Dietary Polyphenols: Luteolin, Quercetin, and Apigenin as Potential Therapeutic Agents in the Treatment of Gliomas
Abstract
1. Introduction
2. Characteristics of Gliomas and the Potential Place for Polyphenols in Their Treatment
3. Natural Sources, Absorption, Metabolism, and Novel Pharmaceutical Measures to Increase Polyphenol Bioavailability
3.1. Luteolin
3.2. Quercetin
3.3. Apigenin
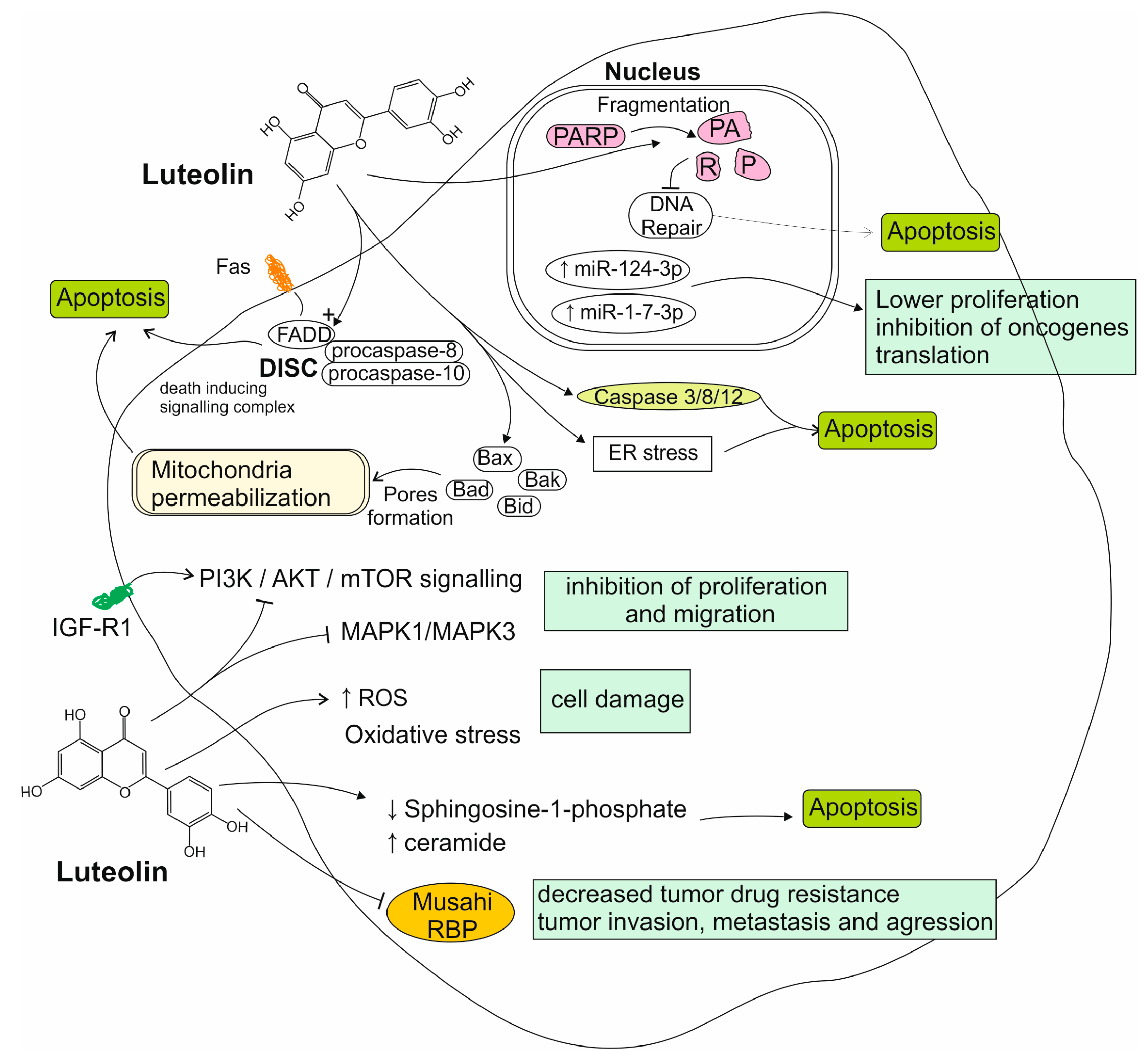
4. Luteolin’s Role in the Treatment of Gliomas
4.1. Reports from In Vitro Studies on Luteolin Activity to Glioma Cells
4.2. Reports from Animal Models on Luteolin Activity
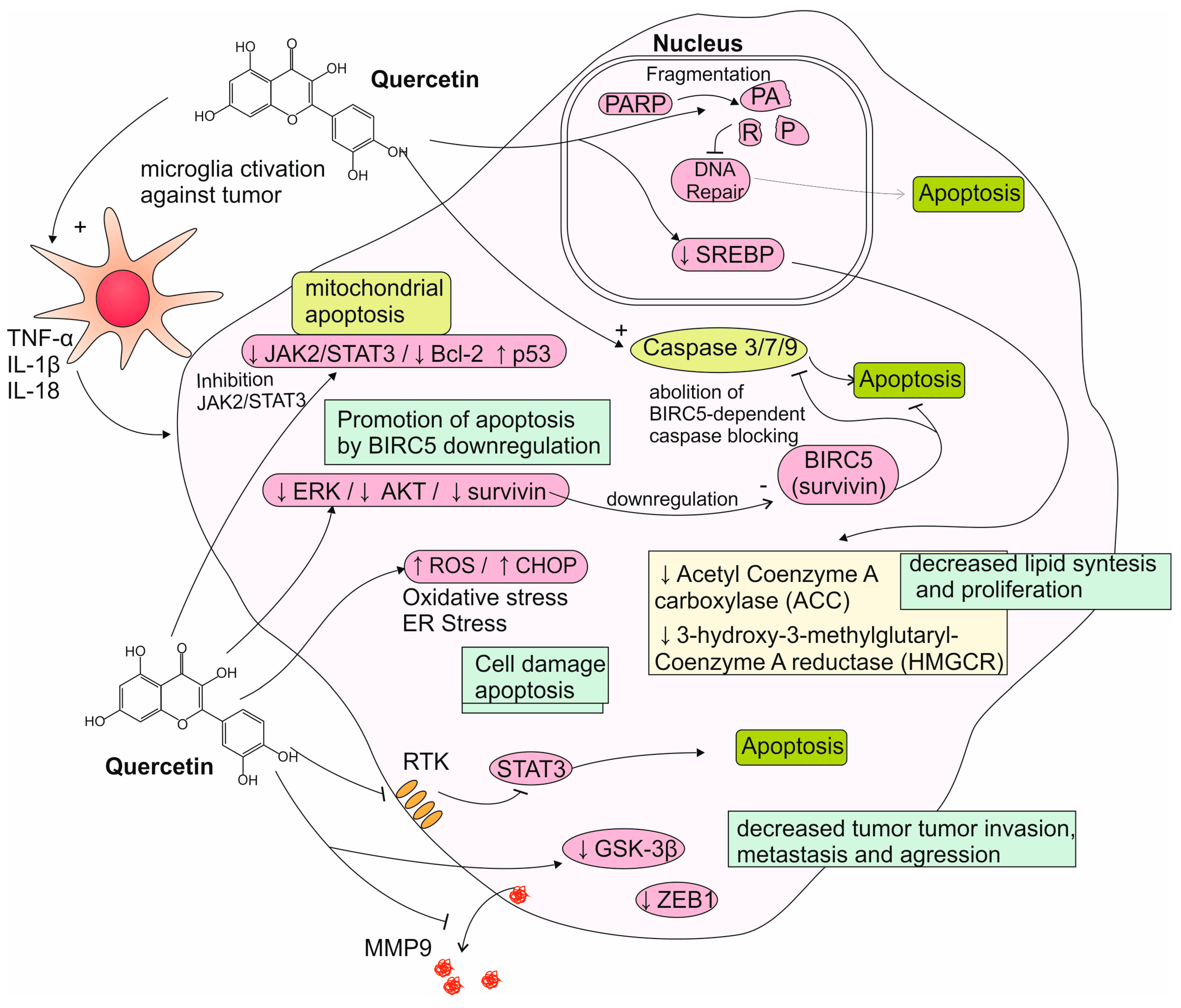
5. Quercetin’s Role in the Treatment of Gliomas
5.1. Reports from In Vitro Studies on Quercetin Activity to Glioma Cells
5.2. Reports from Animal Models on Quercetin Activity
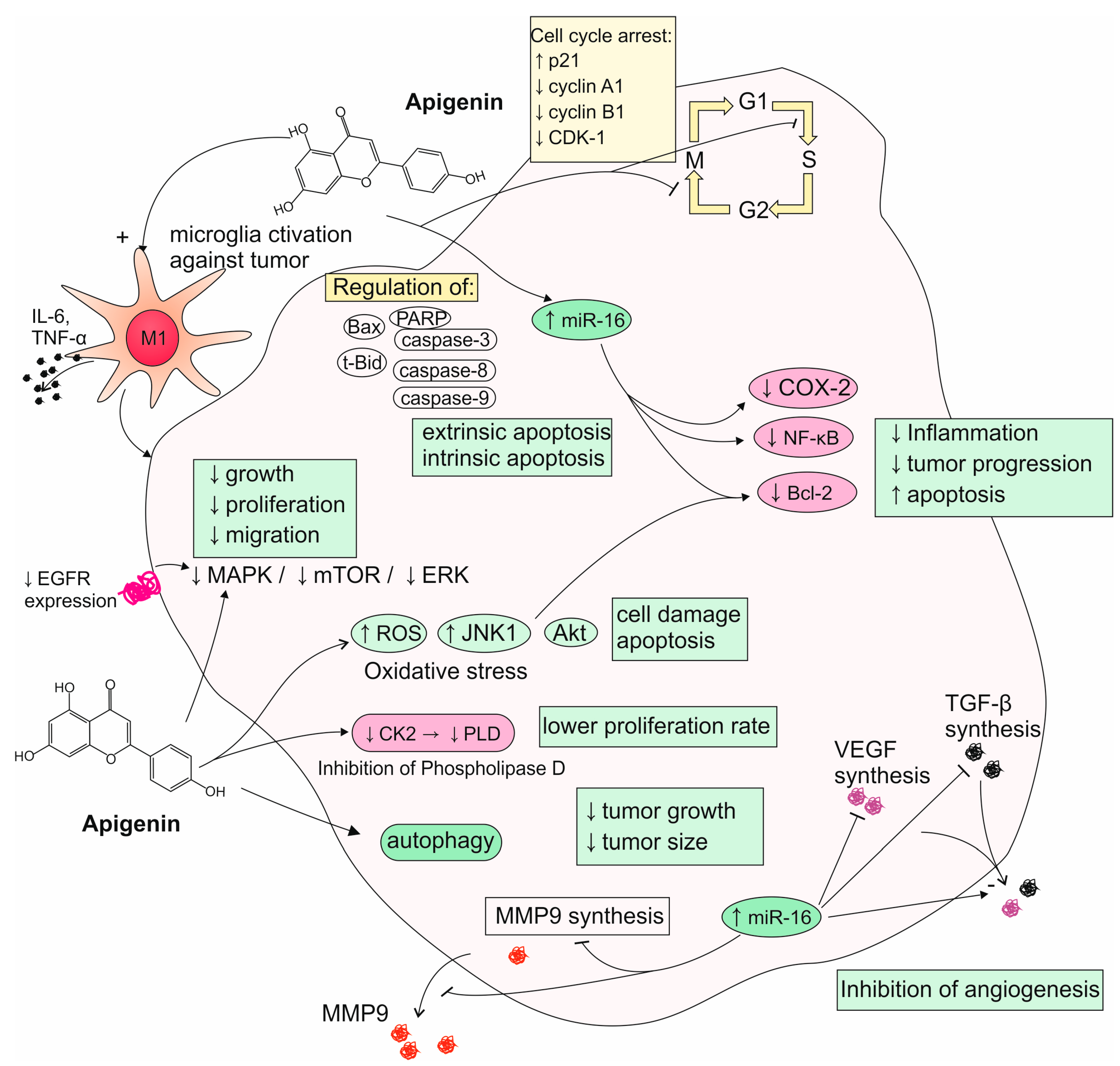
6. Apigenin’s Role in the Treatment of Gliomas
6.1. Reports from In Vitro Studies on Apigenin Activity to Glioma Cells
6.2. Reports from Animal Models on Apigenin Activity
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Tomas-Barberan, F.A.; Yoshida, K. Polyphenols: From Plants to a Variety of Food and Nonfood Uses. J. Agric. Food Chem. 2015, 63, 7589–7594. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrnčič, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phyther. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Lyubitelev, A.; Studitsky, V. Inhibition of Cancer Development by Natural Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2023, 24, 10663. [Google Scholar] [CrossRef]
- Dayi, T.; Oniz, A. Effects of the Mediterranean diet polyphenols on cancer development. J. Prev. Med. Hyg. 2022, 63, E74–E80. [Google Scholar] [CrossRef]
- Hu, Z.; Li, M.; Cao, Y.; Akan, O.D.; Guo, T.; Luo, F. Targeting AMPK Signaling by Dietary Polyphenols in Cancer Prevention. Mol. Nutr. Food Res. 2022, 66, 2100732. [Google Scholar] [CrossRef]
- Vingrys, K.; Mathai, M.L.; McAinch, A.J.; Bassett, J.K.; de Courten, M.; Stojanovska, L.; Millar, L.; Giles, G.G.; Hodge, A.M.; Apostolopoulos, V. Intake of polyphenols from cereal foods and colorectal cancer risk in the Melbourne Collaborative Cohort Study. Cancer Med. 2023, 12, 19188–19202. [Google Scholar] [CrossRef] [PubMed]
- Fike, L.T.; Munro, H.; Yu, D.; Dai, Q.; Shrubsole, M.J. Dietary polyphenols and the risk of colorectal cancer in the prospective Southern Community Cohort Study. Am. J. Clin. Nutr. 2022, 115, 1155–1165. [Google Scholar] [CrossRef]
- Woźniak, M.; Krajewski, R.; Makuch, S.; Agrawal, S. Phytochemicals in Gynecological Cancer Prevention. Int. J. Mol. Sci. 2021, 22, 1219. [Google Scholar] [CrossRef]
- Guerreiro, Í.; Ferreira-Pêgo, C.; Carregosa, D.; Santos, C.N.; Menezes, R.; Fernandes, A.S.; Costa, J.G. Polyphenols and Their Metabolites in Renal Diseases: An Overview. Foods 2022, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Zhang, J.; Okolo, P.I. Liver cancer wars: Plant-derived polyphenols strike back. Med. Oncol. 2024, 41, 116. [Google Scholar] [CrossRef]
- Ding, S.; Xu, S.; Fang, J.; Jiang, H. The Protective Effect of Polyphenols for Colorectal Cancer. Front. Immunol. 2020, 11, 1407. [Google Scholar] [CrossRef]
- Upadhyay, P.K.; Singh, S.; Vishwakarma, V.K. Natural Polyphenols in Cancer Management: Promising Role, Mechanisms, and Chemistry. Curr. Pharm. Biotechnol. 2024, 25, 694–712. [Google Scholar] [CrossRef]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef]
- Li, J.; Zhong, X.; Zhao, Y.; Shen, J.; Pilapong, C.; Xiao, Z. Polyphenols as Lung Cancer Chemopreventive Agents by Targeting microRNAs. Molecules 2022, 27, 5903. [Google Scholar] [CrossRef]
- Costea, T.; Nagy, P.; Ganea, C.; Szöllősi, J.; Mocanu, M.-M. Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1062. [Google Scholar] [CrossRef] [PubMed]
- Billowria, K.; Rouchan, A.; Kumar, R.N.; Ram, K.; and Chawla, P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Crit. Rev. Anal. Chem. 2024, 54, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.-W.; Wang, Q.-L.; Luo, M.; Zhu, M.-D.; Liang, H.-M.; Li, W.-J.; Cai, H.; Zhou, Z.-B.; Wang, H.; Tong, S.-Q.; et al. Phytochemistry and pharmacology of natural prenylated flavonoids. Arch. Pharm. Res. 2023, 46, 207–272. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Ali, F.; Falaq, N.; Smita, J.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Zhang, Y.; Zheng, X.; Cepeda, C.; Wang, Y.; Duan, S.; Tong, X. Interactions of glial cells with neuronal synapses, from astrocytes to microglia and oligodendrocyte lineage cells. Glia 2023, 71, 1383–1401. [Google Scholar] [CrossRef]
- Perry, A.; Wesseling, P. Chapter 5—Histologic classification of gliomas. In Gliomas; Berger, M.S., Weller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 134, pp. 71–95. ISBN 0072-9752. [Google Scholar]
- Smith, H.L.; Wadhwani, N.; Horbinski, C. Major Features of the 2021 WHO Classification of CNS Tumors. Neurotherapeutics 2022, 19, 1691–1704. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Francis, S.S.; Barnholtz-Sloan, J.S. Epidemiology of Brain and Other CNS Tumors. Curr. Neurol. Neurosci. Rep. 2021, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Redjal, N.; Venteicher, A.S.; Dang, D.; Sloan, A.; Kessler, R.A.; Baron, R.R.; Hadjipanayis, C.G.; Chen, C.C.; Ziu, M.; Olson, J.J.; et al. Guidelines in the management of CNS tumors. J. Neurooncol. 2021, 151, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Tomar, M.S.; Kumar, A.; Srivastava, C.; Shrivastava, A. Elucidating the mechanisms of Temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188616. [Google Scholar] [CrossRef]
- Zheng, S.; Cheng, Y.; Teng, Y.; Liu, X.; Yu, T.; Wang, Y.; Liu, J.; Hu, Y.; Wu, C.; Wang, X.; et al. Application of luteolin nanomicelles anti-glioma effect with improvement in vitro and in vivo. Oncotarget 2017, 8, 61146–61162. [Google Scholar] [CrossRef]
- Soriano-Ursúa, M.A.; Vega-García, A.; Buzoianu-Anguiano, V.; Ocampo-Nestor, A.L.; Manjarrez-Marmolejo, J.; Feria-Romero, I.A. In vitro and in vivo evaluation of nanoliposomes loading quercetin and 3-bromopyruvate against glioma. Futur. J. Pharm. Sci. 2024, 10, 7. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Q.; Chen, X.; Liu, J. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int. J. Nanomedicine 2019, 14, 7515–7531. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, R.; Shao, N.; Zhi, F.; Yang, Y. Luteolin suppresses tumor proliferation through inducing apoptosis and autophagy via MAPK activation in glioma. Onco. Targets. Ther. 2019, 12, 2383–2396. [Google Scholar] [CrossRef]
- Shendge, A.K.; Chaudhuri, D.; Mandal, N. The natural flavones, acacetin and apigenin, induce Cdk-Cyclin mediated G2/M phase arrest and trigger ROS-mediated apoptosis in glioblastoma cells. Mol. Biol. Rep. 2021, 48, 539–549. [Google Scholar] [CrossRef]
- Jakubowicz-Gil, J.; Langner, E.; Badziul, D.; Wertel, I.; Rzeski, W. Silencing of Hsp27 and Hsp72 in glioma cells as a tool for programmed cell death induction upon temozolomide and quercetin treatment. Toxicol. Appl. Pharmacol. 2013, 273, 580–589. [Google Scholar] [CrossRef]
- Coelho, P.L.C.; Amparo, J.A.O.; da Silva, A.B.; da Silva, K.C.; Braga-de-Souza, S.; Barbosa, P.R.; Lopes, G.P.d.F.; Costa, S.L. Apigenin from Croton betulaster Müll restores the immune profile of microglia against glioma cells. Phyther. Res. 2019, 33, 3191–3202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Dietary Luteolin: A Narrative Review Focusing on Its Pharmacokinetic Properties and Effects on Glycolipid Metabolism. J. Agric. Food Chem. 2021, 69, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Razif, M.R.M.; Chan, S.Y.; Chew, Y.-L.; Hassan, M.; Hisham, S.A.; Rahman, S.A.; Mai, C.-W.; Teo, M.Y.; Kee, P.E.; Khoo, K.S.; et al. Recent Developments in Luteolin-Loaded Nanoformulations for Enhanced Anti-Carcinogenic Activities: Insights from In Vitro and In Vivo Studies. Sci 2024, 6, 68. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Okda, T.M.; Atwa, G.M.K.; Omran, G.A.; Elbaky, A.E.A.; Ramadan, A.E. Design and Optimization of Orally Administered Luteolin Nanoethosomes to Enhance Its Anti-Tumor Activity against Hepatocellular Carcinoma. Pharmaceutics 2021, 13, 648. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Cheng, M.; Liu, Q.; Liu, W.; Gao, C.; Feng, J.; Jin, Y.; Tu, L. Improving Oral Bioavailability of Luteolin Nanocrystals by Surface Modification of Sodium Dodecyl Sulfate. AAPS PharmSciTech 2021, 22, 133. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; El-Feky, Y.A.; Al-Sawahli, M.M.; Ali, M.E.; Fayez, A.M.; Abbas, H. A Brain-Targeted Approach to Ameliorate Memory Disorders in a Sporadic Alzheimer’s Disease Mouse Model via Intranasal Luteolin-Loaded Nanobilosomes. Pharmaceutics 2022, 14, 576. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Sayed, N.S.; Youssef, N.A.; Gaafar, P.M.E.; Mousa, M.R.; Fayez, A.M.; Elsheikh, M.A. Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceutics 2022, 14, 1003. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phyther. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-F.; Wong, W.-T. Design and optimization of quercetin-based functional foods. Crit. Rev. Food Sci. Nutr. 2022, 62, 7319–7335. [Google Scholar] [CrossRef]
- Zang, X.; Cheng, M.; Zhang, X.; Chen, X. Quercetin nanoformulations: A promising strategy for tumor therapy. Food Funct. 2021, 12, 6664–6681. [Google Scholar] [CrossRef]
- Liu, L.; Barber, E.; Kellow, N.J.; Williamson, G. Improving quercetin bioavailability: A systematic review and meta-analysis of human intervention studies. Food Chem. 2025, 477, 143630. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Pavlović, N.; Milošević Sopta, N.; Mitrović, D.; Zaklan, D.; Tomas Petrović, A.; Stilinović, N.; Vukmirović, S. Principal Component Analysis (PCA) of Molecular Descriptors for Improving Permeation through the Blood–Brain Barrier of Quercetin Analogues. Int. J. Mol. Sci. 2024, 25, 192. [Google Scholar] [CrossRef]
- Manta, K.; Papakyriakopoulou, P.; Nikolidaki, A.; Balafas, E.; Kostomitsopoulos, N.; Banella, S.; Colombo, G.; Valsami, G. Comparative Serum and Brain Pharmacokinetics of Quercetin after Oral and Nasal Administration to Rats as Lyophilized Complexes with β-Cyclodextrin Derivatives and Their Blends with Mannitol/Lecithin Microparticles. Pharmaceutics 2023, 15, 2036. [Google Scholar] [CrossRef]
- Ambele, M.A.; Maebele, L.T.; Mulaudzi, T.V.; Kungoane, T.; Damane, B.P. Advances in nano-delivery of phytochemicals for glioblastoma treatment. Discov. Nano 2024, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Yu, P.; Yang, C.; Xia, C.; Deng, J.; Yu, M.; Xiang, Z.; Gan, L.; Zhu, B.; et al. A Novel Quercetin Encapsulated Glucose Modified Liposome and Its Brain-Target Antioxidative Neuroprotection Effects. Molecules 2024, 29, 607. [Google Scholar] [CrossRef]
- Jian, C.; Hong, Y.; Liu, H.; Yang, Q.; Zhao, S. ROS-responsive quercetin-based polydopamine nanoparticles for targeting ischemic stroke by attenuating oxidative stress and neuroinflammation. Int. J. Pharm. 2025, 669, 125087. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hong, H.; Xue, J.; Luo, J.; Liu, Q.; Chen, X.; Pan, Y.; Zhou, J.; Liu, Z.; Chen, T. Near-Infrared Radiation-Assisted Drug Delivery Nanoplatform to Realize Blood–Brain Barrier Crossing and Protection for Parkinsonian Therapy. ACS Appl. Mater. Interfaces 2021, 13, 37746–37760. [Google Scholar] [CrossRef]
- Fossatelli, L.; Maroccia, Z.; Fiorentini, C.; Bonucci, M. Resources for Human Health from the Plant Kingdom: The Potential Role of the Flavonoid Apigenin in Cancer Counteraction. Int. J. Mol. Sci. 2024, 25, 251. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Guo, Z.; Lei, J.; Zhou, B. Recent advancement in bioeffect, metabolism, stability, and delivery systems of apigenin, a natural flavonoid compound: Challenges and perspectives. Front. Nutr. 2023, 10, 1221227. [Google Scholar] [CrossRef]
- DeRango-Adem, E.F.; Blay, J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef]
- Wong, T.-Y.; Tsai, M.-S.; Hsu, L.-C.; Lin, S.-W.; Liang, P.-H. Traversal of the Blood–Brain Barrier by Cleavable l-Lysine Conjugates of Apigenin. J. Agric. Food Chem. 2018, 66, 8124–8131. [Google Scholar] [CrossRef]
- Wu, S.; Wang, S.-T.; Chen, G.-Y.; Hsu, C.; Chen, Y.-H.; Tsai, H.-Y.; Weng, T.-I.; Chen, C.-L.; Wu, Y.-F.; Su, N.-W. Monophosphate Derivatives of Luteolin and Apigenin as Efficient Precursors with Improved Oral Bioavailability in Rats. Antioxidants 2024, 13, 1530. [Google Scholar] [CrossRef]
- Tsai, Y.D.; Chen, H.J.; Hsu, H.F.; Lu, K.; Liang, C.L.; Liliang, P.C.; Wang, K.W.; Wang, H.K.; Wang, C.P.; Houng, J.Y. Luteolin inhibits proliferation of human glioblastoma cells via induction of cell cycle arrest and apoptosis. J. Taiwan Inst. Chem. Eng. 2013, 44, 837–845. [Google Scholar] [CrossRef]
- Yuan, X.; Ouyang, J.; Long, C. Effects and Mechanism of Luteolin on Proliferation and Apoptosis of Glioma. Altern. Ther. Health Med. 2024, 30. [Google Scholar]
- Pirvu, L.C.; Pintilie, L.; Albulescu, A.; Stefaniu, A.; Neagu, G. Anti-Proliferative Potential of Cynaroside and Orientin—In Silico (DYRK2) and In Vitro (U87 and Caco-2) Studies. Int. J. Mol. Sci. 2023, 24, 16555. [Google Scholar] [CrossRef]
- Anson, D.M.; Wilcox, R.M.; Huseman, E.D.; Stump, T.A.; Paris, R.L.; Darkwah, B.O.; Lin, S.; Adegoke, A.O.; Gryka, R.J.; Jean-Louis, D.S.; et al. Luteolin Decreases Epidermal Growth Factor Receptor-Mediated Cell Proliferation and Induces Apoptosis in Glioblastoma Cell Lines. Basic Clin. Pharmacol. Toxicol. 2018, 123, 678–686. [Google Scholar] [CrossRef]
- Han, W.; Yu, F.; Wang, R.; Guan, W.; Zhi, F. Valproic Acid Sensitizes Glioma Cells to Luteolin Through Induction of Apoptosis and Autophagy via Akt Signaling. Cell. Mol. Neurobiol. 2021, 41, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Powe, E.; Parschauer, D.; Istifan, J.; Lin, S.; Duan, H.; Gryka, R.; Jean-Louis, D.; Tiwari, A.K.; Amos, S. Luteolin enhances erlotinib’s cell proliferation inhibitory and apoptotic effects in glioblastoma cell lines. Front. Pharmacol. 2022, 13, 952169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, H.; Jia, Y.; Pan, H.; Ding, H. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother. Pharmacol. 2017, 79, 1031–1041. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Jia, Y.; Ding, H.; Zhang, L.; Pan, H. Luteolin reduces migration of human glioblastoma cell lines via inhibition of the p-IGF-1R/PI3K/AKT/mTOR signaling pathway. Oncol. Lett. 2017, 14, 3545–3551. [Google Scholar] [CrossRef]
- Franco, Y.E.M.; Carolina, A.d.L.; Marcela, N.R.; Viviane, A.O.S.; Rui, M.R.; Denise, G.P.; Patricia, O.C.; Jessyane, R.d.N.; Cláudia, Q.d.R.; Longato, G.B. Investigation of U-251 cell death triggered by flavonoid luteolin: Towards a better understanding on its anticancer property against glioblastomas. Nat. Prod. Res. 2021, 35, 4807–4813. [Google Scholar] [CrossRef]
- Sejda, A.; Grajkowska, W.; Trubicka, J.; Szutowicz, E.; Wojdacz, T.; Kloc, W.; Iżycka-Świeszewska, E. WHO CNS5 2021 classification of gliomas: A practical review and road signs for diagnosing pathologists and proper patho-clinical and neuro-oncological cooperation. Folia Neuropathol. 2022, 60, 137–152. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ray, S.K. Anti-tumor activities of luteolin and silibinin in glioblastoma cells: Overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis 2016, 21, 312–328. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ray, S.K. Synergistic anti-tumor actions of luteolin and silibinin prevented cell migration and invasion and induced apoptosis in glioblastoma SNB19 cells and glioblastoma stem cells. Brain Res. 2015, 1629, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Dong, R.; Wang, N.; Lan, B.; Zhao, H.; Gao, Y. Exploring the Antiglioma Mechanisms of Luteolin Based on Network Pharmacology and Experimental Verification. Evid. Based Complement. Altern. Med. 2021, 2021, 7765658. [Google Scholar] [CrossRef]
- Yi, C.; Li, G.; Ivanov, D.N.; Wang, Z.; Velasco, M.X.; Hernández, G.; Kaundal, S.; Villarreal, J.; Gupta, Y.K.; Qiao, M.; et al. Luteolin inhibits Musashi1 binding to RNA and disrupts cancer phenotypes in glioblastoma cells. RNA Biol. 2018, 15, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, B.S.; Kang, H.M.; Kim, J.H.; Shin, S.H.; Kim, I.R. Role of luteolin-induced apoptosis and autophagy in human glioblastoma cell lines. Medicina 2021, 57, 879. [Google Scholar] [CrossRef] [PubMed]
- Navone, S.E.; Guarnaccia, L.; Rizzaro, M.D.; Begani, L.; Barilla, E.; Alotta, G.; Garzia, E.; Caroli, M.; Ampollini, A.; Violetti, A.; et al. Role of Luteolin as Potential New Therapeutic Option for Patients with Glioblastoma through Regulation of Sphingolipid Rheostat. Int. J. Mol. Sci. 2024, 25, 130. [Google Scholar] [CrossRef]
- Braganhol, E.; Zamin, L.L.; Delgado Canedo, A.; Horn, F.; Tamajusuku, A.S.K.; Wink, M.R.; Salbego, C.; Battastini, A.M.O. Antiproliferative effect of quercetin in the human U138MG glioma cell line. Anticancer. Drugs 2006, 17, 663–671. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, C.H.; Park, J.Y.; Kang, S.K.; Kim, Y.K. Underlying mechanism of quercetin-induced cell death in human glioma cells. Neurochem. Res. 2008, 33, 971–979. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Reuss, D.E.; Habel, A.; Rami, A.; Von Deimling, A. Quercetin promotes degradation of survivin and thereby enhances death-receptor- mediated apoptosis in glioma cells. Neuro. Oncol. 2009, 11, 122–131. [Google Scholar] [CrossRef]
- Kusaczuk, M.; Krętowski, R.; Naumowicz, M.; Stypułkowska, A.; Cechowska-Pasko, M. A Preliminary Study of the Effect of Quercetin on Cytotoxicity, Apoptosis, and Stress Responses in Glioblastoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 1345. [Google Scholar] [CrossRef]
- Kusaczuk, M.; Tovar-Ambel, E.; Martín-Cabrera, P.; Lorente, M.; Salvador-Tormo, N.; Mikłosz, A.; Chabowski, A.; Velasco, G.; Naumowicz, M. Cytotoxicity, Proapoptotic Activity and Drug-like Potential of Quercetin and Kaempferol in Glioblastoma Cells: Preclinical Insights. Int. J. Mol. Sci. 2024, 25, 10740. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Chen, X.L.; Du, S.M.; Li, D.S.; Pei, Z.J.; Lan, H.; Wu, L.B. The JAK2/STAT3 and mitochondrial pathways are essential for quercetin nanoliposome-induced C6 glioma cell death. Cell Death Dis. 2013, 4, e746. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Lee, S.J.; Choi, Y.J.; Kim, M.J.; Kim, T.Y.; Ko, S.G. Quercetin Induces Apoptosis in Glioblastoma Cells by Suppressing Axl/IL-6/STAT3 Signaling Pathway. Am. J. Chin. Med. 2021, 49, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Jiang, Q.; Yu, Y.; Mei, J.P.; Cui, Y.K.; Zhao, W.J. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem. Int. 2015, 80, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Langner, E.; Bądziul, D.; Wertel, I.; Rzeski, W. Quercetin and sorafenib as a novel and effective couple in programmed cell death induction in human gliomas. Neurotox. Res. 2014, 26, 64–77. [Google Scholar] [CrossRef]
- Jakubowicz-Gil, J.; Langner, E.; Rzeski, W. Kinetic studies of the effects of Temodal and quercetin on astrocytoma cells. Pharmacol. Rep. 2011, 63, 403–416. [Google Scholar] [CrossRef]
- Sang, D.P.; Li, R.J.; Lan, Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol. Sin. 2014, 35, 832–838. [Google Scholar] [CrossRef]
- Albani, P.D.; Marco, B.D.; Grasso, S.; Rocco, C.; Foti, M.C. Quercetin derivatives as potent inducers of selective cytotoxicity in glioma cells. Eur. J. Pharm. Sci. 2017, 101, 56–65. [Google Scholar] [CrossRef]
- Damiano, F.; Giannotti, L.; Gnoni, G.V.; Siculella, L.; Gnoni, A. Quercetin inhibition of SREBPs and ChREBP expression results in reduced cholesterol and fatty acid synthesis in C6 glioma cells. Int. J. Biochem. Cell Biol. 2019, 117, 105618. [Google Scholar] [CrossRef]
- da Silva, A.B.; Coelho, P.L.C.; Oliveira, M.d.N.; Oliveira, J.L.; Amparo, J.A.O.; da Silva, K.C.; Soares, J.R.P.; Pitanga, B.P.S.; Souza, C.d.S.; Lopes, G.P.d.F.; et al. The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav. Immun. 2020, 85, 170–185. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Chen, X.L.; Du, L.; Li, F. Quercetin-loaded freeze-dried nanomicelles: Improving absorption and anti-glioma efficiency in vitro and in vivo. J. Control. Release 2016, 235, 276–290. [Google Scholar] [CrossRef]
- Taylor, M.A.; Khathayer, F.; Ray, S.K. Quercetin and Sodium Butyrate Synergistically Increase Apoptosis in Rat C6 and Human T98G Glioblastoma Cells Through Inhibition of Autophagy. Neurochem. Res. 2019, 44, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Tsiailanis, A.D.; Renziehausen, A.; Kiriakidi, S.; Vrettos, E.I.; Markopoulos, G.S.; Sayyad, N.; Hirmiz, B.; Aguilar, M.-I.; Del Borgo, M.P.; Kolettas, E.; et al. Enhancement of glioblastoma multiforme therapy through a novel Quercetin-Losartan hybrid. Free Radic. Biol. Med. 2020, 160, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.C.d.; Silva, N.O.; Espinelli, J.B.d.S.; Marinho, M.A.G.; Borges, Z.V.; Branco, N.B.C.; Faita, F.L.; Soares, B.M.; Horn, A.P.; Parize, A.L.; et al. Molecular interactions and physico-chemical characterization of quercetin-loaded magnetoliposomes. Chem. Phys. Lipids 2019, 218, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Ersoz, M.; Erdemir, A.; Derman, S.; Arasoglu, T.; Mansuroglu, B. Quercetin-loaded nanoparticles enhance cytotoxicity and antioxidant activity on C6 glioma cells. Pharm. Dev. Technol. 2020, 25, 757–766. [Google Scholar] [CrossRef]
- Zamin, L.L.; Filippi-Chiela, E.C.; Vargas, J.; Demartini, D.R.; Meurer, L.; Souza, A.P.; Bonorino, C.; Salbego, C.; Lenz, G. Quercetin promotes glioma growth in a rat model. Food Chem. Toxicol. 2014, 63, 205–211. [Google Scholar] [CrossRef]
- Zamin, L.L.; Filippi-Chiela, E.C.; Dillenburg-Pilla, P.; Horn, F.; Salbego, C.; Lenz, G. Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. 2009, 100, 1655–1662. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Wu, L.; Zhou, D.; Song, Y.; Zhang, L.; Wu, Q.; He, Q.; Wang, G.; Liu, X.; et al. Quercetin Suppresses Human Glioblastoma Migration and Invasion via GSK3β/β-catenin/ZEB1 Signaling Pathway. Front. Pharmacol. 2022, 13, 963614. [Google Scholar] [CrossRef]
- Liu, F.; Peng, B.; Li, M.; Ma, J.; Deng, G.; Zhang, S.; Sheu, W.C.; Zou, P.; Wu, H.; Liu, J.; et al. Targeted disruption of tumor vasculature via polyphenol nanoparticles to improve brain cancer treatment. Cell Rep. Phys. Sci. 2022, 3, 100691. [Google Scholar] [CrossRef]
- Coelho, P.L.C.; Oliveira, M.N.; da Silva, A.B.; Pitanga, B.P.S.; Silva, V.D.A.; Faria, G.P.; Sampaio, G.P.; Costa, M.d.F.D.; Braga-de-Souza, S.; Costa, S.L. The flavonoid apigenin from Croton betulaster Mull inhibits proliferation, induces differentiation and regulates the inflammatory profile of glioma cells. Anticancer. Drugs 2016, 27, 960–969. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Bauer, D.; Mendonca, P.; Taka, E.; Soliman, K.F.A. Natural product HTP screening for attenuation of cytokine-induced neutrophil chemo attractants (CINCs) and NO2−in LPS/IFNγ activated glioma cells. J. Neuroimmunol. 2017, 302, 10–19. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.S.; Yin, L.H.; Xu, L.N.; Peng, J.Y.; Zhou, H.; Kang, W. Synergistic anti-glioma effect of Hydroxygenkwanin and Apigenin In vitro. Chem. Biol. Interact. 2013, 206, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Seibert, H.; Maser, E.; Schweda, K.; Seibert, S.; Gülden, M. Cytoprotective activity against peroxide-induced oxidative damage and cytotoxicity of flavonoids in C6 rat glioma cells. Food Chem. Toxicol. 2011, 49, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Blot, E.; Panis, Y.; Bauer, S.; Trochon, V.; Nagy, H.J.; Lu, H.; Soria, C. Apigenin—Strong cytostatic and anti-angiogenic action in vitro contrasted by lack of efficacy in vivo. Phytomedicine 2002, 9, 489–495. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 2010, 116, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Stump, T.A.; Santee, B.N.; Williams, L.P.; Kunze, R.A.; Heinze, C.E.; Huseman, E.D.; Gryka, R.J.; Simpson, D.S.; Amos, S. The antiproliferative and apoptotic effects of apigenin on glioblastoma cells. J. Pharm. Pharmacol. 2017, 69, 907–916. [Google Scholar] [CrossRef]
- Ahn, B.-H.; Min, G.; Bae, Y.-S.; Bae, Y.-S.; Min, D.S. Phospholipase D is activated and phosphorylated by casein kinase-II in human U87 astroglioma cells. Exp. Mol. Med. 2006, 38, 55–62. [Google Scholar] [CrossRef]
- Jeremic, I.; Isakovic, A.; Trajkovic, V.; Markovic, I.; Redzic, Z.; Isakovic, A.; Tadic, V. The Mechanisms of In Vitro Cytotoxicity of Mountain Tea, Sideritis scardica, against the C6 Glioma Cell Line. Planta Med. 2013, 79, 1516–1524. [Google Scholar] [CrossRef]
- Wätjen, W.; Weber, N.; Lou, Y.-J.; Wang, Z.-Q.; Chovolou, Y.; Kampkötter, A.; Kahl, R.; Proksch, P. Prenylation enhances cytotoxicity of apigenin and liquiritigenin in rat H4IIE hepatoma and C6 glioma cells. Food Chem. Toxicol. 2007, 45, 119–124. [Google Scholar] [CrossRef]
- Freitas, S.; Costa, S.; Azevedo, C.; Carvalho, G.; Freire, S.; Barbosa, P.; Velozo, E.; Schaer, R.; Tardy, M.; Meyer, R.; et al. Flavonoids inhibit angiogenic cytokine production by human glioma cells. Phyther. Res. 2011, 25, 916–921. [Google Scholar] [CrossRef]
- Schindler, R.; Mentlein, R. Flavonoids and Vitamin E Reduce the Release of the Angiogenic Peptide Vascular Endothelial Growth Factor from Human Tumor Cells. J. Nutr. 2006, 136, 1477–1482. [Google Scholar] [CrossRef]
- Chen, X.; Wu, M.; Li, D.; You, J. Apigenin inhibits glioma cell growth through promoting microRNA-16 and suppression of BCL-2 and nuclear factor-κB/MMP-9. Mol. Med. Rep. 2016, 14, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.L.; Oliveira, M.N.; Coelho, P.L.C.; Pitanga, B.P.S.; da Silva, A.B.; Adelita, T.; Silva, V.D.A.; Costa, M.d.F.D.; El-Bachá, R.S.; Tardy, M.; et al. Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem. Biol. Interact. 2015, 242, 123–138. [Google Scholar] [CrossRef]
- Kim, B.; Jung, N.; Lee, S.; Sohng, J.K.; Jung, H.J. Apigenin Inhibits Cancer Stem Cell-Like Phenotypes in Human Glioblastoma Cells via Suppression of c-Met Signaling. Phyther. Res. 2016, 30, 1833–1840. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, Q.; Liu, C.; Tao, M.-L. Drug screening study using glioma stem-like cells. Mol. Med. Rep. 2012, 6, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, H.; Jia, C.-H.; Fan, K.; Xie, T.; Zhu, Z.-Y.; Xie, M.-L. Apigenin increases radiosensitivity of glioma stem cells by attenuating HIF-1α-mediated glycolysis. Med. Oncol. 2021, 38, 131. [Google Scholar] [CrossRef] [PubMed]
- Kroonen, J.; Artesi, M.; Capraro, V.; Nguyen-Khac, M.-T.; Willems, M.; Chakravarti, A.; Bours, V.; Robe, A.P. Casein kinase 2 inhibition modulates the DNA damage response but fails to radiosensitize malignant glioma cells. Int. J. Oncol. 2012, 41, 776–782. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Dai, X.; Zhang, L.; Li, M. Apigenin and Temozolomide Synergistically Inhibit Glioma Growth Through the PI3K/AKT Pathway. Cancer Biother. Radiopharm. 2021, 39, 125–132. [Google Scholar] [CrossRef]
- Chen, L.-J.; Hsu, T.-C.; Yeh, P.-J.; Yow, J.L.; Chang, C.L.; Lin, C.-H.; Tzang, B.S. Differential Effects of Wedelia chinensis on Human Glioblastoma Multiforme Cells. Integr. Cancer Ther. 2021, 20, 15347354211000120. [Google Scholar] [CrossRef]
- Parajuli, P.; Joshee, N.; Rimando, A.M.; Mittal, S.; Yadav, A.K. In vitro Antitumor Mechanisms of Various Scutellaria Extracts and Constituent Flavonoids. Planta Med. 2009, 75, 41–48. [Google Scholar] [CrossRef]
- Teles, Y.C.F.; Horta, C.C.; Agra, M.D.; Siheri, W.; Boyd, M.; Igoli, J.O.; Gray, A.I.; De Souza, M.D. New Sulphated Flavonoids from Wissadula periplocifolia (L.) C. Presl (Malvaceae). Molecules 2015, 20, 20161–20172. [Google Scholar] [CrossRef]
- Jia, C.; Zhao, Y.; Huang, H.; Fan, K.; Xie, T.; Xie, M. Apigenin sensitizes radiotherapy of mouse subcutaneous glioma through attenuations of cell stemness and DNA damage repair by inhibiting NF-κB/HIF-1α-mediated glycolysis. J. Nutr. Biochem. 2022, 107, 109038. [Google Scholar] [CrossRef] [PubMed]
| Dosage | Animal Species | Tumor Type | Way of Administration | Time Period | Results | Ref. |
|---|---|---|---|---|---|---|
| 50 mg/kg | Female nude BALB/c mice | Glioma (C6 cell line) Glioblastoma (U87 cell line) | Intravenous injection (1 time a day) | The administration of luteolin was continued for 15 days | C6 glioma group: ↓ tumor volume U87 glioma group: ↓ tumor volume | [30] |
| 5 μg/mL | Zebrafish | Glioblastoma (U87 cell line) | Maintaining proper luteolin concentration in zebrafish incubating solution | Proper concentration of luteolin in zebrafish incubating solution was maintained for 5 days | ↓ tumor volume | [30] |
| 50 mg/kg | C57 mice | Glioma (GL261 cell line) | Intravenous injection (1 time a day) | The administration of luteolin was continued for 13 days | ↓ tumor volume ↓ CD31 expression in tumor cells ↓ angiogenesis in tumor tissue ↑ apoptosis of tumor cells | [32] |
| 10 mg/kg | Male BALB/c athymic nude mice | Glioblastoma (U87MG cell line) | Intraperitoneal injection (1 time every 2 days) | The administration of luteolin was started once tumors reached volumes of 70–100 cm3 and continued until day 35 of the whole experiment | ↓ tumor growth through the activation of caspase-3 and cleaved capsase-12 in tumor cells ↑ endoplasmic reticulum stress through ATF4 and CHOP proteins in tumor cells | [67] |
| Dosage | Animal Species | Tumor Type | Way of Administration | Time Period | Results | Ref. |
|---|---|---|---|---|---|---|
| 100 mg/kg | Male nude mice | Glioblastoma (U87 cell line) | Intraperitoneal injection (1 time a day) | The administration of quercetin was started once tumors reached volume of 100 cm3 and continued until the day 21 of whole experiment | ↓ tumor volume ↓ Ki67-positive cells number in tumor tissue ↓ expression of N-cadherin, vimentin, p-GSK-3β, β-catenin, and ZEB1 in tumor cells ↑ expression of E-cadherin in tumor cells ↓ mice weight loss | [98] |
| 1.5 mg/kg (encapsulated in liposomes with 3-BP | Male Sprague–Dawley rats | Glioma (C6 cell line) | Intraperitoneal injection (1 time every 3 days) | The administration of quercetin was continued for 6 days | ↓ angiogenesis in tumor tissue ↓ tumor volume | [31] |
| 50 mg/kg | Male Wistar rats | Glioma (C6 cell line) | Intraperitoneal injection (1 time a day) | The administration of quercetin was continued for 15 days | ↓ lymphocytic infiltration in tumor tissue ↓ T-cell proliferation | [96] |
| 25 mg/kg (encapsulated in FD-NMs or NLs) | BALB/c nude mice | Glioma (C6 cell line) | Intragastric administration (1 time every 7 days) | The administration of quercetin was continued for 28 days | FD-NMs group: ↓ tumor growth rate ↓ tumor volume ↑ survival time ↓ Bcl-2 expression in tumor cells NLs group: ↓ tumor growth rate ↓ tumor volume | [91] |
| 25 mg/kg (in form of polyphenol nanoparticles) | Female C57BL/6 mice Athymic NCr-nu/nu mice | Glioma (GL261 cell line) Glioblastoma (PS30 cell line) | Intravenous injections (2 times per week) | The administration of quercetin was continued for 21 days | ↓ tumor growth rate ↑ survival time ↑ vessel loss and cellular apoptosis in tumor tissues | [99] |
| Dosage | Animal Species | Tumor Type | Way of Administration | Time Period | Results | Ref. |
|---|---|---|---|---|---|---|
| 50 mg/kg | Adult athymic mice | Glioma (C6 cell line) | Intratumoral injection (3 times per day) | The administration of apigenin was continued for 12 days | ↓ tumor volume (modest) | [104] |
| 20 mg/kg | Mice | Glioblastoma (SU3-5R cell line) | Intraperitoneal injection (1 time a day) | The administration of apigenin was continued for 12 days | ↓ expression of NF-κB, HIF-1α, GLUT-1, GLUT-3, PKM2 ↓ activity of glycolytic enzymes ↑ susceptibility to radiation at dose of 8 gray | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Justyńska, W.; Grabarczyk, M.; Smolińska, E.; Szychowska, A.; Glabinski, A.; Szpakowski, P. Dietary Polyphenols: Luteolin, Quercetin, and Apigenin as Potential Therapeutic Agents in the Treatment of Gliomas. Nutrients 2025, 17, 2202. https://doi.org/10.3390/nu17132202
Justyńska W, Grabarczyk M, Smolińska E, Szychowska A, Glabinski A, Szpakowski P. Dietary Polyphenols: Luteolin, Quercetin, and Apigenin as Potential Therapeutic Agents in the Treatment of Gliomas. Nutrients. 2025; 17(13):2202. https://doi.org/10.3390/nu17132202
Chicago/Turabian StyleJustyńska, Weronika, Mikołaj Grabarczyk, Ewa Smolińska, Aleksandra Szychowska, Andrzej Glabinski, and Piotr Szpakowski. 2025. "Dietary Polyphenols: Luteolin, Quercetin, and Apigenin as Potential Therapeutic Agents in the Treatment of Gliomas" Nutrients 17, no. 13: 2202. https://doi.org/10.3390/nu17132202
APA StyleJustyńska, W., Grabarczyk, M., Smolińska, E., Szychowska, A., Glabinski, A., & Szpakowski, P. (2025). Dietary Polyphenols: Luteolin, Quercetin, and Apigenin as Potential Therapeutic Agents in the Treatment of Gliomas. Nutrients, 17(13), 2202. https://doi.org/10.3390/nu17132202







