Carnitine Deficiency in Chronic Kidney Disease: Pathophysiology, Clinical Implications, and Therapeutic Perspectives
Abstract
1. Introduction
2. Physiological Roles and Metabolism of Carnitine
2.1. Structure of Carnitine
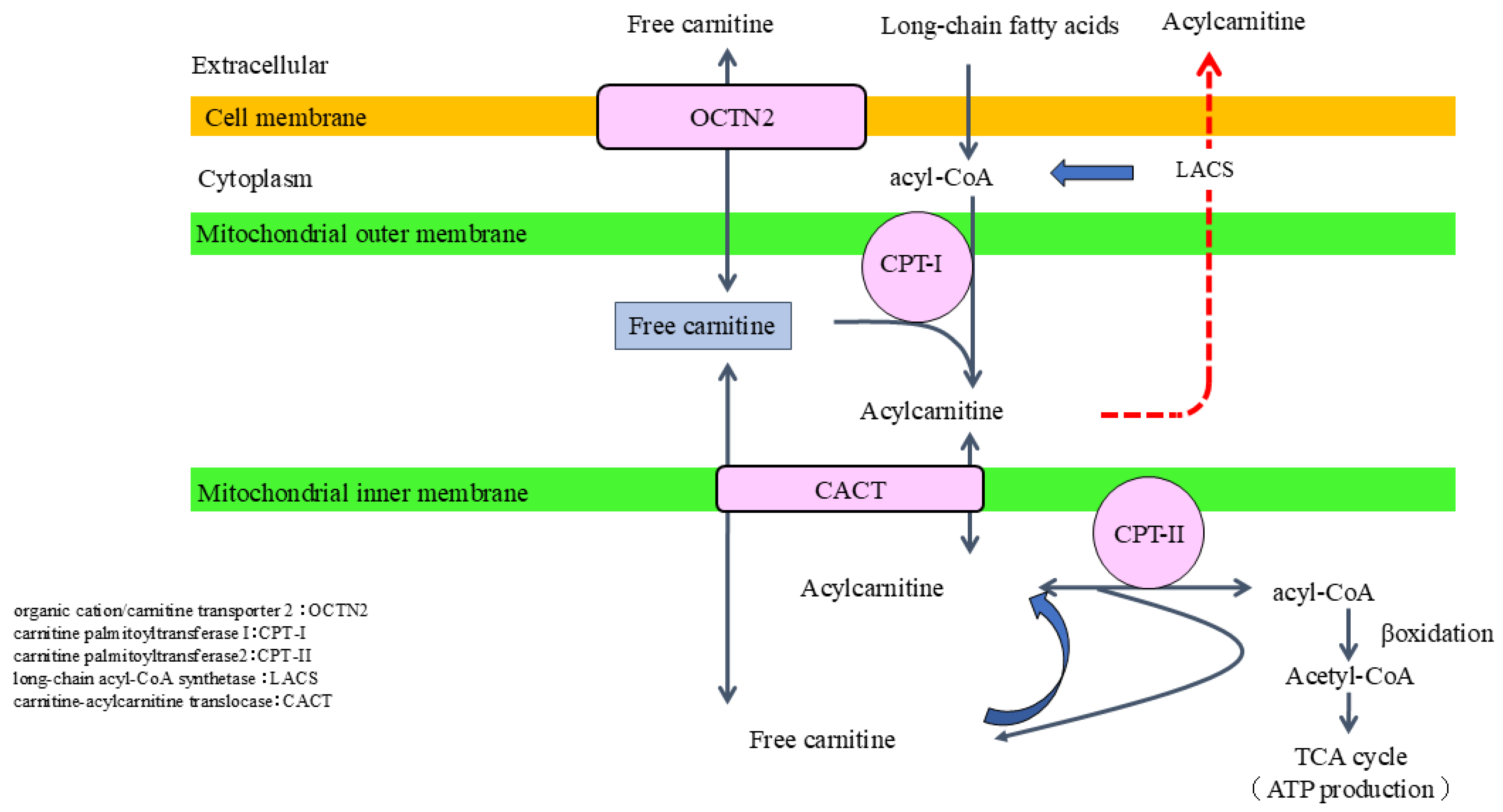
2.2. Role of Carnitine in Fatty Acid Metabolism
2.3. Auxiliary Role of Carnitine in Metabolic Regulation
2.4. Carnitine Synthesis and Metabolism
3. Impaired Carnitine Homeostasis in the Context of CKD
3.1. Impaired Endogenous Synthesis of Carnitine
3.2. Insufficient Intake and Absorption Defects
3.3. Renal Excretion and Reabsorption of Carnitine
3.4. Increased Loss of Carnitine During Hemodialysis Process
4. Clinical Implications of Carnitine Deficiency
4.1. ESA Resistance and Its Impact on Anemia Treatment
4.2. Cardiovascular Effects
4.3. Impact on Quality of Life
4.4. Impact on Clinical Parameters
4.5. Meta-Analysis of L-Carnitine Supplementation in Patients with Dialysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bremer, J. Carnitine–metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J.; Seim, H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu. Rev. Nutr. 1998, 18, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann. N. Y. Acad. Sci. 2004, 1033, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Hurot, J.M.; Cucherat, M.; Haugh, M.; Fouque, D. Effects of L-carnitine supplementation in maintenance hemodialysis patients: A systematic review. J. Am. Soc. Nephrol. 2002, 13, 708–714. [Google Scholar] [CrossRef]
- Huang, H.; Song, L.; Zhang, H.; Zhang, J.; Zhao, W. Influence of L-carnitine supplementation on serum lipid profile in hemodialysis patients: A systematic review and meta-analysis. Kidney Blood Press. Res. 2013, 38, 31–41. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Lynch, K.E.; Feldman, H.I.; Berlin, J.A.; Flory, J.; Rowan, C.G.; Brunelli, S.M. Effects of L-carnitine on dialysis-related hypotension and muscle cramps: A meta-analysis. Am. J. Kidney Dis. 2008, 52, 962–971. [Google Scholar] [CrossRef]
- Calvani, M.; Benatti, P.; Mancinelli, A.; D’Iddio, S.; Giordano, V.; Koverech, A.; Amato, A.; Brass, E.P. Carnitine replacement in end-stage renal disease and hemodialysis. Ann. N. Y. Acad. Sci. 2004, 1033, 52–66. [Google Scholar] [CrossRef]
- Zhu, Y.; Xue, C.; Ou, J.; Xie, Z.; Deng, J. Effect of L-carnitine supplementation on renal anemia in patients on hemodialysis: A meta-analysis. Int. Urol. Nephrol. 2021, 53, 2149–2158. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, T. The efficacy of L-carnitine in improving malnutrition in patients on maintenance hemodialysis: A meta-analysis. Biosci. Rep. 2020, 40, BSR20201639. [Google Scholar] [CrossRef] [PubMed]
- Chewcharat, A.; Chewcharat, P.; Liu, W.; Cellini, J.; Phipps, E.A.; Melendez Young, J.A.; Nigwekar, S.U. The effect of levocarnitine supplementation on dialysis-related hypotension: A systematic review, meta-analysis, and trial sequential analysis. PLoS ONE 2022, 17, e0271307. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G. The discovery of a vitamin role for carnitine: The first 50 years. J. Nutr. 2006, 136, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Vergani, L.; Martinuzzi, A. Clinical and biochemical aspects of carnitine deficiency and insufficiency: Transport defects and inborn errors of beta-oxidation. Crit. Rev. Clin. Lab. Sci. 1992, 29, 217–242. [Google Scholar] [CrossRef]
- Tein, I. Role of carnitine and fatty acid oxidation and its defects in infantile epilepsy. J. Child. Neurol. 2002, 17 (Suppl. S3), 3S57–3S82; discussion 3S82–3S83. [Google Scholar]
- Ramsay, R.R.; Zammit, V.A. Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol. Aspects Med. 2004, 25, 475–493. [Google Scholar] [CrossRef]
- Hoppel, C. The role of carnitine in normal and altered fatty acid metabolism. Am. J. Kidney Dis. 2003, 41, S4–S12. [Google Scholar] [CrossRef]
- Foster, D.W. The role of the carnitine system in human metabolism. Ann. N. Y. Acad. Sci. 2004, 1033, 1–16. [Google Scholar] [CrossRef]
- Stanley, C.A. Carnitine deficiency disorders in children. Ann. N. Y. Acad. Sci. 2004, 1033, 42–51. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Pochini, L.; Scalise, M.; Galluccio, M.; Amelio, L.; Indiveri, C. Reconstitution in liposomes of the functionally active human OCTN1 (SLC22A4) transporter overexpressed in Escherichia coli. Biochem. J. 2011, 439, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.A. New genetic defects in mitochondrial fatty acid oxidation and carnitine deficiency. Adv. Pediatr. 1987, 34, 59–88. [Google Scholar] [CrossRef] [PubMed]
- Borum, P.R. Carnitine. Annu. Rev. Nutr. 1983, 3, 233–259. [Google Scholar] [CrossRef]
- Hagen, T.M.; Moreau, R.; Suh, J.H.; Visioli, F. Mitochondrial decay in the aging rat heart: Evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann. N. Y. Acad. Sci. 2002, 959, 491–507. [Google Scholar] [CrossRef]
- Cao, Y.; Qu, H.J.; Li, P.; Wang, C.B.; Wang, L.X.; Han, Z.W. Single dose administration of L-carnitine improves antioxidant activities in healthy subjects. Tohoku J. Exp. Med. 2011, 224, 209–213. [Google Scholar] [CrossRef]
- Lee, B.J.; Lin, J.S.; Lin, Y.C.; Lin, P.T. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: A randomized, placebo-controlled trial. Nutr. J. 2014, 13, 79. [Google Scholar] [CrossRef]
- Arockia Rani, P.J.; Panneerselvam, C. Carnitine as a free radical scavenger in aging. Exp. Gerontol. 2001, 36, 1713–1726. [Google Scholar] [CrossRef]
- Vardiyan, R.; Ezati, D.; Anvari, M.; Ghasemi, N.; Talebi, A. Effect of L-carnitine on the expression of the apoptotic genes Bcl-2 and Bax. Clin. Exp. Reprod. Med. 2020, 47, 155–160. [Google Scholar] [CrossRef]
- Lee, Y.G.; Chou, H.C.; Chen, Y.T.; Tung, S.Y.; Ko, T.L.; Buyandelger, B.; Wen, L.L.; Juan, S.H. L-Carnitine reduces reactive oxygen species/endoplasmic reticulum stress and maintains mitochondrial function during autophagy-mediated cell apoptosis in perfluorooctanesulfonate-treated renal tubular cells. Sci. Rep. 2022, 12, 4673. [Google Scholar] [CrossRef]
- Xiang, F.; Zhang, Z.; Xie, J.; Xiong, S.; Yang, C.; Liao, D.; Xia, B.; Lin, L. Comprehensive review of the expanding roles of the carnitine pool in metabolic physiology: Beyond fatty acid oxidation. J. Transl. Med. 2025, 23, 324. [Google Scholar] [CrossRef]
- Virmani, M.A.; Cirulli, M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 2022, 23, 2717. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Black, S.M. Carnitine homeostasis, mitochondrial function, and cardiovascular disease. Drug Discov. Today Dis. Mech. 2009, 6, e31–e39. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Luan, H.; Chen, X.; Han, Y.; Wang, C. l-carnitine protects human hepatocytes from oxidative stress-induced toxicity through Akt-mediated activation of Nrf2 signaling pathway. Can. J. Physiol. Pharmacol. 2016, 94, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Mancuso, C.; Pennisi, G.; Calafato, S.; Bellia, F.; Bates, T.E.; Giuffrida Stella, A.M.; Schapira, T.; Dinkova Kostova, A.T.; et al. Cellular stress response: A novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem. Res. 2008, 33, 2444–2471. [Google Scholar] [CrossRef]
- Yang, T.; Liu, S.; Ma, H.; Lai, H.; Wang, C.; Ni, K.; Lu, Y.; Li, W.; Hu, X.; Zhou, Z.; et al. Carnitine functions as an enhancer of NRF2 to inhibit osteoclastogenesis via regulating macrophage polarization in osteoporosis. Free Radic. Biol. Med. 2024, 213, 174–189. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109 (Suppl. 1), S81–S96. [Google Scholar] [CrossRef]
- Mahfouz, R.; H El-Rewini, S.; I Ghoneim, A.; Sheta, E.; A Ali, M.; Ibrahim, S.S.A. L-Carnitine augments probenecid anti-inflammatory effect in monoiodoacetate-induced knee osteoarthritis in rats: Involvement of miRNA-373/P2X7/NLRP3/NF-κB milieu. Inflammopharmacology 2024, 32, 715–731. [Google Scholar] [CrossRef]
- Zambrano, S.; Blanca, A.J.; Ruiz-Armenta, M.V.; Miguel-Carrasco, J.L.; Arévalo, M.; Vázquez, M.J.; Mate, A.; Vázquez, C.M. L-Carnitine protects against arterial hypertension-related cardiac fibrosis through modulation of PPAR-γ expression. Biochem. Pharmacol. 2013, 85, 937–944. [Google Scholar] [CrossRef]
- Mansour, H.H.; El Kiki, S.M.; Ibrahim, A.B.; Omran, M.M. Effect of l-carnitine on cardiotoxicity and apoptosis induced by imatinib through PDGF/PPARγ/MAPK pathways. Arch. Biochem. Biophys. 2021, 704, 108866. [Google Scholar] [CrossRef]
- Taguchi, K.; Sugahara, S.; Elias, B.C.; Pabla, N.S.; Canaud, G.; Brooks, C.R. IL-22 is secreted by proximal tubule cells and regulates DNA damage response and cell death in acute kidney injury. Kidney Int. 2024, 105, 99–114. [Google Scholar] [CrossRef]
- Koohpeyma, F.; Siri, M.; Allahyari, S.; Mahmoodi, M.; Saki, F.; Dastghaib, S. The effects of L-carnitine on renal function and gene expression of caspase-9 and Bcl-2 in monosodium glutamate-induced rats. BMC Nephrol. 2021, 22, 162. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Guo, Y.; Cong, P.; Tian, Y.; Gao, X. Liver Lipidomics Analysis Revealed the Novel Ameliorative Mechanisms of L-Carnitine on High-Fat Diet-Induced NAFLD Mice. Nutrients 2023, 15, 1359. [Google Scholar] [CrossRef] [PubMed]
- Jamali-Raeufy, N.; Alizadeh, F.; Mehrabi, Z.; Mehrabi, S.; Goudarzi, M. Acetyl-L-carnitine confers neuroprotection against lipopolysaccharide (LPS) -induced neuroinflammation by targeting TLR4/NFκB, autophagy, inflammation and oxidative stress. Metab. Brain Dis. 2021, 36, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, B.; Bachmann, C.; Colombo, J.P.; Gitzelmann, R. The renal handling of carnitine in patients with selective tubulopathy and with Fanconi syndrome. Pediatr. Res. 1987, 21, 201–204. [Google Scholar] [CrossRef]
- Gahl, W.A.; Bernardini, I.; Dalakas, M.; Rizzo, W.B.; Harper, G.S.; Hoeg, J.M.; Hurko, O.; Bernar, J. Oral carnitine therapy in children with cystinosis and renal Fanconi syndrome. J. Clin. Invest. 1988, 81, 549–560. [Google Scholar] [CrossRef]
- Ito, S.; Taguchi, K.; Kodama, G.; Kubo, S.; Moriyama, T.; Yamashita, Y.; Yokota, Y.; Nakayama, Y.; Kaida, Y.; Shinohara, M.; et al. Involvement of impaired carnitine-induced fatty acid oxidation in experimental and human diabetic kidney disease. JCI Insight, 2025; in press. [Google Scholar] [CrossRef]
- Miguel, V.; Tituaña, J.; Herrero, J.I.; Herrero, L.; Serra, D.; Cuevas, P.; Barbas, C.; Puyol, D.R.; Márquez-Expósito, L.; Ruiz-Ortega, M.; et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Invest. 2021, 131, e140695. [Google Scholar] [CrossRef]
- Steiber, A.; Kerner, J.; Hoppel, C.L. Carnitine: A nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 2004, 25, 455–473. [Google Scholar] [CrossRef]
- Krähenbühl, S. Carnitine metabolism in chronic liver disease. Life Sci. 1996, 59, 1579–1599. [Google Scholar] [CrossRef]
- Hoppel, C.L.; Davis, A.T. Inter-tissue relationships in the synthesis and distribution of carnitine. Biochem. Soc. Trans. 1986, 14, 673–674. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Engel, A.G. Tissue distribution of carnitine biosynthetic enzymes in man. Biochim. Biophys. Acta 1980, 630, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.L.; Rebouche, C.J. gamma-Butyrobetaine hydroxylase activity is not rate limiting for carnitine biosynthesis in the human infant. J. Nutr. 1987, 117, 1024–1031. [Google Scholar] [CrossRef]
- Wanner, C.; Hörl, W.H. Carnitine abnormalities in patients with renal insufficiency. Pathophysiological and therapeutical aspects. Nephron 1988, 50, 89–102. [Google Scholar] [CrossRef]
- Borum, P.R.; Bennett, S.G. Carnitine as an essential nutrient. J. Am. Coll. Nutr. 1986, 5, 177–182. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Engel, A.G. Kinetic compartmental analysis of carnitine metabolism in the human carnitine deficiency syndromes. Evidence for alterations in tissue carnitine transport. J. Clin. Invest. 1984, 73, 857–867. [Google Scholar] [CrossRef]
- Crentsil, V. Mechanistic contribution of carnitine deficiency to geriatric frailty. Ageing Res. Rev. 2010, 9, 265–268. [Google Scholar] [CrossRef]
- Sawicka, A.K.; Hartmane, D.; Lipinska, P.; Wojtowicz, E.; Lysiak-Szydlowska, W.; Olek, R.A. l-Carnitine Supplementation in Older Women. A Pilot Study on Aging Skeletal Muscle Mass and Function. Nutrients 2018, 10, 255. [Google Scholar] [CrossRef]
- Borum, P.R. Carnitine in parenteral nutrition. Gastroenterology 2009, 137, S129–S134. [Google Scholar] [CrossRef]
- Schmidt-Sommerfeld, E.; Penn, D.; Wolf, H. Carnitine deficiency in premature infants receiving total parenteral nutrition: Effect of L-carnitine supplementation. J. Pediatr. 1983, 102, 931–935. [Google Scholar] [CrossRef]
- Zhou, L.T.; Lv, L.L.; Qiu, S.; Yin, Q.; Li, Z.L.; Tang, T.T.; Ni, L.H.; Feng, Y.; Wang, B.; Ma, K.L.; et al. Bioinformatics-based discovery of the urinary BBOX1 mRNA as a potential biomarker of diabetic kidney disease. J. Transl. Med. 2019, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Hu, L.; Jia, L.; Zhou, J.; Wang, T.; Kim, K.; Zhong, H.; Yao, H.; Dong, L.; Guo, L.; et al. BBOX1 restrains TBK1-mTORC1 oncogenic signaling in clear cell renal cell carcinoma. Nat. Commun. 2025, 16, 1543. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, G.; Lindstedt, S.; Nordin, I. Gamma-butyrobetaine hydroxylase in human kidney. Scand. J. Clin. Lab. Invest. 1982, 42, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.A.; Cardozo, L.F.M.F.; Anjos, J.S.; Black, A.P.; Moraes, C.; Bergman, P.; Lindholm, B.; Stenvinkel, P. Red meat intake in chronic kidney disease patients: Two sides of the coin. Nutrition 2018, 46, 26–32. [Google Scholar] [CrossRef]
- Fornasini, G.; Upton, R.N.; Evans, A.M. A pharmacokinetic model for L-carnitine in patients receiving haemodialysis. Br. J. Clin. Pharmacol. 2007, 64, 335–345. [Google Scholar] [CrossRef]
- Iorember, F.M. Malnutrition in Chronic Kidney Disease. Front. Pediatr. 2018, 6, 161. [Google Scholar] [CrossRef]
- Sguanci, M.; Ferrara, G.; Palomares, S.M.; Parozzi, M.; Godino, L.; Gazineo, D.; Anastasi, G.; Mancin, S. Dysgeusia and Chronic Kidney Disease: A Scoping Review. J. Ren. Nutr. 2024, 34, 374–390. [Google Scholar] [CrossRef]
- Rahbar Saadat, Y.; Abbasi, A.; Hejazian, S.S.; Hekmatshoar, Y.; Ardalan, M.; Farnood, F.; Zununi Vahed, S. Combating chronic kidney disease-associated cachexia: A literature review of recent therapeutic approaches. BMC Nephrol. 2025, 26, 133. [Google Scholar] [CrossRef]
- Isaka, Y. Adaptor protein is a new therapeutic target in chronic kidney disease. Kidney Int. 2017, 92, 1312–1314. [Google Scholar] [CrossRef]
- Engel, A.G.; Rebouche, C.J.; Wilson, D.M.; Glasgow, A.M.; Romshe, C.A.; Cruse, R.P. Primary systemic carnitine deficiency. II. Renal handling of carnitine. Neurology 1981, 31, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Bellinghieri, G.; Santoro, D.; Calvani, M.; Mallamace, A.; Savica, V. Carnitine and hemodialysis. Am. J. Kidney Dis. 2003, 41, S116–S122. [Google Scholar] [CrossRef] [PubMed]
- Nezu, J.; Tamai, I.; Oku, A.; Ohashi, R.; Yabuuchi, H.; Hashimoto, N.; Nikaido, H.; Sai, Y.; Koizumi, A.; Shoji, Y.; et al. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat. Genet. 1999, 21, 91–94. [Google Scholar] [CrossRef]
- Koizumi, A.; Nozaki, J.; Ohura, T.; Kayo, T.; Wada, Y.; Nezu, J.; Ohashi, R.; Tamai, I.; Shoji, Y.; Takada, G.; et al. Genetic epidemiology of the carnitine transporter OCTN2 gene in a Japanese population and phenotypic characterization in Japanese pedigrees with primary systemic carnitine deficiency. Hum. Mol. Genet. 1999, 8, 2247–2254. [Google Scholar] [CrossRef]
- Raskind, J.Y.; El-Chaar, G.M. The role of carnitine supplementation during valproic acid therapy. Ann. Pharmacother. 2000, 34, 630–638. [Google Scholar] [CrossRef]
- Opala, G.; Winter, S.; Vance, C.; Vance, H.; Hutchison, H.T.; Linn, L.S. The effect of valproic acid on plasma carnitine levels. Am. J. Dis. Child. 1991, 145, 999–1001. [Google Scholar] [CrossRef]
- Origlia, N.; Migliori, M.; Panichi, V.; Filippi, C.; Bertelli, A.; Carpi, A.; Giovannini, L. Protective effect of L-propionylcarnitine in chronic cyclosporine-a induced nephrotoxicity. Biomed. Pharmacother. 2006, 60, 77–81. [Google Scholar] [CrossRef]
- Ito, T.; Tsukahara, K.; Sato, H.; Shimizu, A.; Okamoto, I. Changes in carnitine levels through induction chemotherapy in head and neck cancer patients as a potential cause of therapy-related malaise. BMC Cancer 2021, 21, 742. [Google Scholar] [CrossRef]
- Lancaster, C.S.; Hu, C.; Franke, R.M.; Filipski, K.K.; Orwick, S.J.; Chen, Z.; Zuo, Z.; Loos, W.J.; Sparreboom, A. Cisplatin-induced downregulation of OCTN2 affects carnitine wasting. Clin. Cancer Res. 2010, 16, 4789–4799. [Google Scholar] [CrossRef]
- Evans, A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am. J. Kidney Dis. 2003, 41, S13–S26. [Google Scholar] [CrossRef]
- Guarnieri, G.; Biolo, G.; Vinci, P.; Massolino, B.; Barazzoni, R. Advances in carnitine in chronic uremia. J. Ren. Nutr. 2007, 17, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, G.; Situlin, R.; Biolo, G. Carnitine metabolism in uremia. Am. J. Kidney Dis. 2001, 38, S63–S67. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Kitsu, A.; Ibarra-Cazares, P.; Mendoza-Guevara, L.; Villasis-Keever, M.A.; Perez Andrade, M.E.; Castillo-Romero, L.; Morales-Nava, A.; Rodriguez-Leyva, F.; Sanchez-Barbosa, L. Frequency of low carnitine levels in children on dialysis. Adv. Perit. Dial. 2006, 22, 208–210. [Google Scholar] [PubMed]
- Naseri, M.; Mottaghi Moghadam Shahri, H.; Horri, M.; Esmaeeli, M.; Ghaneh Sherbaf, F.; Jahanshahi, S.; Moeenolroayaa, G.; Rasoli, Z.; Salemian, F.; Pour Hasan, M. Absolute and Relative Carnitine Deficiency in Patients on Hemodialysis and Peritoneal Dialysis. Iran. J. Kidney Dis. 2016, 10, 36–43. [Google Scholar]
- Calò, L.A.; Vertolli, U.; Davis, P.A.; Savica, V. L carnitine in hemodialysis patients. Hemodial. Int. 2012, 16, 428–434. [Google Scholar] [CrossRef]
- Akizawa, T.; Okumura, H.; Alexandre, A.F.; Fukushima, A.; Kiyabu, G.; Dorey, J. Burden of Anemia in Chronic Kidney Disease Patients in Japan: A Literature Review. Ther. Apher. Dial. 2018, 22, 444–456. [Google Scholar] [CrossRef]
- Bonomini, M.; Zammit, V.; Pusey, C.D.; De Vecchi, A.; Arduini, A. Pharmacological use of L-carnitine in uremic anemia: Has its full potential been exploited? Pharmacol. Res. 2011, 63, 157–164. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Amano, I.; Hirose, S.; Tsuruta, Y.; Hara, S.; Murata, M.; Imai, T. Effects of L-carnitine supplementation on renal anemia in poor responders to erythropoietin. Blood Purif. 2001, 19, 24–32. [Google Scholar] [CrossRef]
- Kuwasawa-Iwasaki, M.; Io, H.; Muto, M.; Ichikawa, S.; Wakabayashi, K.; Kanda, R.; Nakata, J.; Nohara, N.; Tomino, Y.; Suzuki, Y. Effects of L-Carnitine Supplementation in Patients Receiving Hemodialysis or Peritoneal Dialysis. Nutrients 2020, 12, 3371. [Google Scholar] [CrossRef]
- Kaneko, S.; Hirai, K.; Morino, J.; Minato, S.; Yanai, K.; Mutsuyoshi, Y.; Ishii, H.; Matsuyama, M.; Kitano, T.; Shindo, M.; et al. Association between carnitine deficiency and the erythropoietin resistance index in patients undergoing peritoneal dialysis: A cross-sectional observational study. Ren. Fail. 2020, 42, 146–153. [Google Scholar] [CrossRef]
- Kaneko, S.; Yanai, K.; Kitano, T.; Miyazawa, H.; Hirai, K.; Ookawara, S.; Morishita, Y. Change in Anemia by Carnitine Supplementation in Patients Undergoing Peritoneal Dialysis: A Retrospective Observational Study. Front. Med. 2021, 8, 767945. [Google Scholar] [CrossRef] [PubMed]
- Verrina, E.; Caruso, U.; Calevo, M.G.; Emma, F.; Sorino, P.; De Palo, T.; Lavoratti, G.; Turrini Dertenois, L.; Cassanello, M.; Cerone, R.; et al. Effect of carnitine supplementation on lipid profile and anemia in children on chronic dialysis. Pediatr. Nephrol. 2007, 22, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Lilien, M.R.; Duran, M.; Quak, J.M.; Frankhuisen, J.J.; Schröder, C.H. Oral L-carnitine does not decrease erythropoietin requirement in pediatric dialysis. Pediatr. Nephrol. 2000, 15, 17–20. [Google Scholar] [CrossRef]
- Pauly, D.F.; Pepine, C.J. The role of carnitine in myocardial dysfunction. Am. J. Kidney Dis. 2003, 41, S35–S43. [Google Scholar] [CrossRef]
- van der Vusse, G.J.; van Bilsen, M.; Glatz, J.F. Cardiac fatty acid uptake and transport in health and disease. Cardiovasc. Res. 2000, 45, 279–293. [Google Scholar] [CrossRef]
- Higuchi, T.; Abe, M.; Yamazaki, T.; Okawa, E.; Ando, H.; Hotta, S.; Oikawa, O.; Kikuchi, F.; Okada, K.; Soma, M. Levocarnitine Improves Cardiac Function in Hemodialysis Patients With Left Ventricular Hypertrophy: A Randomized Controlled Trial. Am. J. Kidney Dis. 2016, 67, 260–270. [Google Scholar] [CrossRef]
- Sakurabayashi, T.; Miyazaki, S.; Yuasa, Y.; Sakai, S.; Suzuki, M.; Takahashi, S.; Hirasawa, Y. L-carnitine supplementation decreases the left ventricular mass in patients undergoing hemodialysis. Circ. J. 2008, 72, 926–931. [Google Scholar] [CrossRef]
- Kazmi, W.H.; Obrador, G.T.; Sternberg, M.; Lindberg, J.; Schreiber, B.; Lewis, V.; Pereira, B.J. Carnitine therapy is associated with decreased hospital utilization among hemodialysis patients. Am. J. Nephrol. 2005, 25, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Abe, M.; Yamazaki, T.; Mizuno, M.; Okawa, E.; Ando, H.; Oikawa, O.; Okada, K.; Kikuchi, F.; Soma, M. Effects of levocarnitine on brachial-ankle pulse wave velocity in hemodialysis patients: A randomized controlled trial. Nutrients 2014, 6, 5992–6004. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Miller, M.J.; Bostwick, B.L.; Kennedy, A.D.; Donti, T.R.; Sun, Q.; Sutton, V.R.; Elsea, S.H. Chronic Oral L-Carnitine Supplementation Drives Marked Plasma TMAO Elevations in Patients with Organic Acidemias Despite Dietary Meat Restrictions. JIMD Rep. 2016, 30, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhang, B.; Liu, J.; Li, K.; Jia, J.; Fan, F.; Jiang, J.; Wang, X.; Zhang, Y. Relationship Between Level of Trimethylamine Oxide and the Risk of Recurrent Cardiovascular Events in Patients with Acute Myocardial Infarction. Nutrients 2025, 17, 1664. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Yamagishi, S.; Sakai, K.; Kaida, Y.; Yokoro, M.; Ueda, S.; Wada, Y.; Takeuchi, M.; Shimizu, M.; Yamazaki, H.; et al. Oral L-carnitine supplementation increases trimethylamine-N-oxide but reduces markers of vascular injury in hemodialysis patients. J. Cardiovasc. Pharmacol. 2015, 65, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Takashima, H.; Maruyama, T.; Abe, M. Significance of Levocarnitine Treatment in Dialysis Patients. Nutrients 2021, 13, 1219. [Google Scholar] [CrossRef]
- Siami, G.; Clinton, M.E.; Mrak, R.; Griffis, J.; Stone, W. Evaluation of the effect of intravenous L-carnitine therapy on function, structure and fatty acid metabolism of skeletal muscle in patients receiving chronic hemodialysis. Nephron 1991, 57, 306–313. [Google Scholar] [CrossRef]
- Ahmad, S.; Robertson, H.T.; Golper, T.A.; Wolfson, M.; Kurtin, P.; Katz, L.A.; Hirschberg, R.; Nicora, R.; Ashbrook, D.W.; Kopple, J.D. Multicenter trial of L-carnitine in maintenance hemodialysis patients. II. Clinical and biochemical effects. Kidney Int. 1990, 38, 912–918. [Google Scholar] [CrossRef]
- Maruyama, T.; Maruyama, N.; Higuchi, T.; Nagura, C.; Takashima, H.; Kitai, M.; Utsunomiya, K.; Tei, R.; Furukawa, T.; Yamazaki, T.; et al. Efficacy of L-carnitine supplementation for improving lean body mass and physical function in patients on hemodialysis: A randomized controlled trial. Eur. J. Clin. Nutr. 2019, 73, 293–301. [Google Scholar] [CrossRef]
- Yano, J.; Kaida, Y.; Maeda, T.; Hashida, R.; Tonan, T.; Nagata, S.; Hazama, T.; Nakayama, Y.; Ito, S.; Kurokawa, Y.; et al. l-carnitine supplementation vs cycle ergometer exercise for physical activity and muscle status in hemodialysis patients: A randomized clinical trial. Ther. Apher. Dial. 2021, 25, 304–313. [Google Scholar] [CrossRef]
- Ibarra-Sifuentes, H.R.; Del Cueto-Aguilera, Á.; Gallegos-Arguijo, D.A.; Castillo-Torres, S.A.; Vera-Pineda, R.; Martínez-Granados, R.J.; Atilano-Díaz, A.; Cuellar-Monterrubio, J.E.; Pezina-Cantú, C.O.; Martínez-Guevara, E.J.; et al. Levocarnitine Decreases Intradialytic Hypotension Episodes: A Randomized Controlled Trial. Ther. Apher. Dial. 2017, 21, 459–464. [Google Scholar] [CrossRef]
- Rathod, R.; Baig, M.S.; Khandelwal, P.N.; Kulkarni, S.G.; Gade, P.R.; Siddiqui, S. Results of a single blind, randomized, placebo-controlled clinical trial to study the effect of intravenous L-carnitine supplementation on health-related quality of life in Indian patients on maintenance hemodialysis. Indian J. Med. Sci. 2006, 60, 143–153. [Google Scholar]
- Sakurauchi, Y.; Matsumoto, Y.; Shinzato, T.; Takai, I.; Nakamura, Y.; Sato, M.; Nakai, S.; Miwa, M.; Morita, H.; Miwa, T.; et al. Effects of L-carnitine supplementation on muscular symptoms in hemodialyzed patients. Am. J. Kidney Dis. 1998, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Fukami, K.; Yamagishi, S.; Kaida, Y.; Adachi, T.; Ando, R.; Manabe, R.; Otsuka, A.; Sugi, K.; Ueda, S.; et al. Evidence for a positive association between serum carnitine and free testosterone levels in uremic men with hemodialysis. Rejuvenation Res. 2013, 16, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Sloan, R.S.; Kastan, B.; Rice, S.I.; Sallee, C.W.; Yuenger, N.J.; Smith, B.; Ward, R.A.; Brier, M.E.; Golper, T.A. Quality of life during and between hemodialysis treatments: Role of L-carnitine supplementation. Am. J. Kidney Dis. 1998, 32, 265–272. [Google Scholar] [CrossRef]
- Chen, Y.; Abbate, M.; Tang, L.; Cai, G.; Gong, Z.; Wei, R.; Zhou, J.; Chen, X. L-Carnitine supplementation for adults with end-stage kidney disease requiring maintenance hemodialysis: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 99, 408–422. [Google Scholar] [CrossRef]
- Naini, A.E.; Sadeghi, M.; Mortazavi, M.; Moghadasi, M.; Harandi, A.A. Oral carnitine supplementation for dyslipidemia in chronic hemodialysis patients. Saudi J. Kidney Dis. Transpl. 2012, 23, 484–488. [Google Scholar]
- Hamedi-Kalajahi, F.; Zarezadeh, M.; Mojtahedi, S.Y.; Shabbidar, S.; Fahimi, D.; Imani, H. Effect of L-carnitine supplementation on lipid profile and apolipoproteins in children on hemodialysis: A randomized placebo-controlled clinical trial. Pediatr. Nephrol. 2021, 36, 3741–3747. [Google Scholar] [CrossRef]
- Duranay, M.; Akay, H.; Yilmaz, F.M.; Senes, M.; Tekeli, N.; Yücel, D. Effects of L-carnitine infusions on inflammatory and nutritional markers in haemodialysis patients. Nephrol. Dial. Transplant. 2006, 21, 3211–3214. [Google Scholar] [CrossRef]
- Shakeri, A.; Tabibi, H.; Hedayati, M. Effects of L-carnitine supplement on serum inflammatory cytokines, C-reactive protein, lipoprotein (a), and oxidative stress in hemodialysis patients with Lp (a) hyperlipoproteinemia. Hemodial. Int. 2010, 14, 498–504. [Google Scholar] [CrossRef]
- Suchitra, M.M.; Ashalatha, V.L.; Sailaja, E.; Rao, A.M.; Reddy, V.S.; Bitla, A.R.; Sivakumar, V.; Rao, P.V. The effect of L-carnitine supplementation on lipid parameters, inflammatory and nutritional markers in maintenance hemodialysis patients. Saudi J. Kidney Dis. Transpl. 2011, 22, 1155–1159. [Google Scholar]
- Fukami, K.; Yamagishi, S.; Sakai, K.; Kaida, Y.; Adachi, T.; Ando, R.; Okuda, S. Potential inhibitory effects of L-carnitine supplementation on tissue advanced glycation end products in patients with hemodialysis. Rejuvenation Res. 2013, 16, 460–466. [Google Scholar] [CrossRef]
- Tashiro, K.; Kaida, Y.; Yamagishi, S.I.; Tanaka, H.; Yokoro, M.; Yano, J.; Sakai, K.; Kurokawa, Y.; Taguchi, K.; Nakayama, Y.; et al. L-Carnitine Supplementation Improves Self-Rating Depression Scale Scores in Uremic Male Patients Undergoing Hemodialysis. Lett. Drug Des. Discov. 2017, 14, 737–742. [Google Scholar] [CrossRef]
- Abrahamsen, R.K.; Lund, A.M.; Rasmussen, J. Patients with primary carnitine deficiency treated with L-carnitine are alive and doing well-A 10-year follow-up in the Faroe Islands. JIMD Rep. 2023, 64, 453–459. [Google Scholar] [CrossRef]

| Author | Objective | Assessed Effects | Key Findings | Conclusion |
|---|---|---|---|---|
| Chewcharat et al., 2022 [12] | To evaluate the effect of oral or IV levocarnitine on dialysis-related hypotension and muscle cramps in HD patients | Dialysis-related hypotension, muscle cramps | ↓ Hypotension (OR = 0.26); ↓ Muscle cramps (OR = 0.22); Oral route > IV; Effective dose > 4200 mg/week, duration ≥ 12 weeks | Oral L-carnitine ≥ 12 weeks reduces dialysis-related hypotension; may reduce muscle cramps. |
| Zhu et al., 2021 [10] | To evaluate the effect of L-carnitine supplementation on renal anemia in HD patients | Plasma L-carnitine levels, ESA dose, ERI, Hb, Ht | ↑ Plasma free L-carnitine; ↓ ESA dose and ERI; No significant change in Hb and Ht | L-carnitine improves ESA responsiveness and reduces ESA dose but does not raise Hb or Ht. |
| Zhou et al., 2020 [11] | To evaluate the efficacy of L-carnitine in improving malnutrition in HD patients | Albumin, prealbumin, total protein, transferrin | ↑ Albumin (SMD: 2.51); ↑ Prealbumin (MD: 70.86); ↑ Total protein (MD: 3.83); ↑ Transferrin (MD: 0.35); all p < 0.001 | L-carnitine significantly improves nutritional biomarkers; it may be a beneficial treatment option for malnutrition |
| Chen et al., 2014 [114] | To reevaluate the effects of L-carnitine in adults with ESKD on maintenance HD | LDL, CRP, triglycerides, cholesterol, HDL, Hb, Ht, albumin, erythropoietin dose | L-carnitine significantly decreased LDL (MD: −5.82 mg/dL) and CRP (MD: −3.65 mg/L). No significant changes in triglycerides, cholesterol, HDL, hemoglobin, hematocrit, albumin, or erythropoietin dose | The study confirmed a significant reduction in CRP and LDL with L-carnitine, although only the CRP reduction may be clinically meaningful. No effect on anemia parameters or erythropoietin requirement was found. |
| Huang et al., 2013 [5] | To assess the effect of L-carnitine supplementation on serum lipid profile in HD patients | Total cholesterol, HDL, LDL, VLDL, triglycerides | No significant effects on total cholesterol (SMD −0.11), HDL (SMD 0.01), VLDL (SMD 0.54), or triglycerides (SMD −0.12); significant decrease in LDL (SMD −0.29; 95% CI −0.53 to −0.06) | L-carnitine significantly reduced LDL-cholesterol levels in HD patients, especially in those receiving intravenous administration and with longer treatment duration. No effect was observed on other lipid parameters. |
| Lynch et al., 2008 [8] | To assess the effects of L-carnitine supplementation on dialysis-related hypotension and muscle cramps in HD patients | Intradialytic muscle cramps and hypotension | L-carnitine reduced the odds of muscle cramps (OR: 0.30; 95% CI: 0.09–1.00; p = 0.05). No significant effect on hypotension (OR: 0.28; 95% CI: 0.04–2.23; p = 0.2) | Evidence suggests a potential benefit for muscle cramping, but not confirmed. No clear benefit for hypotension. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaida, Y.; Taguchi, K.; Fukami, K. Carnitine Deficiency in Chronic Kidney Disease: Pathophysiology, Clinical Implications, and Therapeutic Perspectives. Nutrients 2025, 17, 2084. https://doi.org/10.3390/nu17132084
Kaida Y, Taguchi K, Fukami K. Carnitine Deficiency in Chronic Kidney Disease: Pathophysiology, Clinical Implications, and Therapeutic Perspectives. Nutrients. 2025; 17(13):2084. https://doi.org/10.3390/nu17132084
Chicago/Turabian StyleKaida, Yusuke, Kensei Taguchi, and Kei Fukami. 2025. "Carnitine Deficiency in Chronic Kidney Disease: Pathophysiology, Clinical Implications, and Therapeutic Perspectives" Nutrients 17, no. 13: 2084. https://doi.org/10.3390/nu17132084
APA StyleKaida, Y., Taguchi, K., & Fukami, K. (2025). Carnitine Deficiency in Chronic Kidney Disease: Pathophysiology, Clinical Implications, and Therapeutic Perspectives. Nutrients, 17(13), 2084. https://doi.org/10.3390/nu17132084






