The Controversial Issue of Hypervitaminosis B12 as Prognostic Factor of Mortality: Global Lessons from a Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Information Sources and Search Strategy
2.4. Selection Process
2.5. Data Extraction
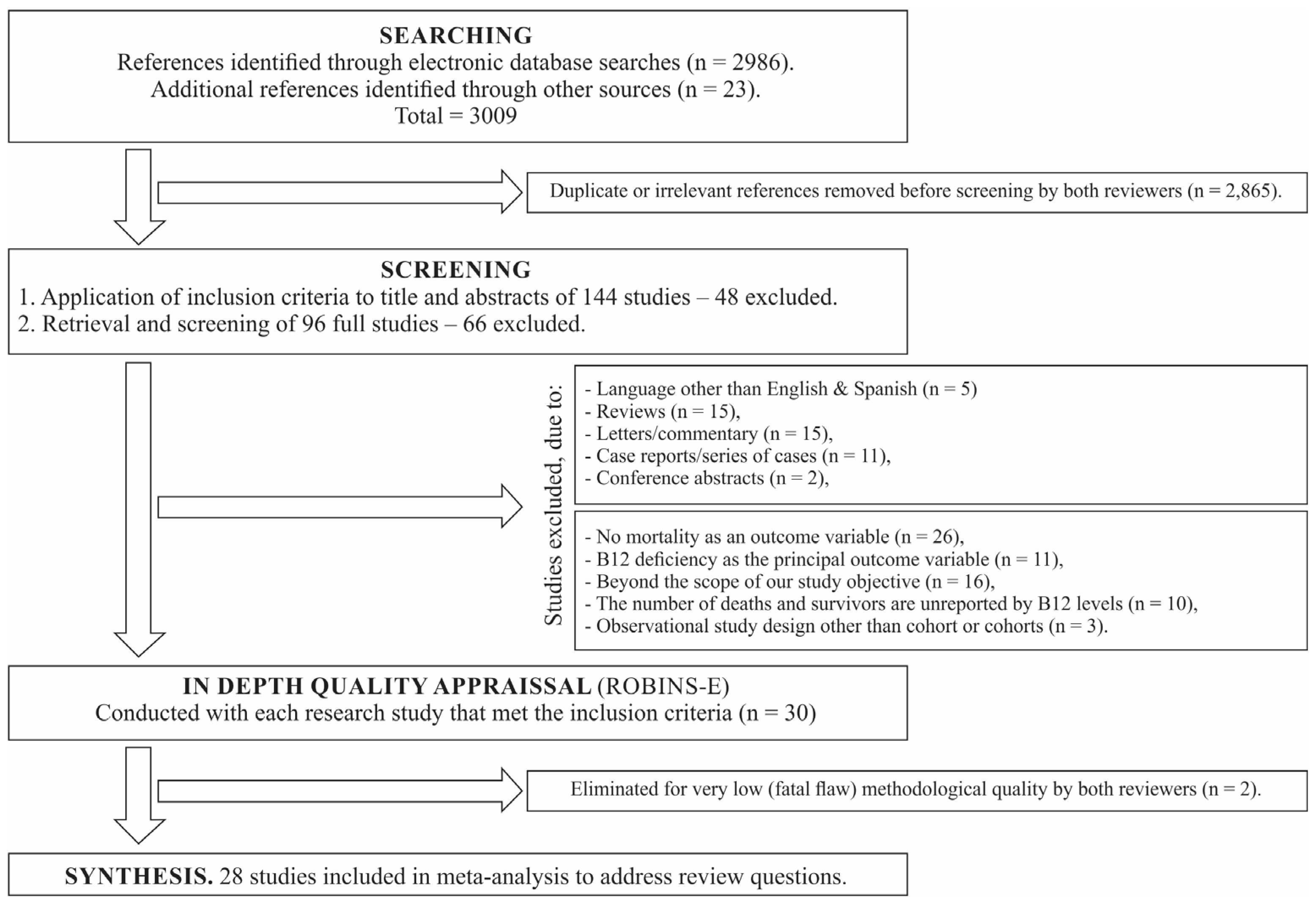
2.6. Search Outcome
2.7. Quality Assessment
2.8. Statistical Analysis
3. Results
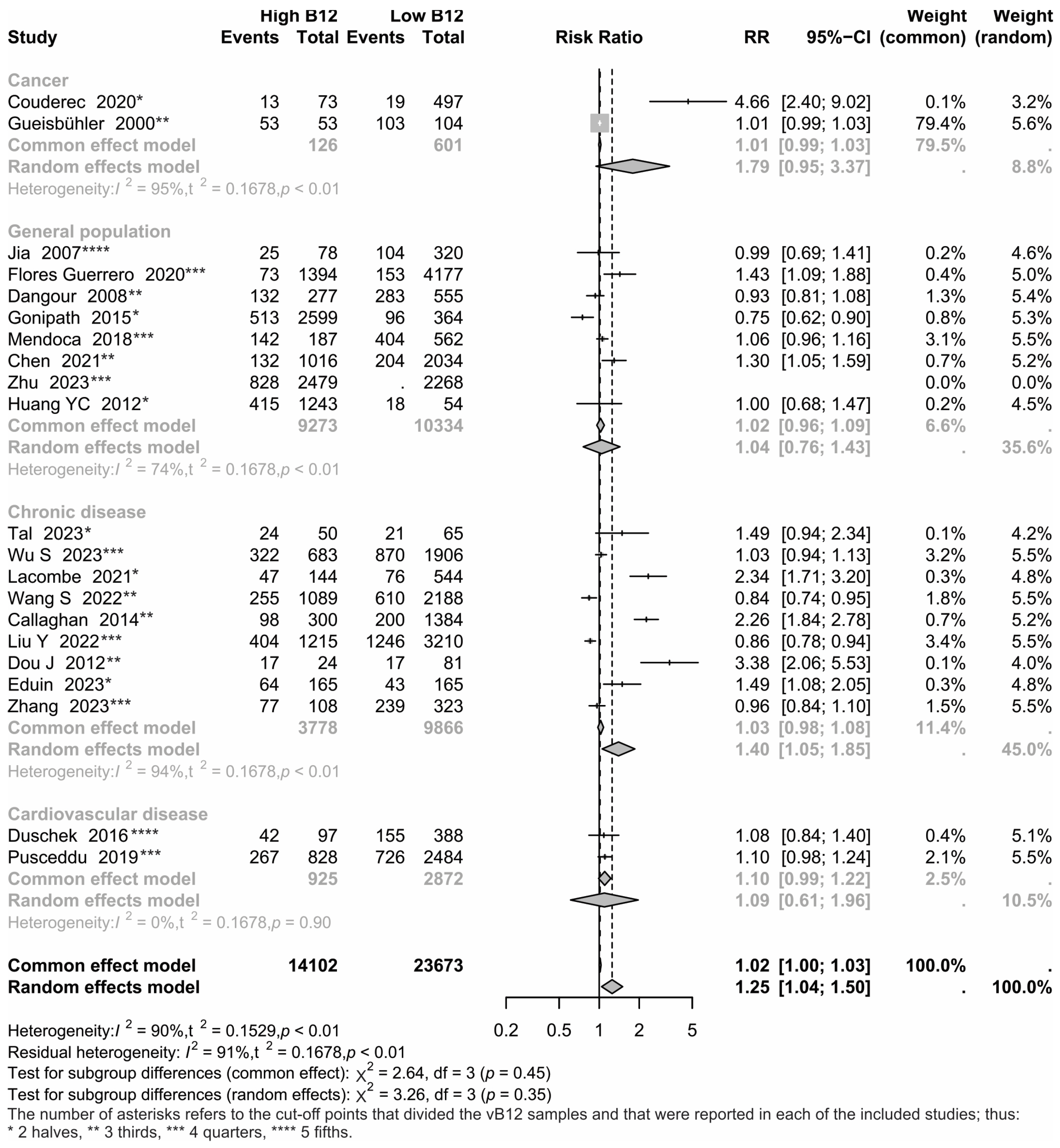
3.1. Characteristics of Included Studies
3.2. Means Difference
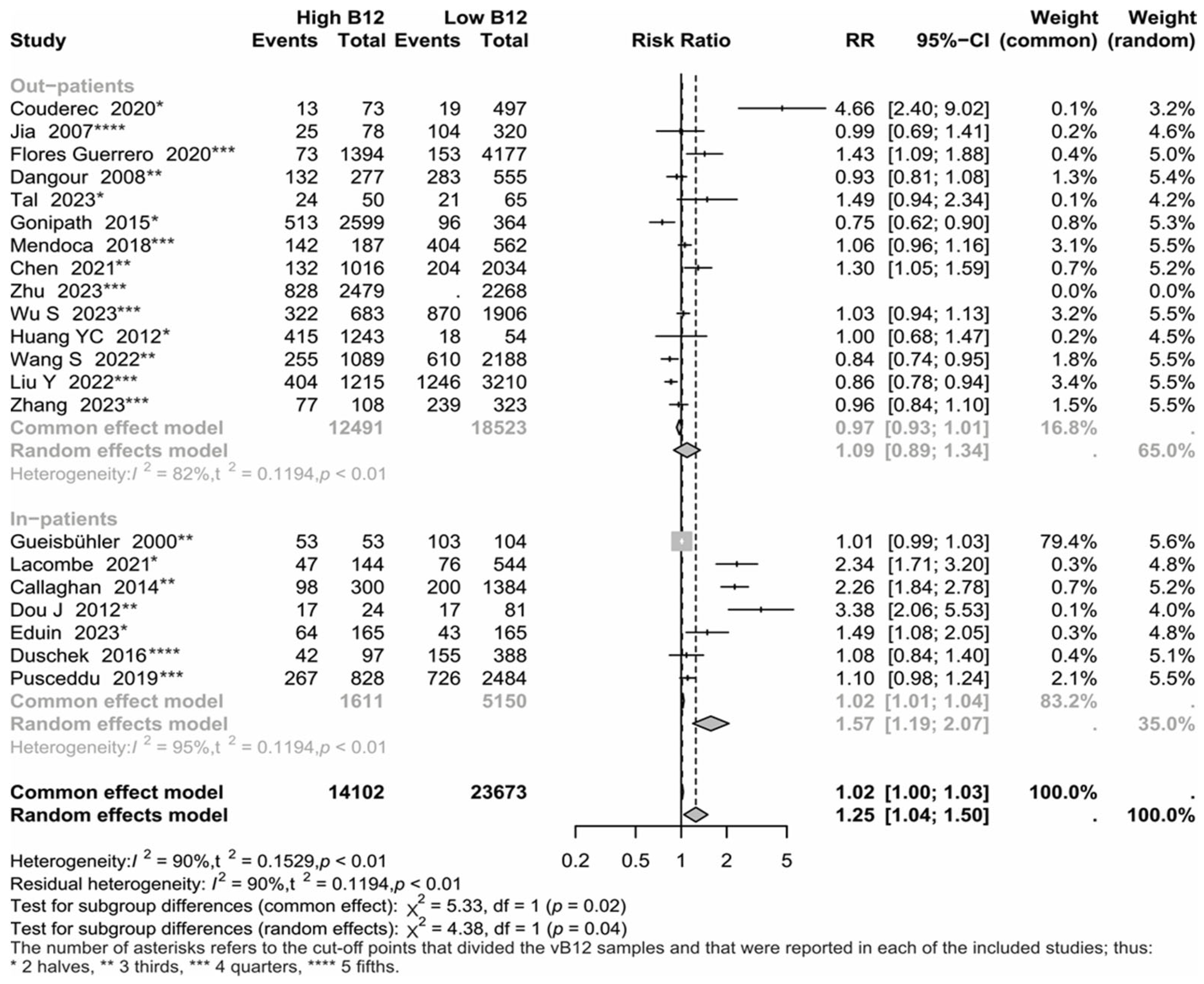
3.3. Group Analyses
3.4. Meta-Regression
3.5. Sensitivity Analyses
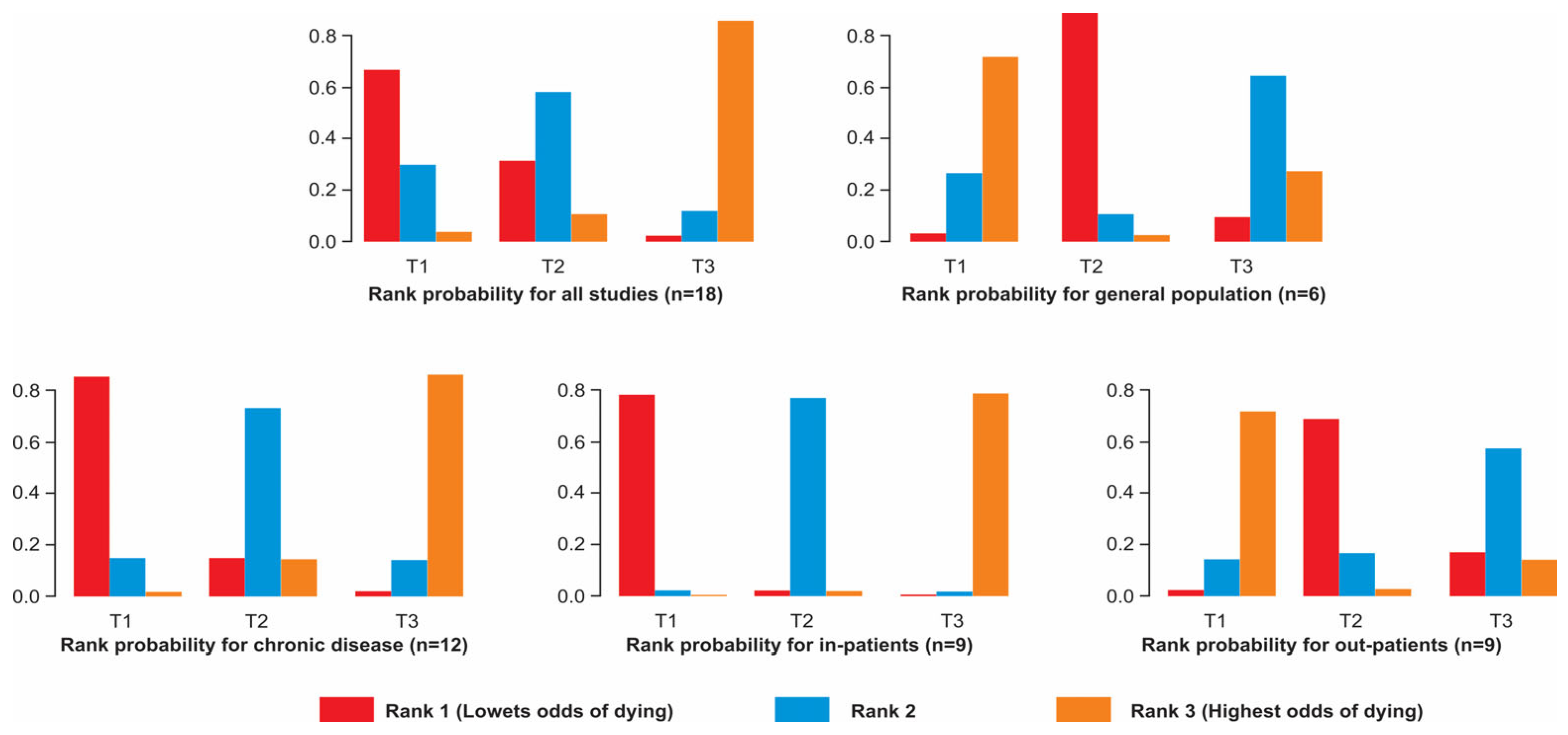
3.6. Networks
4. Discussion
Comparison with Earlier Reviews
5. Limitations and Strengths
6. What Next?
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halczuk, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T.; Karmańska, A.; Cieślak, M. Vitamin B12—Multifaceted in vivo functions and in vitro applications. Nutrients 2023, 15, 2734. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Kus, F.; Cysewski, D.; Smolenski, R.T.; Tomczyk, M. Vitamin B12 metabolism: A network of multi-protein mediated processes. Int. J. Mol. Sci. 2024, 25, 8021. [Google Scholar] [CrossRef]
- Couderc, A.-L.; Puchades, E.; Villani, P.; Arcani, R.; Farnault, L.; Daumas, A.; Courcier, A.; Greillier, L.; Barlesi, F.; Duffaud, F.; et al. High serum vitamin B12 levels associated with c-reactive protein in older patients with cancer. Oncol. 2020, 25, e1980–e1989. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Xu, W.; Ye, B.; Zhang, Y.; Mao, W. Serum vitamin B12 levels as indicators of disease severity and mortality of patients with acute-on-chronic liver failure. Clin. Chim. Acta 2012, 413, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Duschek, N.; Basic, J.; Falkensammer, J.; Taher, F.; Assadian, A. B-vitamin serum concentrations predicting long-term overall and stroke-free survival after carotid endarterectomy. J. Stroke Cerebrovasc. Dis. 2016, 25, 1235–1243. [Google Scholar] [CrossRef]
- Eduin, B.; Roubille, C.; Badiou, S.; Cristol, J.P.; Fesler, P. Association between elevated plasma vitamin B12 and short-term mortality in elderly patients hospitalized in an internal medicine unit. Int. J. Clin. Pr. 2023, 2023, 1–7. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Minovic, I.; Groothof, D.; Gruppen, E.G.; Riphagen, I.J.; Kootstra-Ros, J.; Kobold, A.M.; Hak, E.; Navis, G.; Gansevoort, R.T.; et al. Association of plasma concentration of vitamin B12 with all-cause mortality in the general population in the Netherlands. JAMA Netw. Open 2020, 3, e1919274. [Google Scholar] [CrossRef]
- Geissbühler, P.; Mermillod, B.; Rapin, C.-H. Elevated serum vitamin B12 levels associated with CRP as a predictive factor of mortality in palliative care cancer patients: A prospective study over five years. J. Pain Symptom Manag. 2000, 20, 93–103. [Google Scholar] [CrossRef]
- Lacombe, V.; Chabrun, F.; Lacout, C.; Ghali, A.; Capitain, O.; Patsouris, A.; Lavigne, C.; Urbanski, G. Persistent elevation of plasma vitamin B12 is strongly associated with solid cancer. Sci. Rep. 2021, 11, 13361. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, T.; Wan, Z.; Lu, Q.; Zhang, X.; Qiu, Z.; Li, L.; Zhu, K.; Liu, L.; Pan, A.; et al. Associations of serum folate and vitamin b12 levels with cardiovascular disease mortality among patients with type 2 diabetes. JAMA Netw. Open 2022, 5, e2146124. [Google Scholar] [CrossRef]
- Mendonça, N.; Jagger, C.; Granic, A.; Martin-Ruiz, C.; Mathers, J.C.; Seal, C.J.; Hill, T.R. Elevated Total Homocysteine in All Participants and Plasma Vitamin B12 Concentrations in Women Are Associated With All-Cause and Cardiovascular Mortality in the Very Old: The Newcastle 85+ Study. J. Gerontol. Ser. A 2018, 73, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Sviri, S.; Khalaila, R.; Daher, S.; Bayya, A.; Linton, D.; Stav, I.; van Heerden, P. Increased vitamin B12 levels are associated with mortality in critically ill medical patients. Clin. Nutr. 2012, 31, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tal, S. Mortality in the oldest-old adults after discharge from acute geriatric ward. Gerontol. Geriatr. Med. 2023, 9, 23337214231156300. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, G.; Navarrete, C.; Oñate, A.; Schmidt, B.; Fuentes, R.; Espejo, E.; Enos, D.; Fernandez-Bussy, I.; Labarca, G. Asociación entre niveles de vitamina B-12 y mortalidad en pacientes hospitalizados adultos mayores. Rev. Medica De Chile 2020, 148, 46–53. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.R.; Heiner-Fokkema, M.R.; Green, R.; Gans, R.O.B. Relationship between serum B12 concentrations and mortality: Experience in NHANES. BMC Med. 2020, 18, 307. [Google Scholar] [CrossRef]
- Zeitlin, A.; Frishman, W.H.; Chang, C.J. The association of vitamin b 12 and folate blood levels with mortality and cardiovascular morbidity incidence in the old old: The Bronx aging study. Am. J. Ther. 1997, 4, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tang, Y.; Cheang, I.; Gao, R.; Liao, S.; Yao, W.; Zhou, Y.; Zhang, H.; Li, X. Nonlinear associations of serum cobalamin with risk of all-cause and cardiovascular mortality in hypertensive adults. Hypertens. Res. 2023, 46, 1276–1286. [Google Scholar] [CrossRef]
- Argan, O.; Ural, D.; Karauzum, K.; Bozyel, S.; Aktaş, M.; Karauzum, I.Y.; Kozdag, G.; Agir, A.A. Elevated levels of vitamin B12 in chronic stable heart failure: A marker for subclinical liver damage and impaired prognosis. Ther. Clin. Risk Manag. 2018, 14, 1067–1073. [Google Scholar] [CrossRef]
- Callaghan, F.M.; Leishear, K.; Abhyankar, S.; Demner-Fushman, D.; McDonald, C.J. High vitamin B12 levels are not associated with increased mortality risk for ICU patients after adjusting for liver function: A cohort study. ESPEN J. 2014, 9, e76–e83. [Google Scholar] [CrossRef]
- Chen, S.; Honda, T.; Hata, J.; Sakata, S.; Furuta, Y.; Yoshida, D.; Shibata, M.; Ohara, T.; Hirakawa, Y.; Oishi, E.; et al. High serum folate concentrations are associated with decreased risk of mortality among Japanese adults. J. Nutr. 2021, 151, 657–665. [Google Scholar] [CrossRef]
- Dangour, A.D.; Breeze, E.; Clarke, R.; Shetty, P.S.; Uauy, R.; Fletcher, A.E. Plasma homocysteine, but not folate or vitamin b-12, predicts mortality in older people in the United Kingdom. J. Nutr. 2008, 138, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Gamarra-Morales, Y.; Molina-López, J.; Herrera-Quintana, L.; Vázquez-Lorente, H.; Planells, E. Folic acid and vitamin B12 as biomarkers of morbidity and mortality in patients with septic shock. Nutr. Hosp. 2022, 39, 247–255. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Huerta, J.M.; Fernández, S.; Patterson, Á.M.; Lasheras, C. Homocysteine increases the risk of mortality in elderly individuals. Br. J. Nutr. 2007, 97, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; Flood, V.M.; Rochtchina, E.; Thiagalingam, A.; Mitchell, P. Serum homocysteine and folate but not vitamin B12 are predictors of CHD mortality in older adults. Eur. J. Prev. Cardiol. 2015, 19, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Lee, M.-S.; Wahlqvist, M.L. Prediction of all-cause mortality by B group vitamin status in the elderly. Clin. Nutr. 2012, 31, 191–198. [Google Scholar] [CrossRef]
- Jia, X.; Aucott, L.S.; McNeill, G. Nutritional status and subsequent all-cause mortality in men and women aged 75 years or over living in the community. Br. J. Nutr. 2007, 98, 593–599. [Google Scholar] [CrossRef]
- Pusceddu, I.; Herrmann, W.; Kleber, M.E.; Scharnagl, H.; März, W.; Herrmann, M. Telomere length, vitamin B12 and mortality in persons undergoing coronary angiography: The Ludwigshafen risk and cardiovascular health study. Aging 2019, 11, 7083–7097. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Wan, X.; Guo, J.; Zhang, Y.; Tian, M.; Fang, S.; Yu, B. Cobalamin intake and related biomarkers: Examining associations with mortality risk among adults with type 2 diabetes in NHANES. Diabetes Care 2022, 45, 276–284. [Google Scholar] [CrossRef]
- Wu, S.; Chang, W.; Xie, Z.; Yao, B.; Wang, X.; Yang, C. Association of serum vitamin B12 and circulating methylmalonic acid levels with all-cause and cardiovascular disease mortality among individuals with chronic kidney disease. Nutrients 2023, 15, 2980. [Google Scholar] [CrossRef]
- Zhang, P.; Xie, X.; Zhang, Y. Associations between homocysteine, vitamin B12, and folate and the risk of all-cause mortality in American adults with stroke. Front. Nutr. 2023, 10, 1279207. [Google Scholar] [CrossRef]
- Liu, K.; Yang, Z.; Lu, X.; Zheng, B.; Wu, S.; Kang, J.; Sun, S.; Zhao, J. The origin of vitamin B12 levels and risk of all-cause, cardiovascular and cancer specific mortality: A systematic review and dose-response meta-analysis. Arch. Gerontol. Geriatr. 2024, 117, 105230. [Google Scholar] [CrossRef] [PubMed]
- Amado-Garzon, S.B.; Molina-Pimienta, L.; Vejarano-Pombo, A.; Vélez-Bonilla, M.; Moreno-Chaparro, J.; Buitrago-Lopez, A. Elevated Vitamin B12, risk of cancer, and mortality: A systematic review. Cancer Investig. 2024, 42, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Booth, A. Formulating Answerable Questions. In Evidence Based Practice for Information Professionals. A Handbook; Booth, A., Brice, A., Eds.; Facet: London, UK, 2004; pp. 61–70. [Google Scholar]
- National Institute for Health and Clinical Excellence. Methods for the Development of NICE Public Health Guidance; National Institute for Health and Clinical Excellence: London, UK, 2009. [Google Scholar]
- Higgins, J.P.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Lemeris, C.; Akl, E.A.; Bateson, T.F.; Berkman, N.D.; et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef]
- Schwarzer, G.C.J.R.R.G. Meta-Analysis with R.; Springer: Cham, Switzerland, 2015; pp. 187–216. [Google Scholar] [CrossRef]
- Chen, D.G.; Peace, K.E. Applied Meta-Analysis with R and Stata, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- van Valkenhoef, G.; Lu, G.; de Brock, B.; Hillege, H.; Ades, A.E.; Welton, N.J. Automating network meta-analysis. Res. Synth. Methods 2012, 3, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Ramspek, C.L.; Steyerberg, E.W.; Riley, R.D.; Rosendaal, F.R.; Dekkers, O.M.; Dekker, F.W.; van Diepen, M. Prediction or causality? A scoping review of their conflation within current observational research. Eur. J. Epidemiol. 2021, 36, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Madure, M.; Greenland, S. Tests for trend and dose response: Misinterpretations and alternatives. Am. J. Epidemiol. 1992, 135, 96–104. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Estimate | S.E. | Z-Value | p-Value | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Intercept | 0.03 | 0.12 | 0.27 | 0.79 | −0.2 | 0.26 |
| Chronic diseases | −0.07 | 0.15 | −0.44 | 0.66 | −0.35 | 0.22 |
| Elderly population | 0.01 | 0.12 | 0.10 | 0.92 | −0.23 | 0.26 |
| In hospital | 0.27 | 0.15 | 1.77 | 0.08 | −0.03 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez-Martínez, E.; Márquez-González, H.; Ramírez-Aldana, R.; Bedolla, M. The Controversial Issue of Hypervitaminosis B12 as Prognostic Factor of Mortality: Global Lessons from a Systematic Review and Meta-Analysis. Nutrients 2025, 17, 2184. https://doi.org/10.3390/nu17132184
Valdez-Martínez E, Márquez-González H, Ramírez-Aldana R, Bedolla M. The Controversial Issue of Hypervitaminosis B12 as Prognostic Factor of Mortality: Global Lessons from a Systematic Review and Meta-Analysis. Nutrients. 2025; 17(13):2184. https://doi.org/10.3390/nu17132184
Chicago/Turabian StyleValdez-Martínez, Edith, Horacio Márquez-González, Ricardo Ramírez-Aldana, and Miguel Bedolla. 2025. "The Controversial Issue of Hypervitaminosis B12 as Prognostic Factor of Mortality: Global Lessons from a Systematic Review and Meta-Analysis" Nutrients 17, no. 13: 2184. https://doi.org/10.3390/nu17132184
APA StyleValdez-Martínez, E., Márquez-González, H., Ramírez-Aldana, R., & Bedolla, M. (2025). The Controversial Issue of Hypervitaminosis B12 as Prognostic Factor of Mortality: Global Lessons from a Systematic Review and Meta-Analysis. Nutrients, 17(13), 2184. https://doi.org/10.3390/nu17132184






