Associations of Serum Thiamine Levels with Blood Pressure Among Middle-Aged and Elderly Women in Eastern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Anthropometric Measurements and Questionnaire Survey
2.3. Blood Pressure Measurement and Definition of Hypertension
2.4. Laboratory Measurements and Definition of Disease
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of Participants
3.2. Associations Between Serum Thiamine and Hypertension Prevalence in Women
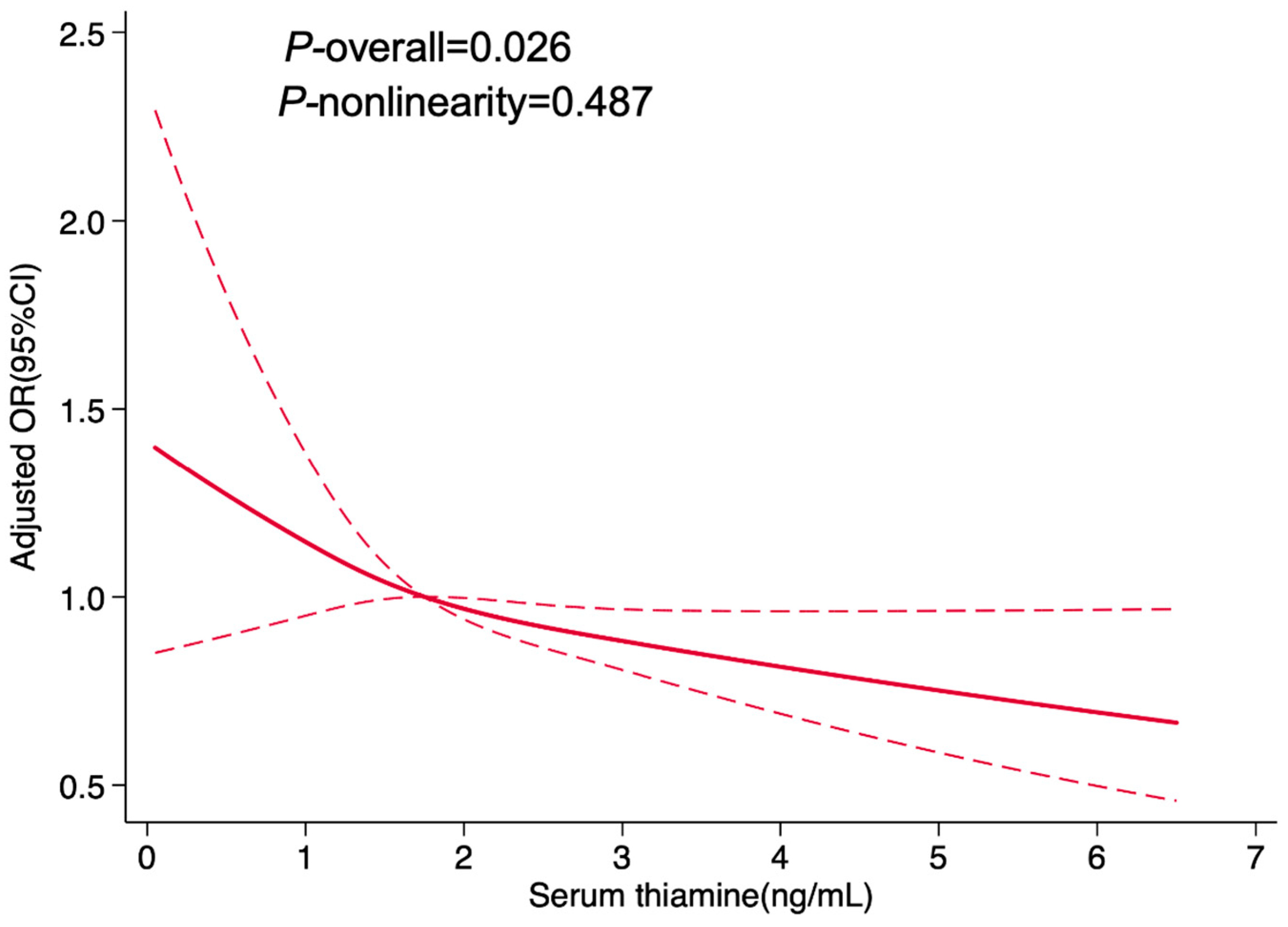
3.3. Dose–Response Relationship Between Serum Thiamine and Hypertension Prevalence in Women
3.4. Associations Between Serum Thiamine and BP in Women
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| RCS | Restricted cubic spline |
| SD | Standard deviation |
| IQR | Interquartile range |
| OR | Odds ratio |
| CI | Confidence interval |
| Coef | Coefficient |
| BMI | Body mass index |
| SES | Socioeconomic status |
| TC | Cholesterol |
| TG | Triglyceride |
| LDL-c | Low density lipoprotein |
| HDL-c | High-density lipoprotein cholesterol |
| FPG | Fasting plasma glucose |
| Ref | Reference |
| NHANES | National Health and Nutrition Examination Survey |
| KNHANES | Korean National Health and Nutrition Examination Survey |
| SHR | Spontaneous hypertensive rats |
| ETKA | Erythrocyte transketolase activity assay |
| HRT | Hormone replacement therapy |
Appendix A
| Serum Levels of Thiamine | n | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | ||
| Continuous variable | ||||||||||
| Every SD increase | 1899 | 0.88 | 0.79, 0.97 | 0.012 | 0.89 | 0.80, 0.98 | 0.020 | 0.88 | 0.80, 0.97 | 0.013 |
| Categorical variable | ||||||||||
| Lowest tertile | 622 | 1.00 | Ref | - | 1.00 | Ref | - | 1.00 | Ref | - |
| Middle tertile | 637 | 0.86 | 0.68, 1.08 | 0.188 | 0.89 | 0.70, 1.13 | 0.336 | 0.88 | 0.69, 1.11 | 0.280 |
| Highest tertile | 640 | 0.70 | 0.56, 0.88 | 0.002 | 0.77 | 0.61, 0.98 | 0.035 | 0.75 | 0.59, 0.96 | 0.021 |
| Serum Levels of Thiamine | n | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coef | 95% CI | p | Coef | 95% CI | p | Coef | 95% CI | p | ||
| Continuous variable | ||||||||||
| Every SD increase | 1899 | −1.44 | −2.21, −0.67 | <0.001 | −1.31 | −2.05, −0.57 | 0.001 | −1.35 | −2.09, −0.61 | <0.001 |
| Categorical variable | ||||||||||
| Lowest tertile | 622 | 0.00 | Ref | - | 0.00 | Ref | - | 0.00 | Ref | - |
| Middle tertile | 637 | −1.12 | −3.22, 0.98 | 0.295 | −0.62 | −2.64, 1.40 | 0.548 | −0.66 | −2.67, 1.36 | 0.524 |
| Highest tertile | 640 | −5.06 | −7.15, −2.96 | <0.001 | −3.81 | −5.84, −1.77 | <0.001 | −3.90 | −5.93, −1.86 | <0.001 |
References
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension global hypertension practice guidelines. J. Hypertens. 2020, 38, 982–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, Y.; Zhou, B.; Huang, Z.; Zhao, Z.; Li, C.; Zhang, X.; Han, G.; Peng, K.; Li, X.; et al. Prevalence, awareness, treatment, and control of hypertension in China, 2004–2018: Findings from six rounds of a national survey. BMJ 2023, 380, e071952. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.; Zeng, X.; Yin, P.; Zhu, J.; Chen, W.; Li, X.; Wang, L.; Wang, L.; Liu, Y.; et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 394, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Wenger, N.K.; Arnold, A.; Bairey Merz, C.N.; Cooper-DeHoff, R.M.; Ferdinand, K.C.; Fleg, J.L.; Gulati, M.; Isiadinso, I.; Itchhaporia, D.; Light-McGroary, K.; et al. Hypertension Across a Woman’s Life Cycle. J. Am. Coll. Cardiol. 2018, 71, 1797–1813. [Google Scholar] [CrossRef]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Bairey Merz, C.N.; Cheng, S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020, 5, 19–26. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Levy, D.; Larson, M.G.; Vasan, R.S.; Kannel, W.B.; Ho, K.K. The progression from hypertension to congestive heart failure. JAMA 1996, 275, 1557–1562. [Google Scholar] [CrossRef]
- Li, M.; Ren, R.; Wang, K.; Wang, S.; Chow, A.; Yang, A.K.; Lu, Y.; Leo, C. Effects of B Vitamins on Homocysteine Lowering and Thrombotic Risk Reduction-A Review of Randomized Controlled Trials Published Since January 1996. Nutrients 2025, 17, 1122. [Google Scholar] [CrossRef]
- Wu, S.; Chang, W.; Xie, Z.; Yao, B.; Wang, X.; Yang, C. Association of Serum Vitamin B(12) and Circulating Methylmalonic Acid Levels with All-Cause and Cardiovascular Disease Mortality among Individuals with Chronic Kidney Disease. Nutrients 2023, 15, 2980. [Google Scholar] [CrossRef]
- Herrmann, W.; Herrmann, M. The Controversial Role of HCY and Vitamin B Deficiency in Cardiovascular Diseases. Nutrients 2022, 14, 1412. [Google Scholar] [CrossRef]
- Ysphaneendramallimoggala; Biswas, M.; Anburaj, S.E.; Iqbal, F.; A, S.; Suryakanth, V.B.; Lewis, L.E.S. Thiamine: An indispensable regulator of paediatric neuro-cardiovascular health and diseases. Eur. J. Pediatr. 2024, 183, 4597–4610. [Google Scholar] [CrossRef] [PubMed]
- Strobbe, S.; Verstraete, J.; Stove, C.; Van Der Straeten, D. Metabolic engineering provides insight into the regulation of thiamin biosynthesis in plants. Plant Physiol. 2021, 186, 1832–1847. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Dragan, G.; Majsterek, I. The importance of thiamine (vitamin B1) in humans. Biosci. Rep. 2023, 43, BSR20230374. [Google Scholar] [CrossRef] [PubMed]
- Manzetti, S.; Zhang, J.; van der Spoel, D. Thiamin function, metabolism, uptake, and transport. Biochemistry 2014, 53, 821–835. [Google Scholar] [CrossRef]
- Marrs, C.; Lonsdale, D. Hiding in Plain Sight: Modern Thiamine Deficiency. Cells 2021, 10, 2595. [Google Scholar] [CrossRef]
- Martin, P.R.; Singleton, C.K.; Hiller-Sturmhöfel, S. The role of thiamine deficiency in alcoholic brain disease. Alcohol. Res. Health 2003, 27, 134–142. [Google Scholar]
- Schostak, T.; San Millan, I.; Jani, A.; Johnson, R.J. Thiamine deficiency: A commonly unrecognised but easily treatable condition. Postgrad. Med. J. 2023, 99, 844–848. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Williams, D.B.; Aher, C.V.; Spann, M.D.; English, W.J. Prevalence of thiamine deficiency is significant in patients undergoing primary bariatric surgery. Surg. Obes. Relat. Dis. 2021, 17, 653–658. [Google Scholar] [CrossRef]
- Eshak, E.S.; Arafa, A.E. Thiamine deficiency and cardiovascular disorders. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 965–972. [Google Scholar] [CrossRef]
- Wen, H.; Niu, X.; Zhao, R.; Wang, Q.; Sun, N.; Ma, L.; Li, Y.; Zhang, W. Association of vitamin B1 with cardiovascular diseases, all-cause and cardiovascular mortality in US adults. Front. Nutr. 2023, 10, 1175961. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Oh, H.; Kim, M.S. An increased intake of thiamine diminishes the risk of metabolic syndrome in the Korean population with various comorbidities. Diabetes Metab. Syndr. 2022, 16, 102443. [Google Scholar] [CrossRef]
- Ziegler, D.; Reiners, K.; Strom, A.; Obeid, R. Association between diabetes and thiamine status—A systematic review and meta-analysis. Metabolism 2023, 144, 155565. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ying, T.; Xu, M.; Chen, Q.; Wu, M.; Liu, Y.; He, G. Joint B Vitamin Intake and Type 2 Diabetes Risk: The Mediating Role of Inflammation in a Prospective Shanghai Cohort. Nutrients 2024, 16, 1901. [Google Scholar] [CrossRef]
- Syed, A.R.S.; Syed, A.A.; Akram, A.; Azam, M.S.; Muzammil, M.A.; Deepak; Ansari, A.I.; Eqbal, F.; Farooq, M.H.; Khatri, M.; et al. Does thiamine supplementation affect heart failure? A systematic review and meta-analysis of randomized control trials. Heart Lung 2023, 61, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Freisling, H.; Leitzmann, M.F.; Taljaard-Krugell, C.; Jacobs, I.; Kruger, H.S.; Smuts, C.M.; Pieters, M. Diet and sedentary behaviour in relation to cancer survival. A report from the national health and nutrition examination survey linked to the U.S. mortality registry. Clin. Nutr. 2020, 39, 3489–3496. [Google Scholar] [CrossRef]
- Lin, H.; Gao, Z.; Ni, H.; Li, J.; Liu, H.; Qin, B.; He, Z.; Jin, Z. Exploring the link between dietary thiamine and type 2 diabetes mellitus risk in US adults aged 45 years and older: Insights from a cross-sectional investigation. PLoS ONE 2024, 19, e0313114. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ji, J.; Lu, Q.; Gong, J.; Luo, Z.; Zhu, L. The effects of thiamine supplementation on patients with heart failure: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2022, 70, 102853. [Google Scholar] [CrossRef]
- Mahdavifar, B.; Hosseinzadeh, M.; Salehi-Abargouei, A.; Mirzaei, M.; Vafa, M. Dietary intake of B vitamins and their association with depression, anxiety, and stress symptoms: A cross-sectional, population-based survey. J. Affect. Disord. 2021, 288, 92–98. [Google Scholar] [CrossRef]
- Fukunaga, T.; Ohta, R.; Sano, C. The Association Between Vitamin B1 Deficiency and Anemia Among Elderly Patients at a Rural Hospital in Japan: A Cross-Sectional Study. Cureus 2023, 15, e47173. [Google Scholar] [CrossRef]
- Tanaka, T.; Sohmiya, K.; Kono, T.; Terasaki, F.; Horie, R.; Ohkaru, Y.; Muramatsu, M.; Takai, S.; Miyazaki, M.; Kitaura, Y. Thiamine attenuates the hypertension and metabolic abnormalities in CD36-defective SHR: Uncoupling of glucose oxidation from cellular entry accompanied with enhanced protein O-GlcNAcylation in CD36 deficiency. Mol. Cell. Biochem. 2007, 299, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Yang, S.; Ye, Z.; Wu, Q.; Liu, M.; Zhou, C.; He, P.; Jiang, J.; Liang, M.; et al. U-Shaped Relation of Dietary Thiamine Intake and New-Onset Hypertension. Nutrients 2022, 14, 3251. [Google Scholar] [CrossRef]
- Tong, W.W.; Tong, G.H.; Yang, M.H.; Qin, X.S. Age and seasonal variation and establishment of reference intervals for water-soluble vitamins determined by liquid chromatography tandem mass spectrometry. Nutrition 2022, 95, 111490. [Google Scholar] [CrossRef]
- Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 2007, 35, 390–419.
- Zoungas, S.; Zhou, Z.; Owen, A.J.; Curtis, A.J.; Espinoza, S.E.; Ernst, M.E.; Woods, R.L.; Orchard, S.G.; McNeil, J.J.; Murray, A.M.; et al. Daily low-dose aspirin and incident type 2 diabetes in community-dwelling healthy older adults: A post-hoc analysis of efficacy and safety in the ASPREE randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2024, 12, 98–106. [Google Scholar] [CrossRef]
- Frank, E.; Harrell, J. Regression Modeling Strategies; Springer Nature: Berlin, Germany, 2015. [Google Scholar]
- Sun, Y.; Zhang, H.; Qi, G.; Tian, W. Nutrient deficiency patterns and all-cause and cardiovascular mortality in older adults with hypertension: A latent class analysis. BMC Public Health 2024, 24, 1551. [Google Scholar] [CrossRef]
- Duc, H.N.; Oh, H.; Yoon, I.M.; Kim, M.S. Association between levels of thiamine intake, diabetes, cardiovascular diseases and depression in Korea: A national cross-sectional study. J. Nutr. Sci. 2021, 10, e31. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Meng, Q.; Wei, Y.; Su, X.; Zhang, Y.; He, P.; Zhou, C.; Liu, M.; Ye, Z.; Qin, X. J-shaped association between dietary thiamine intake and the risk of cognitive decline in cognitively healthy, older Chinese individuals. Gen. Psychiatr. 2024, 37, e101311. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Choi, J.H. Genetic Variations in Thiamin Transferase SLC35F3 and the Risk of Hypertension in Koreans. Clin. Nutr. Res. 2021, 10, 140–149. [Google Scholar] [CrossRef]
- Wiley, K.D.; Gupta, M. Vitamin B1 (Thiamine) Deficiency. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- DiNicolantonio, J.J.; Liu, J.; O’Keefe, J.H. Thiamine and Cardiovascular Disease: A Literature Review. Prog. Cardiovasc. Dis. 2018, 61, 27–32. [Google Scholar] [CrossRef]
- Zhang, K.; Huentelman, M.J.; Rao, F.; Sun, E.I.; Corneveaux, J.J.; Schork, A.J.; Wei, Z.; Waalen, J.; Miramontes-Gonzalez, J.P.; Hightower, C.M.; et al. Genetic implication of a novel thiamine transporter in human hypertension. J. Am. Coll. Cardiol. 2014, 63, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Coutinho, T. Arterial stiffness and its clinical implications in women. Can. J. Cardiol. 2014, 30, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Dong, X.; Zhao, X.; Cai, J. Sex Differences in Achieved Diastolic Blood Pressure and Cardiovascular Outcomes in Elderly Patients with Hypertension. JACC Adv. 2025, 4, 101675. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Chen, H.; Sun, Q.; Shi, L.; Sun, J.; Li, G.; Xing, L.; Liu, S. Prevalence of isolated diastolic hypertension and the risk of cardiovascular mortality among adults aged 40 years and older in northeast China: A prospective cohort study. BMJ Open 2022, 12, e061762. [Google Scholar] [CrossRef]
- Shibayama, J.; Tada, H.; Morita, M.; Yoshida, S.; Sakata, K.; Usui, S.; Kawashiri, M.A.; Takamura, M. A Case of Pulmonary Hypertension in a 67-Year-Old Woman with Thiamine Deficiency Following Partial Gastrectomy and Exacerbated by Diuretics. Am. J. Case Rep. 2022, 23, e935308. [Google Scholar] [CrossRef]



| Variables | Overall (n = 1906) | Participants Without Hypertension (n = 740) | Participants with Hypertension (n = 1166) | t/χ2/z | p |
|---|---|---|---|---|---|
| Age, mean ± SD, years | 59.93 ± 6.64 | 58.47 ± 6.94 | 60.86 ± 6.28 | 60.34 a | <0.001 |
| Educational level, n (%) | 24.17 b | <0.001 | |||
| Without any formal schooling | 858 (45.02) | 293 (39.59) | 565 (48.46) | ||
| Primary school | 634 (33.26) | 251 (33.92) | 383 (32.85) | ||
| Junior high school | 351 (18.42) | 163 (22.03) | 188 (16.12) | ||
| Senior high school | 57 (2.99) | 28 (3.78) | 29 (2.49) | ||
| College and above | 6 (0.31) | 5 (0.68) | 1 (0.09) | ||
| BMI, mean ± SD, kg/m2 | 24.45 ± 3.59 | 23.66 ± 3.07 | 24.95 ± 3.79 | 61.01 a | <0.001 |
| Menopausal status, n (%) | 11.52 b | 0.001 | |||
| Pre-menopausal | 180 (9.44) | 91 (12.30) | 89 (7.63) | ||
| Post-menopausal | 1726 (90.56) | 649 (87.70) | 1077 (92.37) | ||
| Drinking status, n (%) | 0.12 b | 0.941 | |||
| Never | 1642 (86.15) | 635 (85.81) | 1007 (86.36) | ||
| Occasionally | 125 (6.56) | 50 (6.76) | 75 (6.43) | ||
| Frequently | 139 (7.29) | 55 (7.43) | 84 (7.20) | ||
| TC, mean ± SD, mmol/L | 5.04 ± 0.95 | 5.03 ± 1.00 | 5.04 ± 0.93 | 0.12 a | 0.728 |
| TG, median (IQR), mmol/L | 1.38 (1.01–1.94) | 1.28 (0.91–1.76) | 1.46 (1.08–2.06) | −6.97 c | <0.001 |
| LDL-c, mean ± SD, mmol/L | 2.89 ± 0.72 | 2.84 ± 0.68 | 2.92 ± 0.74 | 6.31 a | 0.012 |
| HDL-c, median (IQR), mmol/L | 1.60 (1.36–1.84) | 1.67 (1.43–1.90) | 1.55 (1.33–1.8) | 5.91 c | <0.001 |
| Lipid-lowering medications use, n (%) | 44 (18.49) | 8 (13.79) | 36 (20.00) | 1.22 b | 0.749 |
| Dyslipidemia, n (%) | 525 (27.54) | 159 (21.49) | 366 (31.39) | 22.24 b | <0.001 |
| FPG, median (IQR), mmol/L | 5.48 (5.07–6.10) | 5.37 (4.98–5.87) | 5.56 (5.13–6.23) | −5.93 c | <0.001 |
| Antidiabetic medication use, n (%) | 186 (83.41) | 60 (83.33) | 126 (83.44) | 0.00 b | 0.983 |
| Abnormal blood glucose, n (%) | 296 (15.53) | 88 (11.89) | 208 (17.84) | 12.20 b | <0.001 |
| Thiamine, median (IQR), ng/mL | 1.75 (1.31–2.32) | 1.81 (1.38–2.36) | 1.73 (1.27–2.28) | 3.04 c | 0.002 |
| Thiamine tertiles, n (%) | 10.23 b | 0.006 | |||
| Lowest | 625 (32.79) | 216 (29.19) | 409 (35.08) | ||
| Middle | 639 (33.53) | 246 (33.24) | 393 (33.70) | ||
| Highest | 642 (33.68) | 278 (37.57) | 364 (31.22) | ||
| Systolic BP, mean ± SD, mmHg | 139.87 ± 19.08 | 124.20 ± 10.68 | 149.81 ± 16.38 | 1424.56 a | <0.001 |
| Diastolic BP, mean ± SD, mmHg | 81.24 ± 10.60 | 75.50 ± 8.04 | 84.89 ± 10.41 | 435.89 a | <0.001 |
| Hypertension Prevalence or BP | Thiamine Tertiles | p For Trend | ||
|---|---|---|---|---|
| Lowest (n = 625) | Middle (n = 639) | Highest (n = 642) | ||
| Hypertension, n (%) | 409 (65.44) | 393 (61.50) | 364 (56.70) | 0.001 |
| Systolic BP, mean ± SD, mmHg | 141.97 ± 18.57 | 140.80 ± 19.05 | 136.89 ± 19.27 | <0.001 |
| Diastolic BP, mean ± SD, mmHg | 81.45 ± 10.88 | 81.55 ± 10.67 | 80.73 ± 10.24 | 0.314 |
| Serum Levels of Thiamine | n | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | ||
| Continuous variable | ||||||||||
| Every SD increase | 1906 | 0.86 | 0.77, 0.97 | 0.011 | 0.88 | 0.78, 0.98 | 0.019 | 0.87 | 0.77, 0.97 | 0.012 |
| Categorical variable | ||||||||||
| Lowest tertile | 625 | 1.00 | Ref | - | 1.00 | Ref | - | 1.00 | Ref | - |
| Middle tertile | 639 | 0.84 | 0.67, 1.06 | 0.146 | 0.87 | 0.69, 1.11 | 0.266 | 0.86 | 0.68, 1.09 | 0.219 |
| Highest tertile | 642 | 0.68 | 0.54, 0.85 | 0.001 | 0.77 | 0.60, 0.97 | 0.028 | 0.75 | 0.59, 0.95 | 0.016 |
| Serum Levels of Thiamine | n | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coef | 95% CI | p | Coef | 95% CI | p | Coef | 95% CI | p | ||
| Continuous variable | ||||||||||
| Every SD increase | 1906 | −1.61 | −2.46, −0.75 | <0.001 | −1.46 | −2.29, −0.64 | 0.001 | −1.51 | −2.33, −0.68 | <0.001 |
| Categorical variable | ||||||||||
| Lowest tertile | 625 | 0.00 | Ref | - | 0.00 | Ref | - | 0.00 | Ref | - |
| Middle tertile | 639 | −1.17 | −3.27, 0.92 | 0.272 | −0.68 | −2.70, 1.33 | 0.506 | −0.72 | −2.73, 1.29 | 0.485 |
| Highest tertile | 642 | −5.08 | −7.17, −2.99 | <0.001 | −3.85 | −5.88, −1.82 | <0.001 | −3.94 | −5.97, −1.92 | <0.001 |
| Serum Levels of Thiamine | n | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coef | 95% CI | p | Coef | 95% CI | p | Coef | 95% CI | p | ||
| Continuous variable | ||||||||||
| Every SD increase | 1906 | −0.41 | −0.84, 0.02 | 0.059 | −0.47 | −0.90, −0.05 | 0.029 | −0.53 | −0.95, −0.10 | 0.015 |
| Categorical variable | ||||||||||
| Lowest tertile | 625 | 0.00 | Ref | / | 0.00 | Ref | / | 0.00 | Ref | / |
| Middle tertile | 639 | 0.10 | −1.07, 1.27 | 0.866 | 0.01 | −1.15, 1.17 | 0.984 | −0.09 | −1.25, 1.07 | 0.880 |
| Highest tertile | 642 | −0.73 | −1.89, 0.44 | 0.223 | −0.90 | −2.07, 0.27 | 0.132 | −1.02 | −2.19, 0.15 | 0.087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Lin, J.; Chen, X.; Ma, Z.; Du, X.; Wang, M.; Chen, R.; Zhong, J. Associations of Serum Thiamine Levels with Blood Pressure Among Middle-Aged and Elderly Women in Eastern China. Nutrients 2025, 17, 2210. https://doi.org/10.3390/nu17132210
Chen L, Lin J, Chen X, Ma Z, Du X, Wang M, Chen R, Zhong J. Associations of Serum Thiamine Levels with Blood Pressure Among Middle-Aged and Elderly Women in Eastern China. Nutrients. 2025; 17(13):2210. https://doi.org/10.3390/nu17132210
Chicago/Turabian StyleChen, Lijin, Jingjing Lin, Xiangyu Chen, Zhimin Ma, Xiaofu Du, Meng Wang, Rong Chen, and Jieming Zhong. 2025. "Associations of Serum Thiamine Levels with Blood Pressure Among Middle-Aged and Elderly Women in Eastern China" Nutrients 17, no. 13: 2210. https://doi.org/10.3390/nu17132210
APA StyleChen, L., Lin, J., Chen, X., Ma, Z., Du, X., Wang, M., Chen, R., & Zhong, J. (2025). Associations of Serum Thiamine Levels with Blood Pressure Among Middle-Aged and Elderly Women in Eastern China. Nutrients, 17(13), 2210. https://doi.org/10.3390/nu17132210






