Imbalance of ω3 and ω6 Fatty Acids in Breast Milk of Overweight/Obese Women
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Profile of Study Women
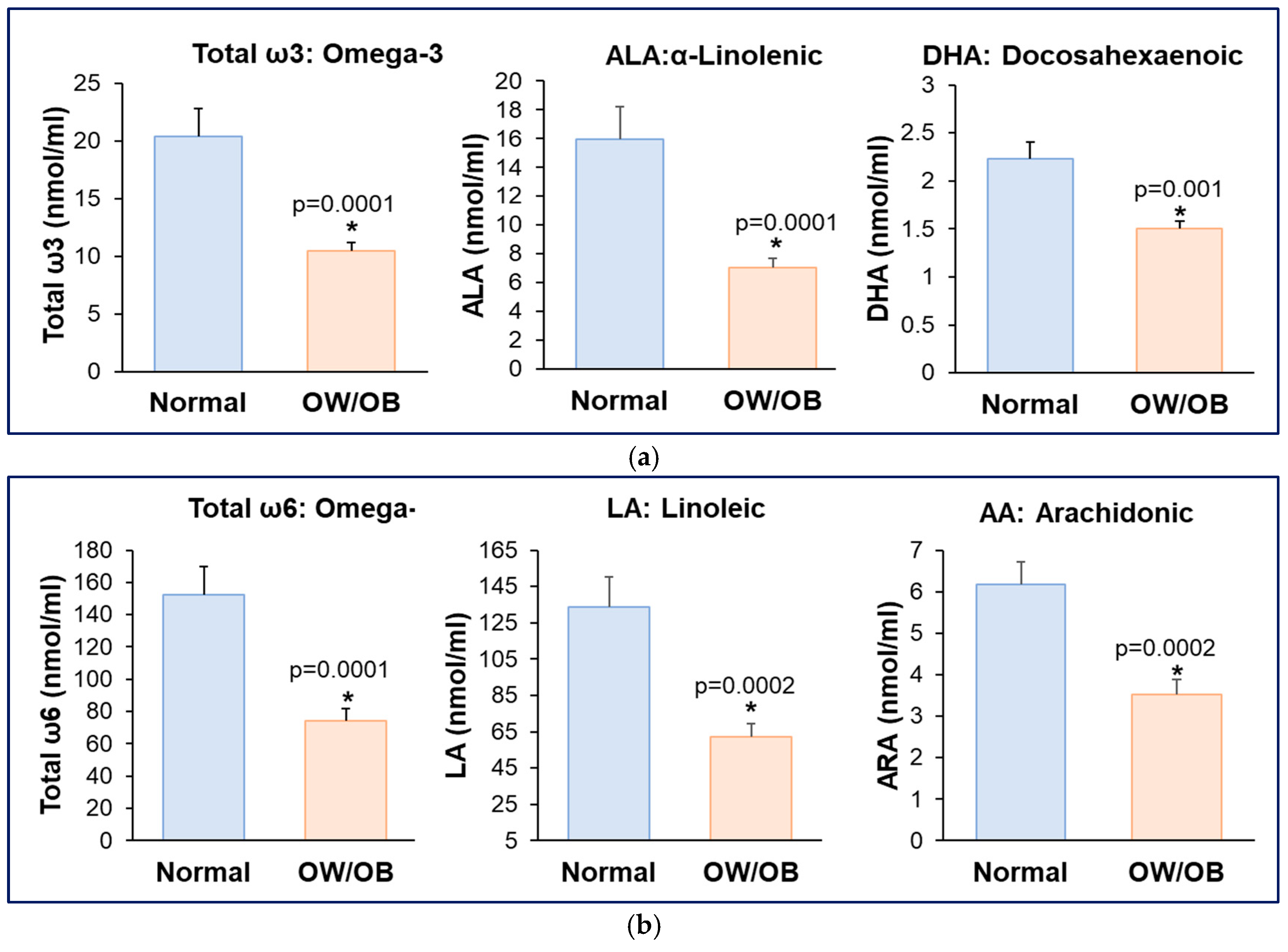
3.2. Reduced Maternal Plasma ω3 and ω6 Fatty Acids in Women with OW/OB
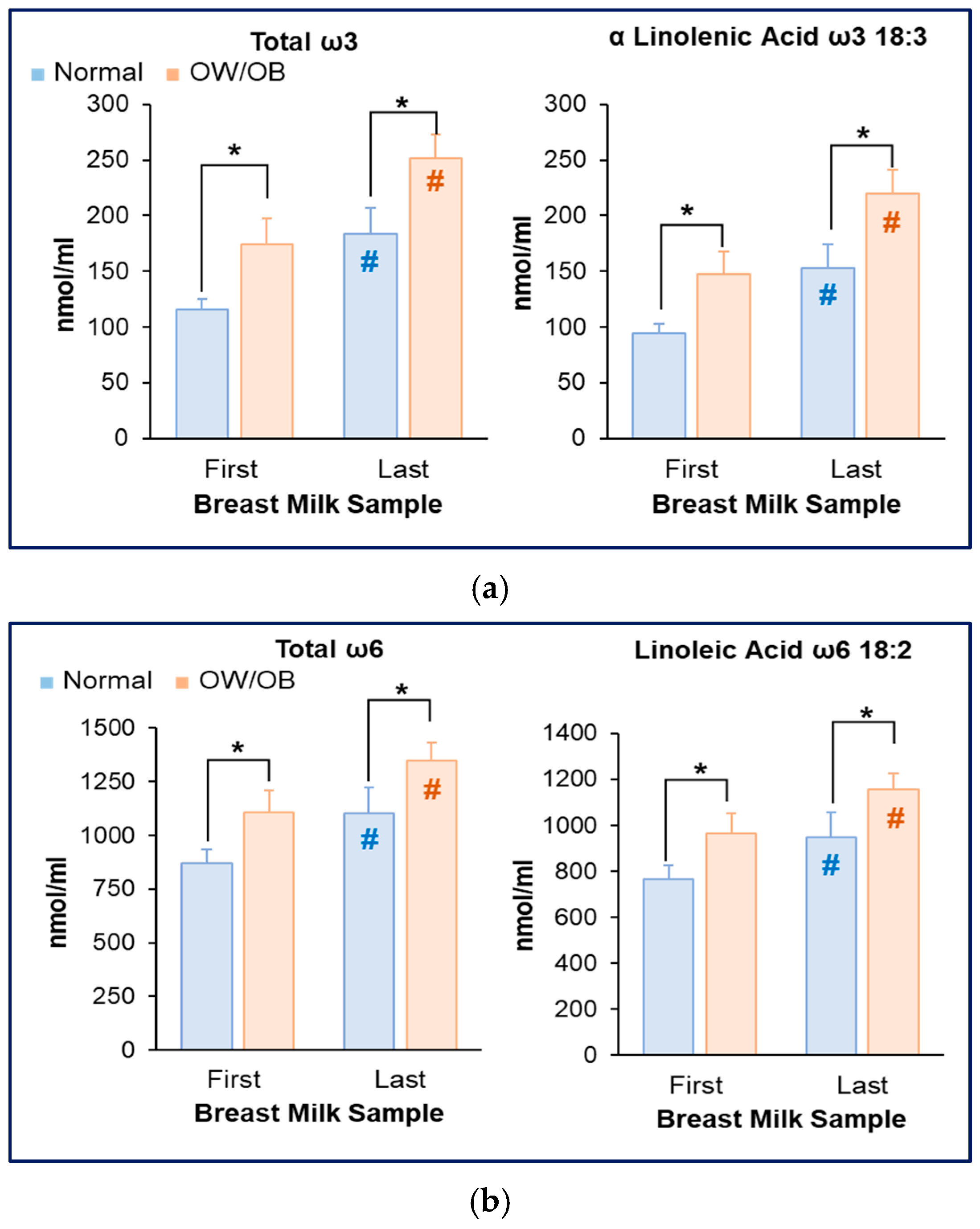
3.3. Increased Milk ω3 and ω6 Fatty Acids in Women with OW/OB
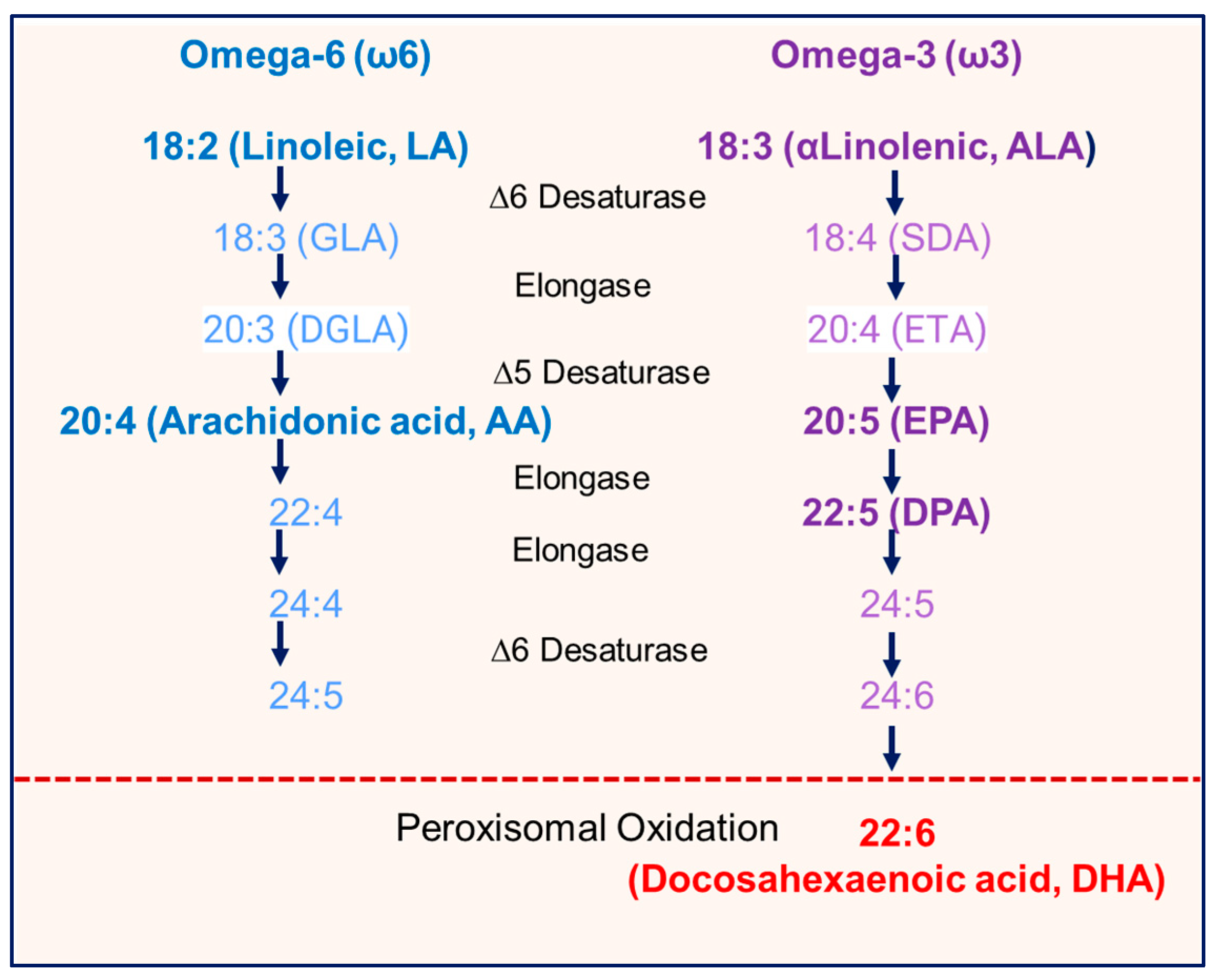
3.4. Reduced Milk DHA and Increased AA Content in Women with OW/OB
3.5. Increased AA Content in Women with OW/OB
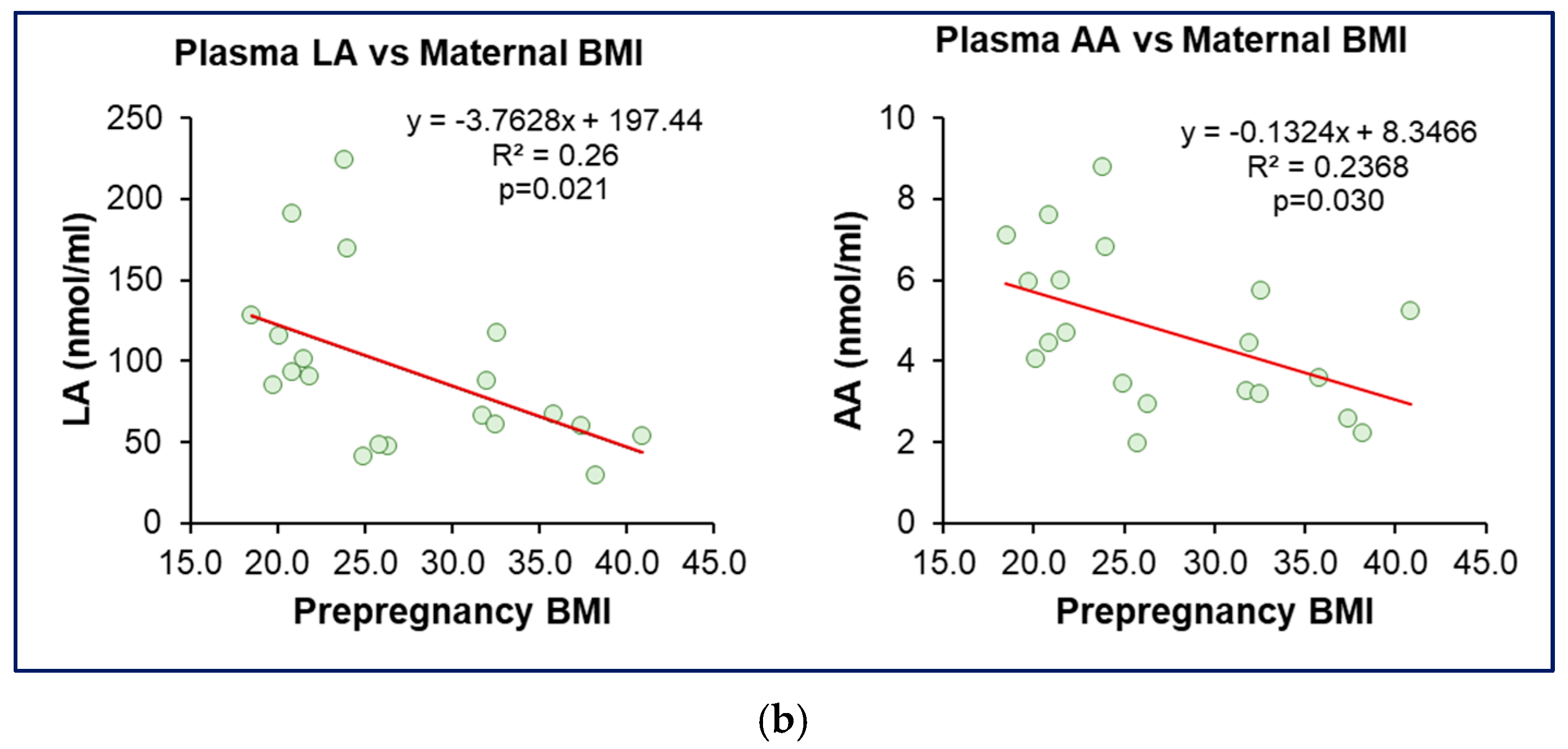
3.6. Association Between Milk Content and Maternal Plasma Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OW/OB | Overweight/obese |
| FA | Fatty Acid |
| PUFAs | Polyunsaturated Fatty Acids |
| LA | 18:2n-6 linoleic |
| ALA | 18:3n-3 α-linolenic acid |
| AA | Arachidonic Acid |
| DHA | Docosahexaenoic Acid |
| LCFA | Long-chain fatty acid |
| ω3 | Omega-3 fatty acids |
| ω6 | Omega 6-fatty acids |
| BMI | Body mass index |
References
- Tan, J.; Zhang, Z.; Yan, L.L.; Xu, X. The developmental origins of health and disease and intergenerational inheritance: A scoping review of multigenerational cohort studies. J. Dev. Orig. Health Dis. 2024, 15, e1. [Google Scholar] [CrossRef]
- Desai, M.; Jellyman, J.K.; Han, G.; Beall, M.; Lane, R.H.; Ross, M.G. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am. J. Obstet. Gynecol. 2014, 211, 237.e1–237.e13. [Google Scholar] [CrossRef]
- Desai, M.; Han, G.; Ross, M.G. Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite 2016, 99, 193–199. [Google Scholar] [CrossRef]
- Picó, C.; Reis, F.; Egas, C.; Mathias, P.; Matafome, P. Lactation as a programming window for metabolic syndrome. Eur. J. Clin. Investig. 2021, 51, e13482. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.K.; Bode, L. Obesogenic Programming Effects during Lactation: A Narrative Review and Conceptual Model Focusing on Underlying Mechanisms and Promising Future Research Avenues. Nutrients 2021, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Infant and Young Child Feeding. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 20 December 2023).
- Meek, J.Y.; Noble, L. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef]
- Dieterich, C.M.; Felice, J.P.; O’Sullivan, E.; Rasmussen, K.M. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr. Clin. N. Am. 2013, 60, 31–48. [Google Scholar] [CrossRef]
- Anstey, E.H.; Chen, J.; Elam-Evans, L.D.; Perrine, C.G. Racial and Geographic Differences in Breastfeeding—United States, 2011–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, J.L.; Hamner, H.C.; Chen, J.; Avila-Rodriguez, W.; Elam-Evans, L.D.; Perrine, C.G. Racial Disparities in Breastfeeding Initiation and Duration Among U.S. Infants Born in 2015. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 745–748. [Google Scholar] [CrossRef]
- McGuire, S. Centers for Disease Control and Prevention. 2013. Strategies to Prevent Obesity and Other Chronic Diseases: The CDC Guide to Strategies to Support Breastfeeding Mothers and Babies. Atlanta, GA: U.S. Department of Health and Human Services, 2013. Adv. Nutr. 2014, 5, 291–292. [Google Scholar] [CrossRef]
- Pham, Q.; Patel, P.; Baban, B.; Yu, J.; Bhatia, J. Factors Affecting the Composition of Expressed Fresh Human Milk. Breastfeed. Med. 2020, 15, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Jenness, R. The composition of human milk. Semin. Perinatol. 1979, 3, 225–239. [Google Scholar]
- Jensen, R.G.; Ferris, A.M.; Lammi-Keefe, C.J.; Henderson, R.A. Lipids of bovine and human milks: A comparison. J. Dairy Sci. 1990, 73, 223–240. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Oledzka, G.; Szostak-Wegierek, D.; Weker, H.; Wesolowska, A. Maternal Nutrition and Body Composition During Breastfeeding: Association with Human Milk Composition. Nutrients 2018, 10, 1379. [Google Scholar] [CrossRef]
- Brown, K.H.; Akhtar, N.A.; Robertson, A.D.; Ahmed, M.G. Lactational capacity of marginally nourished mothers: Relationships between maternal nutritional status and quantity and proximate composition of milk. Pediatrics 1986, 78, 909–919. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Sunehag, A.L.; Haymond, M.W. De novo synthesis of milk triglycerides in humans. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E838–E847. [Google Scholar] [CrossRef] [PubMed]
- Insull, W., Jr.; Hirsch, J.; James, T.; Ahrens, E.H., Jr. The fatty acids of human milk, I.I. Alterations produced by manipulation of caloric balance and exchange of dietary fats. J. Clin. Investig. 1959, 38, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Bzikowska, A.; Czerwonogrodzka-Senczyna, A.; Weker, H.; Wesołowska, A. Correlation between human milk composition and maternal nutritional status. Rocz. Państwowego Zakładu Hig. 2018, 69, 363–367. [Google Scholar] [CrossRef]
- Grote, V.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2016, 70, 250–256. [Google Scholar] [CrossRef]
- Nommsen, L.A.; Lovelady, C.A.; Heinig, M.J.; Lönnerdal, B.; Dewey, K.G. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: The DARLING Study. Am. J. Clin. Nutr. 1991, 53, 457–465. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.; Jung, B.M.; Yi, H.; Jung, J.A.; Chang, N. Breast milk fatty acid composition and fatty acid intake of lactating mothers in South Korea. Br. J. Nutr. 2017, 117, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F.; Skafte, L.; Badsberg, J.H.; Jørgensen, M. Variation in macronutrients in human bank milk: Influencing factors and implications for human milk banking. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 229–239. [Google Scholar] [CrossRef]
- Yuhas, R.; Pramuk, K.; Lien, E.L. Human milk fatty acid composition from nine countries varies most in DHA. Lipids 2006, 41, 851–858. [Google Scholar] [CrossRef]
- Li, J.; Pora, B.L.R.; Dong, K.; Hasjim, J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Sci. Nutr. 2021, 9, 5229–5243. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Lattka, E.; Rzehak, P.; Steer, C.; Koletzko, B. Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Matern. Child Nutr. 2011, 7 (Suppl. 2), 27–40. [Google Scholar] [CrossRef] [PubMed]
- Keim, S.A.; Daniels, J.L.; Siega-Riz, A.M.; Herring, A.H.; Dole, N.; Scheidt, P.C. Breastfeeding and long-chain polyunsaturated fatty acid intake in the first 4 post-natal months and infant cognitive development: An observational study. Matern. Child Nutr. 2012, 8, 471–482. [Google Scholar] [CrossRef]
- Fan, L.; Wang, X.; Szeto, I.M.; Liu, B.; Sinclair, A.J.; Li, D. Dietary intake of different ratios of ARA/DHA in early stages and its impact on infant development. Food Funct. 2024, 15, 3259–3273. [Google Scholar] [CrossRef]
- Tomedi, L.E.; Chang, C.C.; Newby, P.K.; Evans, R.W.; Luther, J.F.; Wisner, K.L.; Bodnar, L.M. Pre-pregnancy obesity and maternal nutritional biomarker status during pregnancy: A factor analysis. Public Health Nutr. 2013, 16, 1414–1418. [Google Scholar] [CrossRef]
- Hashemi Javaheri, F.S.; Karbin, K.; Senobari, M.A.; Hakim, H.G.; Hashemi, M. The association between maternal body mass index and breast milk composition: A systematic review. Nutr. Rev. 2024, 83, 83–111. [Google Scholar] [CrossRef]
- Ross, M.G.; Kavasery, M.P.; Cervantes, M.K.; Han, G.; Horta, B.; Coca, K.P.; Costa, S.O.; Desai, M. High-Fat, High-Calorie Breast Milk in Women with Overweight or Obesity and Its Association with Maternal Serum Insulin Concentration and Triglycerides Levels. Children 2024, 11, 141. [Google Scholar] [CrossRef]
- Ross, M.G.; Coca, K.P.; Rocha, A.C.L.; Camargo, B.T.S.; de Castro, L.S.; Horta, B.L.; Desai, M. Composition of Breast Milk in Women with Obesity. J. Clin. Med. 2024, 13, 6947. [Google Scholar] [CrossRef] [PubMed]
- Takumi, H.; Kato, K.; Nakanishi, H.; Tamura, M.; Ohto, N.T.; Nagao, S.; Hirose, J. Comprehensive Analysis of Lipid Composition in Human Foremilk and Hindmilk. J. Oleo Sci. 2022, 71, 947–957. [Google Scholar] [CrossRef]
- Karatas, Z.; Durmus Aydogdu, S.; Dinleyici, E.C.; Colak, O.; Dogruel, N. Breastmilk ghrelin, leptin, and fat levels changing foremilk to hindmilk: Is that important for self-control of feeding? Eur. J. Pediatr. 2011, 170, 1273–1280. [Google Scholar] [CrossRef]
- Hsieh, W.Y.; Williams, K.J.; Su, B.; Bensinger, S.J. Profiling of mouse macrophage lipidome using direct infusion shotgun mass spectrometry. STAR Protoc. 2021, 2, 100235. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Bettcher, L.F.; Hsieh, W.Y.; Hornburg, D.; Pearson, M.J.; Blomberg, N.; Giera, M.; Snyder, M.P.; Raftery, D.; Bensinger, S.J.; et al. A DMS Shotgun Lipidomics Workflow Application to Facilitate High-Throughput, Comprehensive Lipidomics. J. Am. Soc. Mass Spectrom. 2021, 32, 2655–2663. [Google Scholar] [CrossRef]
- Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/ (accessed on 24 April 2025).
- Gázquez, A.; Larqué, E. Towards an Optimized Fetal DHA Accretion: Differences on Maternal DHA Supplementation Using Phospholipids vs. Triglycerides during Pregnancy in Different Models. Nutrients 2021, 13, 511. [Google Scholar] [CrossRef]
- Sherry, C.L.; Oliver, J.S.; Marriage, B.J. Docosahexaenoic acid supplementation in lactating women increases breast milk and plasma docosahexaenoic acid concentrations and alters infant omega 6:3 fatty acid ratio. Prostaglandins Leukot. Essent. Fat. Acids 2015, 95, 63–69. [Google Scholar] [CrossRef]
- Mida, K.; Shamay, A.; Argov-Argaman, N. Elongation and desaturation pathways in mammary gland epithelial cells are associated with modulation of fat and membrane composition. J. Agric. Food Chem. 2012, 60, 10657–10665. [Google Scholar] [CrossRef] [PubMed]
- Bobiński, R.; Bobińska, J. Fatty acids of human milk—A review. Int. J. Vitam. Nutr. Res. 2022, 92, 280–291. [Google Scholar] [CrossRef]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef]
- Uauy, R.; Mena, P.; Rojas, C. Essential fatty acids in early life: Structural and functional role. Proc. Nutr. Soc. 2000, 59, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Hamel, F.G.; Bennett, R.G.; Upward, J.L.; Duckworth, W.C. Insulin inhibits peroxisomal fatty acid oxidation in isolated rat hepatocytes. Endocrinology 2001, 142, 2702–2706. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Dorotea, D.; Jiang, S.; Koh, E.H.; Oh, G.T.; Ha, H. Impaired Peroxisomal Fitness in Obese Mice, a Vicious Cycle Exacerbating Adipocyte Dysfunction via Oxidative Stress. Antioxid. Redox Signal. 2019, 31, 1339–1351. [Google Scholar] [CrossRef]
- Brenna, J.T. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 fatty acids eicosapentaenoic (EPA) and docosahexaenoic (DHA) as modulatory and anti-inflammatory agents in noncommunicable diet-related diseases—Reports from the last 10 years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505s–1519s. [Google Scholar] [CrossRef]
- Jerab, D.; Blangero, F.; da Costa, P.C.T.; de Brito Alves, J.L.; Kefi, R.; Jamoussi, H.; Morio, B.; Eljaafari, A. Beneficial Effects of Omega-3 Fatty Acids on Obesity and Related Metabolic and Chronic Inflammatory Diseases. Nutrients 2025, 17, 1253. [Google Scholar] [CrossRef]
- Shulhan, J.; Dicken, B.; Hartling, L.; Larsen, B.M. Current Knowledge of Necrotizing Enterocolitis in Preterm Infants and the Impact of Different Types of Enteral Nutrition Products. Adv. Nutr. 2017, 8, 80–91. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Autoimmune Diseases, Asthma, and Allergies. Mo. Med. 2021, 118, 453–459. [Google Scholar]
- Fleith, M.; Clandinin, M.T. Dietary PUFA for preterm and term infants: Review of clinical studies. Crit. Rev. Food Sci. Nutr. 2005, 45, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Mitguard, S.; Doucette, O.; Miklavcic, J. Human milk polyunsaturated fatty acids are related to neurodevelopmental, anthropometric, and allergic outcomes in early life: A systematic review. J. Dev. Orig. Health Dis. 2023, 14, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Holbrook, J.; Fish, A.; Glynn, L.M. Human Milk Omega-3 Fatty Acid Composition is Associated with Infant Temperament. Nutrients 2019, 11, 2964. [Google Scholar] [CrossRef] [PubMed]
- de la Garza Puentes, A.; Martí Alemany, A.; Chisaguano, A.M.; Montes Goyanes, R.; Castellote, A.I.; Torres-Espínola, F.J.; García-Valdés, L.; Escudero-Marín, M.; Segura, M.T.; Campoy, C.; et al. The Effect of Maternal Obesity on Breast Milk Fatty Acids and Its Association with Infant Growth and Cognition-The PREOBE Follow-Up. Nutrients 2019, 11, 2154. [Google Scholar] [CrossRef]
- Miklavcic, J.J.V.E.; MacDonald, I.M.; Urichuk, L.; Mazurak, V.C.; Rinaldi, C.; Clandinin, M.T. AA and DHA are Decreased in Paediatric AD/HD and Inattention is Ameliorated by Increased Plasma DHA. Hum. Nutr. Metab. 2023, 31, 200183. [Google Scholar] [CrossRef]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef]
- Helland, I.B.; Smith, L.; Blomén, B.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Effect of supplementing pregnant and lactating mothers with n-3 very-long-chain fatty acids on children’s IQ and body mass index at 7 years of age. Pediatrics 2008, 122, e472–e479. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Simmer, K.; Dixon, G.; Prescott, S.L. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F45–F50. [Google Scholar] [CrossRef]
- Donahue, S.M.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal fatty acid status and child adiposity at age 3 y: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef]
- Davidson, M.H.; Johnson, J.; Rooney, M.W.; Kyle, M.L.; Kling, D.F. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: The ECLIPSE (Epanova(®) compared to Lovaza(®) in a pharmacokinetic single-dose evaluation) study. J. Clin. Lipidol. 2012, 6, 573–584. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]




| Normal (BMI 18.9–24.9) n = 9 | OW/OB (BMI ≥ 25) n = 13 | p-Value OW/OB vs. Normal | |
|---|---|---|---|
| Age | 29.8 ± 2.6 | 29 ± 1.9 | NS |
| Pre-pregnancy Height (m) | 1.60 ± 0.02 | 1.59 ± 0.01 | NS |
| Pregnancy Weight (kg) | 54.2 ± 1.5 | 81.9 ± 3.3 | p < 0.001 |
| Pre-pregnancy BMI (kg/m2) | 21.2 ± 0.6 | 32.5 ± 1.5 | p < 0.001 |
| Gestational Weight Gain (kg) | 14.8 ± 1.8 | 11.8 ± 1.1 | p = 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, M.G.; Kavasery, M.P.; Han, G.; Cervantes, M.K.; Bora, L.; Williams, K.J.; Desai, M. Imbalance of ω3 and ω6 Fatty Acids in Breast Milk of Overweight/Obese Women. Nutrients 2025, 17, 2158. https://doi.org/10.3390/nu17132158
Ross MG, Kavasery MP, Han G, Cervantes MK, Bora L, Williams KJ, Desai M. Imbalance of ω3 and ω6 Fatty Acids in Breast Milk of Overweight/Obese Women. Nutrients. 2025; 17(13):2158. https://doi.org/10.3390/nu17132158
Chicago/Turabian StyleRoss, Michael G., Manasa P. Kavasery, Guang Han, MacKenzie K. Cervantes, Lihiri Bora, Kevin J. Williams, and Mina Desai. 2025. "Imbalance of ω3 and ω6 Fatty Acids in Breast Milk of Overweight/Obese Women" Nutrients 17, no. 13: 2158. https://doi.org/10.3390/nu17132158
APA StyleRoss, M. G., Kavasery, M. P., Han, G., Cervantes, M. K., Bora, L., Williams, K. J., & Desai, M. (2025). Imbalance of ω3 and ω6 Fatty Acids in Breast Milk of Overweight/Obese Women. Nutrients, 17(13), 2158. https://doi.org/10.3390/nu17132158







