dL | 100.62 (92.70–109.44) | 103.50 (95.76–114.84) | <0.001 | 101.70 (93.60–110.79) | 104.94 (96.48–117.36) | <0.001 |
| TG, mg/dL | 88.50 (65.49–127.44) | 121.25 (87.61–181.43) | <0.001 | 93.81 (69.03–138.06) | 123.46 (88.50–184.08) | <0.001 |
| TC, mg/dL | 185.37 (163.15–209.54) | 193.30 (169.72–219.40) | <0.001 | 191.56 (166.82–212.63) | 196.78 (174.36–225.00) | <0.001 |
| HDL-c, mg/dL | 53.35 (44.46–63.79) | 45.62 (37.50–54.51) | <0.001 | 53.93 (44.65–63.40) | 46.01 (37.89–54.12) | <0.001 |
| LDL-c, mg/dL | 109.79 (89.30–131.06) | 117.14 (95.10–140.34) | <0.001 | 113.66 (94.52–133.76) | 121.01 (99.36–145.75) | <0.001 |
| HbA1c, % | 5.10 (4.80–5.30) | 5.20 (4.90–5.50) | <0.001 | 5.10 (4.90–5.40) | 5.20 (4.90–5.50) | <0.001 |
| BMI, kg/m2 | 21.07 (19.60–22.38) | 25.61 (24.29–27.54) | <0.001 | 21.26 (19.77–22.39) | 26.07 (24.45–28.32) | <0.001 |
| WC, cm | 78.10 (74.00–82.20) | 91.60 (88.00–96.40) | <0.001 | 79.00 (74.55–82.60) | 93.00 (89.00–98.30) | <0.001 |
| TyG | 8.42 (8.09–8.80) | 8.77 (8.40–9.22) | <0.001 | 8.49 (8.13–8.86) | 8.79 (8.41–9.25) | <0.001 |
| Cumulative average TyG | 8.44 (8.16–8.78) | 8.81 (8.48–9.21) | <0.001 | 8.51 (8.21–8.84) | 8.85 (8.48–9.26) | <0.001 |
| Cumulative average TyG-BMI | 178.76 (165.22–194.35) | 228.32 (210.68–249.62) | <0.001 | 182.41 (166.32–196.61) | 233.34 (212.21–256.90) | <0.001 |
| Cumulative average TyG-WC | 666.74 (620.88–713.27) | 812.56 (757.11–877.93) | <0.001 | 681.62 (630.02–723.07) | 826.17 (769.45–898.88) | <0.001 |
| Cumulative average TyG-WHtR | 4.21 (3.91–4.55) | 5.17 (4.78–5.59) | <0.001 | 4.30 (4.01–4.64) | 5.27 (4.86–5.73) | <0.001 |
| Cumulative average TyG-ABSI | 0.69 (0.66–0.73) | 0.74 (0.70–0.79) | <0.001 | 0.70 (0.67–0.75) | 0.75 (0.71–0.79) | <0.001 |
| Cumulative average TyG-WWI | 91.41 (86.48–97.29) | 101.71 (95.81–108.13) | <0.001 | 93.57 (87.85–99.36) | 103.29 (96.81–109.82) | <0.001 |
| Cumulative average TyG-CI | 10.55 (10.03–11.20) | 11.71 (11.08–12.41) | <0.001 | 10.77 (10.17–11.38) | 11.86 (11.17–12.62) | <0.001 |
| Cumulative average TyG-BRI | 28.24 (23.72–33.25) | 44.56 (38.80–51.84) | <0.001 | 29.77 (24.95–34.20) | 47.08 (40.20–55.22) | <0.001 |
| Cumulative average TyG-RFM | 225.08 (186.72–306.90) | 344.96 (266.51–384.29) | <0.001 | 241.35 (197.89–315.39) | 354.44 (281.09–393.70) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, X.; Niu, H.; Wang, N.; Wang, Y.; Xu, H.; Wang, H.; Wu, L.; Li, W.; Han, L. Triglyceride–Glucose-Based Anthropometric Indices for Predicting Incident Cardiovascular Disease: Relative Fat Mass (RFM) as a Robust Indicator. Nutrients 2025, 17, 2212. https://doi.org/10.3390/nu17132212
Chu X, Niu H, Wang N, Wang Y, Xu H, Wang H, Wu L, Li W, Han L. Triglyceride–Glucose-Based Anthropometric Indices for Predicting Incident Cardiovascular Disease: Relative Fat Mass (RFM) as a Robust Indicator. Nutrients. 2025; 17(13):2212. https://doi.org/10.3390/nu17132212
Chicago/Turabian StyleChu, Xinlei, Haozhi Niu, Ning Wang, Yu Wang, Hongkai Xu, Huiying Wang, Liting Wu, Wei Li, and Lei Han. 2025. "Triglyceride–Glucose-Based Anthropometric Indices for Predicting Incident Cardiovascular Disease: Relative Fat Mass (RFM) as a Robust Indicator" Nutrients 17, no. 13: 2212. https://doi.org/10.3390/nu17132212
APA StyleChu, X., Niu, H., Wang, N., Wang, Y., Xu, H., Wang, H., Wu, L., Li, W., & Han, L. (2025). Triglyceride–Glucose-Based Anthropometric Indices for Predicting Incident Cardiovascular Disease: Relative Fat Mass (RFM) as a Robust Indicator. Nutrients, 17(13), 2212. https://doi.org/10.3390/nu17132212






