Obesity-Associated NAFLD Coexists with a Chronic Inflammatory Kidney Condition That Is Partially Mitigated by Short-Term Oral Metformin
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design
2.3. Histology Analysis
2.4. Immunohistochemistry Analysis
2.5. Western Blot Analysis
2.6. Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
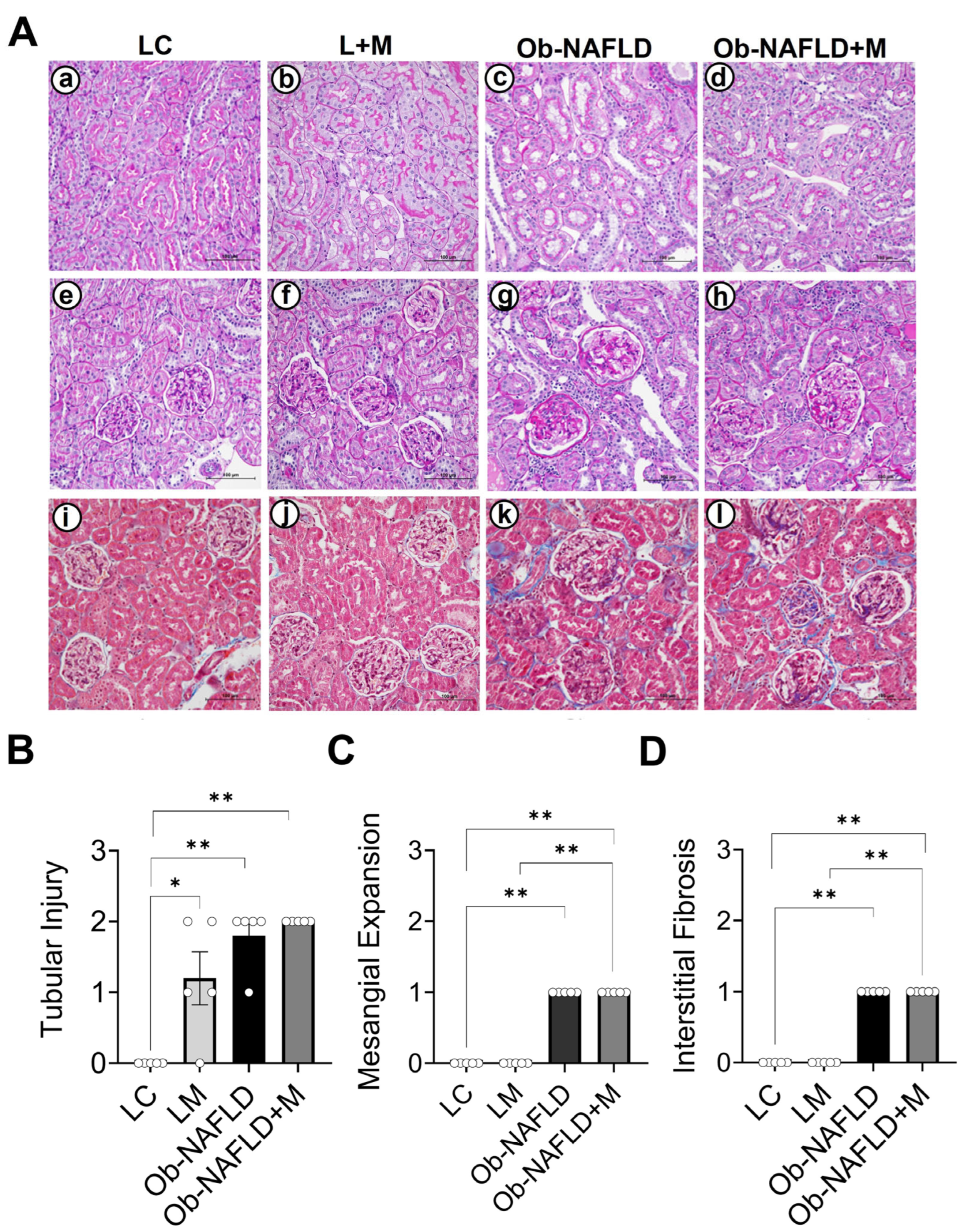
3.1. Ob-NAFLD Induces Tubular Injury, Glomerular Sclerosis, and Interstitial Fibrosis, While Short-Term Metformin Treatment Did Not Notably Ameliorate Kidney Pathology
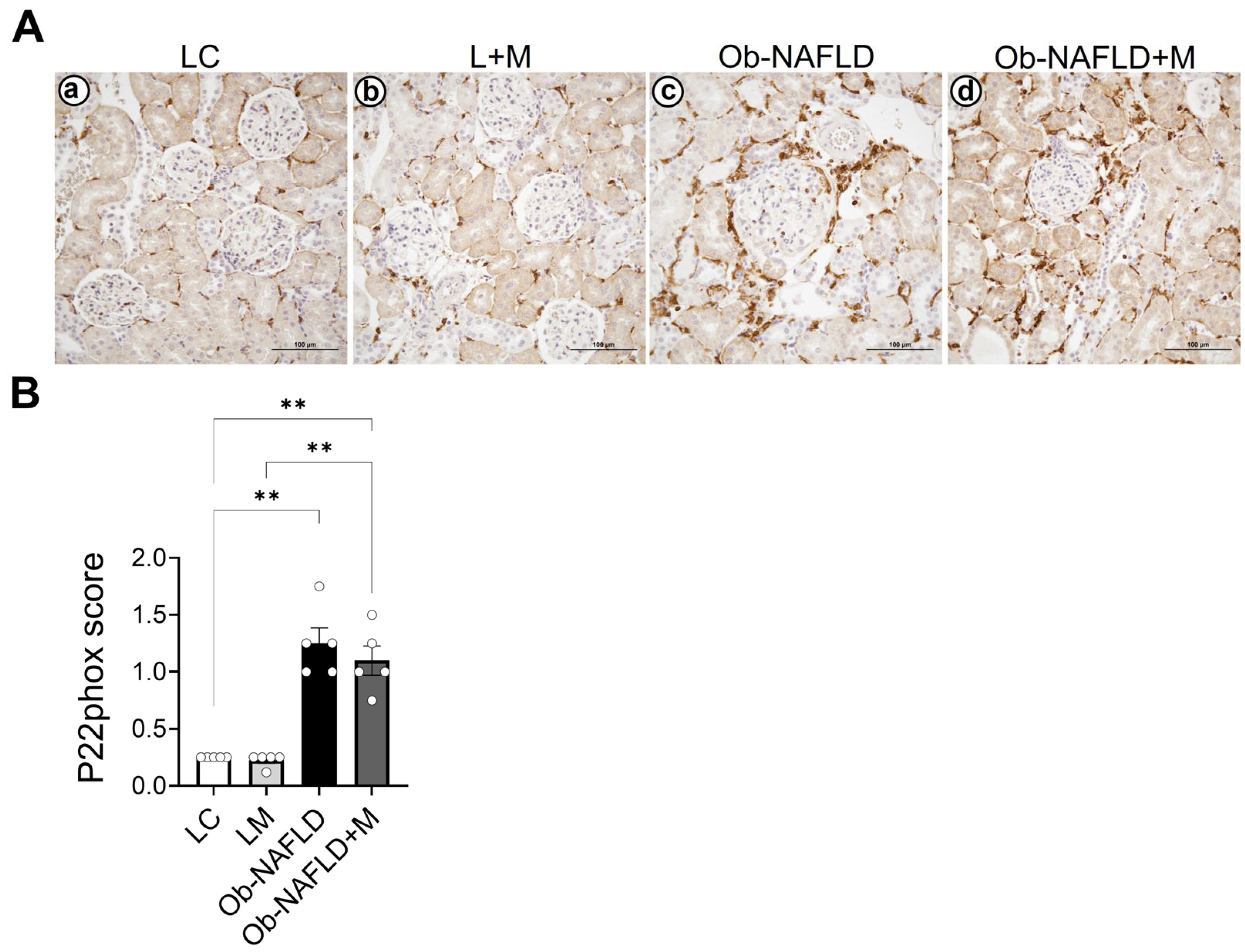
3.2. Ob-NAFLD Increases Nitrotyrosine Levels in the Kidneys, Which Were Reduced by Short-Term Oral Metformin
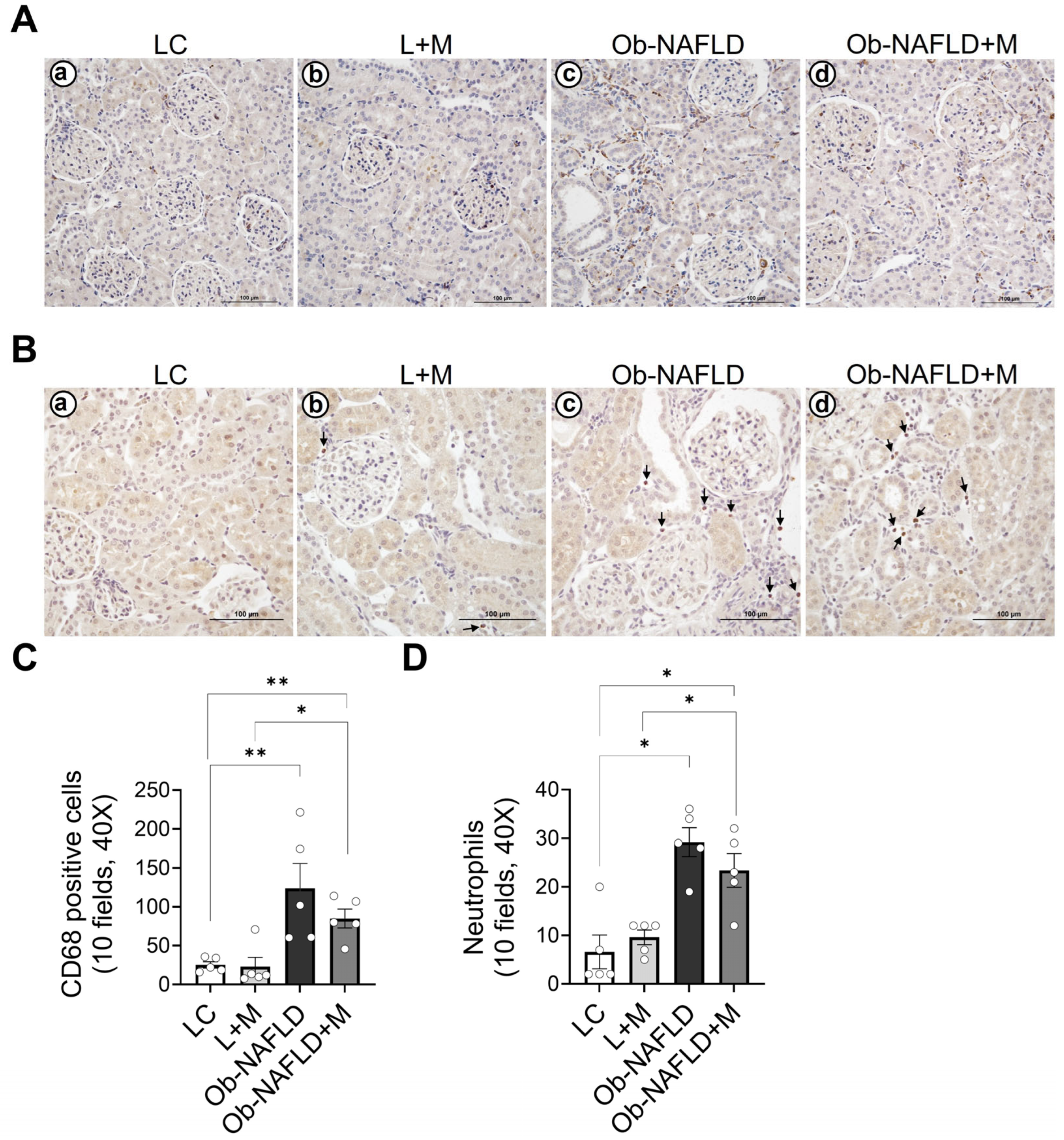
3.3. Ob-NAFLD Increases Macrophage and Neutrophil Infiltration in the Kidneys, Which Is Mildly Reduced by Short-Term Metformin Treatment
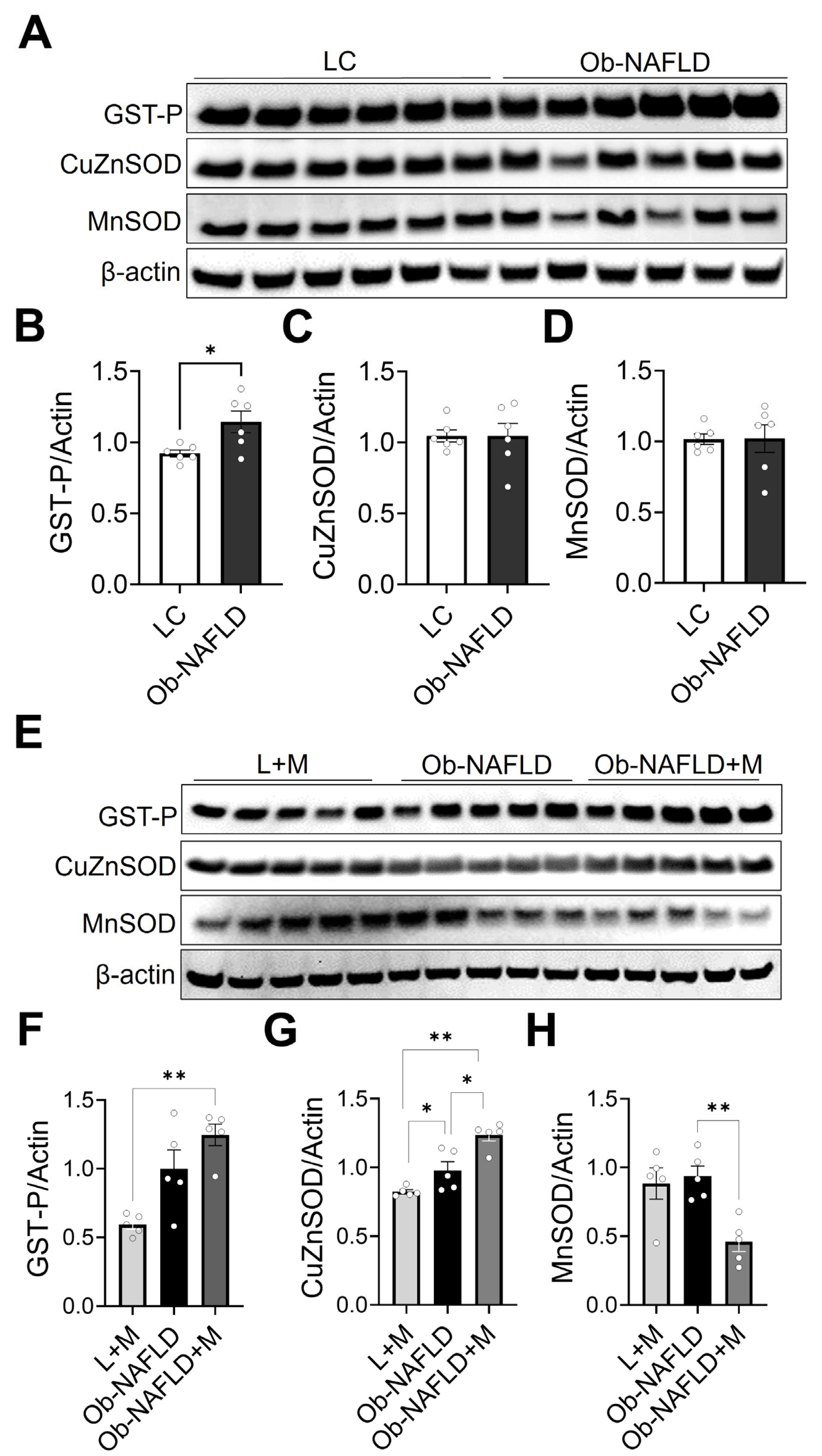
3.4. Ob-NAFLD Selectively Modulates Renal Antioxidant Enzymes, and Metformin Differentially Affects GST-P, SOD1, and SOD2 Levels
3.5. Ob-NAFLD Upregulates the Gene Expression of Pro-Inflammatory Markers TNF-α, MCP-1, and CXCR2 in the Kidney, While Metformin Treatment Specifically Reduces TNF-α Expression
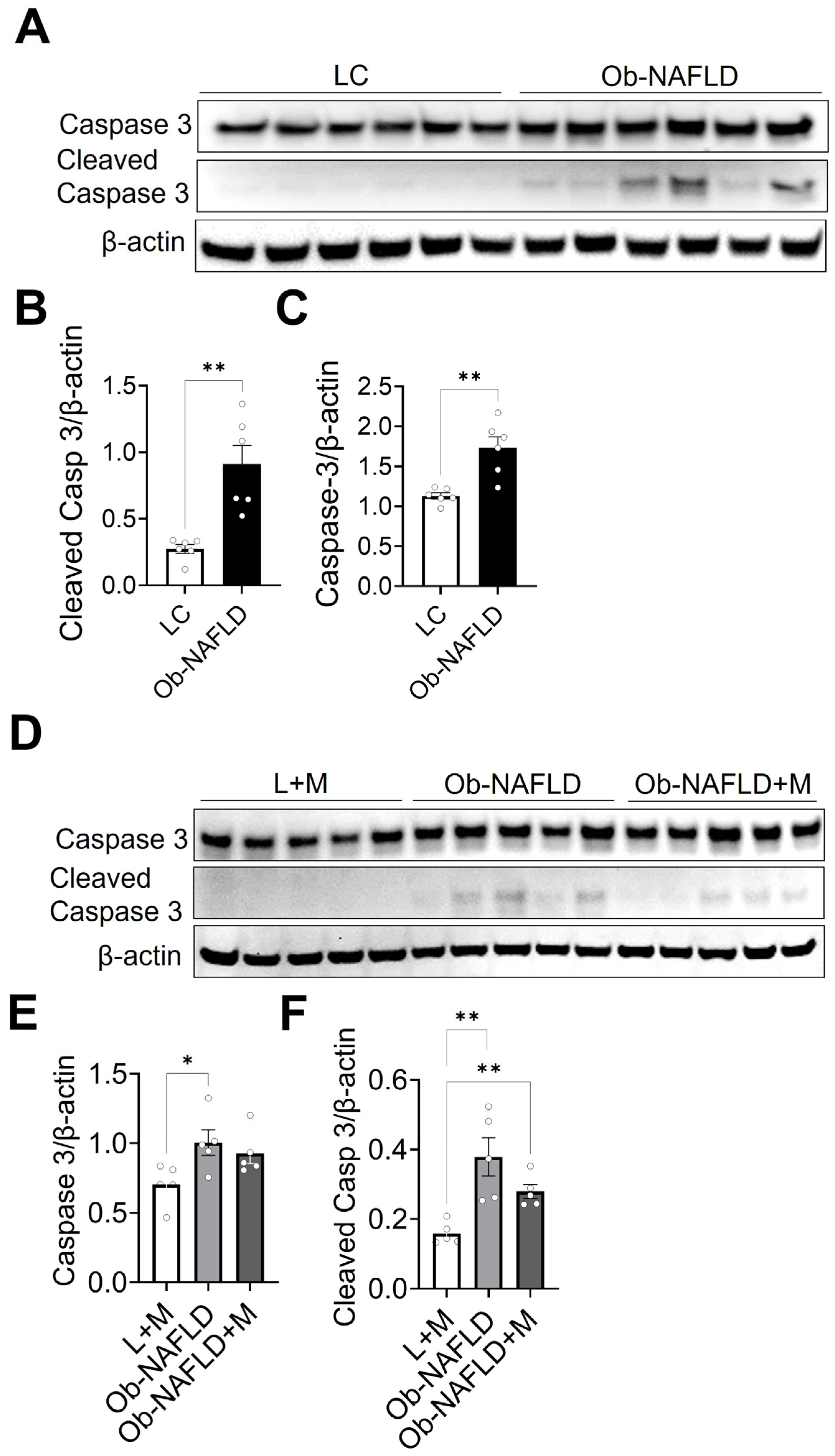
3.6. Ob-NAFLD Enhances Apoptosis in Kidneys; Short-Term Oral Metformin Offers Modest Modulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NAFLD | Non-alcoholic fatty liver disease |
| Ob | Obesity |
| CKD | Chronic kidney disease |
| LC | Lean control |
| M | Metformin |
| TBS | Tris buffered saline |
| TNF-a | Tumor necrosis factor alpha |
| Ccl2 | The chemokine (c-c motif) ligand 2 |
| RIPA | Radio immune-absorbent assay |
| SOD | Superoxide dismutase |
| GSTP | Glutathione S-transferase P (GST1) gene |
| CXCR2 | CXC chemokine receptor 2 |
| FSGS | Focal segmental glomerulosclerosis |
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Cao, Y.; Deng, Y.; Wang, J.; Zhao, H.; Zhang, J.; Xie, W. The association between NAFLD and risk of chronic kidney disease: A cross-sectional study. Ther. Adv. Chronic. Dis. 2021, 12, 20406223211048649. [Google Scholar] [CrossRef]
- Ghazy, F.; Ebrahimi, N.; Ebadinejad, A.; Barzin, M.; Mahdavi, M.; Valizadeh, M.; Azizi, F.; Hosseinpanah, F. Association of obesity severity and duration with incidence of chronic kidney disease. BMC Nephrol. 2024, 25, 320. [Google Scholar] [CrossRef]
- Pacifico, L.; Bonci, E.; Andreoli, G.M.; Di Martino, M.; Gallozzi, A.; De Luca, E.; Chiesa, C. The impact of nonalcoholic fatty liver disease on renal function in children with overweight/obesity. Int. J. Mol. Sci. 2016, 17, 1218. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Schattenberg, J.M.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: An updated meta-analysis. Gut 2022, 71, 156–162. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagstrom, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar] [CrossRef]
- De Taeye, B.M.; Novitskaya, T.; McGuinness, O.P.; Gleaves, L.; Medda, M.; Covington, J.W.; Vaughan, D.E. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E713–E725. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Luo, J.; Pan, X.; Wei, J.; Xiao, X.; Li, J.; Luo, M. Association between inflammatory markers and non-alcoholic fatty liver disease in obese children. Front. Public Health 2022, 10, 991393. [Google Scholar] [CrossRef]
- Fontes-Cal, T.C.M.; Mattos, R.T.; Medeiros, N.I.; Pinto, B.F.; Belchior-Bezerra, M.; Roque-Souza, B.; Dutra, W.O.; Ferrari, T.C.A.; Vidigal, P.V.T.; Faria, L.C.; et al. Crosstalk between plasma cytokines, inflammation, and liver damage as a new strategy to monitoring nafld progression. Front. Immunol. 2021, 12, 708959. [Google Scholar] [CrossRef]
- Kumar, R.; Prakash, S.; Chhabra, S.; Singla, V.; Madan, K.; Gupta, S.D.; Panda, S.K.; Khanal, S.; Acharya, S.K. Association of pro-inflammatory cytokines, adipokines & oxidative stress with insulin resistance & non-alcoholic fatty liver disease. Indian J. Med. Res. 2012, 136, 229–236. [Google Scholar] [PubMed]
- Schattenberg, J.M.; Singh, R.; Wang, Y.; Lefkowitch, J.H.; Rigoli, R.M.; Scherer, P.E.; Czaja, M.J. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology 2006, 43, 163–172. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hakkak, R.; Rose, S.; Spray, B.; Kozaczek, M.; Korourian, S. Effects of obesity and 10 weeks metformin treatment on liver steatosis. Biomed. Rep. 2021, 14, 49. [Google Scholar] [CrossRef]
- Woo, S.L.; Xu, H.; Li, H.; Zhao, Y.; Hu, X.; Zhao, J.; Guo, X.; Guo, T.; Botchlett, R.; Qi, T.; et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS ONE 2014, 9, e91111. [Google Scholar] [CrossRef]
- Kim, M.H.; Oh, H.J.; Kwon, S.H.; Jeon, J.S.; Noh, H.; Han, D.C.; Kim, H.; Ryu, D.R. Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: A nationwide cohort study. Kidney Res. Clin. Pract. 2021, 40, 660–672. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, Y.C.; Park, J.Y.; Lee, J.; An, J.N.; Kim, C.T.; Oh, S.; Park, S.; Kim, D.K.; Oh, Y.K.; et al. The long-term effects of metformin on patients with type 2 diabetic kidney disease. Diabetes Care 2020, 43, 948–955. [Google Scholar] [CrossRef]
- Melnyk, S.; Hakkak, R. Metabolic status of lean and obese zucker rats based on untargeted and targeted metabolomics analysis of serum. Biomedicines 2022, 10, 153. [Google Scholar] [CrossRef]
- Melnyk, S.; Hakkak, R. Effect of metformin treatment on serum metabolic profile changes in lean and obese zucker rat model for fatty liver disease. Biomolecules 2023, 13, 1234. [Google Scholar] [CrossRef]
- Bhattarai, D.; Lee, S.O.; Joshi, N.; Jun, S.R.; Lo, S.; Jiang, L.; Gokden, N.; Parajuli, N. Cold storage followed by transplantation induces immunoproteasome in rat kidney allografts: Inhibition of immunoproteasome does not improve function. Kidney360 2024, 5, 743–752. [Google Scholar] [CrossRef]
- Lo, S.; Byrum, S.D.; Tackett, A.J.; Parajuli, N. Cold storage increases albumin and advanced glycation-end product-albumin levels in kidney transplants: A possible cause for exacerbated renal damage. Transplant. Direct. 2019, 5, e454. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.; Jiang, L.; Stacks, S.; Lin, H.; Parajuli, N. Aberrant activation of the complement system in renal grafts is mediated by cold storage. Am. J. Physiol. Renal. Physiol. 2021, 320, F1174–F1190. [Google Scholar] [CrossRef]
- Sharma, A.; Jun, S.R.; Bhattarai, D.; Panday, S.; Venugopal, G.; Panawan, O.; Washam, C.; Mackintosh, S.; Byrum, S.; Udaondo, Z.; et al. Cold storage disrupts the proteome and phosphoproteome landscape in rat kidney transplants. Transplantation 2025, 109, 806–822. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, N.; Muller-Holzner, E.; Bock, G.; Werner, E.R.; Villunger, A.; Doppler, W. Infiltrating CD11b+CD11c+ cells have the potential to mediate inducible nitric oxide synthase-dependent cell death in mammary carcinomas of HER-2/neu transgenic mice. Int. J. Cancer 2010, 126, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Simsek, O.; Baris, E.; Ural, C.; Incir, C.; Aydemir, S.; Gumustekin, M.; Cavdar, Z.; Yilmaz, O.; Ozbal, S.; Arici, M.A. Preventative effect of montelukast in mild to moderate contrast-induced acute kidney injury in rats via NADPH oxidase 4, p22phox and nuclear factor kappa-B expressions. Int. Urol. Nephrol. 2025, 57, 2313–2325. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, S.H.; Park, K.H.; Zhi-Wei, Y.; Jie, Z.; Townsend, D.M.; Tew, K.D. Glutathione S-Transferase P influences redox homeostasis and response to drugs that induce the unfolded protein response in zebrafish. J. Pharmacol. Exp. Ther. 2021, 377, 121–132. [Google Scholar] [CrossRef]
- Crapo, J.D.; Oury, T.; Rabouille, C.; Slot, J.W.; Chang, L.Y. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc. Natl. Acad. Sci. USA 1992, 89, 10405–10409. [Google Scholar] [CrossRef]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St Clair, D.K. Manganese superoxide dismutase: Guardian of the powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef]
- Behrens, K.A.; Jania, L.A.; Snouwaert, J.N.; Nguyen, M.; Moy, S.S.; Tikunov, A.P.; Macdonald, J.M.; Koller, B.H. Beyond detoxification: Pleiotropic functions of multiple glutathione S-transferase isoforms protect mice against a toxic electrophile. PLoS ONE 2019, 14, e0225449. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A. Antiviral immune responses in the genital tract: Clues for vaccines. Nat. Rev. Immunol. 2010, 10, 699–711. [Google Scholar] [CrossRef]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- Awad, A.S.; Kinsey, G.R.; Khutsishvili, K.; Gao, T.; Bolton, W.K.; Okusa, M.D. Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. Am. J. Physiol. Renal. Physiol. 2011, 301, F1358–F1366. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Harris, D.C.; Wang, Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology 2015, 30, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.T.; Xue, J.Y.; Lin, M.C.; Spokas, E.G.; Sun, F.F.; Wong, P.Y. Peroxynitrite induces apoptosis of HL-60 cells by activation of a caspase-3 family protease. Am. J. Physiol. 1998, 274, C855–C860. [Google Scholar] [CrossRef]
- Thongnak, L.; Chatsudthipong, V.; Lungkaphin, A. Mitigation of renal inflammation and endoplasmic reticulum stress by vildagliptin and statins in high-fat high-fructose diet-induced insulin resistance and renal injury in rats. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2020, 1865, 158755. [Google Scholar] [CrossRef]
- Abbate, M.; Mascaro, C.M.; Montemayor, S.; Casares, M.; Gomez, C.; Ugarriza, L.; Tejada, S.; Abete, I.; Zulet, M.A.; Sureda, A.; et al. Non-alcoholic fatty liver disease is associated with kidney glomerular hyperfiltration in adults with metabolic syndrome. J. Clin. Med. 2021, 10, 1717. [Google Scholar] [CrossRef]
- Nam, G.E.; Hwang, S.Y.; Chung, H.S.; Choi, J.H.; Lee, H.J.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; et al. Implication of nonalcoholic fatty liver disease, metabolic syndrome, and subclinical inflammation on mild renal insufficiency. Int. J. Endocrinol. 2018, 2018, 1835486. [Google Scholar] [CrossRef] [PubMed]
- Cantero-Navarro, E.; Rayego-Mateos, S.; Orejudo, M.; Tejedor-Santamaria, L.; Tejera-Munoz, A.; Sanz, A.B.; Marquez-Exposito, L.; Marchant, V.; Santos-Sanchez, L.; Egido, J.; et al. Role of macrophages and related cytokines in kidney disease. Front. Med. 2021, 8, 688060. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, S.C.; Ko, Y.S.; Lee, H.Y.; Jo, S.K.; Cho, W. The role of m2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS ONE 2015, 10, e0143961. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Shen, A.; Tang, T.; Li, N.; Xu, C.; Liu, B.; Lv, L. Mincle receptor in macrophage and neutrophil contributes to the unresolved inflammation during the transition from acute kidney injury to chronic kidney disease. Front. Immunol. 2024, 15, 1385696. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, L.; Sung, S.S.; Vergis, A.L.; Rosin, D.L.; Rose, C.E., Jr.; Lobo, P.I.; Okusa, M.D. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008, 74, 1526–1537. [Google Scholar] [CrossRef]
- Segerer, S.; Nelson, P.J.; Schlondorff, D. Chemokines, chemokine receptors, and renal disease: From basic science to pathophysiologic and therapeutic studies. J. Am. Soc. Nephrol. 2000, 11, 152–176. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Kanwar, Y.S. Relevance of TNF-alpha in the context of other inflammatory cytokines in the progression of diabetic nephropathy. Kidney Int. 2015, 88, 662–665. [Google Scholar] [CrossRef]
- Das, S.; Alhasson, F.; Dattaroy, D.; Pourhoseini, S.; Seth, R.K.; Nagarkatti, M.; Nagarkatti, P.S.; Michelotti, G.A.; Diehl, A.M.; Kalyanaraman, B.; et al. NADPH Oxidase-derived peroxynitrite drives inflammation in mice and human nonalcoholic steatohepatitis via tlr4-lipid raft recruitment. Am. J. Pathol. 2015, 185, 1944–1957. [Google Scholar] [CrossRef]
- Gonzalez, A.; Huerta-Salgado, C.; Orozco-Aguilar, J.; Aguirre, F.; Tacchi, F.; Simon, F.; Cabello-Verrugio, C. Role of oxidative stress in hepatic and extrahepatic dysfunctions during Nonalcoholic Fatty Liver Disease (NAFLD). Oxid. Med. Cell. Longev. 2020, 2020, 1617805. [Google Scholar] [CrossRef]
- Hou, X.; Song, J.; Li, X.N.; Zhang, L.; Wang, X.; Chen, L.; Shen, Y.H. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem. Biophys. Res. Commun. 2010, 396, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ouslimani, N.; Peynet, J.; Bonnefont-Rousselot, D.; Therond, P.; Legrand, A.; Beaudeux, J.L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 2005, 54, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Hakkak, R.; Zeng, H.; Dhakal, I.B.; Korourian, S. Short- and long-term soy diet versus casein protects liver steatosis independent of the arginine content. J. Med. Food 2015, 18, 1274–1280. [Google Scholar] [CrossRef] [PubMed]


| Name | Company | Catalog No. | Host | Dilution |
|---|---|---|---|---|
| Cu/ZnSOD | MilliporeSigma | HPA001401 | Rabbit | 1:1000 (WB) |
| Ccl2 | LSBio | C201039 | Rabbit | 1:1000 (WB) |
| MnSOD | MilliporeSigma | 6984 | Rabbit | 1:1000 (WB) |
| GST-P | MBL | 311 | Rabbit | 1:1000 (WB) |
| Caspase 3 | Cell Signaling | 14220 | Rabbit | 1:1000 (WB) |
| Cleaved Caspase 3 | Cell Signaling | 14220 | Rabbit | 1:1000 (WB) |
| Actin | Invitrogen | MA5-15739 | Mouse | 1:1000 (WB) |
| CD68 | Abcam | ab125212 | Rabbit | 1:200 (IHC) |
| Nitrotyrosine | Millipore | 06-284 | Rabbit | 1:2000 (IHC) |
| Neutrophil Elastase | Cell Signaling | 44030 | Rabbit | 1:400 (IHC) |
| P22phox | Cell Signaling | 37570 | Rabbit | 1:1000 (IHC) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Hakkak, R.; Gokden, N.; Joshi, N.; Parajuli, N. Obesity-Associated NAFLD Coexists with a Chronic Inflammatory Kidney Condition That Is Partially Mitigated by Short-Term Oral Metformin. Nutrients 2025, 17, 2115. https://doi.org/10.3390/nu17132115
Sharma A, Hakkak R, Gokden N, Joshi N, Parajuli N. Obesity-Associated NAFLD Coexists with a Chronic Inflammatory Kidney Condition That Is Partially Mitigated by Short-Term Oral Metformin. Nutrients. 2025; 17(13):2115. https://doi.org/10.3390/nu17132115
Chicago/Turabian StyleSharma, Amod, Reza Hakkak, Neriman Gokden, Neelam Joshi, and Nirmala Parajuli. 2025. "Obesity-Associated NAFLD Coexists with a Chronic Inflammatory Kidney Condition That Is Partially Mitigated by Short-Term Oral Metformin" Nutrients 17, no. 13: 2115. https://doi.org/10.3390/nu17132115
APA StyleSharma, A., Hakkak, R., Gokden, N., Joshi, N., & Parajuli, N. (2025). Obesity-Associated NAFLD Coexists with a Chronic Inflammatory Kidney Condition That Is Partially Mitigated by Short-Term Oral Metformin. Nutrients, 17(13), 2115. https://doi.org/10.3390/nu17132115







