Thiamine (Vitamin B1)—An Essential Health Regulator
Abstract
1. Introduction
2. Effects of Inadequate Intake
3. Energy Metabolism and Cellular Function
4. Oxidative Stress Reduction
5. The Non-Coenzymatic Role of Thiamine
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-HACL | 2-hydroxyacyl-CoA lyase |
| 6PGD | 6-phosphogluconate dehydrogenase |
| CoA | Coenzyme A, acetyl-CoA-acetyl coenzyme A |
| eNOS | Endothelial nitric oxide synthase |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GPI | Glucose-6-phosphate isomerase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSSG | Glutathione disulfide |
| HIF-1 | Hypoxia-induced factor-1α |
| NAD+/NADH | Nicotinamide adenine dinucleotide |
| NADP+/NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-B |
| Non-oxPPP | Non-oxidative branch of pentose–phosphate pathway |
| oxPPP | Oxidative branch of pentose–phosphate pathway |
| PDC | Pyruvate dehydrogenase |
| PdxK | Pyridoxaldehyde kinase |
| PPP | Pentose–phosphate pathway |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| TDP | Thiamine diphosphate, also known as thiamine pyrophosphate |
| TKT | Transketolase |
| TMP | Thiamine monophosphate |
| TNF-α | Tumor necrosis factor-α |
| TRX | Thioredoxin |
| TRX(ox) | Oxidized thioredoxin |
| TRXR | Thioredoxin reductase |
| TTP | Thiamine triphosphate |
| αKGDH | α-ketoglutarate dehydrogenase |
References
- Uchi, T.; Konno, S.; Kihara, H.; Sugimoto, H. Thiamine deficiency unrelated to alcohol consumption presented with urinary retention and Wernicke’s encephalopathy: A case report. Clin. Case Rep. 2023, 11, e7681. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Dietary reference values for thiamin. EFSA J. 2016, 14, 4653. [Google Scholar] [CrossRef]
- Combs, G.F. Thiamin. In The Vitamins; Academic Press: New York, NY, USA, 2012; pp. 261–276. [Google Scholar] [CrossRef]
- Oudman, E.; Wijnia, J.W.; Oey, M.J.; van Dam, M.; Postma, A. Wernicke-Korsakoff syndrome despite no alcohol abuse: A summary of systematic reports. J. Neurol. Sci. 2021, 426, 117482. [Google Scholar] [CrossRef]
- Schoenenberger, A.W.; Schoenenberger-Berzins, R.; der Maur, C.A.; Suter, P.M.; Vergopoulos, A.; Erne, P. Thiamine supplementation in symptomatic chronic heart failure: A randomized, double-blind, placebo-controlled, cross-over pilot study. Clin. Res. Cardiol. 2012, 101, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Keith, M.E.; Walsh, N.A.; Darling, P.B.; Hanninen, S.A.; Thirugnanam, S.; Leong-Poi, H.; Sole, M.J. B-Vitamin Deficiency in Hospitalized Patients with Heart Failure. J. Am. Diet. Assoc. 2009, 109, 1406–1410. [Google Scholar] [CrossRef]
- Mates, E.; Alluri, D.; Artis, T.; Riddle, M.S. A Retrospective Case Series of Thiamine Deficiency in Non-Alcoholic Hospitalized Veterans: An Important Cause of Delirium and Falling? J. Clin. Med. 2021, 10, 1449. [Google Scholar] [CrossRef] [PubMed]
- Rakotoambinina, B.; Hiffler, L.; Gomes, F. Pediatric thiamine deficiency disorders in high-income countries between 2000 and 2020: A clinical reappraisal. Ann. N. Y. Acad. Sci. 2021, 1498, 57–76. [Google Scholar] [CrossRef]
- Kim, Y.N.; Cho, Y.O. Prevalent Low Thiamine Status Among Adults Living in Seoul Metropolitan Area (South Korea). Int. J. Vitam. Nutr. Res. 2019, 89, 314–320. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Thiamin. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; Chapter 4; National Academies Press (US): Washington, DC, USA, 1998; Available online: https://www.ncbi.nlm.nih.gov/books/NBK114331 (accessed on 12 February 2025).
- Elmadfa, I.; Majchrzak, D.; Rust, P.; Genser, D. The thiamine status of adult humans depends on carbohydrate intake. Int. J. Vitam. Nutr. Res. 2001, 71, 217–221. [Google Scholar] [CrossRef]
- Duc, H.N.; Oh, H.; Yoon, I.M.; Kim, M.S. Association between levels of thiamine intake, diabetes, cardiovascular diseases and depression in Korea: A national cross-sectional study. J. Nutr. Sci. 2021, 10, e31. [Google Scholar] [CrossRef]
- Alaei-Shahmiri, F.; Soares, M.J.; Zhao, Y.; Sherriff, J. The impact of thiamine supplementation on blood pressure, serum lipids and C-reactive protein in individuals with hyperglycemia: A randomised, double-blind cross-over trial. Diabetes Metab. Syndr. 2015, 9, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Alaei Shahmiri, F.; Soares, M.J.; Zhao, Y.; Sherriff, J. High-dose thiamine supplementation improves glucose tolerance in hyperglycemic individuals: A randomized, double-blind cross-over trial. Eur. J. Nutr. 2013, 52, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- Ardhi, M.S.; Hamdan, M.; Romdhoni, A.C. Effect of Thiamine on Serum Glutamate in Ischemic Stroke Animal Model. Pharmacogn. J. 2023, 15, 390–392. [Google Scholar] [CrossRef]
- Oudman, E. Wernicke encephalopathy in patients with depression: A systematic review. Psychiatry Clin. Neurosci. 2020, 74, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ding, H.; Chen, H.; Ye, X.; Li, H.; Lin, X.; Ke, Z. Thiamine nutritional status and depressive symptoms are inversely associated among older Chinese adults. J. Nutr. 2013, 143, 53–58. [Google Scholar] [CrossRef]
- Martel, J.L.; Kerndt, C.C.; Doshi, H.; Sina, R.E.; Franklin, D.S. Vitamin B1 (Thiamine). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6042, Vitamin B1. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/vitamin-B1 (accessed on 23 June 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1132, Thiamine-Pyrophosphate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thiamine-pyrophosphate (accessed on 23 June 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 511, Thiamine Triphosphate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thiamine-Triphosphate (accessed on 23 June 2025).
- Bettendorff, L.; Wins, P. Biochemistry of Thiamine and Thiamine Phosphate Compounds. In Encyclopedia of Biological Chemistry III; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Patra, K.C.; Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, R.S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef]
- Ge, T.; Yang, J.; Zhou, S.; Wang, Y.; Li, Y.; Tong, X. The Role of the Pentose Phosphate Pathway in Diabetes and Cancer. Front. Endocrinol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Pekovich, S.; Martin, P.; Singleton, K. Thiamine Deficiency Decreases Steady-State Transketolase Pyruvate Dehydrogenase but not α-Ketoglutarate Dehydrogenase mRNA Levels in Three Human Cell Types. J. Nutr. 1998, 128, 683–687. [Google Scholar] [CrossRef]
- Pekovich, S.R.; Martin, P.R.; Singleton, C.K. Thiamine pyrophosphate–requiring enzymes are altered during pyrithiamine-induced thiamine deficiency in cultured human lymphoblasts. J. Nutr. 1996, 126, 1791–1798. [Google Scholar] [CrossRef]
- Hostetler, K.Y.; Landau, B.R. Estimation of the Pentose Cycle Contribution to Glucose Metabolism in Tissue in Vivo. Biochemistry 1967, 6, 2961–2964. [Google Scholar] [CrossRef] [PubMed]
- Kloska, S.M.; Pałczyński, K.; Marciniak, T.; Talaśka, T.; Miller, M.; Wysocki, B.J.; Davis, P.; Wysocki, T.A. Queueing theory model of pentose phosphate pathway. Sci. Rep. 2022, 12, 4601. [Google Scholar] [CrossRef] [PubMed]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The pentose phosphate pathway in health and disease. Nat. Metab. 2023, 8, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, C.; Grieco, D.; Costanzo, V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011, 30, 546–555. [Google Scholar] [CrossRef]
- Efferth, T.; Fabry, U.; Osieka, R. DNA damage and apoptosis in mononuclear cells from glucose-6-phosphate dehydrogenase-deficient patients (G6PD Aachen variant) after UV irradiation. J. Leukoc. Biol. 2016, 9, 340–342. [Google Scholar] [CrossRef]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Aldieri, E.; Ghigo, D. The pentose phosphate pathway: An antioxidant defense and a crossroad in tumor cell fate. Free Radical Biol. Med. 2012, 53, 421–436. [Google Scholar] [CrossRef]
- Milanese, C.; Mastroberardino, P.G. A perspective on DNA damage-induced potentiation of the pentose phosphate shunt and reductive stress in chemoresistance. Mol. Cell Oncol. 2020, 7, 1733383. [Google Scholar] [CrossRef]
- Jiang, P.; Du, W.; Wang, X.; Mancuso, A.; Gao, X.; Wu, M.; Yang, X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 2011, 13, 310–316. [Google Scholar] [CrossRef]
- Deshpande, G.P.; Patterton, H.G.; Faadiel Essop, M. The human transketolase-like proteins TKTL1 and TKTL2 are bona fide transketolases. BMC Struct. Biol. 2019, 19, 2. [Google Scholar] [CrossRef]
- McLure, K.G.; Takagi, M.; Kastan, M.B. NAD+ modulates p53 DNA binding specificity and function. Mol. Cell Biol. 2004, 24, 9958–9967. [Google Scholar] [CrossRef]
- Shin, B.H.; Choi, S.H.; Cho, E.Y.; Shin, M.J.; Hwang, K.C.; Cho, H.K.; Chung, J.H.; Jang, Y. Thiamine attenuates hypoxia-induced cell death in cultured neonatal rat cardiomyocytes. Mol. Cells. 2004, 18, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Nauton, L.; Hecquet, L.; Théry, V. QM/MM Study of Human Transketolase: Thiamine Diphosphate Activation Mechanism and Complete Catalytic Cycle. J. Chem. Inf. Model. 2021, 61, 3502–3515. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Q.; Lin, J.F.; Tian, T.; Xie, D.; Xu, R.H. NADPH homeostasis in cancer: Functions, mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2020, 5, 231. [Google Scholar] [CrossRef] [PubMed]
- Xu, I.M.; Lai, R.K.; Lin, S.H.; Tse, A.P.; Chiu, D.K.; Koh, H.Y.; Law, C.T.; Wong, C.M.; Cai, Z.; Wong, C.C.; et al. Transketolase counteracts oxidative stress to drive cancer development. Proc. Natl. Acad. Sci. USA 2016, 113, E725–E734. [Google Scholar] [CrossRef]
- Bozic, I.; Lavrnja, I. Thiamine and benfotiamine: Focus on their therapeutic potential. Heliyon 2023, 9, e21839. [Google Scholar] [CrossRef]
- Fukui, K.; Wakamatsu, T.; Agari, Y.; Masui, R.; Kuramitsu, S. Inactivation of the DNA repair genes mutS, mutL or the anti-recombination gene mutS2 leads to activation of vitamin B1 biosynthesis genes. PLoS ONE 2011, 6, e19053. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K.; FSato, E.; Konaka, R.; Inoue, M. Potent radical-scavenging activities of thiamin and thiamin diphosphate. J. Clin. Biochem. Nutr. 2007, 40, 42–48. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Quang, D.D. Unraveling the antioxidant potential of thiamine: Thermochemical and kinetics studies in aqueous phase using DFT. Viet J. Chem. 2019, 57, 485–490. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef]
- Panov, A.V.; Dikalov, S.I. Cardiolipin, Perhydroxyl Radicals, and Lipid Peroxidation in Mitochondrial Dysfunctions and Aging. Oxid. Med. Cell Longev. 2020, 2020, 1323028. [Google Scholar] [CrossRef]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2019, 119, e25665. [Google Scholar] [CrossRef]
- Carreon-Gonzalez, M.; Vivier-Bunge, A.; Alvarez-Idaboy, J.R. Thiophenols Promising Scavengers of Peroxyl Radicals: Mechanisms kinetics. J. Comput. Chem. 2019, 40, 2103–2110. [Google Scholar] [CrossRef]
- Gliszczynska-Swiglo, A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Sambon, M.; Napp, A.; Demelenne, A.; Vignisse, J.; Wins, P.; Fillet, M.; Bettendorff, L. Thiamine and benfotiamine protect neuroblastoma cells against paraquat and β-amyloid toxicity by a coenzyme-independent mechanism. Heliyon 2019, 5, e01710. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Dedina, L.; O’Brien, P.J. Rescuing hepatocytes from iron-catalyzed oxidative stress using vitamins B1 and B6. Toxicol. Vitr. 2011, 25, 1114–1122. [Google Scholar] [CrossRef]
- Sarandol, E.; Tas, S.; Serdar, Z.; Dirican, M. Effects of thiamine treatment on oxidative stress in experimental diabetes. Bratisl. Med. J. 2020, 121, 235–241. [Google Scholar] [CrossRef]
- Dhir, S.; Tarasenko, M.; Napoli, E.; Giulivi, C. Neurological, Psychiatric, and Biochemical Aspects of Thiamine Deficiency in Children and Adults. Front. Psychiatry 2019, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, G.; Li, W.; Fan, Z.; Sun, A.; Luo, J.; Ke, Z.J. Thiamine deficiency increases β-secretase activity and accumulation of β-amyloid peptides. Neurobiol. Aging 2011, 32, 42–53. [Google Scholar] [CrossRef]
- Yang, G.; Meng, Y.; Li, W.; Yong, Y.; Fan, Z.; Ding, H.; Wei, Y.; Luo, J.; Ke, Z.J. Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain Pathol. 2011, 21, 279–297. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Frank, J.A.; Ke, Z.J.; Luo, J. Thiamine deficiency induces endoplasmic reticulum stress and oxidative stress in human neurons derived from induced pluripotent stem cells. Toxicol. Appl. Pharmacol. 2017, 320, 26–31. [Google Scholar] [CrossRef]
- Sweet, R.L.; Zastre, J.A. HIF1-α-mediated gene expression induced by vitamin B1 deficiency. Int. J. Vitam. Nutr. Res. 2013, 83, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, S.; Zuo, A.; Zhang, J.; Wen, W.; Jiang, W.; Chen, H.; Liang, D.; Sun, J.; Wang, M. HIF-1α/JMJD1A signaling regulates inflammation and oxidative stress following hyperglycemia and hypoxia-induced vascular cell injury. Cell Mol. Biol. Lett. 2021, 26, 40. [Google Scholar] [CrossRef]
- Zera, K.; Zastre, J. Stabilization of the hypoxia-inducible transcription Factor-1 alpha (HIF-1α) in thiamine deficiency is mediated by pyruvate accumulation. Toxicol. Appl. Pharmacol. 2018, 355, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bist, R.; Bubber, P. Thiamine deficiency induces oxidative stress in brain mitochondria of Mus musculus. J. Physiol. Biochem. 2013, 69, 539–546. [Google Scholar] [CrossRef]
- Chauhan, A.; Srivastva, N.; Bubber, P. Thiamine Deficiency Induced Dietary Disparity Promotes Oxidative Stress and Neurodegeneration. Indian J. Clin. Biochem. 2017, 33, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Tapias, V.; Jainuddin, S.; Ahuja, M.; Stack, C.; Elipenahli, C.; Vignisse, J.; Gerges, M.; Starkova, N.; Xu, H.; Starkov, A.A.; et al. Benfotiamine treatment activates the Nrf2/ARE pathway and is neuroprotective in a transgenic mouse model of tauopathy. Hum. Mol. Genet. 2018, 27, 2874–2892. [Google Scholar] [CrossRef]
- Portari, G.V.; Ovidio, P.P.; Deminice, R.; Jordão, A.A. Protective effect of treatment with thiamine or benfotiamine on liver oxidative damage in rat model of acute ethanol intoxication. Life Sci. 2016, 162, 21–24. [Google Scholar] [CrossRef]
- Hintz, K.K.; Relling, D.P.; Saari, J.T.; Borgerding, A.J.; Duan, J.; Ren, B.H.; Kato, K.; Epstein, P.N.; Ren, J. Cardiac overexpression of alcohol dehydrogenase exacerbates cardiac contractile dysfunction, lipid peroxidation, and protein damage after chronic ethanol ingestion. Alcohol. Clin. Exp. Res. 2003, 27, 1090–1098. [Google Scholar] [CrossRef]
- Aberle, N.S., 2nd; Burd, L.; Zhao, B.H.; Ren, J. Acetaldehyde-induced cardiac contractile dysfunction may be alleviated by vitamin B1 but not by vitamins B6 or B12. Alcohol Alcohol. 2004, 39, 450–454. [Google Scholar] [CrossRef]
- Duan, J.; McFadden, G.E.; Borgerding, A.J.; Norby, F.L.; Ren, B.H.; Ye, G.; Epstein, P.N.; Ren, J. Overexpression of alcohol dehydrogenase exacerbates ethanol-induced contractile defect in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1216–H1222. [Google Scholar] [CrossRef] [PubMed]
- Zahr, N.M.; Pfefferbaum, A. Alcohol’s Effects on the Brain: Neuroimaging Results in Humans and Animal Models. Alcohol. Res. 2017, 38, 183–206. [Google Scholar] [PubMed] [PubMed Central]
- Xu, H.; Li, H.; Liu, D.; Wen, W.; Xu, M.; Frank, J.A.; Chen, J.; Zhu, H.; Grahame, N.J.; Luo, J. Chronic Voluntary Alcohol Drinking Causes Anxiety-like Behavior, Thiamine Deficiency, and Brain Damage of Female Crossed High Alcohol Preferring Mice. Front. Pharmacol. 2021, 12, 614396. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Liggett, A. Adding an orange to the banana bag: Vitamin C deficiency is common in alcohol use disorders. Crit. Care 2019, 23, 165. [Google Scholar] [CrossRef]
- Nicoll, F.; Gerasimidis, K.; Forrest, E. The Role of Micronutrients in the Pathogenesis of Alcohol-Related Liver Disease. Alcohol Alcohol. 2022, 57, 275–282. [Google Scholar] [CrossRef]
- Ferdouse, A.; Agrawal, R.R.; Gao, M.A.; Jiang, H.; Blaner, W.S.; Clugston, R.D. Alcohol induced hepatic retinoid depletion is associated with the induction of multiple retinoid catabolizing cytochrome P450 enzymes. PLoS ONE 2022, 17, e0261675. [Google Scholar] [CrossRef]
- Schupp, N.; Dette, E.M.; Schmid, U.; Bahner, U.; Winkler, M.; Heidland, A.; Stopper, H. Benfotiamine reduces genomic damage in peripheral lymphocytes of hemodialysis patients. Naunyn-Schmied. Arch. Pharmacol. 2008, 378, 283–291. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709, Erratum in Nat. Rev. Drug Discov. 2021, 20, 652. [Google Scholar] [CrossRef]
- Starkov, A.A. An update on the role of mitochondrial α-ketoglutarate dehydrogenase in oxidative stress. Mol. Cell Neurosci. 2013, 55, 13–16. [Google Scholar] [CrossRef]
- Starkov, A.A.; Fiskum, G.; Chinopoulos, C.; Lorenzo, B.J.; Browne, S.E.; Patel, M.S.; Beal, M.F. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 2004, 24, 7779–7788. [Google Scholar] [CrossRef]
- Kruse, M.; Navarro, D.; Desjardins, P.; Butterworth, R.F. Increased brain endothelial nitric oxide synthase expression in thiamine deficiency: Relationship to selective vulnerability. Neurochem. Int. 2004, 45, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mezzar, S.; De Schryver, E.; Asselberghs, S.; Meyhi, E.; Morvay, P.L.; Baes, M.; Van Veldhoven, P.P. Phytol-induced pathology in 2-hydroxyacyl-CoA lyase (HACL1) deficient mice. Evidence for a second non-HACL1-related lyase. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 972–990. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.G.; Canani, C.R.; Fernandes, C.G.; Zanatta, Â.; Seminotti, B.; Ribeiro, C.A.; Leipnitz, G.; Vargas, C.R.; Wajner, M. Reactive nitrogen species mediate oxidative stress and astrogliosis provoked by in vivo administration of phytanic acid in cerebellum of adolescent rats: A potential contributing pathomechanism of cerebellar injury in peroxisomal disorders. Neuroscience 2015, 304, 122–132. [Google Scholar] [CrossRef]
- Gangolf, M.; Czerniecki, J.; Radermecker, M.; Detry, O.; Nisolle, M.; Jouan, C.; Martin, D.; Chantraine, F.; Lakaye, B.; Wins, P.; et al. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS ONE 2010, 5, e13616. [Google Scholar] [CrossRef]
- Egi, Y.; Koyama, S.; Shioda, T.; Yamada, K.; Kawasaki, T. Identification, purification and reconstitution of thiamin metabolizing enzymes in human red blood cells. Biochim. Biophys. Acta 1992, 1160, 171–178. [Google Scholar] [CrossRef]
- Bettendorff, L. Update on Thiamine Triphosphorylated Derivatives and Metabolizing Enzymatic Complexes. Biomolecules 2021, 11, 1645. [Google Scholar] [CrossRef]
- Nghiem, H.O.; Bettendorff, L.; Changeux, J.P. Specific phosphorylation of Torpedo 43K rapsyn by endogenous kinase(s) with thiamine triphosphate as the phosphate donor. FASEB J. 2000, 14, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Bettendorff, L.; Wins, P. Thiamin diphosphate in biological chemistry: New aspects of thiamin metabolism especially triphosphate derivatives action other than as cofactors. FASEB J. 2009, 276, 2917–2925. [Google Scholar] [CrossRef]
- Bunik, V.; Aleshin, V.; Nogues, I.; Kähne, T.; Parroni, A.; Contestabile, R.; Salvo, M.L.; Graf, A.; Tramonti, A. Thiamine-dependent regulation of mammalian brain pyridoxal kinase in vitro and in vivo. J. Neurochem. 2022, 161, 20–39. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Mkrtchyan, G.V.; Kaehne, T.; Graf, A.V.; Maslova, M.V.; Bunik, V.I. Diurnal regulation of the function of the rat brain glutamate dehydrogenase by acetylation and its dependence on thiamine administration. J. Neurochem. 2020, 153, 80–102. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Artiukhov, A.V.; Kaehne, T.; Graf, A.V.; Bunik, V.I. Daytime Dependence of the Activity of the Rat Brain Pyruvate Dehydrogenase Corresponds to the Mitochondrial Sirtuin 3 Level and Acetylation of Brain Proteins, All Regulated by Thiamine Administration Decreasing Phosphorylation of PDHA Ser293. Int. J. Mol. Sci. 2021, 22, 8006. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Chang, K.; Gunter, M.J.; Rauber, F.; Levy, R.B.; Huybrechts, I.; Kliemann, N.; Millett, C.; Vamos, E.P. Ultra-processed food consumption, cancer risk and cancer mortality: A large-scale prospective analysis within the UK Biobank. EClinicalMedicine 2023, 56, 101840. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Qiu, W.; Li, X.; Li, H.; Zhou, J.; Zhu, H. Transketolase Serves as a Biomarker for Poor Prognosis in Human Lung Adenocarcinoma. J. Cancer 2022, 13, 2584–2593. [Google Scholar] [CrossRef]
- Boopathy, D.; Grahf, D.; Ross, J.; Hawatian, K.; Rammal, J.A.; Alaimo, K.; Miller, J.B. Thiamine Deficiency Is Common and Underrecognized in Emergency Department Oncology Patients. J. Clin. Med. 2025, 14, 257. [Google Scholar] [CrossRef] [PubMed]
- Comín-Anduix, B.; Boren, J.; Martinez, S.; Moro, C.; Centelles, J.J.; Trebukhina, R.; Petushok, N.; Lee, W.N.; Boros, L.G.; Cascante, M. The effect of thiamine supplementation on tumour proliferation. A metabolic control analysis study. Eur. J. Biochem. 2001, 268, 4177–4182. [Google Scholar] [CrossRef]
- Kabat, G.C.; Miller, A.B.; Jain, M.; Rohan, T.E. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br. J. Cancer 2008, 99, 816–821. [Google Scholar] [CrossRef]
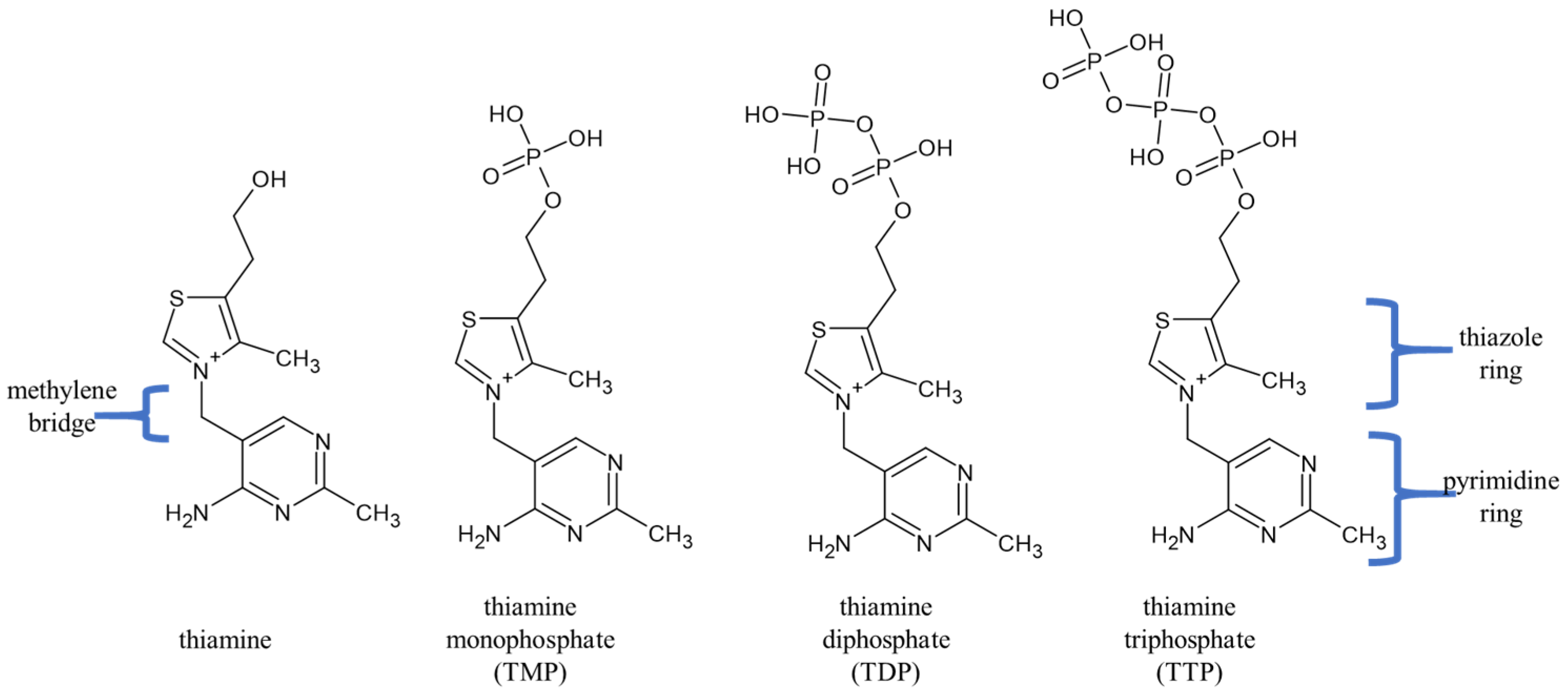
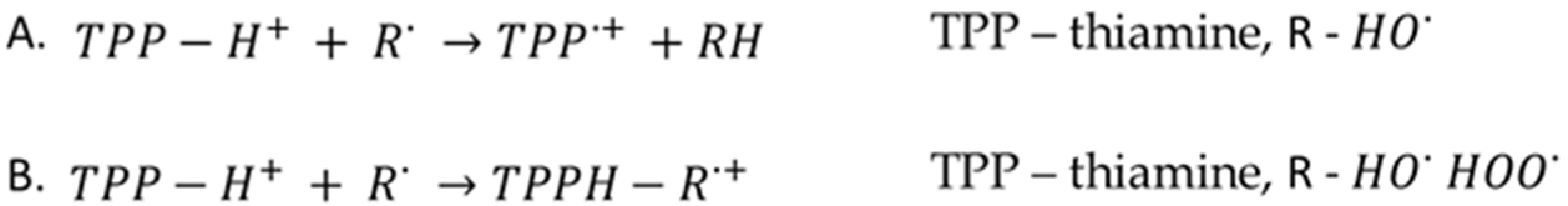
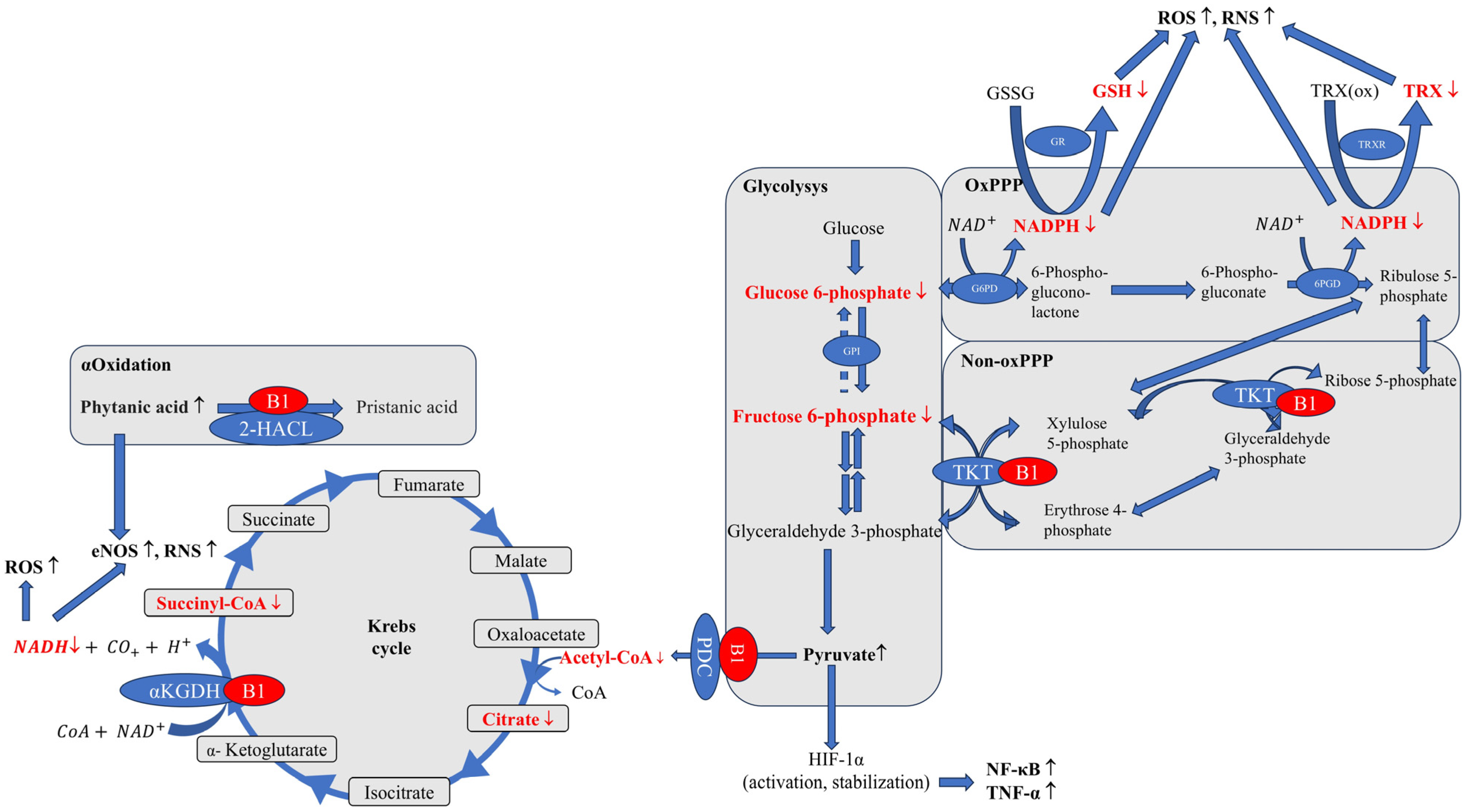
| Aspect of Thiamine | Details & Guidance |
|---|---|
| Properties of Thiamine | Daily Requirements: provided primarily through diet, ~1.1 mg/day for women, ~1.2 mg/day for men; pregnant women have a thiamine intake of up to 1.4 mg/d |
| Food Sources: Whole grains, legumes, lean meats | |
| Stability: Heat sensitive—cooking losses common | |
| Deficiency | At-Risk Groups: Alcoholism, malabsorption, bariatric surgery, chronic diuretic use |
| Related Diseases: Beriberi (wet: cardiovascular; dry: neurological) Wernicke–Korsakoff syndrome (in alcoholics), lactic acidosis, irritability, fatigue, poor memory | |
| Excess | High-Dose Benefits: Investigated in hyperglycemic complications, post-stroke recovery |
| Toxicity: The human body excretes excess thiamine in the urine. Toxicity resulting from high thiamine intake from food or supplements has not been established. There is no established upper limit of thiamine intake that causes toxicity | |
| Specific Patient/Populations | Pregnancy/Lactation: Increased needs—especially in hyperemesis gravidarum |
| Pediatrics: Essential during rapid growth—ensure adequate intake through diet or supplements in cases of mothers malnutrition or exclusive formula feeding Diabetes: Possible benefits in preventing microvascular complications; supplements should be considered (e.g., benfotiamine) Heart failure: Thiamine levels may be decreased in patients with heart failure Elderly: About 20% to 30% of elderly people are thiamine deficient. This may be due to inadequate intake of thiamine-rich foods, chronic health problems, and polypharmacy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierczak-Barańska, J.; Halczuk, K.; Karwowski, B.T. Thiamine (Vitamin B1)—An Essential Health Regulator. Nutrients 2025, 17, 2206. https://doi.org/10.3390/nu17132206
Kaźmierczak-Barańska J, Halczuk K, Karwowski BT. Thiamine (Vitamin B1)—An Essential Health Regulator. Nutrients. 2025; 17(13):2206. https://doi.org/10.3390/nu17132206
Chicago/Turabian StyleKaźmierczak-Barańska, Julia, Krzysztof Halczuk, and Bolesław T. Karwowski. 2025. "Thiamine (Vitamin B1)—An Essential Health Regulator" Nutrients 17, no. 13: 2206. https://doi.org/10.3390/nu17132206
APA StyleKaźmierczak-Barańska, J., Halczuk, K., & Karwowski, B. T. (2025). Thiamine (Vitamin B1)—An Essential Health Regulator. Nutrients, 17(13), 2206. https://doi.org/10.3390/nu17132206







