Twelve-Week Curcumin Supplementation Improves Glucose Homeostasis and Gut Health in Prediabetic Older Adults: A Pilot, Double-Blind, Placebo-Controlled Trial
Highlights
- Curcumin (80 mg/day for 12 weeks) significantly reduces HbA1c in older adults (aged 60+) with prediabetes.
- Curcumin modestly enriches beneficial gut bacteria (e.g., Agathobacter, Bacteroides, and Roseburia).
- This study supports curcumin’s potential as a dietary bioactive for promoting metabolic health in aging populations.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
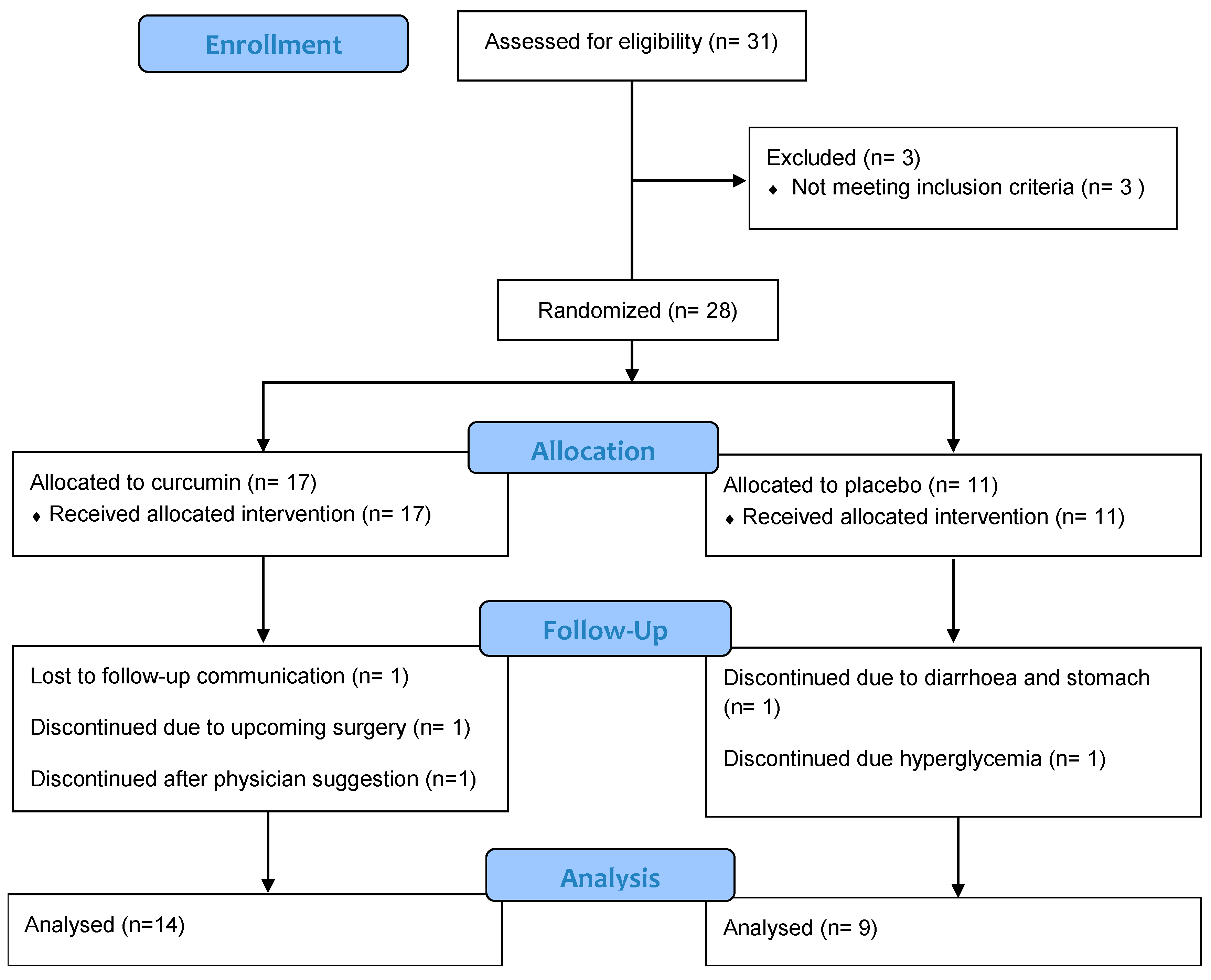
2.2. Sample Size, Randomization, and Allocation
2.3. Data Collection and Outcomes
2.4. Analysis of HbA1c, Lactate, Lipid Panel, Hepatic Enzymes, and Serum Insulin Levels
2.5. Gut Microbiome Analysis
2.6. Statistical Analysis
3. Results
3.1. Participants’ Characteristics and Baseline Measurement
3.2. Effect of Curcumin on Glucose Homeostasis, Lipid Profile, and Hepatic Enzymes
3.3. Effect of Curcumin on Nutrient Intake, Arthropometric Parameters, and Body Composition
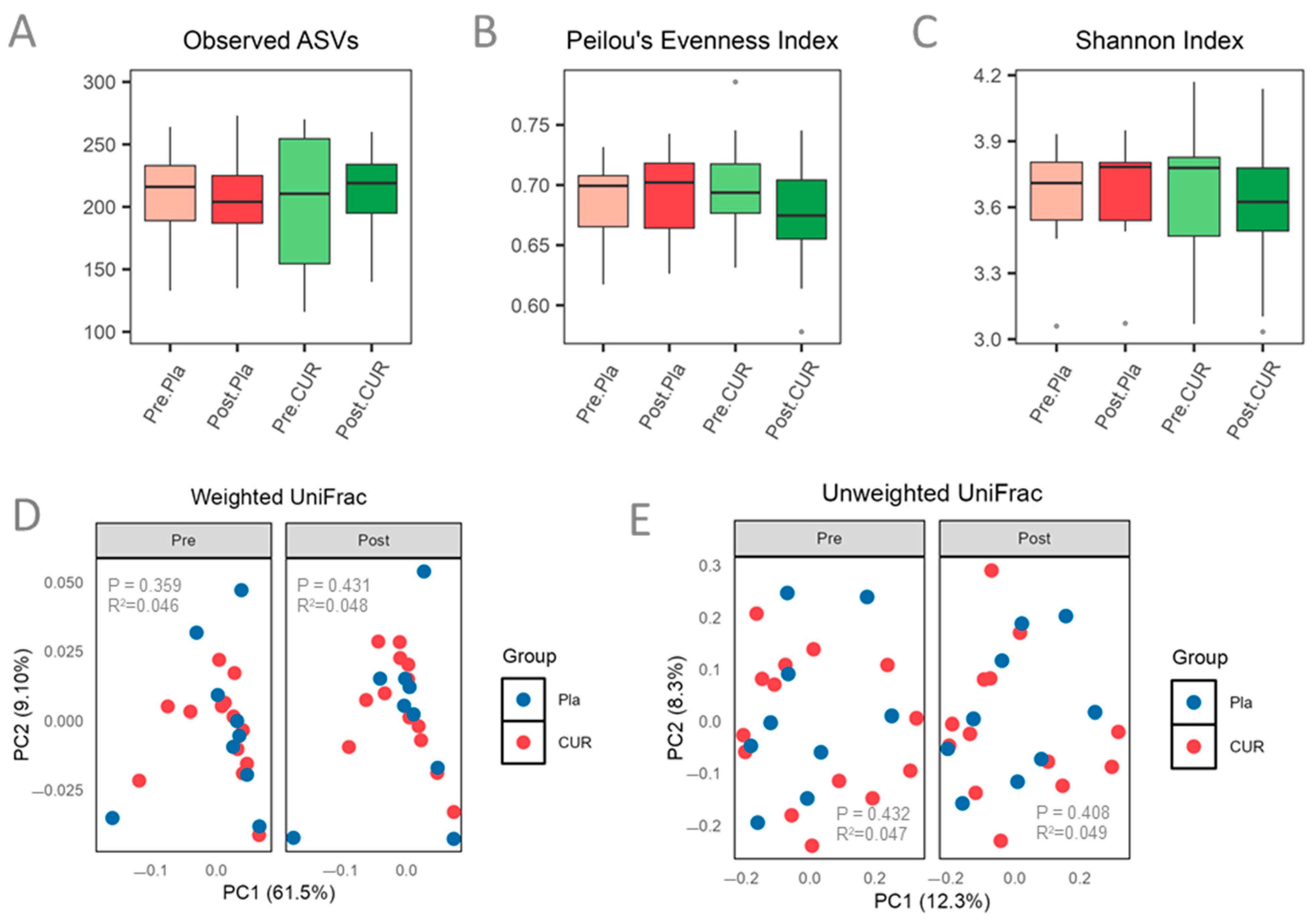
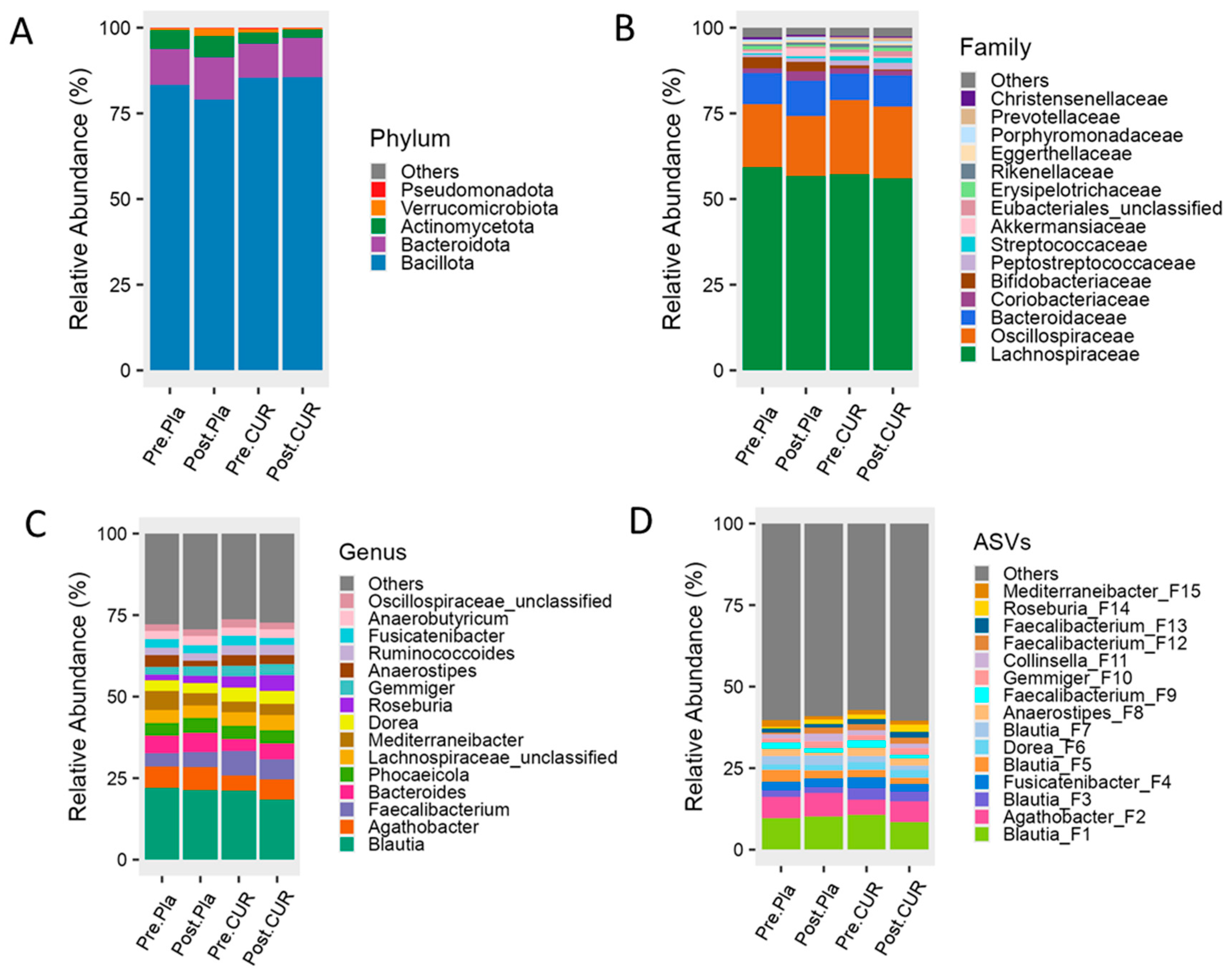
3.4. Effect of Curcumin on Gut Microbiota Diversity and Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine Aminotransferase |
| ANOVA | Analysis of Variance |
| AST | Aspartate Aminotransferase |
| ASVs | Amplicon Sequence Variants |
| BMC | Bone Mineral Content |
| BMD | Bone Mineral Density |
| BMI | Body Mass Index |
| CDC | Centers for Disease Control and Prevention |
| DEXA | Dual-Energy X-ray Absorptiometry |
| GCP | Good Clinical Practice |
| HbA1c | Hemoglobin A1c |
| HDL | High-Density Lipoprotein |
| IRB | Institutional Review Board |
| LDA | Linear Discriminant Analysis |
| LDL | Low-Density Lipoprotein |
| LEfSe | Linear Discriminant Analysis Effect Size |
| Post.CUR | Post-Curcumin |
| Post.Pla | Post-Placebo |
| Pre.CUR | Pre-Curcumin |
| Pre.Pla | Pre-Placebo |
| rRNA | Ribosomal Ribonucleic Acid |
| VLDL | Very Low-Density Lipoprotein |
References
- Mortality in the United States. 2023. Available online: https://www.cdc.gov/nchs/products/databriefs/db521.htm (accessed on 24 January 2025).
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 24 January 2025).
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 Diabetes Mellitus in Older Adults: Clinical Considerations and Management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef] [PubMed]
- CDC National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 24 January 2025).
- Dattani, S.; Rodés-Guirao, L.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M. Life Expectancy. Our World Data. Available online: https://ourworldindata.org/life-expectancy (accessed on 24 January 2025).
- Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2024 | Diabetes Care | American Diabetes Association. Available online: https://diabetesjournals.org/care/article/47/Supplement_1/S158/153955/9-Pharmacologic-Approaches-to-Glycemic-Treatment (accessed on 24 January 2025).
- CDC Prevent Type 2 Diabetes: Talking to Your Patients About Lifestyle Change. Available online: https://www.cdc.gov/diabetes/hcp/lifestyle-change-program/index.html (accessed on 24 January 2025).
- Harris, E. Lifestyle Changes Lowered Blood Glucose in People with Prediabetes. JAMA 2023, 330, 2241. [Google Scholar] [CrossRef]
- Verma, S.; Hussain, M.E. Obesity and Diabetes: An Update. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. GLP-1-Based Therapies for Diabetes, Obesity and Beyond. Nat. Rev. Drug Discov. 2025, 1–20. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, M.; Wang, J.; Jia, H.; Lv, P.; Zeng, J.; Ti, G. Efficacy and Safety of Orlistat in Controlling the Progression of Prediabetes to Diabetes: A Meta-Analysis and Systematic Review. Medicine 2024, 103, e38354. [Google Scholar] [CrossRef]
- Panigrahi, A.; Mohanty, S. Efficacy and Safety of HIMABERB® Berberine on Glycemic Control in Patients with Prediabetes: Double-Blind, Placebo-Controlled, and Randomized Pilot Trial. BMC Endocr. Disord. 2023, 23, 190. [Google Scholar] [CrossRef] [PubMed]
- Nakanekar, A.; Kohli, K.; Tatke, P. Ayurvedic Polyherbal Combination (PDBT) for Prediabetes: A Randomized Double Blind Placebo Controlled Study. J. Ayurveda Integr. Med. 2019, 10, 284–289. [Google Scholar] [CrossRef]
- Yau, J.W.; Thor, S.M.; Ramadas, A. Nutritional Strategies in Prediabetes: A Scoping Review of Recent Evidence. Nutrients 2020, 12, 2990. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Golden, S.H.; Cefalu, W.T. Diabetes and Aging: Unique Considerations and Goals of Care. Diabetes Care 2017, 40, 440–443. [Google Scholar] [CrossRef]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M.; Gummesson, A.; Perkins, R.; Bergström, G.; Bäckhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390.e3. [Google Scholar] [CrossRef]
- Letchumanan, G.; Abdullah, N.; Marlini, M.; Baharom, N.; Lawley, B.; Omar, M.R.; Mohideen, F.B.S.; Addnan, F.H.; Nur Fariha, M.M.; Ismail, Z.; et al. Gut Microbiota Composition in Prediabetes and Newly Diagnosed Type 2 Diabetes: A Systematic Review of Observational Studies. Front. Cell. Infect. Microbiol. 2022, 12, 943427. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, S.M.; Carson, T.; Lowe, J.; Ramaraj, T.; Leff, J.W.; Luo, L.; Bell, C.J.; Shah, V.O. Composition, Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J. Diabetes Obes. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Morrow, C.; Barnes, S.; Wilson, L.; Womack, E.D.; McLain, A.; Yarar-Fisher, C. Gut Microbiome Composition and Serum Metabolome Profile Among Individuals with Spinal Cord Injury and Normal Glucose Tolerance or Prediabetes/Type 2 Diabetes. Arch. Phys. Med. Rehabil. 2022, 103, 702–710. [Google Scholar] [CrossRef]
- Dimba, N.R.; Mzimela, N.; Khathi, A. Improved Gut Health May Be a Potential Therapeutic Approach for Managing Prediabetes: A Literature Review. Biomedicines 2024, 12, 1275. [Google Scholar] [CrossRef]
- Gomes, J.M.G.; Costa, J.d.A.; Alfenas, R.d.C.G. Metabolic Endotoxemia and Diabetes Mellitus: A Systematic Review. Metabolism 2017, 68, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Forlano, R.; Martinez-Gili, L.; Takis, P.; Miguens-Blanco, J.; Liu, T.; Triantafyllou, E.; Skinner, C.; Loomba, R.; Thursz, M.; Marchesi, J.R.; et al. Disruption of Gut Barrier Integrity and Host-Microbiome Interactions Underlie MASLD Severity in Patients with Type-2 Diabetes Mellitus. Gut Microbes 2024, 16, 2304157. [Google Scholar] [CrossRef]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and Inflammation in Non-Alcoholic Fatty Liver Disease: A Randomized, Placebo Controlled Clinical Trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin Extract Improves Beta Cell Functions in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutr. J. 2024, 23, 119. [Google Scholar] [CrossRef] [PubMed]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Li, Y.; Liang, Y.; Hong, T.; Yang, J.; Cao, Z.; Mai, H.; Yao, J.; Zhang, T.; et al. Curcumin Supplementation Alleviates Hepatic Fat Content Associated with Modulation of Gut Microbiota-Dependent Bile Acid Metabolism in Patients with Non-Alcoholic Simple Fatty Liver Disease: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2024, 120, 66–79. [Google Scholar] [CrossRef]
- Heshmati, J.; Moini, A.; Sepidarkish, M.; Morvaridzadeh, M.; Salehi, M.; Palmowski, A.; Mojtahedi, M.F.; Shidfar, F. Effects of Curcumin Supplementation on Blood Glucose, Insulin Resistance and Androgens in Patients with Polycystic Ovary Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Phytomedicine 2021, 80, 153395. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.C.D.S.G.; Alves, A.G.P.; Guillo, L.A.; Sousa, M.A.D.; Trindade, N.R.; Silva, M.S. Curcumin Supplementation Reduces Blood Glucose and Serum Lipids of Brazilian Women with High Waist Circumference: A Randomized Clinical Trial. Arch. Endocrinol. Metab. 2022, 66(6), 800–807. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Lee, D.-Y.; Franks, R.; Olawale, F.; Jin, J.-B.; Egan, J.M.; Kim, Y. Curcumin-Rich Diet Mitigates Non-Alcoholic Fatty Liver Disease (NAFLD) by Attenuating Fat Accumulation and Improving Insulin Sensitivity in Aged Female Mice under Nutritional Stress. Biology 2024, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Chandrasekran, P.; Mazucanti, C.H.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Dietary Curcumin Restores Insulin Homeostasis in Diet-Induced Obese Aged Mice. Aging 2022, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Olawale, F.; Liu, J.; Lee, D.-Y.; Lee, S.-J.; Chaffin, N.; Alake, S.; Lucas, E.A.; Zhang, G.; Egan, J.M.; et al. Curcumin Mitigates Gut Dysbiosis and Enhances Gut Barrier Function to Alleviate Metabolic Dysfunction in Obese, Aged Mice. Biology 2024, 13, 955. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and Cognition: A Randomised, Placebo-Controlled, Double-Blind Study of Community-Dwelling Older Adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef]
- Kisiolek, J.N.; Kheredia, N.; Flores, V.; Ramani, A.; Lisano, J.; Johnston, N.; Stewart, L.K. Short Term, Oral Supplementation with Optimized Curcumin Does Not Impair Performance Improvements Associated with High Intensity Interval Training. J. Diet. Suppl. 2022, 19, 733–746. [Google Scholar] [CrossRef]
- Nahar, P.P.; Slitt, A.L.; Seeram, N.P. Anti-Inflammatory Effects of Novel Standardized Solid Lipid Curcumin Formulations. J. Med. Food 2015, 18, 786–792. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Nathan, D.M.; Kuenen, J.; Borg, R.; Zheng, H.; Schoenfeld, D.; Heine, R.J. For the A1c-Derived Average Glucose (ADAG) Study Group Translating the A1C Assay into Estimated Average Glucose Values. Diabetes Care 2008, 31, 1473–1478. [Google Scholar] [CrossRef]
- Bonora, E.; Tuomilehto, J. The Pros and Cons of Diagnosing Diabetes with A1C. Diabetes Care 2011, 34, S184–S190. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Hekmatdoost, A.; Mirmiran, P. Anti-Hyperglycemic and Insulin Sensitizer Effects of Turmeric and Its Principle Constituent Curcumin. Int. J. Endocrinol. Metab. 2014, 12, e18081. [Google Scholar] [CrossRef] [PubMed]
- Na, L.-X.; Li, Y.; Pan, H.-Z.; Zhou, X.-L.; Sun, D.-J.; Meng, M.; Li, X.-X.; Sun, C.-H. Curcuminoids Exert Glucose-Lowering Effect in Type 2 Diabetes by Decreasing Serum Free Fatty Acids: A Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Kumar, V.; Das, R.; Sharma, V.; Mehta, D.K. Biomarkers of Hepatic Toxicity: An Overview. Curr. Ther. Res. Clin. Exp. 2024, 100, 100737. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Amini, M.R.; Askarpour, M. Effects of Curcumin/Turmeric Supplementation on Liver Function in Adults: A GRADE-Assessed Systematic Review and Dose–Response Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2023, 74, 102952. [Google Scholar] [CrossRef]
- Xuan, Y.; Wu, D.; Zhang, Q.; Yu, Z.; Yu, J.; Zhou, D. Elevated ALT/AST Ratio as a Marker for NAFLD Risk and Severity: Insights from a Cross-Sectional Analysis in the United States. Front. Endocrinol. 2024, 15, 1457598. [Google Scholar] [CrossRef]
- Jia, X.; Xu, W.; Zhang, L.; Li, X.; Wang, R.; Wu, S. Impact of Gut Microbiota and Microbiota-Related Metabolites on Hyperlipidemia. Front. Cell Infect. Microbiol. 2021, 11, 634780. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary Lipids, Gut Microbiota and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut Microbiota in the Pathogenesis and Therapeutic Approaches of Diabetes. eBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef]
- Portincasa, P.; Khalil, M.; Graziani, A.; Frühbeck, G.; Baffy, G.; Garruti, G.; Di Ciaula, A.; Bonfrate, L. Gut Microbes in Metabolic Disturbances. Promising Role for Therapeutic Manipulations? Eur. J. Intern. Med. 2024, 119, 13–30. [Google Scholar] [CrossRef]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2022, 26, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Evidence for the Gut Microbiota Short-Chain Fatty Acids as Key Pathophysiological Molecules Improving Diabetes. Mediat. Inflamm. 2014, 2014, 162021. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Complement. Altern. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, Variability, and Temporal Stability of the Intestinal Microbiota of the Elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Rea, A.; Michel, S. Efficacy of a Curcumin Extract (CurcugenTM) on Gastrointestinal Symptoms and Intestinal Microbiota in Adults with Self-Reported Digestive Complaints: A Randomised, Double-Blind, Placebo-Controlled Study. BMC Complement. Med. Ther. 2021, 21, 40. [Google Scholar] [CrossRef]
| Measures | All Participants (n = 23) | Curcumin (n = 14) | Placebo (n = 9) | p-Value | ||
|---|---|---|---|---|---|---|
| Demographic data | ||||||
| Age (Years) | 66.52 ± 5.76 | 65.5 (61.25–72) | 67 (62–69) | 0.914 b | ||
| Gender | Male | 6 | 4 | 2 | 0.735 c | |
| Female | 17 | 10 | 7 | |||
| Weight (Kg) | 85.44 ± 19.81 | 88.56 ± 21.94 | 80.59 ± 14.69 | 0.369 a | ||
| BMI (Kg/m2) | 31.25 ± 6.45 | 32.36 ± 7.33 | 29.52 ± 4.25 | 0.325 a | ||
| Waist circumference (cm) | 100.72 ± 13.34 | 103.14 ± 15.23 | 96.94 ± 8.38 | 0.298 a | ||
| Blood pressure (mmHg) | Systolic | 126.87 ± 15.61 | 133.88 ± 13.74 | 115.96 ± 11.55 | 0.005 a | |
| Diastolic | 81.75 ± 9.80 | 81 (75–84.75) | 81 (73.67–81.67) | 0.496 b | ||
| Blood glucose (mg/dL) | 106.43 ± 7.93 | 107.07 ± 9.21 | 105.44 ± 5.21 | 0.649 a | ||
| HbA1c (%) | 5.61 ± 0.40 | 5.66 ± 0.47 | 5.52 ± 0.22 | 0.429 a | ||
| Lactate (mmol/L) | 1.28 ± 0.47 | 1.38 ± 0.47 | 1.10 ± 0.42 | 0.203 a | ||
| Triglyceride (mg/dL) | 123.26 ± 69.80 | 84.5 (72.25–157.75) | 98 (78–151) | 0.793 b | ||
| Cholesterol (mg/dL) | 185.09 ± 40.57 | 198.86 ± 38.24 | 163.67 ± 34.32 | 0.044 a | ||
| HDL (mg/dL) | 61.83 ± 12.70 | 65.5 (56.25–68.5) | 60 (58–63) | 0.231 b | ||
| LDL (mg/dL) | 98.43 ± 32.91 | 109.21 ± 29.62 | 81.67 ± 30.68 | 0.053 a | ||
| VLDL (mg/dL) | 24.23 ± 14.23 | 17 (14.25–31.75) | 19.5 (15.75–23.25) | 0.934 b | ||
| ALT (U/L) | 32.82 ± 9.43 | 32.79 ± 10.82 | 32.89 ± 6.71 | 0.981 a | ||
| AST (U/L) | 30.65 ± 6.96 | 31.00 ± 7.82 | 30.11 ± 5.32 | 0.778 a | ||
| Serum Insulin level (µIU/mL) | 9.15 ± 6.74 | 5.93 (4.58–8.32) | 10.50 (7.45–10.68) | 0.125 b | ||
| HOMA-IR | 2.40 ± 1.74 | 1.67 (1.12–2.18) | 2.79 (2.03–2.90) | 0.125 b | ||
| Body composition | ||||||
| BMC (Kg) | 2.22 ± 0.46 | 2.28 ± 0.45 | 2.14 ± 0.46 | 0.481 a | ||
| BMD (g/cm2) | 1.08 ± 0.13 | 1.10 ± 0.12 | 1.06 ± 0.14 | 0.480 a | ||
| Fat mass (Kg) | 33.24 ± 11.12 | 34.71 ± 12.96 | 30.95 ± 6.78 | 0.452 a | ||
| Lean mass (Kg) | 50.78 ± 12.30 | 48 (44.11–54.97) | 50.09 (41.02–54.18) | 0.600 b | ||
| % fat | 38.22 ± 7.33 | 38.38 ± 8.44 | 37.98 ± 5.13 | 0.904 a | ||
| Measures | Curcumin (n = 14) | Placebo (n = 9) | p-Value Post-Intervention Between Groups | p-Value Interaction | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | p-Value Within Group | Pre-Intervention | Post-Intervention | p-Value Within Group | |||
| Blood glucose (mg/dL) | 107.07 ± 9.21 | 110.36 ± 12.73 | 0.154 | 105.44 ± 5.21 | 105.67 ± 7.56 | >0.999 | 0.559 | 0.290 |
| HbA1c (%) | 5.66 ± 0.47 | 5.41 ± 0.45 | 0.044 | 5.52 ± 0.22 | 5.48 ± 0.20 | >0.999 | >0.999 | 0.217 |
| Lactate (mmol/L) | 1.30 (1.03–1.75) | 1.20 (0.90–1.40) | 0.893 | 0.90 (0.85–1.40) | 1.15 (0.93–1.80) | 0.685 | >0.999 | 0.228 |
| Triglyceride (mg/dL) | 84.50 (72.25–157.75) | 92.50 (73.75–157.50) | >0.999 | 98 (78–151) | 109 (99–138) | 0.609 | >0.999 | 0.440 |
| Cholesterol (mg/dL) | 187.5 (171–218.5) | 190 (172.75–197.5) | >0.999 | 176 (145–190) | 177 (164–200) | 0.101 | 0.489 | 0.112 |
| HDL (mg/dL) | 65.5 (56.25–68.50) | 66.50 (59.5–68.75) | >0.999 | 60 (58–63) | 62 (61–65) | 0.306 | >0.999 | 0.233 |
| LDL (mg/dL) | 109.21 ± 29.62 | 108.86 ± 2 5.66 | 0.990 | 81.67 ± 30.68 | 93.22 ± 28.94 | >0.999 | >0.999 | 0.965 |
| VLDL (mg/dL) | 17 (14.25–31.75) | 18.5 (15–31.25) | >0.999 | 19.5 (15.75–23.25) | 17 (14.25–31.75) | 0.332 | >0.999 | 0.291 |
| ALT (U/L) | 32.5 (22.75–38.5) | 27 (22.75–33.75) | 0.224 | 30 (28–38) | 26 (26–30) | 0.028 | >0.999 | 0.404 |
| AST (U/L) | 30.5 (24.25–38) | 36 (25.5–48.5) | 0.028 | 29 (25–33) | 31 (30–35) | 0.191 | >0.999 | 0.758 |
| ALT/AST ratio | 1.05 ± 0.19 | 0.80 ± 0.21 | 0.002 | 1.11 ± 0.24 | 0.84 ± 0.28 | <0.001 | >0.999 | 0.302 |
| Serum Insulin level (µIU/mL) | 5.93 (4.58–8.32) | 6.93 (3.63–12.62) | >0.999 | 10.50 (7.45–10.68) | 8.45 (5.73–12.85) | >0.999 | >0.999 | 0.704 |
| HOMA-IR | 1.67 (1.12–2.18) | 1.85 (0.95–3.57) | >0.999 | 2.79 (0.02–2.90) | 2.15 (1.50–3.57) | >0.999 | >0.999 | 0.648 |
| Measures | Curcumin (n = 14) | Placebo (n = 9) | p-Value Post-Intervention Between Groups | p-Value Interaction | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | p-Value Within Group | Pre-Intervention | Post-Intervention | p-Value Within Group | |||
| Total calories (kcal) | 2075 (1741.25–2348.22) | 1463.47 (1196.32–2413.83) | 0.545 | 1977 (1512.25–2160.44) | 1549.8 (1354.39–2009.28) | 0.486 | >0.999 | 0.818 |
| Calories from fat (kcal) | 785.27 (491.61–815.67) | 595.98 (339.36–809.84) | >0.999 | 728.06 (544.02–1011.54) | 532.2 (458.59–597.07) | 0.207 | 0.510 | 0.325 |
| Protein (g) | 90.17 (72.50–143.13) | 67.14 (43.67–100.99) | 0.169 | 72.87 (65.85–85.47) | 81.53 (69.75–96.63) | 0.890 | 0.999 | 0.444 |
| Carbohydrates (g) | 212.27 ± 68.21 | 203.29 ± 74.73 | >0.999 | 215.70 ± 55.79 | 183.05 ± 57.91 | 0.714 | 0.996 | 0.600 |
| Total dietary fiber (g) | 13.91 (8.79–22.85) | 13.89 (8.57–20.75) | 0.989 | 19.5 (16.52–25.41) | 14.57 (5.64–22.6) | 0.903 | 0.721 | 0.820 |
| Sugar (g) | 87.24 ± 33.52 | 87.35 ± 51.50 | >0.999 | 94.73 ± 37.41 | 75.20 ± 42.34 | 0.649 | >0.999 | 0.438 |
| Fat (g) | 87.45 (54.62–90.78) | 66.22 (37.71–89.98) | 0.850 | 80.9 (60.45–112.39) | 59.13 (50.95–66.34) | 0.196 | 0.509 | 0.325 |
| Saturated fat (g) | 28.22 ± 10.16 | 28.15 ± 19.04 | >0.999 | 25.35 ± 13.08 | 17.95 ± 7.59 | 0.579 | 0.205 | 0.410 |
| Trans-fatty acid (g) | 0.29 (0.09–0.61) | 0.34 (0.09–1.57) | 0.708 | 0.26 (0.07–0.52) | 0.07 (0–0.25) | 0.856 | 0.971 | 0.960 |
| Cholesterol (mg) | 335.99 (183.87–514.02) | 244.39 (192.45–482.09) | 0.917 | 122.16 (76.15–214.83) | 260.75 (115.05–397.77) | 0.251 | >0.999 | 0.159 |
| Measures | Curcumin (n = 14) | Placebo (n = 9) | p-Value Post-Intervention Between Groups | p-Value Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | p-Value Within Group | Pre-Intervention | Post-Intervention | p-Value Within Group | ||||
| Weight (Kg) | 88.56 ± 21.94 | 88.87 ± 22.00 | 0.953 | 80.59 ± 14.69 | 80.74 ± 14.80 | 0.999 | 0.716 | 0.820 | |
| BMI (Kg/m2) | 32.36 ± 7.33 | 32.48 ± 7.35 | 0.926 | 29.89 ± 4.72 | 29.59 ± 4.47 | >0.999 | 0.631 | 0.864 | |
| Waist circumference (cm) | 103.14 ± 15.23 | 104.50 ± 15.64 | 0.507 | 96.94 ± 8.38 | 96.38 ± 9.23 | >0.999 | 0.354 | 0.311 | |
| Blood pressure (mmHg) | Systolic | 133.88 ± 13.74 | 137.30 ± 14.16 | 0.737 | 115.96±11.55 | 117.85 ±16.83 | >0.999 | 0.007 | 0.800 |
| Diastolic | 81 (75–84.75) | 84.25 (75.42–91.17) | 0.938 | 81 (73.67–81.67) | 77 (73.33–81.67) | 0.667 | 0.467 | 0.231 | |
| BMC (Kg) | 2.28 ± 0.45 | 2.27 ± 0.42 | 0.891 | 2.14 ± 0.46 | 2.11 ± 0.44 | 0.249 | 0.458 | 0.454 | |
| BMD (gm/cm2) | 1.10 ± 0.12 | 1.09 ± 0.11 | >0.999 | 1.06 ± 0.14 | 1.05 ± 0.12 | 0.620 | 0.881 | 0.500 | |
| Fat mass (Kg) | 34.71 ± 12.96 | 35.14 ± 13.55 | >0.999 | 30.95 ± 6.78 | 31.07 ± 7.29 | 0.646 | 0.975 | 0.614 | |
| Lean mass (Kg) | 48.0(44.11–54.97) | 47.73 (45.47–55.17) | 0.921 | 50.09 (41.02–54.18) | 50.84 (41.07–56.67) | >0.999 | >0.999 | 0.732 | |
| % fat | 38.38 ± 8.44 | 38.48 ± 8.78 | >0.999 | 37.98 ± 5.13 | 38.00 ± 5.36 | 0.587 | >0.999 | 0.259 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamichhane, G.; Godsey, T.J.; Liu, J.; Franks, R.; Zhang, G.; Emerson, S.R.; Kim, Y. Twelve-Week Curcumin Supplementation Improves Glucose Homeostasis and Gut Health in Prediabetic Older Adults: A Pilot, Double-Blind, Placebo-Controlled Trial. Nutrients 2025, 17, 2164. https://doi.org/10.3390/nu17132164
Lamichhane G, Godsey TJ, Liu J, Franks R, Zhang G, Emerson SR, Kim Y. Twelve-Week Curcumin Supplementation Improves Glucose Homeostasis and Gut Health in Prediabetic Older Adults: A Pilot, Double-Blind, Placebo-Controlled Trial. Nutrients. 2025; 17(13):2164. https://doi.org/10.3390/nu17132164
Chicago/Turabian StyleLamichhane, Gopal, Tyler J. Godsey, Jing Liu, Rienna Franks, Guolong Zhang, Sam R. Emerson, and Yoo Kim. 2025. "Twelve-Week Curcumin Supplementation Improves Glucose Homeostasis and Gut Health in Prediabetic Older Adults: A Pilot, Double-Blind, Placebo-Controlled Trial" Nutrients 17, no. 13: 2164. https://doi.org/10.3390/nu17132164
APA StyleLamichhane, G., Godsey, T. J., Liu, J., Franks, R., Zhang, G., Emerson, S. R., & Kim, Y. (2025). Twelve-Week Curcumin Supplementation Improves Glucose Homeostasis and Gut Health in Prediabetic Older Adults: A Pilot, Double-Blind, Placebo-Controlled Trial. Nutrients, 17(13), 2164. https://doi.org/10.3390/nu17132164







