Effects of 12-Week Dietary Inflammatory Index-Based Dietary Education on Frailty Status in Frail Patients with Colorectal Cancer: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design Overview
2.2. Participants
2.3. Sample Size Calculation
2.4. Randomization and Masking
2.5. Intervention
2.6. Sociodemographic and Clinical Information
2.7. Primary Outcome
2.8. Secondary Outcomes
2.8.1. Dietary Intake Assessment and DII Calculation
2.8.2. Plasma Inflammatory Biomarkers
2.8.3. Nutritional Status Assessment
2.8.4. Quality of Life
2.9. Statistical Analysis
3. Results
3.1. Overview
3.2. Baseline Characteristics
3.3. Effects on Frailty Status
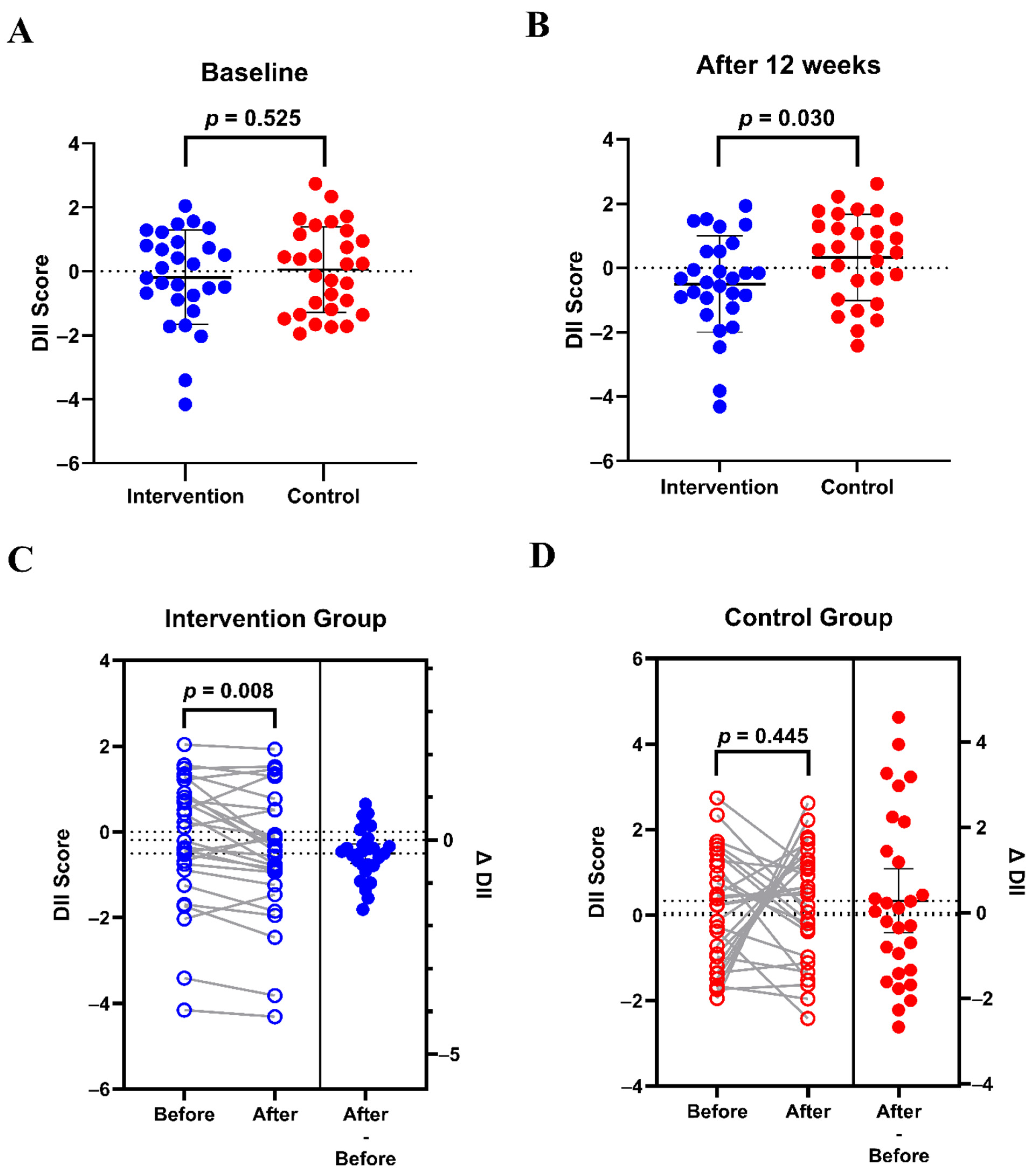
3.4. Effects on Dietary Inflammatory Potential
3.5. Effects on Plasma Inflammatory Biomarkers, BMI, Nutritional Status, and Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| DII | Dietary inflammatory index |
| FP | Fried frailty phenotype |
| BMI | Body mass index |
| QoL | Quality of life |
| MNA | Mini nutritional assessment |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| RCT | Randomized controlled trial |
| IPAQ-SF | International Physical Activity Questionnaire Short Form |
| MUFA | Monounsaturated fatty acids |
| PUFA | Polyunsaturated fatty acids |
| FACT-C | Functional Assessment of Cancer Therapy-Colorectal |
| SD | Standard deviation |
| CNY | Chinese yuan |
| FOLFOX | Chemotherapy regimen consisting of fluorouracil, leucovorin, and oxaliplatin, along with other oxaliplatin-based treatment protocols |
| FOLFIRI | Chemotherapy regimen consisting of fluorouracil, leucovorin, and irinotecan, along with other irinotecan-based treatment protocols |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory: Cancer Tomorrow (Version 1.1). Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/tomorrow (accessed on 13 May 2025).
- Fried, L.P.; Cohen, A.A.; Xue, Q.-L.; Walston, J.; Bandeen-Roche, K.; Varadhan, R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 2021, 1, 36–46. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Dolan, R.D.; Horgan, P.G.; Laird, B.J.; McMillan, D.C. The prevalence and prognostic value of frailty screening measures in patients undergoing surgery for colorectal cancer: Observations from a systematic review. BMC Geriatr. 2022, 22, 260. [Google Scholar] [CrossRef]
- Tamura, K.; Matsuda, K.; Fujita, Y.; Iwahashi, M.; Mori, K.; Yamade, N.; Hotta, T.; Noguchi, K.; Sakata, Y.; Takifuji, K.; et al. Optimal assessment of frailty predicts postoperative complications in older patients with colorectal cancer surgery. World J. Surg. 2021, 45, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Stem, M.; Cerullo, M.; Gearhart, S.L.; Safar, B.; Fang, S.H.; Weiss, M.J.; He, J.; Efron, J.E. The effect of frailty index on early outcomes after combined colorectal and liver resections. J. Gastrointest. Surg. 2018, 22, 640–649. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, W.; Xiao, H.; Liu, H.; Chen, T. Correlation between frailty and adverse outcomes among older community-dwelling Chinese adults: The China Health and Retirement Longitudinal Study. J. Nutr. Health Aging 2020, 24, 752–757. [Google Scholar] [CrossRef]
- Chu, W.; Chang, S.-F.; Ho, H.-Y. Adverse health effects of frailty: Systematic review and meta-analysis of middle-aged and older adults with implications for evidence-based practice. Worldviews Evid.-Based Nurs. 2021, 18, 282–289. [Google Scholar] [CrossRef]
- Kojima, G.; Taniguchi, Y.; Iliffe, S.; Jivraj, S.; Walters, K. Transitions between frailty states among community-dwelling older people: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 50, 81–88. [Google Scholar] [CrossRef]
- Mendonca, N.; Kingston, A.; Yadegarfar, M.; Hanson, H.; Duncan, R.; Jagger, C.; Robinson, L. Transitions between frailty states in the very old: The influence of socioeconomic status and multi-morbidity in the Newcastle 85+ cohort study. Age Ageing 2020, 49, 974–981. [Google Scholar] [CrossRef]
- Dent, E.; Hanlon, P.; Sim, M.; Jylhava, J.; Liu, Z.; Vetrano, D.L.; Stolz, E.; Perez-Zepeda, M.U.; Crabtree, D.R.; Nicholson, C.; et al. Recent developments in frailty identification, management, risk factors and prevention: A narrative review of leading journals in geriatrics and gerontology. Ageing Res. Rev. 2023, 91, 102082. [Google Scholar] [CrossRef]
- Perez-Ros, P.; Vila-Candel, R.; Lopez-Hernandez, L.; Martinez-Arnau, F.M. Nutritional status and risk factors for frailty in community-dwelling older people: A cross-sectional study. Nutrients 2020, 12, 1041. [Google Scholar] [CrossRef]
- Park, Y.; Choi, J.E.; Hwang, H.S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Twizeyemariya, A.; Zarnowiecki, D.; Niyonsenga, T.; Bogomolova, S.; Wilson, A.; O’Dea, K.; Parletta, N. Cost effectiveness and cost-utility analysis of a group-based diet intervention for treating major depression—The HELFIMED trial. Nutr. Neurosci. 2020, 23, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Casals, C.; Ávila-Cabeza-de-Vaca, L.; González-Mariscal, A.; Marín-Galindo, A.; Costilla, M.; Ponce-Gonzalez, J.G.; Vázquez-Sánchez, M.; Corral-Pérez, J. Effects of an educational intervention on frailty status, physical function, physical activity, sleep patterns, and nutritional status of older adults with frailty or pre-frailty: The FRAGSALUD study. Front. Public Health 2023, 11, 1267666. [Google Scholar] [CrossRef]
- Wu, S.Y.; Hsu, L.L.; Hsu, C.C.; Hsieh, T.J.; Su, S.C.; Peng, Y.W.; Guo, T.M.; Kang, Y.W.; Pan, W.H. Dietary education with customised dishware and food supplements can reduce frailty and improve mental well-being in elderly people: A single-blind randomized controlled study. Asia Pac. J. Clin. Nutr. 2018, 27, 1018–1030. [Google Scholar] [CrossRef]
- Han, C.Y.; Miller, M.; Yaxley, A.; Baldwin, C.; Woodman, R.; Sharma, Y. Effectiveness of combined exercise and nutrition interventions in prefrail or frail older hospitalised patients: A systematic review and meta-analysis. BMJ Open 2020, 10, e040146. [Google Scholar] [CrossRef]
- Hsieh, T.-J.; Su, S.-C.; Chen, C.-W.; Kang, Y.-W.; Hu, M.-H.; Hsu, L.-L.; Wu, S.-Y.; Chen, L.; Chang, H.-Y.; Chuang, S.-Y.; et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Pansarasa, O.; Pistono, C.; Davin, A.; Bordoni, M.; Mimmi, M.C.; Guaita, A.; Cereda, C. Altered immune system in frailty: Genetics and diet may influence inflammation. Ageing Res. Rev. 2019, 54, 100935. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Nitin, S.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar]
- Xia, S.F.; Liu, Y.; Chen, Y.; Li, Z.Y.; Cheng, L.; He, J.Y.; Hang, L.; Maitiniyazi, G.; Cheng, X.X.; Sun, S.R.; et al. Association between dietary inflammatory potential and frailty is mediated by inflammation among patients with colorectal cancer: A cross-sectional study. Nutr. Res. 2024, 125, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Kenkhuis, M.-F.; Mols, F.; van Roekel, E.H.H.; Breedveld-Peters, J.J.L.; Breukink, S.; Janssen-Heijnen, M.; Keulen, E.; van Duijnhoven, F.J.; Weijenberg, M.P.P.; Bours, M. Longitudinal associations of fast foods, red and processed meat, alcohol and sugar-sweetened drinks with quality of life and symptoms in colorectal cancer survivors up to 24 months post-treatment. Br. J. Nutr. 2023, 130, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Piaseu, N.; Phumonsakul, S.; Thadakant, S. Effects of a comprehensive dietary intervention program, promoting nutrition literacy, eating behavior, dietary quality, and gestational weight gain in Chinese urban women with normal body mass index during pregnancy. Nutrients 2024, 16, 217. [Google Scholar] [CrossRef]
- Li, J.; Lee, D.H.; Hu, J.; Tabung, F.K.; Li, Y.; Bhupathiraju, S.N.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; et al. Dietary inflammatory potential and risk of cardiovascular disease among men and women in the US. JACC Adv. 2020, 76, 2181–2193. [Google Scholar] [CrossRef] [PubMed]
- Kehler, D.S.; Theou, O. The impact of physical activity and sedentary behaviors on frailty levels. Mech. Ageing Dev. 2019, 180, 29–41. [Google Scholar] [CrossRef]
- Zhang, N.; Jia, Z.; Gu, T.; Zheng, Y.; Zhang, Y.; Song, W.; Chen, Z.; Li, G.; Tse, G.; Liu, T. Associations between modifiable risk factors and frailty: A Mendelian randomisation study. J. Epidemiol. Community Health 2023, 77, 782–790. [Google Scholar] [CrossRef]
- Bassett, D.R. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1396. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Li, J.J.; Jiang, S.; Zhu, M.L.; Liu, X.H.; Sun, X.H.; Zhao, S.Q. Comparison of three frailty scales for prediction of adverse outcomes among older adults: A prospective cohort study. J. Nutr. Health Aging 2021, 25, 419–424. [Google Scholar] [CrossRef]
- Balogh, M.; Kahn, H.A.; Medalie, J.H. Random repeat 24-hour dietary recalls. Am. J. Clin. Nutr. 1971, 24, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-lessons learned, improvements made, and future directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 1996, 54, S59–S65. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Kim, J.C.; Eremenco, S.; Han, O.S. Quality of life in colorectal cancer patients with colectomy and the validation of the functional assessment of cancer Therapy-Colorectal (FACT-C), version 4. J. Pain Symptom Manag. 2005, 30, 24–32. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, Y.B.; Li, Y.F.; Wan, C.H.; Luo, J.H.; Meng, Q.; Zhang, X.Q. Evaluation of the Functional Assessment of Cancer Therapy-Colorectal (V4.0) in Chinese version. J. Clin. Rehabil. Tissue Eng. Res. 2007, 11, 8753–8756. [Google Scholar]
- Cruz-Jentoft, A.J.; Woo, J. Nutritional interventions to prevent and treat frailty. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 191–195. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Shin, H.R.; Kim, Y.S.; Park, Y.K.; Koo, S.K.; Son, W.H.; Han, J.W.; Son, E.H.; Kang, H.J.; Choi, K.H.; Han, J.S.; et al. Nutritional status and frailty improvement through senior-friendly diet among community-dwelling older adults in South Korea. Nutrients 2023, 15, 1381. [Google Scholar] [CrossRef]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Y.; He, J.; Cheng, X.; Wang, Y.; Lin, X.; Huang, Z.; Miao, X.; Xia, S. Effects of 12-week anti-inflammatory dietary education on depressive symptoms among depressed patients with breast cancer undergoing adjuvant chemotherapy: A randomized controlled trial. Nutrients 2025, 17, 957. [Google Scholar] [CrossRef]
- Barlow, K.H.; van der Pols, J.C.; Ekberg, S.; Johnston, E.A. Cancer survivors’ perspectives of dietary information provision after cancer treatment: A scoping review of the Australian context. Health Promot. J. Aust. 2022, 33, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.R.; Raji, D.; Olaniran, M.; Alick, C.; Nichols, D.; Allicock, M. A systematic scoping review of post-treatment lifestyle interventions for adult cancer survivors and family members. J. Cancer Surviv. 2022, 16, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Mantzorou, M.; Tolia, M.; Poultsidi, A.; Vasios, G.K.; Papandreou, D.; Theocharis, S.; Kavantzas, N.; Troumbis, A.Y.; Giaginis, C. Adherence to Mediterranean diet and nutritional status in women with breast cancer: What is their impact on disease progression and recurrence-free patients’ survival? Curr. Oncol. 2022, 29, 7482–7497. [Google Scholar] [CrossRef] [PubMed]
- Welch, A. Micronutrient malnutrition across the life course, sarcopenia and frailty. Proc. Nutr. Soc. 2021, 80, 279–282. [Google Scholar] [CrossRef]
- Kavyani, Z.; Musazadeh, V.; Fathi, S.; Faghfouri, A.H.; Dehghan, P.; Sarmadi, B. Efficacy of the omega-3 fatty acids supplementation on inflammatory biomarkers: An umbrella meta-analysis. Int. Immunopharmacol. 2022, 111, 109104. [Google Scholar] [CrossRef]
- Therdyothin, A.; Phiphopthatsanee, N.; Isanejad, M. The effect of omega-3 fatty acids on sarcopenia: Mechanism of action and potential efficacy. Mar. Drugs 2023, 21, 399. [Google Scholar] [CrossRef]
- Zhang, F.; Li, W. Vitamin D and sarcopenia in the senior people: A review of mechanisms and comprehensive prevention and treatment strategies. Br. J. Anaesth. 2024, 20, 577–595. [Google Scholar] [CrossRef]
- Rodrigues Junior, C.F.; Murata, G.M.; Gerlinger-Romero, F.; Nachbar, R.T.; Marzuca-Nassr, G.N.; Gorjão, R.; Vitzel, K.F.; Hirabara, S.M.; Pithon-Curi, T.C.; Curi, R. Changes in skeletal muscle protein metabolism signaling induced by glutamine supplementation and exercise. Nutrients 2023, 15, 4711. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, X.; Hu, Q.; Chen, L.; Zuo, H. The effect of leucine supplementation on sarcopenia-related measures in older adults: A systematic review and meta-analysis of 17 randomized controlled trials. Front. Nutr. 2022, 9, 929891. [Google Scholar] [CrossRef]
- Millar, C.L.; Dufour, A.B.; Shivappa, N.; Habtemariam, D.; Murabito, J.M.; Benjamin, E.J.; Hebert, J.R.; Kiel, D.P.; Hannan, M.T.; Sahni, S. A proinflammatory diet is associated with increased odds of frailty after 12-year follow-up in a cohort of adults. Am. J. Clin. Nutr. 2022, 115, 334–343. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western diet and the immune system: An inflammatory connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.S.; Han, C.Y.; Sukumaran, S.; Delaney, C.L.; Miller, M.D. Effect of anti-inflammatory diets on inflammation markers in adult human populations: A systematic review of randomized controlled trials. Nutr. Rev. 2022, 81, 55–74. [Google Scholar] [CrossRef]
- Shin, P.-K.; Park, S.-J.; Kim, M.S.; Kwon, D.Y.; Kim, M.J.; Kim, K.; Chun, S.; Lee, H.-J.; Choi, S.-W. A traditional Korean diet with a low dietary inflammatory index increases anti-inflammatory IL-10 and decreases pro-inflammatory NF-κB in a small dietary intervention study. Nutrients 2020, 12, 2468. [Google Scholar] [CrossRef]
- Marcos-Perez, D.; Sanchez-Flores, M.; Proietti, S.; Bonassi, S.; Costa, S.; Teixeira, J.P.; Fernandez-Tajes, J.; Pasaro, E.; Laffon, B.; Valdiglesias, V. Association of inflammatory mediators with frailty status in older adults: Results from a systematic review and meta-analysis. Geroscience 2020, 42, 1451–1473. [Google Scholar] [CrossRef]
- Kochlik, B.; Franz, K.; Henning, T.; Weber, D.; Wernitz, A.; Herpich, C.; Jannasch, F.; Aykac, V.; Mueller-Werdan, U.; Schulze, M.B.; et al. Frailty is characterized by biomarker patterns reflecting inflammation or muscle catabolism in multi-morbid patients. J. Cachexia Sarcopenia Muscle 2023, 14, 157–166. [Google Scholar] [CrossRef]
- Amoore, B.Y.; Gaa, P.K.; Amalba, A.; Mogre, V. Nutrition education intervention improves medical students’ dietary habits and their competency and self-efficacy in providing nutrition care: A pre, post and follow-up quasi-experimental study. Front. Nutr. 2023, 10, 1063316. [Google Scholar] [CrossRef]
- Wu, S.Y.; Cheng, Y.Y.; Chang, H.Y.; Wang, P.H.; Hsieh, I.C.; Yeh, N.H.; Huang, K.C.; Pan, W.H. Efficacy of Dietary Intervention with Group Activities on Dietary Intakes, Frailty Status, and Working Memory: A Cluster-Randomized Controlled Trial in Community Strongholds. Nutrients 2023, 15, 1976. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwenhuizen, A.J.; Buffart, L.M.; Langendijk, J.A.; Vergeer, M.R.; Voortman, J.; Leemans, C.R.; Verdonck-de Leeuw, I.M. Health-related quality of life and overall survival: A prospective study in patients with head and neck cancer treated with radiotherapy. Qual. Life Res. 2021, 30, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, G.; Xiu, Y.; Zhao, M. The effect of nutritional support based on the dietary anti-inflammatory index on cancer-related fatigue in lung cancer patients undergoing chemotherapy. Cancer Nurs. 2022, 46, 394–404. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Agudo, A. The role of diet in prognosis among cancer survivors: A systematic review and meta-analysis of dietary patterns and diet interventions. Nutrients 2022, 14, 348. [Google Scholar] [CrossRef]
- Parma, D.A.L.; Reynolds, G.L.; Munoz, E.; Ramirez, A.G. Effect of an anti-inflammatory dietary intervention on quality of life among breast cancer survivors. Support. Care Cancer 2022, 30, 5903–5910. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, A.; Belnap, B.H.; Rothenberger, S.D.; Feldman, R.; Rollman, B.L.; Celano, C.M. Psychosocial predictors of health behavior adherence in heart-failure patients with comorbid depression: A secondary analysis of the Hopeful Heart trial. BMC Psychol. 2024, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Banu, B.; Khan, M.M.H.; Ali, L.; Barnighausen, T.; Sauerborn, R.; Souares, A. Pattern and predictors of non-adherence to diabetes self-management recommendations among patients in peripheral district of Bangladesh. Trop. Med. Int. Health 2024, 29, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Ajani, K.; Gowani, A.; Gul, R.; Petrucka, P. Levels and Predictors of Self-Care Among Patients with Hypertension in Pakistan. Int. J. Gen. Med. 2021, 14, 1023–1032. [Google Scholar] [CrossRef]
- Harvey, B.I.; Youngblood, S.M.; Kleckner, A.S. Barriers and Facilitators to Adherence to a Mediterranean Diet Intervention during Chemotherapy Treatment: A Qualitative Analysis. Nutr. Cancer 2023, 75, 1349–1360. [Google Scholar] [CrossRef]
- Owolabi, E.O.; Ajayi, A.I. Adherence to medication, dietary and physical activity recommendations: Findings from a multicenter cross-sectional study among adults with diabetes in rural South Africa. J. Eval. Clin. Pract. 2024, 30, 1261–1271. [Google Scholar] [CrossRef]
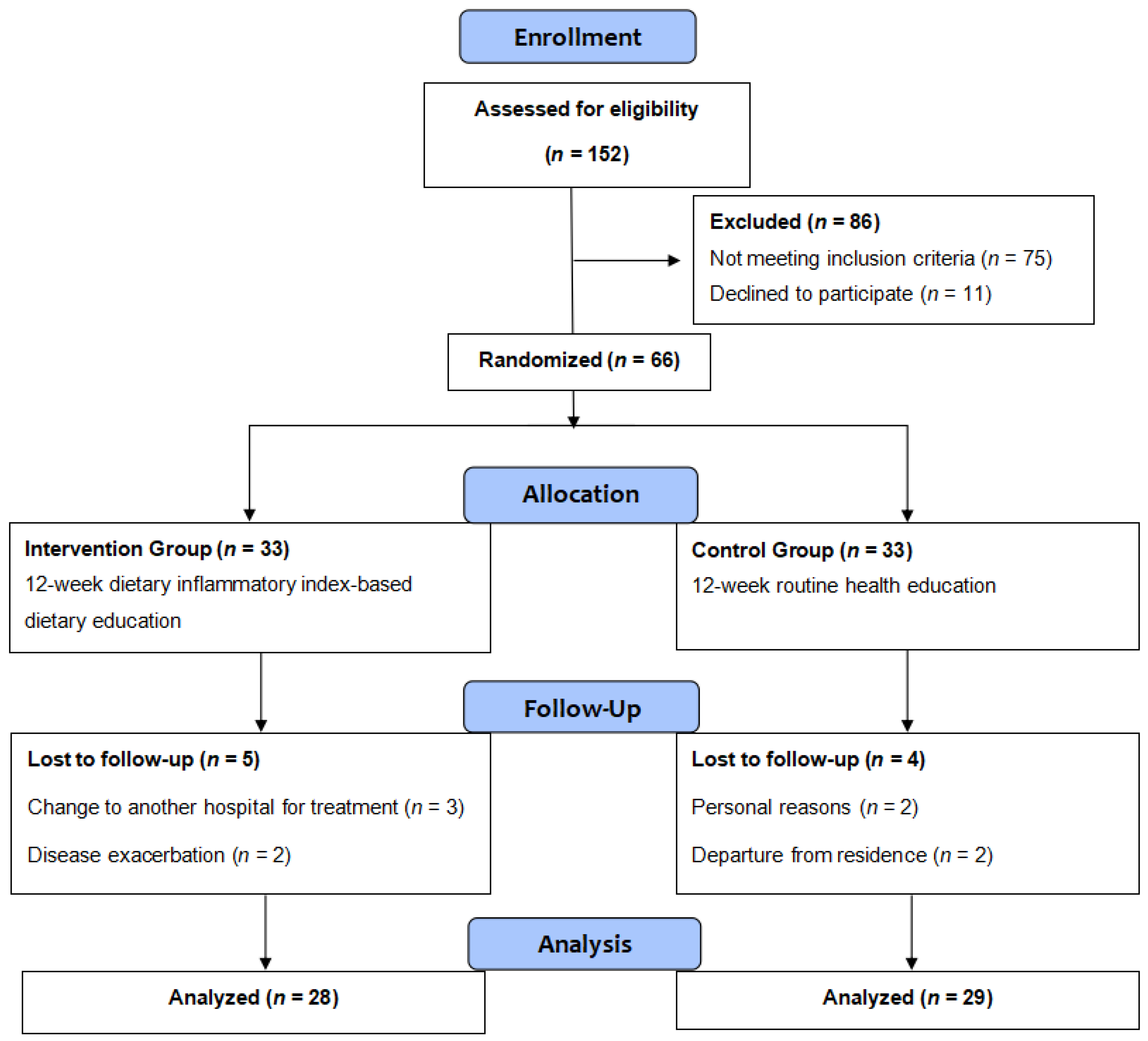

| Variables | Overall (n = 57) | Intervention (n = 28) | Control (n = 29) | t/χ2/Z | p |
|---|---|---|---|---|---|
| Age (year) a | 68.75 ± 6.13 | 70.14 ± 6.30 | 67.41 ± 5.76 | 1.708 | 0.093 |
| BMI (kg/m2) a | 21.37 ± 2.85 | 21.67 ± 2.72 | 21.08 ± 2.99 | 0.788 | 0.434 |
| Sex b | |||||
| Male | 33 (57.9) | 16 (57.1) | 17 (58.6) | 0.013 | 0.910 |
| Female | 24 (42.1) | 12 (42.9) | 12 (41.4) | ||
| Marital status c | |||||
| Married | 47 (82.5) | 25 (89.3) | 22 (75.9) | 0.968 | 0.325 |
| Widowed/divorced/single | 10 (17.5) | 3 (10.7) | 7 (24.1) | ||
| Education level d | |||||
| Primary school or lower | 23 (40.3) | 12 (42.9) | 11 (38.0) | 1.298 | 0.912 |
| Middle school | 22 (38.6) | 10 (35.7) | 12 (41.4) | ||
| High/secondary school | 11 (19.3) | 6 (21.4) | 5 (17.2) | ||
| Junior college or higher | 1 (1.8) | 0 (0.0) | 1 (3.4) | ||
| Employment d | |||||
| Employed | 2 (3.5) | 1 (3.6) | 1 (3.4) | 2.309 | 0.355 |
| Unemployed | 8 (14.0) | 2 (7.1) | 6 (20.7) | ||
| Retired | 47 (82.5) | 25 (89.3) | 22 (75.9) | ||
| Residence d | |||||
| Rural areas | 15 (26.3) | 9 (32.1) | 6 (20.7) | 2.222 | 0.373 |
| Towns | 6 (10.5) | 4 (14.3) | 2 (6.9) | ||
| Urban areas | 36 (63.2) | 15 (53.6) | 21 (72.4) | ||
| Family monthly income d | |||||
| <2000 CNY | 24 (42.1) | 11 (39.3) | 13 (44.8) | 3.562 | 0.154 |
| 2000~5000 CNY | 27 (47.4) | 16 (57.1) | 11 (38.0) | ||
| >5000 CNY | 6 (10.5) | 1 (3.6) | 5 (17.2) |
| Variables | Overall (n = 57) | Intervention (n = 28) | Control (n = 29) | χ2/Z | p |
|---|---|---|---|---|---|
| Presence of comorbidities a | |||||
| No | 29 (50.9) | 12 (42.9) | 17 (58.6) | 1.416 | 0.234 |
| Yes | 28 (49.1) | 16 (57.1) | 12 (41.4) | ||
| Cancer stage a | |||||
| I | 5 (8.8) | 3 (10.7) | 2 (6.9) | 0.522 | 0.771 |
| II | 21 (36.8) | 11 (39.3) | 10 (34.5) | ||
| III | 31 (54.4) | 14 (50.0) | 17 (58.6) | ||
| Number of chemotherapy cycles completed c | |||||
| 0 | 28 (49.1) | 14 (50.0) | 14 (48.3) | 1.151 | 0.819 |
| 1 | 18 (31.6) | 8 (28.6) | 10 (34.5) | ||
| 2 | 3 (5.3) | 1 (3.6) | 2 (6.9) | ||
| 3 | 8 (14.0) | 5 (17.8) | 3 (10.3) | ||
| Type of surgery b | |||||
| Laparoscopic surgery | 51 (89.5) | 24 (85.7) | 27 (93.1) | 0.228 | 0.633 |
| Laparotomy | 6 (10.5) | 4 (14.3) | 2 (6.9) | ||
| Chemotherapy regimen c | |||||
| FOLFOX | 24 (42.1) | 12 (42.8) | 12 (41.4) | 1.392 | 1.000 |
| FOLFIRI | 3 (5.3) | 1 (3.6) | 2 (6.9) | ||
| Capecitabine | 1 (1.8) | 0 (0.0) | 1 (3.4) | ||
| Others | 29 (50.9) | 15 (53.6) | 14 (48.3) | ||
| Smoking status a | |||||
| Never | 28 (49.1) | 16 (57.1) | 12 (41.4) | 1.416 | 0.234 |
| Former/Current | 29 (50.9) | 12 (42.9) | 17 (58.6) | ||
| Drinking status a | |||||
| Never | 34 (59.6) | 19 (67.9) | 15 (51.7) | 1.540 | 0.215 |
| Former/Current | 23 (40.4) | 9 (32.1) | 14 (48.3) | ||
| Physical activity level a | |||||
| Low | 20 (35.1) | 12 (42.9) | 8 (27.6) | 1.459 | 0.227 |
| Moderate | 37 (64.9) | 16 (57.1) | 21 (72.4) | ||
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Variables | Overall (n = 57) | Intervention (n = 28) | Control (n = 29) | χ2 | p |
|---|---|---|---|---|---|
| Smoking status | |||||
| Never | 28 (49.1) | 16 (57.1) | 12 (41.4) | 1.416 | 0.234 |
| Former/Current | 29 (50.9) | 12 (42.9) | 17 (58.6) | ||
| Drinking status | |||||
| Never | 34 (59.6) | 19 (67.9) | 15 (51.7) | 1.540 | 0.215 |
| Former/Current | 23 (40.4) | 9 (32.1) | 14 (48.3) | ||
| Physical activity level | |||||
| Low | 20 (35.1) | 12 (42.9) | 8 (27.6) | 1.459 | 0.227 |
| Moderate | 37 (64.9) | 16 (57.1) | 21 (72.4) | ||
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Variable | Intervention | Control | t/Z | p | ||
|---|---|---|---|---|---|---|
| IL-6 (pg/mL) | ||||||
| T1 | 7.35 ± 0.63 | 7.16 ± 0.98 | 0.885 | 0.380 a | ||
| T2 | 6.96 ± 0.88 | 7.28 ± 1.08 | –1.242 | 0.220 a | ||
| ∆IL-6 | –0.40 ± 0.65 | 0.12 ± 0.40 | –3.634 | 0.001 a | ||
| t | 3.258 | 1.639 | ||||
| p | 0.003 c | 0.113 c | ||||
| IL-10 (pg/mL) | ||||||
| T1 | 17.59 ± 0.87 | 17.64 ± 0.82 | –0.239 | 0.812 a | ||
| T2 | 17.90 ± 0.93 | 17.49 ± 0.81 | 1.767 | 0.083 a | ||
| ∆IL-10 | 0.31 ± 0.68 | –0.15 ± 0.46 | 2.996 | 0.004 b | ||
| t | –2.456 | –1.716 | ||||
| p | 0.021 c | 0.098 c | ||||
| Variable | Intervention | Control | t/Z | p |
|---|---|---|---|---|
| BMI | ||||
| T1 | 21.67 ± 2.72 | 21.08 ± 2.99 | 0.788 | 0.434 a |
| T2 | 22.40 ± 2.79 | 20.68 ± 2.19 | 2.608 | 0.012 a |
| ∆BMI | 0.32 (0.00, 1.65) | 0.00 (–0.75, 0.66) | –2.143 | 0.032 b |
| Z | –1.272 | 0.635 | ||
| p | 0.203 d | 0.525 d | ||
| MNA | ||||
| T1 | 22.33 ± 2.31 | 21.85 ± 3.00 | 0.681 | 0.383 b |
| T2 | 23.17 ± 2.36 | 21.79 ± 2.21 | 2.276 | 0.027 a |
| ∆MNA | 0.84 ± 1.60 | –0.05 ± 1.55 | 2.141 | 0.037 a |
| Z/t | –2.562 | 0.180 | ||
| p | 0.010 d | 0.859 c |
| Intervention | Control | t/Z | p | |
|---|---|---|---|---|
| T1 | 92.91 ± 14.75 | 84.92 ± 19.07 | 1.765 | 0.083 a |
| T2 | 97.52 ± 15.76 | 86.47 ± 16.91 | 2.551 | 0.014 a |
| ∆FACT-C scores | 4.61 ± 6.34 | 1.55 ± 5.57 | 1.940 | 0.058 a |
| t | –3.849 | −1.500 | ||
| p | <0.001 b | 0.145 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Y.; Cheng, L.; He, J.; Cheng, X.; Lin, X.; Miao, X.; Huang, Z.; Xia, S. Effects of 12-Week Dietary Inflammatory Index-Based Dietary Education on Frailty Status in Frail Patients with Colorectal Cancer: A Randomized Controlled Trial. Nutrients 2025, 17, 2203. https://doi.org/10.3390/nu17132203
Wang Y, Liu Y, Cheng L, He J, Cheng X, Lin X, Miao X, Huang Z, Xia S. Effects of 12-Week Dietary Inflammatory Index-Based Dietary Education on Frailty Status in Frail Patients with Colorectal Cancer: A Randomized Controlled Trial. Nutrients. 2025; 17(13):2203. https://doi.org/10.3390/nu17132203
Chicago/Turabian StyleWang, Yuting, Yuan Liu, Lan Cheng, Jianyun He, Xinxin Cheng, Xiaoxia Lin, Xinyi Miao, Zhenzhen Huang, and Shufang Xia. 2025. "Effects of 12-Week Dietary Inflammatory Index-Based Dietary Education on Frailty Status in Frail Patients with Colorectal Cancer: A Randomized Controlled Trial" Nutrients 17, no. 13: 2203. https://doi.org/10.3390/nu17132203
APA StyleWang, Y., Liu, Y., Cheng, L., He, J., Cheng, X., Lin, X., Miao, X., Huang, Z., & Xia, S. (2025). Effects of 12-Week Dietary Inflammatory Index-Based Dietary Education on Frailty Status in Frail Patients with Colorectal Cancer: A Randomized Controlled Trial. Nutrients, 17(13), 2203. https://doi.org/10.3390/nu17132203





