Zinc Deficiency in Chronic Kidney Disease and Hemodialysis: Insights from Basic Research to Clinical Implications
Abstract
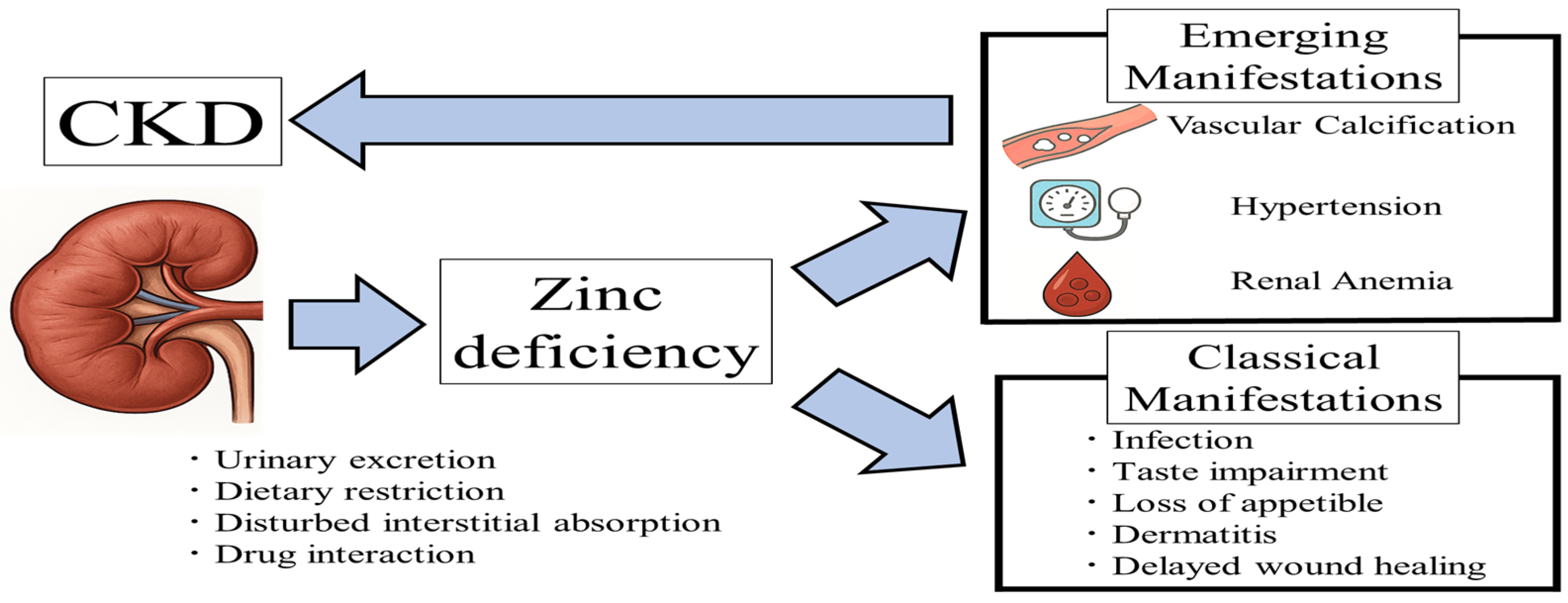
1. Introduction
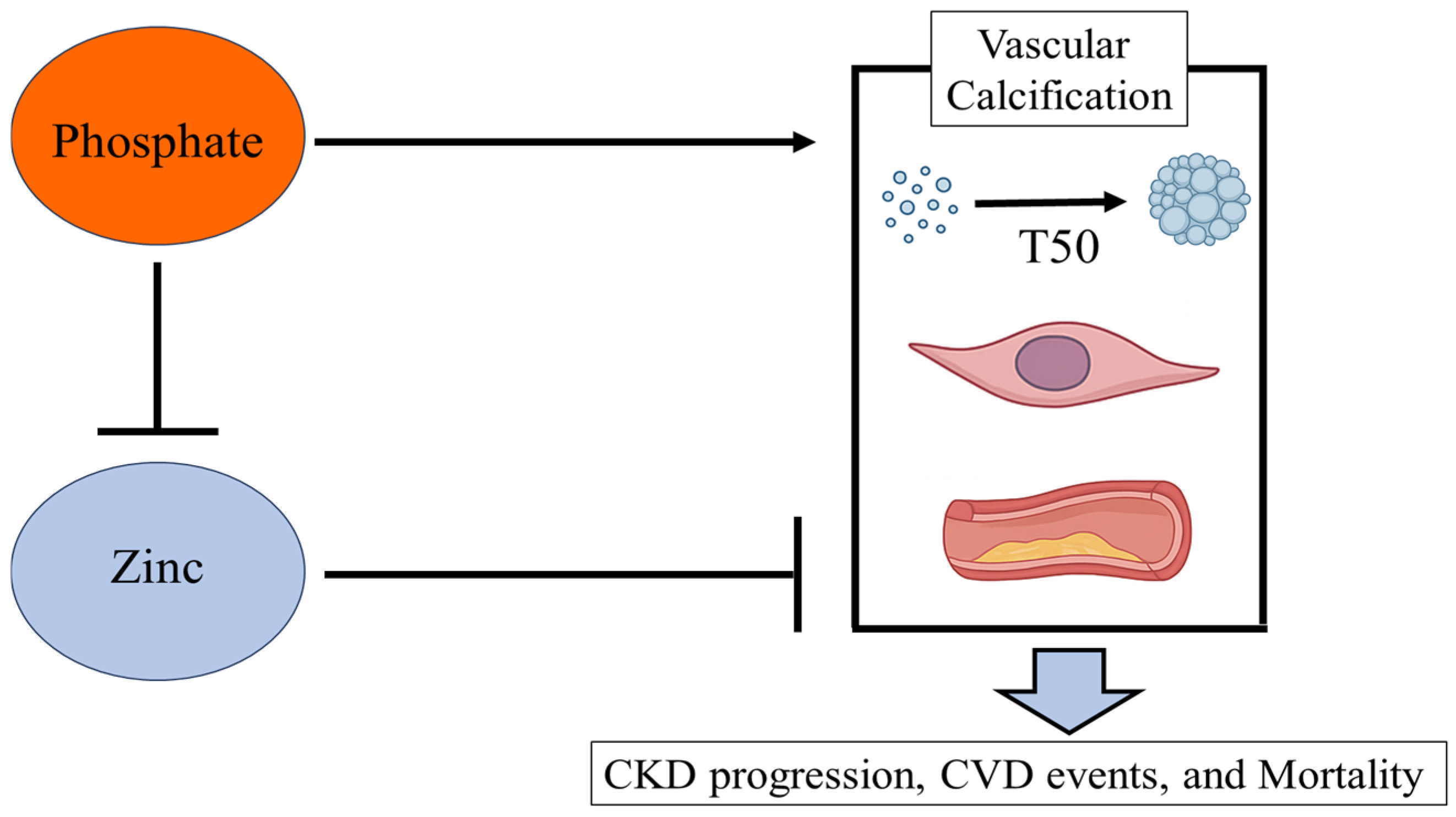
2. Zinc and Calcification
2.1. Vasculature Smooth Muscle Cells
2.2. Serum Calcification Propensity: T50
2.3. Vasculature Change
3. Zinc and Blood Pressure
4. Zinc and Anemia
5. Zinc and Other Diseases
5.1. Diabetes
5.2. Infection
5.3. Cardiac Diastolic Dysfunction
6. Zinc and Progression of Kidney Disease
7. Zinc and CVD Events
8. Zinc and Mortality in Patients with Hemodialysis
9. Clinical Consideration in Zinc Supplementation
10. Future Clinical Trials
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAC | Abdominal aortic calcification |
| ALP | Alkaline Phosphatase |
| BMI | Body mass index |
| BMSCs | Bone Marrow-Derived Mesenchymal Stem Cells |
| CIMT | Carotid intima-media thickness |
| CKD | Chronic kidney disease |
| eGFR | Estimated glomerular filtration rate |
| CPP | Calciprotein particle |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| ESA | Erythropoiesis-stimulating agent |
| HbA1c | Glycosylated hemoglobin |
| HIF | Hypoxia-inducible factor |
| HIF-PHIs | Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors |
| HOMAIR | Homeostasis model assessment-estimated insulin resistance |
| Nrf2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| NF-κB | nuclear factor kappa light chain enhancer of activated B |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| OCN | Osteocalcin |
| PWV | Pulse wave velocity |
| RCT | Randomized controlled trial |
| RUNX2 | Runt-Related Transcription Factor 2 |
| SOD | Superoxide Dismutase |
| SOX9 | SRY-Box Transcription Factor 9 |
| T50 | Serum calcification propensity |
| TNF | Tumor necrosis factor |
| TNFAIP3 | TNFα-induced protein 3 |
| VSMC | vasculature smooth muscle cell |
References
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef]
- Mahajan, S.K.; Bowersox, E.M.; Rye, D.L.; Abu-Hamdan, D.K.; Prasad, A.S.; McDonald, F.D.; Biersack, K.L. Factors underlying abnormal zinc metabolism in uremia. Kidney Int. Suppl. 1989, 27, S269–S273. [Google Scholar] [PubMed]
- Damianaki, K.; Lourenco, J.M.; Braconnier, P.; Ghobril, J.P.; Devuyst, O.; Burnier, M.; Lenglet, S.; Augsburger, M.; Thomas, A.; Pruijm, M. Renal handling of zinc in chronic kidney disease patients and the role of circulating zinc levels in renal function decline. Nephrol. Dial. Transpl. 2020, 35, 1163–1170. [Google Scholar] [CrossRef]
- Toida, T.; Toida, R.; Ebihara, S.; Takahashi, R.; Komatsu, H.; Uezono, S.; Sato, Y.; Fujimoto, S. Association between Serum Zinc Levels and Clinical Index or the Body Composition in Incident Hemodialysis Patients. Nutrients 2020, 12, 3187. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Abe, K.; Sakai, S.; Kamei, Y.; Mori, Y.; Adachi, Y.; Takikawa, M.; Kitamura, A.; Ohminami, H.; Ohnishi, K.; et al. Elevated luminal inorganic phosphate suppresses intestinal Zn absorption in 5/6 nephrectomized rats. Am. J. Physiol. Ren. Physiol. 2024, 326, F411–F419. [Google Scholar] [CrossRef]
- Kodama, H.; Tanaka, M.; Naito, Y.; Katayama, K.; Moriyama, M. Japan’s Practical Guidelines for Zinc Deficiency with a Particular Focus on Taste Disorders, Inflammatory Bowel Disease, and Liver Cirrhosis. Int. J. Mol. Sci. 2020, 21, 2941. [Google Scholar] [CrossRef]
- Nakatani, S.; Mori, K.; Shoji, T.; Emoto, M. Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients 2021, 13, 1680. [Google Scholar] [CrossRef]
- Elgenidy, A.; Amin, M.A.; Awad, A.K.; Husain-Syed, F.; Aly, M.G. Serum Zinc Levels in Chronic Kidney Disease Patients, Hemodialysis Patients, and Healthy Controls: Systematic Review and Meta-Analysis. J. Ren. Nutr. 2023, 33, 103–115. [Google Scholar] [CrossRef]
- Gibson, R.S.; King, J.C.; Lowe, N. A Review of Dietary Zinc Recommendations. Food Nutr. Bull. 2016, 37, 443–460. [Google Scholar] [CrossRef]
- Inoue, Y. Dietary reference intakes of trace elements for Japanese and problems in clinical fields. Nihon Rinsho. Jpn. J. Clin. Med. 2016, 74, 1066–1073. [Google Scholar]
- Chen, W.; Eisenberg, R.; Mowrey, W.B.; Wylie-Rosett, J.; Abramowitz, M.K.; Bushinsky, D.A.; Melamed, M.L. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol. Dial. Transpl. 2020, 35, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- Hosseini, R.; Montazerifar, F.; Shahraki, E.; Karajibani, M.; Mokhtari, A.M.; Dashipour, A.R.; Ferns, G.A.; Jalali, M. The Effects of Zinc Sulfate Supplementation on Serum Copeptin, C-Reactive Protein and Metabolic Markers in Zinc-Deficient Diabetic Patients on Hemodialysis: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2022, 200, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Haddadian-Khouzani, S.; Shahidi, S.; Askari, G.; Clark, C.C.T.; Rouhani, M.H. The efficacy and safety of zinc gluconate supplementation on quality of life, sleep quality, and serum albumin in hemodialysis patients: A randomized clinical trial. Eur. J. Integr. Med. 2022, 55, 102183. [Google Scholar] [CrossRef]
- Okamoto, T.; Hatakeyama, S.; Konishi, S.; Okita, K.; Tanaka, Y.; Imanishi, K.; Takashima, T.; Saitoh, F.; Suzuki, T.; Ohyama, C. Comparison of zinc acetate hydrate and polaprezinc for zinc deficiency in patients on maintenance hemodialysis: A single-center, open-label, prospective randomized study. Ther. Apher. Dial. 2020, 24, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Ayala-Macedo, G.; Sakihara, G.; Peralta, S.; Almaraz-Gomez, A.; Barrado, E.; Marugan-Miguelsanz, J.M. Effects of Zinc Supplementation on Nutritional Status in Children with Chronic Kidney Disease: A Randomized Trial. Nutrients 2019, 11, 2671. [Google Scholar] [CrossRef]
- Wang, L.J.; Wang, M.Q.; Hu, R.; Yang, Y.; Huang, Y.S.; Xian, S.X.; Lu, L. Effect of Zinc Supplementation on Maintenance Hemodialysis Patients: A Systematic Review and Meta-Analysis of 15 Randomized Controlled Trials. Biomed. Res. Int. 2017, 2017, 1024769. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Thompson, S.; Kinniburgh, D.; Klarenbach, S.W.; Walsh, M.; Bello, A.K.; Faruque, L.; Field, C.; Manns, B.J.; et al. Trace element supplementation in hemodialysis patients: A randomized controlled trial. BMC Nephrol. 2015, 16, 52. [Google Scholar] [CrossRef]
- Abdollahi, S.; Toupchian, O.; Jayedi, A.; Meyre, D.; Tam, V.; Soltani, S. Zinc Supplementation and Body Weight: A Systematic Review and Dose-Response Meta-analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 398–411. [Google Scholar] [CrossRef]
- Kobayashi, H.; Abe, M.; Okada, K.; Tei, R.; Maruyama, N.; Kikuchi, F.; Higuchi, T.; Soma, M. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 2015, 7, 3783–3795. [Google Scholar] [CrossRef]
- El-Shazly, A.N.; Ibrahim, S.A.; El-Mashad, G.M.; Sabry, J.H.; Sherbini, N.S. Effect of zinc supplementation on body mass index and serum levels of zinc and leptin in pediatric hemodialysis patients. Int. J. Nephrol. Renov. Dis. 2015, 8, 159–163. [Google Scholar] [CrossRef]
- Argani, H.; Mahdavi, R.; Ghorbani-haghjo, A.; Razzaghi, R.; Nikniaz, L.; Gaemmaghami, S.J. Effects of zinc supplementation on serum zinc and leptin levels, BMI, and body composition in hemodialysis patients. J. Trace Elem. Med. Biol. 2014, 28, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Pakfetrat, M.; Shahroodi, J.R.; Zolgadr, A.A.; Larie, H.A.; Nikoo, M.H.; Malekmakan, L. Effects of zinc supplement on plasma homocysteine level in end-stage renal disease patients: A double-blind randomized clinical trial. Biol. Trace Elem. Res. 2013, 153, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Mazani, M.; Argani, H.; Rashtchizadeh, N.; Ghorbanihaghjo, A.; Hamdi, A.; Estiar, M.A.; Nezami, N. Effects of zinc supplementation on antioxidant status and lipid peroxidation in hemodialysis patients. J. Ren. Nutr. 2013, 23, 180–184. [Google Scholar] [CrossRef]
- Guo, C.H.; Wang, C.L. Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int. J. Med. Sci. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Rahimi-Ardabili, B.; Argani, H.; Ghorbanihaghjo, A.; Rashtchizadeh, N.; Naghavi-Behzad, M.; Ghorashi, S.; Nezami, N. Paraoxonase enzyme activity is enhanced by zinc supplementation in hemodialysis patients. Ren. Fail. 2012, 34, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Roozbeh, J.; Hedayati, P.; Sagheb, M.M.; Sharifian, M.; Hamidian Jahromi, A.; Shaabani, S.; Jalaeian, H.; Raeisjalali, G.A.; Behzadi, S. Effect of zinc supplementation on triglyceride, cholesterol, LDL, and HDL levels in zinc-deficient hemodialysis patients. Ren. Fail. 2009, 31, 798–801. [Google Scholar] [CrossRef]
- Rashidi, A.A.; Salehi, M.; Piroozmand, A.; Sagheb, M.M. Effects of zinc supplementation on serum zinc and C-reactive protein concentrations in hemodialysis patients. J. Ren. Nutr. 2009, 19, 475–478. [Google Scholar] [CrossRef]
- Nava, H.J.; Amato, D. Effect of zinc supplements on the levels of pre-albumin and transferrin in patients with dialysis. Rev. Investig. Clínica 2005, 57, 123–125. [Google Scholar]
- Matson, A.; Wright, M.; Oliver, A.; Woodrow, G.; King, N.; Dye, L.; Blundell, J.; Brownjohn, A.; Turney, J. Zinc supplementation at conventional doses does not improve the disturbance of taste perception in hemodialysis patients. J. Ren. Nutr. 2003, 13, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, C.A.; Liepa, G.; Murphy, M.D.; Suneson, J.; Vanbeber, A.D.; Gorman, M.A.; Cochran, C. The effects of zinc supplementation on serum zinc and cholesterol concentrations in hemodialysis patients. J. Ren. Nutr. 2002, 12, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Candan, F.; Gultekin, F.; Candan, F. Effect of vitamin C and zinc on osmotic fragility and lipid peroxidation in zinc-deficient haemodialysis patients. Cell Biochem. Funct. 2002, 20, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Jern, N.A.; VanBeber, A.D.; Gorman, M.A.; Weber, C.G.; Liepa, G.U.; Cochran, C.C. The effects of zinc supplementation on serum zinc concentration and protein catabolic rate in hemodialysis patients. J. Ren. Nutr. 2000, 10, 148–153. [Google Scholar] [CrossRef]
- Brodersen, H.P.; Holtkamp, W.; Larbig, D.; Beckers, B.; Thiery, J.; Lautenschlager, J.; Probst, H.J.; Ropertz, S.; Yavari, A. Zinc supplementation and hepatitis B vaccination in chronic haemodialysis patients: A multicentre study. Nephrol. Dial. Transpl. 1995, 10, 1780. [Google Scholar]
- Prasad, A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 646–652. [Google Scholar] [CrossRef]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Henkin, R.I. Zinc in taste function: A critical review. Biol. Trace Elem. Res. 1984, 6, 263–280. [Google Scholar] [CrossRef]
- Lask, B.; Fosson, A.; Rolfe, U.; Thomas, S. Zinc deficiency and childhood-onset anorexia nervosa. J. Clin. Psychiatry 1993, 54, 63–66. [Google Scholar]
- Gray, N.A.; Dhana, A.; Stein, D.J.; Khumalo, N.P. Zinc and atopic dermatitis: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Clinical manifestations of zinc deficiency. Annu. Rev. Nutr. 1985, 5, 341–363. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wu, M.L.; Chou, Y.Y.; Li, S.Y.; Deng, J.F.; Yang, W.C.; Ng, Y.Y. Essential trace element status and clinical outcomes in long-term dialysis patients: A two-year prospective observational cohort study. Clin. Nutr. 2012, 31, 630–636. [Google Scholar] [CrossRef]
- Cardozo, L.; Mafra, D. Don’t forget the zinc. Nephrol. Dial. Transpl. 2020, 35, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Nagler, E.V.; Verbeke, F.; Van Biesen, W.; Vanholder, R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am. J. Med. 2013, 126, 825–831. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wong, C.S.; Chung, C.J.; Wu, M.Y.; Huang, Y.L.; Ao, P.L.; Lin, Y.F.; Lin, Y.C.; Shiue, H.S.; Su, C.T.; et al. The association between plasma selenium and chronic kidney disease related to lead, cadmium and arsenic exposure in a Taiwanese population. J. Hazard. Mater. 2019, 375, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.Z.; Huang, Y.; Zheng, X.F.; Feng, R.; Li, X.Y.; Zheng, Z.G.; Jiang, B.J.; Du, S.; Chen, H.G.; Xu, Y. The association between serum magnesium and chronic kidney disease in Chinese adults: A cross-sectional study. BMC Public Health 2024, 24, 187. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Isaka, Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014, 85, 174–181. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Bello, A.; Field, C.J.; Gill, J.S.; Hemmelgarn, B.R.; Holmes, D.T.; Jindal, K.; Klarenbach, S.W.; Manns, B.J.; et al. Concentrations of Trace Elements and Clinical Outcomes in Hemodialysis Patients: A Prospective Cohort Study. Clin. J. Am. Soc. Nephrol. 2018, 13, 907–915. [Google Scholar] [CrossRef]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef]
- Kircelli, F.; Peter, M.E.; Sevinc Ok, E.; Celenk, F.G.; Yilmaz, M.; Steppan, S.; Asci, G.; Ok, E.; Passlick-Deetjen, J. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol. Dial. Transpl. 2012, 27, 514–521. [Google Scholar] [CrossRef]
- Louvet, L.; Buchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z.A. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol. Dial. Transpl. 2013, 28, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Petho, D.; Gall, T.; Zavaczki, E.; Nyitrai, M.; Posta, J.; Zarjou, A.; Agarwal, A.; Balla, G.; Balla, J. Zinc Inhibits HIF-Prolyl Hydroxylase Inhibitor-Aggravated VSMC Calcification Induced by High Phosphate. Front. Physiol. 2019, 10, 1584. [Google Scholar] [CrossRef]
- Voelkl, J.; Tuffaha, R.; Luong, T.T.D.; Zickler, D.; Masyout, J.; Feger, M.; Verheyen, N.; Blaschke, F.; Kuro, O.M.; Tomaschitz, A.; et al. Zinc Inhibits Phosphate-Induced Vascular Calcification through TNFAIP3-Mediated Suppression of NF-κB. J. Am. Soc. Nephrol. 2018, 29, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gordillo-Martinez, F.; Jiang, L.; He, P.; Hong, W.; Wei, X.; Staines, K.A.; Macrae, V.E.; Zhang, C.; Yu, D.; et al. Zinc ameliorates human aortic valve calcification through GPR39 mediated ERK1/2 signalling pathway. Cardiovasc. Res. 2021, 117, 820–835. [Google Scholar] [CrossRef]
- Henze, L.A.; Estepa, M.; Pieske, B.; Lang, F.; Eckardt, K.U.; Alesutan, I.; Voelkl, J. Zinc Ameliorates the Osteogenic Effects of High Glucose in Vascular Smooth Muscle Cells. Cells 2021, 10, 3083. [Google Scholar] [CrossRef]
- Alcantara, E.H.; Kwon, J.H.; Kang, M.K.; Cho, Y.E.; Kwun, I.S. Zinc Deficiency Promotes Calcification in Vascular Smooth Muscle Cells Independent of Alkaline Phosphatase Action and Partly Impacted by Pit1 Upregulation. Nutrients 2024, 16, 291. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Toth, A.; Csiki, D.M.; Jeney, V. Zinc Ameliorates High Pi and Ca-Mediated Osteogenic Differentiation of Mesenchymal Stem Cells. Nutrients 2024, 16, 4012. [Google Scholar] [CrossRef]
- Heiss, A.; Jahnen-Dechent, W.; Endo, H.; Schwahn, D. Structural dynamics of a colloidal protein-mineral complex bestowing on calcium phosphate a high solubility in biological fluids. Biointerphases 2007, 2, 16–20. [Google Scholar] [CrossRef]
- Heiss, A.; DuChesne, A.; Denecke, B.; Grotzinger, J.; Yamamoto, K.; Renne, T.; Jahnen-Dechent, W. Structural basis of calcification inhibition by α2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J. Biol. Chem. 2003, 278, 13333–13341. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Smith, E.R. Nature’s remedy to phosphate woes: Calciprotein particles regulate systemic mineral metabolism. Kidney Int. 2020, 97, 648–651. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Pasch, A. Solving the insoluble: Calciprotein particles mediate bulk mineral transport. Kidney Int. 2023, 103, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Kuro, O.M. Phosphate as a Pathogen of Arteriosclerosis and Aging. J. Atheroscler. Thromb. 2021, 28, 203–213. [Google Scholar] [CrossRef]
- Aghagolzadeh, P.; Bachtler, M.; Bijarnia, R.; Jackson, C.; Smith, E.R.; Odermatt, A.; Radpour, R.; Pasch, A. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-alpha. Atherosclerosis 2016, 251, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Pasch, A.; Farese, S.; Graber, S.; Wald, J.; Richtering, W.; Floege, J.; Jahnen-Dechent, W. Nanoparticle-based test measures overall propensity for calcification in serum. J. Am. Soc. Nephrol. 2012, 23, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Pluquet, M.; Kamel, S.; Choukroun, G.; Liabeuf, S.; Laville, S.M. Serum Calcification Propensity Represents a Good Biomarker of Vascular Calcification: A Systematic Review. Toxins 2022, 14, 637. [Google Scholar] [CrossRef]
- Bundy, J.D.; Cai, X.; Mehta, R.C.; Scialla, J.J.; de Boer, I.H.; Hsu, C.Y.; Go, A.S.; Dobre, M.A.; Chen, J.; Rao, P.S.; et al. Serum Calcification Propensity and Clinical Events in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 1562–1571. [Google Scholar] [CrossRef]
- Mori, K.; Shoji, T.; Nakatani, S.; Uedono, H.; Ochi, A.; Yoshida, H.; Imanishi, Y.; Morioka, T.; Tsujimoto, Y.; Kuro, O.M.; et al. Differential associations of fetuin-A and calcification propensity with cardiovascular events and subsequent mortality in patients undergoing hemodialysis. Clin. Kidney J. 2024, 17, sfae042. [Google Scholar] [CrossRef]
- Pasch, A.; Block, G.A.; Bachtler, M.; Smith, E.R.; Jahnen-Dechent, W.; Arampatzis, S.; Chertow, G.M.; Parfrey, P.; Ma, X.; Floege, J. Blood Calcification Propensity, Cardiovascular Events, and Survival in Patients Receiving Hemodialysis in the EVOLVE Trial. Clin. J. Am. Soc. Nephrol. 2017, 12, 315–322. [Google Scholar] [CrossRef]
- Lorenz, G.; Steubl, D.; Kemmner, S.; Pasch, A.; Koch-Sembdner, W.; Pham, D.; Haller, B.; Bachmann, Q.; Mayer, C.C.; Wassertheurer, S.; et al. Worsening calcification propensity precedes all-cause and cardiovascular mortality in haemodialyzed patients. Sci. Rep. 2017, 7, 13368. [Google Scholar] [CrossRef]
- Smith, E.R.; Ford, M.L.; Tomlinson, L.A.; Bodenham, E.; McMahon, L.P.; Farese, S.; Rajkumar, C.; Holt, S.G.; Pasch, A. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J. Am. Soc. Nephrol. 2014, 25, 339–348. [Google Scholar] [CrossRef]
- Nakatani, S.; Mori, K.; Sonoda, M.; Nishide, K.; Uedono, H.; Tsuda, A.; Emoto, M.; Shoji, T. Association between Serum Zinc and Calcification Propensity (T50) in Patients with Type 2 Diabetes Mellitus and In Vitro Effect of Exogenous Zinc on T50. Biomedicines 2020, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Obereigner, J.; Mitter, G.; Schmid, T.; Hofer, A.S.; Schuster, G.; Hugl, A.; Dorninger, A.H.; Mandl, M.; Pasch, A.; et al. Association of serum zinc with mineral stress in chronic kidney disease. Clin. Kidney J. 2024, 17, sfae258. [Google Scholar] [CrossRef]
- Schweikle, M.; Bjornoy, S.H.; van Helvoort, A.T.J.; Haugen, H.J.; Sikorski, P.; Tiainen, H. Stabilisation of amorphous calcium phosphate in polyethylene glycol hydrogels. Acta Biomater. 2019, 90, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.A.; Komarovska, L.; Viksna, A. Efficient zinc incorporation into hydroxyapatite through crystallization of an amorphous phase could extend the properties of zinc apatites. J. Aust. Ceram. Soc. 2013, 49, 129–135. [Google Scholar]
- Candidato, R.T., Jr.; Thouzellier, C.; Pawlowski, L. Evaluation of the in-vitro behavior of nanostructured hydroxyapatite and zinc doped hydroxyapatite coatings obtained using solution precursor plasma spraying. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2101–2108. [Google Scholar] [CrossRef]
- Kestenbaum, B.R.; Adeney, K.L.; de Boer, I.H.; Ix, J.H.; Shlipak, M.G.; Siscovick, D.S. Incidence and progression of coronary calcification in chronic kidney disease: The Multi-Ethnic Study of Atherosclerosis. Kidney Int. 2009, 76, 991–998. [Google Scholar] [CrossRef]
- Chen, J.; Budoff, M.J.; Reilly, M.P.; Yang, W.; Rosas, S.E.; Rahman, M.; Zhang, X.; Roy, J.A.; Lustigova, E.; Nessel, L.; et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients With Chronic Kidney Disease. JAMA Cardiol. 2017, 2, 635–643. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, Y.; Li, H.; Wang, Y.; Niu, Z.; Zhou, W.; Wang, D. Associations of Whole Blood Zinc Levels with Coronary Artery Calcification and Future Cardiovascular Events in CKD Patients. Biol. Trace Elem. Res. 2024, 202, 46–55. [Google Scholar] [CrossRef]
- Wilson, P.W.; Kauppila, L.I.; O’Donnell, C.J.; Kiel, D.P.; Hannan, M.; Polak, J.M.; Cupples, L.A. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 2001, 103, 1529–1534. [Google Scholar] [CrossRef]
- Peeters, M.J.; van den Brand, J.A.; van Zuilen, A.D.; Koster, Y.; Bots, M.L.; Vervloet, M.G.; Blankestijn, P.J.; Wetzels, J.F.; the MASTERPLAN Study Group. Abdominal aortic calcification in patients with CKD. J. Nephrol. 2017, 30, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sayed, O.; Ahmed, S.; Ismail, H.; Elfeshawy, M.; Ibrahim, M. Zinc and Abdominal Aortic Calcification in Patients under Regular Hemodialysis. Al-Azhar Int. Med. J. 2022, 3, 205–211. [Google Scholar] [CrossRef]
- Yang, C.W.; Guo, Y.C.; Li, C.I.; Liu, C.S.; Lin, C.H.; Liu, C.H.; Wang, M.C.; Yang, S.Y.; Li, T.C.; Lin, C.C. Subclinical Atherosclerosis Markers of Carotid Intima-Media Thickness, Carotid Plaques, Carotid Stenosis, and Mortality in Community-Dwelling Adults. Int. J. Env. Res. Public Health 2020, 17, 4745. [Google Scholar] [CrossRef]
- Yang, Y.J.; Choi, B.Y.; Chun, B.Y.; Kweon, S.S.; Lee, Y.H.; Park, P.S.; Kim, M.K. Dietary zinc intake is inversely related to subclinical atherosclerosis measured by carotid intima-media thickness. Br. J. Nutr. 2010, 104, 1202–1211. [Google Scholar] [CrossRef]
- Ari, E.; Kaya, Y.; Demir, H.; Asicioglu, E.; Keskin, S. The correlation of serum trace elements and heavy metals with carotid artery atherosclerosis in maintenance hemodialysis patients. Biol. Trace Elem. Res. 2011, 144, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Emoto, M.; Shinohara, K.; Kakiya, R.; Tsujimoto, Y.; Kishimoto, H.; Ishimura, E.; Tabata, T.; Nishizawa, Y. Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J. Am. Soc. Nephrol. 2001, 12, 2117–2124. [Google Scholar] [CrossRef]
- Ishioka, K.; Hidaka, S.; Fujiwara, N.; Yamano, M.; Mochida, Y.; Oka, M.; Maesato, K.; Moriya, H.; Ohtake, T.; Kobayashi, S. Association between zinc deficiency and aorta stiffness in non-diabetic hemodialysis patients. PLoS ONE 2023, 18, e0268875. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liu, H.; Li, S.; Zhang, D. The association of serum zinc and copper with hypertension: A meta-analysis. J. Trace Elem. Med. Biol. 2019, 53, 41–48. [Google Scholar] [CrossRef]
- Sato, M.; Yanagisawa, H.; Nojima, Y.; Tamura, J.; Wada, O. Zn deficiency aggravates hypertension in spontaneously hypertensive rats: Possible role of Cu/Zn-superoxide dismutase. Clin. Exp. Hypertens. 2002, 24, 355–370. [Google Scholar] [CrossRef]
- Dimitrova, A.A.; Strashimirov, D.; Betova, T.; Russeva, A.; Alexandrova, M. Zinc content in the diet affects the activity of Cu/ZnSOD, lipid peroxidation and lipid profile of spontaneously hypertensive rats. Acta Biol. Hung. 2008, 59, 305–314. [Google Scholar] [CrossRef]
- Zalewski, P.D.; Beltrame, J.F.; Wawer, A.A.; Abdo, A.I.; Murgia, C. Roles for endothelial zinc homeostasis in vascular physiology and coronary artery disease. Crit. Rev. Food Sci. Nutr. 2019, 59, 3511–3525. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Mofrad, M.D.; Borges do Nascimento, I.J.; Milajerdi, A.; Mokhtari, T.; Esmaillzadeh, A. Correction to: The effect of zinc supplementation on blood pressure: A systematic review and dose-response meta-analysis of randomized-controlled trials. Eur. J. Nutr. 2020, 59, 1829. [Google Scholar] [CrossRef] [PubMed]
- Nakazono, K.; Watanabe, N.; Matsuno, K.; Sasaki, J.; Sato, T.; Inoue, M. Does superoxide underlie the pathogenesis of hypertension? Proc. Natl. Acad. Sci. USA 1991, 88, 10045–10048. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Hong, N.J.; Yang, N.; Cabral, P.D.; Berthiaume, J.M.; Dominici, F.P.; Garvin, J.L. Dietary Fructose Increases the Sensitivity of Proximal Tubules to Angiotensin II in Rats Fed High-Salt Diets. Nutrients 2018, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takata, T.; Hanada, H.; Taniguchi, S.; Hamada, S.; Mae, Y.; Iyama, T.; Kanda, T.; Isomoto, H. Zinc deficiency induces hypertension by paradoxically amplifying salt sensitivity under high salt intake in mice. Clin. Exp. Nephrol. 2024, 28, 728–739. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, F.; Zhang, X.; Jin, Y.; Li, Q.; Shen, H.; Fu, H.; Mao, J. Benefits and risks of essential trace elements in chronic kidney disease: A narrative review. Ann. Transl. Med. 2022, 10, 1400. [Google Scholar] [CrossRef]
- Jeng, S.S.; Chen, Y.H. Association of Zinc with Anemia. Nutrients 2022, 14, 4918. [Google Scholar] [CrossRef]
- Xia, J.; Browning, J.D.; O’Dell, B.L. Decreased plasma membrane thiol concentration is associated with increased osmotic fragility of erythrocytes in zinc-deficient rats. J. Nutr. 1999, 129, 814–819. [Google Scholar] [CrossRef]
- Tanimura, N.; Liao, R.; Wilson, G.M.; Dent, M.R.; Cao, M.; Burstyn, J.N.; Hematti, P.; Liu, X.; Zhang, Y.; Zheng, Y.; et al. GATA/Heme Multi-omics Reveals a Trace Metal-Dependent Cellular Differentiation Mechanism. Dev. Cell 2018, 46, 581–594.e4. [Google Scholar] [CrossRef]
- King, L.E.; Fraker, P.J. Zinc deficiency in mice alters myelopoiesis and hematopoiesis. J. Nutr. 2002, 132, 3301–3307. [Google Scholar] [CrossRef]
- Konomi, A.; Yokoi, K. Zinc deficiency decreases plasma erythropoietin concentration in rats. Biol. Trace Elem. Res. 2005, 107, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.L.; Chen, Y.H.; Jeng, S.S. Effect of Zinc Supplementation on Renal Anemia in 5/6-Nephrectomized Rats and a Comparison with Treatment with Recombinant Human Erythropoietin. Int. J. Mol. Sci. 2019, 20, 4985. [Google Scholar] [CrossRef]
- Fukasawa, H.; Furuya, R.; Kaneko, M.; Nakagami, D.; Ishino, Y.; Kitamoto, S.; Omata, K.; Yasuda, H. Clinical Significance of Trace Element Zinc in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 1667. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Horike, H.; Fujiki, S.; Kitada, S.; Sasaki, T.; Kashihara, N. Zinc deficiency anemia and effects of zinc therapy in maintenance hemodialysis patients. Ther. Apher. Dial. 2009, 13, 213–219. [Google Scholar] [CrossRef]
- Haase, V.H. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial. Int. 2017, 21 (Suppl. S1), S110–S124. [Google Scholar] [CrossRef] [PubMed]
- Mokas, S.; Lariviere, R.; Lamalice, L.; Gobeil, S.; Cornfield, D.N.; Agharazii, M.; Richard, D.E. Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int. 2016, 90, 598–609. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, W.Q.; Han, X.Q.; Wang, Y.; Wang, X.; Liu, N.F. Advanced glycation end products accelerate calcification in VSMCs through HIF-1alpha/PDK4 activation and suppress glucose metabolism. Sci. Rep. 2018, 8, 13730. [Google Scholar] [CrossRef]
- Toth, A.; Csiki, D.M.; Nagy, B., Jr.; Balogh, E.; Lente, G.; Ababneh, H.; Szoor, A.; Jeney, V. Daprodustat Accelerates High Phosphate-Induced Calcification Through the Activation of HIF-1 Signaling. Front. Pharmacol. 2022, 13, 798053. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kuragano, T. Growing concerns about using hypoxia-inducible factor prolyl hydroxylase inhibitors for the treatment of renal anemia. Clin. Kidney J. 2024, 17, sfae051. [Google Scholar] [CrossRef]
- Farooq, M. Zinc Deficiency is Associated with Poor Glycemic Control. J. Coll. Physicians Surg. Pak. 2019, 29, 253–257. [Google Scholar] [CrossRef]
- Tamura, Y. The Role of Zinc Homeostasis in the Prevention of Diabetes Mellitus and Cardiovascular Diseases. J. Atheroscler. Thromb. 2021, 28, 1109–1122. [Google Scholar] [CrossRef]
- Daneshvar, M.; Ghaheri, M.; Safarzadeh, D.; Karimi, F.; Adib-Hajbagheri, P.; Ahmadzade, M.; Haedi, A. Effect of zinc supplementation on glycemic biomarkers: An umbrella of interventional meta-analyses. Diabetol. Metab. Syndr. 2024, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ichikawa, T.; Li, J.; Si, Q.; Yang, H.; Chen, X.; Goldblatt, C.S.; Meyer, C.J.; Li, X.; Cai, L.; et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 2011, 60, 625–633. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Q.; Lu, J.; Zhang, X.; Suen, D.; Tan, Y.; Jin, L.; Xiao, J.; Xie, R.; Rane, M.; et al. Zinc supplementation partially prevents renal pathological changes in diabetic rats. J. Nutr. Biochem. 2010, 21, 237–246. [Google Scholar] [CrossRef]
- Farvid, M.S.; Jalali, M.; Siassi, F.; Hosseini, M. Comparison of the effects of vitamins and/or mineral supplementation on glomerular and tubular dysfunction in type 2 diabetes. Diabetes Care 2005, 28, 2458–2464. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, H.M.; Ismail, S.H.; Hussein, K.I.; Bakir, I.H.; Sahib, A.S.; Khalaf, B.H.; Hussain, S.A. Effects of melatonin and zinc on lipid profile and renal function in type 2 diabetic patients poorly controlled with metformin. J. Pineal Res. 2006, 41, 189–193. [Google Scholar] [CrossRef]
- Parham, M.; Amini, M.; Aminorroaya, A.; Heidarian, E. Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: A double blind, randomized, placebo-controlled, cross-over trial. Rev. Diabet. Stud. 2008, 5, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Giacconi, R.; Muzzioli, M.; Cipriano, C. Zinc, infections and immunosenescence. Mech. Ageing Dev. 2000, 121, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.W.; Prasad, A.S.; Kaplan, J.; Fitzgerald, J.T.; Brewer, G.J. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. 1997, 272, E1002–E1007. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Meydani, S.N.; Barnett, J.B.; Dallal, G.E.; Fine, B.C.; Jacques, P.F.; Leka, L.S.; Hamer, D.H. Serum zinc and pneumonia in nursing home elderly. Am. J. Clin. Nutr. 2007, 86, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Saka, Y.; Naruse, T.; Matsumoto, J.; Takeda, Y.; Onogi, C.; Yokoi, J.; Kato, A.; Tawada, N.; Noda, Y.; Niwa, S.; et al. Low Serum Zinc Concentration Is Associated With Infection Particularly in Patients With Stage 5 Chronic Kidney Disease Medicated with Proton Pump Inhibitors. J. Ren. Nutr. 2021, 31, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.B.; Dao, M.C.; Hamer, D.H.; Kandel, R.; Brandeis, G.; Wu, D.; Dallal, G.E.; Jacques, P.F.; Schreiber, R.; Kong, E.; et al. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2016, 103, 942–951. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: A meta-analysis of randomized, double-blind and placebo-controlled trials. Clin. Respir. J. 2018, 12, 857–864. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Djafarian, K.; Mojtahed, A.; Varkaneh, H.K.; Shab-Bidar, S. The effect of zinc supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2018, 834, 10–16. [Google Scholar] [CrossRef]
- Huang, J.C.; Su, H.M.; Wu, P.Y.; Lee, J.J.; Lee, W.H.; Chen, S.C.; Chiu, Y.W.; Hsu, Y.L.; Chang, J.M.; Chen, H.C. Ratio of Early Mitral Inflow Velocity to the Global Diastolic Strain Rate and Global Left Ventricular Longitudinal Systolic Strain Predict Overall Mortality and Major Adverse Cardiovascular Events in Hemodialysis Patients. Dis. Markers 2019, 2019, 7512805. [Google Scholar] [CrossRef]
- Ogawa, T.; Nitta, K. Clinical Impact of Left Ventricular Diastolic Dysfunction in Chronic Kidney Disease. Contrib. Nephrol. 2018, 195, 81–91. [Google Scholar] [CrossRef]
- Escoli, R.; Carvalho, M.J.; Cabrita, A.; Rodrigues, A. Diastolic Dysfunction, an Underestimated New Challenge in Dialysis. Ther. Apher. Dial. 2019, 23, 108–117. [Google Scholar] [CrossRef]
- Yu, X.; Huang, L.; Zhao, J.; Wang, Z.; Yao, W.; Wu, X.; Huang, J.; Bian, B. The Relationship between Serum Zinc Level and Heart Failure: A Meta-Analysis. Biomed. Res. Int. 2018, 2018, 2739014. [Google Scholar] [CrossRef]
- Mohtashamian, A.; Soleimani, A.; Gilasi, H.R.; Kheiripour, N.; Moeini Taba, S.M.; Sharifi, N. Association of Zinc Status with Matrix Metalloproteinases, Advanced Glycation End-Products, and Blood Pressure in Patients with Chronic Kidney Disease. Biol. Trace Elem. Res. 2023, 201, 4275–4285. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-T.; Shiu, Y.-L.; Chen, C.-A.; Lin, H.-Y.; Huang, Y.-L.; Lin, C.-C. Changes in levels of copper, iron, zinc, and selenium in patients at different stages of chronic kidney disease. Genom. Med. Biomark. Health Sci. 2012, 4, 128–130. [Google Scholar] [CrossRef][Green Version]
- Kung, W.J.; Shih, C.T.; Lee, C.H.; Lin, C.C. The Divalent Elements Changes in Early Stages of Chronic Kidney Disease. Biol. Trace Elem. Res. 2018, 185, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, A.; Kanda, E.; Itano, S.; Kondo, M.; Wada, Y.; Kadoya, H.; Kidokoro, K.; Nagasu, H.; Sasaki, T.; Kashihara, N. Effect of zinc deficiency on chronic kidney disease progression and effect modification by hypoalbuminemia. PLoS ONE 2021, 16, e0251554. [Google Scholar] [CrossRef]
- Huang, Z.; Liao, Y.; Zheng, Y.; Ye, S.; Zhang, Q.; Yu, X.; Liu, X.; Li, N. Zinc Deficiency Causes Glomerulosclerosis and Renal Interstitial Fibrosis Through Oxidative Stress and Increased Lactate Metabolism in Rats. Biol. Trace Elem. Res. 2024, 203, 2084–2098. [Google Scholar] [CrossRef]
- Maiguma, M.; Suzuki, Y.; Suzuki, H.; Okazaki, K.; Aizawa, M.; Muto, M.; Tomino, Y. Dietary zinc is a key environmental modifier in the progression of IgA nephropathy. PLoS ONE 2014, 9, e90558. [Google Scholar] [CrossRef]
- Mbanefo, N.R.; Uwaezuoke, S.N.; Eneh, C.I.; Odimegwu, C.L.; Chikani, U.N.; Muoneke, U.V.; Nwolisa, C.E.; Odo, K.E.; Ogbuka, F.N.; Akwue, A.T. Can Oral Zinc Supplementation Reduce Relapses in Childhood Steroid-Sensitive Nephrotic Syndrome? A Systematic Review. Int. J. Nephrol. Renov. Dis. 2023, 16, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Yoshigai, E.; Ohashi, T.; Fukada, T. Zinc in Cardiovascular Functions and Diseases: Epidemiology and Molecular Mechanisms for Therapeutic Development. Int. J. Mol. Sci. 2023, 24, 7152. [Google Scholar] [CrossRef]
- Chu, A.; Foster, M.; Samman, S. Zinc Status and Risk of Cardiovascular Diseases and Type 2 Diabetes Mellitus-A Systematic Review of Prospective Cohort Studies. Nutrients 2016, 8, 707. [Google Scholar] [CrossRef]
- Pilz, S.; Dobnig, H.; Winklhofer-Roob, B.M.; Renner, W.; Seelhorst, U.; Wellnitz, B.; Boehm, B.O.; Marz, W. Low serum zinc concentrations predict mortality in patients referred to coronary angiography. Br. J. Nutr. 2009, 101, 1534–1540. [Google Scholar] [CrossRef]
- Soinio, M.; Marniemi, J.; Laakso, M.; Pyorala, K.; Lehto, S.; Ronnemaa, T. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 2007, 30, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Knehtl, M.; Piko, N.; Ekart, R.; Hojs, R.; Bevc, S. Serum zinc values, ankle brachial index and mortality in hemodialysis patients. BMC Nephrol. 2022, 23, 355. [Google Scholar] [CrossRef]
- Prasad, A.S.; Oberleas, D. Binding of zinc to amino acids and serum proteins in vitro. J. Lab. Clin. Med. 1970, 76, 416–425. [Google Scholar]
- Nakatani, S.; Shoji, T.; Morioka, F.; Nakaya, R.; Ueda, M.; Uedono, H.; Tsuda, A.; Morioka, T.; Fujii, H.; Yoshida, H.; et al. Association between Serum Zinc and All-Cause Mortality in Patients Undergoing Maintenance Hemodialysis: The Osaka Dialysis Complication Study (ODCS). Nutrients 2024, 16, 3270. [Google Scholar] [CrossRef]
- Garagarza, C.; Valente, A.; Caetano, C.; Ramos, I.; Sebastiao, J.; Pinto, M.; Oliveira, T.; Ferreira, A.; Sousa Guerreiro, C. Zinc Deficient Intake in Hemodialysis Patients: A Path to a High Mortality Risk. J. Ren. Nutr. 2022, 32, 87–93. [Google Scholar] [CrossRef] [PubMed]
| Author, Year [Reference] | Number of Subjects | Age (Years) † | Elemental Zinc Dose (mg/day) | Administration Duration (Days) | Outcomes |
|---|---|---|---|---|---|
| Haddadian-Khouzani S et al., 2022 [15] | 44 | 48.1 ± 14.8 | NA (30 mg of zinc gluconate) | 84 | Improve: sleep quality and albumin Not significant: CRP and QOL |
| Hosseibi R et al., 2022 [14] | 46 | 54.1 ± 5.4 | 50 | 56 | Increase: serum zinc, BMI Decrease: hs-CRP, BUN, FBG |
| Okamoto T et al., 2019 [16] | 91 | 68 | 34 (polaprezinc) /50 (zinc acetate hydrate) | 180 | Increase: serum zinc Decrease: serum copper (zinc acetate hydrate vs. polaprezinc) |
| Escobedo-Monge et al., 2019 [17] | 48 (children) | 12.8 ± 4 | 15/30 | 365 | Increase: BMI (30 mg/day group only) |
| Tonelli et al., 2015 [19] | 150 | 62 | 25 and 50 | 90 and 180 | Not significant |
| Kobayashi et al., 2015 [21] | 70 | 69 ± 10 | 34 | 90/180/270/360 | Increase: serum zinc Decrease: serum copper, ferritin |
| El-Shazly et al., 2015 [22] | 30 | 13.2 ± 2.1 | 16.5 | 90 | Increase: serum zinc, BMI Decrease: serum leptin |
| Argani et al., 2014 [23] | 60 | (50, 60) | 90 | 60 | Increase: serum zinc, albumin, hemoglobin, BMI Decrease: serum leptin |
| Pakfetrat et al., 2013 [24] | 97 | 51.6 ± 16.8 | 50 | 43 | Increase: serum zinc Decrease: homocysteine |
| Mazani et al., 2013 [25] | 65 | 52.7 ± 12.6 | 100 | 60 | Increase: serum zinc, GSH, MDA, SOD, TAC |
| Guo and Wang, 2013 [26] | 65 | 59.7 ± 9.2 | 11 | 56 | Increase: plasma zinc, albumin, hemoglobin, hematocrit, nPNA, SOD, vitamin C, vitamin E, CD4, D19 Decrease: plasma copper, CRP, MDA INF-b, TNF-α, |
| Rahimi-Ardabili et al., 2012 [27] | 60 | 52.7 ± 12.7 | 100 | 60 | Increase: Apo-AI, HDL-C, PON |
| Roozbeh et al., 2009 [28] | 53 | 55.7 | 45 | 42 | Increase: serum zinc, TC, HDL-C, LDL-C, TG |
| Rashidi et al., 2009 [29] | 55 | 57.6 | 45 | 42 | Increase: serum zinc |
| Nava-Hernandez and Amato, 2005 [30] | 25 | 16.6 | 100 | 90 | NA |
| Matson et al., 2003 [31] | 15 | 60 (31, 76) | 45 | 42 | Not significant |
| Chevalier et al., 2002 [32] | 27 | 51.9 | 50 | 40/90/90 | Increase: serum zinc, LDL-C |
| Candan et al., 2002 [33] | 34 | 45.6 (28, 64) | 20 | 90 | Increase: serum zinc Decrease: lipid peroxidation osmotic fragility |
| Jern et al., 2000 [34] | 14 | 56.5 (23, 80) | 45 | 40/90 | Increase: serum zinc, nPNA |
| Brodersen et al., 1995 [35] | 40 | 60 | 60 | 112 | Increase: serum zinc |
| Author, Year [Reference] | Number of Subjects | Number of Deaths (Follow-Up Period) | Conclusions |
|---|---|---|---|
| Yang CY, et al., 2012 [43] | Prevalent patients on maintenance hemodialysis (n = 43) and peritoneal dialysis (n = 68) | 14 deaths (2 years) | Old age, hypoalbuminemia, and zinc deficiency were independent predictors of mortality. |
| Tonelli M, et al., 2018 [49] | Incident hemodialysis (n = 1278) | 260 deaths (2 years) | Lower level of zinc was not associated with higher risk of death. |
| Toida T, et al., 2020 [5] | Incident hemodialysis (n = 142) | 15 deaths (2.5 years) | The association between serum zinc levels and all-cause mortality was not clear after adjustments for potential confounders. |
| Knehtl M, et al., 2022 [142] | Prevalent patients on maintenance hemodialysis (n = 61) | 11 deaths (2.8 years) | No significant association of serum zinc with mortality. |
| Nakatani S, et al., 2024 [144] | Prevalent patients on Maintenance hemodialysis (n = 1662) | 468 deaths (5 years) | A lower serum zinc level was a significant factor predicting a higher risk of mortality in those with lower serum albumin. |
| Garagarza C, et al., 2022 [145] | Prevalent patients on Maintenance hemodialysis (n = 582) | 29 deaths (1 year) | Lower zinc intake below was a significant factor predicting a higher risk of mortality. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatani, S.; Morioka, T.; Morioka, F.; Mori, K.; Emoto, M. Zinc Deficiency in Chronic Kidney Disease and Hemodialysis: Insights from Basic Research to Clinical Implications. Nutrients 2025, 17, 2191. https://doi.org/10.3390/nu17132191
Nakatani S, Morioka T, Morioka F, Mori K, Emoto M. Zinc Deficiency in Chronic Kidney Disease and Hemodialysis: Insights from Basic Research to Clinical Implications. Nutrients. 2025; 17(13):2191. https://doi.org/10.3390/nu17132191
Chicago/Turabian StyleNakatani, Shinya, Tomoaki Morioka, Fumiyuki Morioka, Katsuhito Mori, and Masanori Emoto. 2025. "Zinc Deficiency in Chronic Kidney Disease and Hemodialysis: Insights from Basic Research to Clinical Implications" Nutrients 17, no. 13: 2191. https://doi.org/10.3390/nu17132191
APA StyleNakatani, S., Morioka, T., Morioka, F., Mori, K., & Emoto, M. (2025). Zinc Deficiency in Chronic Kidney Disease and Hemodialysis: Insights from Basic Research to Clinical Implications. Nutrients, 17(13), 2191. https://doi.org/10.3390/nu17132191






