Dietary Fats Substitution and Blood Pressure Levels: A Longitudinal Study in Mexican Adults
Abstract
1. Introduction
2. Materials and Methods
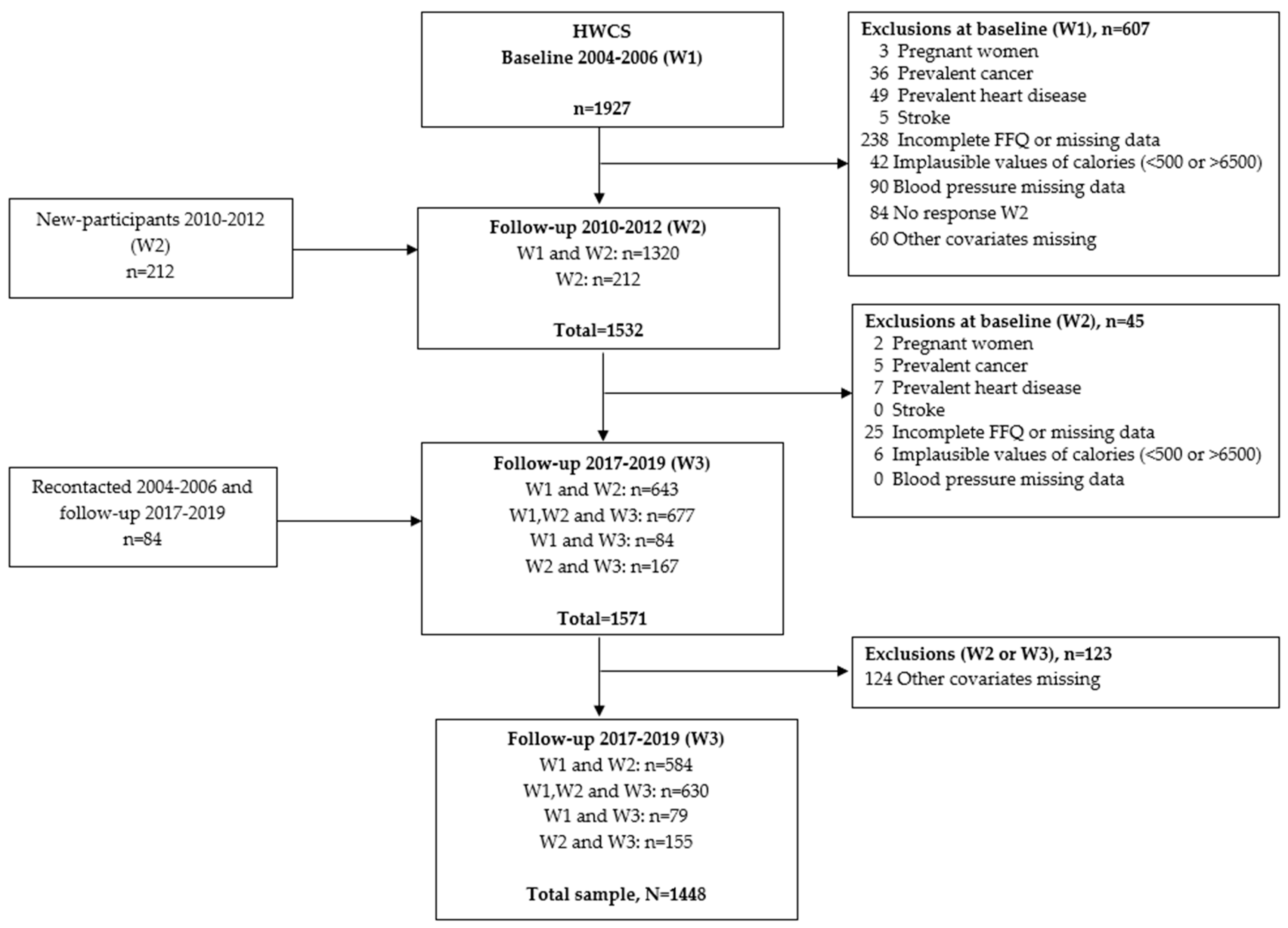
2.1. Study Population
2.2. Dietary Assessment
2.3. Blood Pressure Assessment
2.4. Covariates
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HBP | High Blood Pressure |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| CVD | Cardiovascular Disease |
| PUFA | Polyunsaturated Fat |
| AHA | American Heart Association |
| SFA | Saturated Fat |
| MUFA | Monounsaturated Fat |
| HWCS | Health Workers Cohort Study |
| FFQ | Food Frequency Questionnaire |
| GI | Glycemic Index |
| LGI | Low Glycemic Index |
| HGI | High Glycemic Index |
| EI | Energy Intake |
| WHO | World Health Organization |
| PA | Physical Activity |
| BMI | Body Mass Index |
| T2D | Type 2 Diabetes |
| GEE | Generalized Estimating Equations |
References
- Campos-Nonato, I.; Hernández-Barrera, L.; Pedroza-Tobías, A.; Medina, C.; Barquera, S. Hipertensión arterial en adultos mexicanos: Prevalencia, diagnóstico y tipo de tratamiento. Ensanut MC 2016. Salud Publica Mex. 2018, 60, 233–243. [Google Scholar] [CrossRef]
- Campos-Nonato, I.; Oviedo-Solís, C.; Vargas-Meza, J.; Ramírez-Villalobos, D.; Medina-García, C.; Gómez-Álvarez, E.; Hernández-Barrera, L.; Barquera, S. Prevalencia, tratamiento y control de la hipertensión arterial en adultos mexicanos: Resultados de la Ensanut 2022. Salud Publica Mex. 2023, 65, s169–s180. [Google Scholar] [CrossRef] [PubMed]
- Campos-Nonato, I.; Hernández-Barrera, L.; Rojas-Martínez, R.; Pedroza, A.; Medina-García, C.; Barquera-Cervera, S. [Hypertension: Prevalence, early diagnosis, control and trends in Mexican adults]. Salud Publica Mex. 2013, 55 (Suppl. 2), S144–S150. [Google Scholar] [CrossRef] [PubMed]
- Campos-Nonato, I.; Oviedo-Solís, C.; Hernández-Barrera, L.; Márquez-Murillo, M.; Gómez-Álvarez, E.; Alcocer-Díaz, L.; Puente-Barragán, A.; Ramírez-Villalobos, D.; Basto-Abreu, A.; Rojas-Martínez, R.; et al. Detección, atención y control de hipertensión arterial. Salud Publica Mex. 2024, 66, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Conyer, R.; Alegre-Díaz, J.; Gnatiuc, L.; Wade, R.; Ramirez-Reyes, R.; Herrington, W.G.; Lewington, S.; Clarke, R.; Collins, R.; Peto, R.; et al. Association of Blood Pressure with Cause-Specific Mortality in Mexican Adults. JAMA Netw. Open 2020, 3, e2018141. [Google Scholar] [CrossRef]
- Kolifarhood, G.; Daneshpour, M.; Hadaegh, F.; Sabour, S.; Mozafar Saadati, H.; Akbar Haghdoust, A.; Akbarzadeh, M.; Sedaghati-Khayat, B.; Khosravi, N. Heritability of blood pressure traits in diverse populations: A systematic review and meta-analysis. J. Hum. Hypertens. 2019, 33, 775–785. [Google Scholar] [CrossRef]
- Charchar, F.J.; Prestes, P.R.; Mills, C.; Ching, S.M.; Neupane, D.; Marques, F.Z.; Sharman, J.E.; Vogt, L.; Burrell, L.M.; Korostovtseva, L.; et al. Lifestyle management of hypertension: International Society of Hypertension position paper endorsed by the World Hypertension League and European Society of Hypertension. J. Hypertens. 2024, 42, 23–49. [Google Scholar] [CrossRef]
- Ojangba, T.; Boamah, S.; Miao, Y.; Guo, X.; Fen, Y.; Agboyibor, C.; Yuan, J.; Dong, W. Comprehensive effects of lifestyle reform, adherence, and related factors on hypertension control: A review. J. Clin. Hypertens. 2023, 25, 509–520. [Google Scholar] [CrossRef]
- Zhang, X.; Ouyang, Y.; Huang, F.; Zhang, J.; Su, C.; Jia, X.; Du, W.; Li, L.; Bai, J.; Zhang, B.; et al. Modifiable factors of 20-year blood pressure trajectories among normotensives and their associations with hypertension: A prospective study. Br. J. Nutr. 2022, 128, 252–262. [Google Scholar] [CrossRef]
- Cherfan, M.; Vallée, A.; Kab, S.; Salameh, P.; Goldberg, M.; Zins, M.; Blacher, J. Unhealthy behaviors and risk of uncontrolled hypertension among treated individuals-The CONSTANCES population-based study. Sci. Rep. 2020, 10, 1925. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; de Castro Ruiz Marques, A.; Li, D.; Ho, H.V.T.; Blanco Mejia, S.; Sievenpiper, J.L.; Zurbau, A.; Komishon, A.; Duvnjak, L.; Bazotte, R.B.; et al. Effect of high-carbohydrate or high-monounsaturated fatty acid diets on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2019, 77, 19–31. [Google Scholar] [CrossRef]
- Clifton, P.M.; Keogh, J.B. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1060–1080. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Nestel, P.J. Dietary Fat and Blood Pressure. Curr. Hypertens. Rep. 2019, 21, 17. [Google Scholar] [CrossRef]
- Heileson, J.L. Dietary saturated fat and heart disease: A narrative review. Nutr. Rev. 2020, 78, 474–485. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266S–273S. [Google Scholar] [CrossRef]
- Barquera, S.; Campos, I.; Rivera, J.A. Mexico attempts to tackle obesity: The process, results, push backs and future challenges. Obes. Rev. 2013, 14 (Suppl. 2), 69–78. [Google Scholar] [CrossRef]
- Marrón-Ponce, J.A.; Flores, M.; Cediel, G.; Monteiro, C.A.; Batis, C. Associations between Consumption of Ultra-Processed Foods and Intake of Nutrients Related to Chronic Non-Communicable Diseases in Mexico. J. Acad. Nutr. Diet. 2019, 119, 1852–1865. [Google Scholar] [CrossRef] [PubMed]
- Landa-Gómez, N.; Barragán-Vázquez, S.; Salazar-Piña, A.; Olvera-Mayorga, G.; Méndez Gómez-Humarán, I.; Carriquiry, A.; Da Silva Gomes, F.; Ramírez-Silva, C.I. Intake of trans fats and other fatty acids in Mexican adults: Results from the 2012 and 2016 National Health and Nutrition Surveys. Salud Publica Mex. 2024, 66, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Denova-Gutierrez, E.; Flores, Y.N.; Gallegos-Carrillo, K.; Ramirez-Palacios, P.; Rivera-Paredez, B.; Munoz-Aguirre, P.; Velazquez-Cruz, R.; Torres-Ibarra, L.; Meneses-Leon, J.; Mendez-Hernandez, P.; et al. Health workers cohort study: Methods and study design. Salud Publica Mex. 2016, 58, 708–716. [Google Scholar] [CrossRef]
- Hernández-Avila, M.; Romieu, I.; Parra, S.; Hernández-Avila, J.; Madrigal, H.; Willett, W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998, 40, 133–140. [Google Scholar] [CrossRef]
- Hernández-Avila, M.; Resoles, M.; Parra, S.; Romieu, I. Sistema de Evaluación de Hábitos Nutricionales y Consumo de Nutrimentos (SNUT); INSP: Cuernavaca, México, 2000. [Google Scholar]
- World Health Organization. A global Brief on Hypertension: Silent Killer, Global Public Health Crisis: World Health Day 2013; WHO: Ginebra, Switzerland, 2013. [Google Scholar]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- World Health Organization. Physical Activity; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetesd2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar]
- Allison, P.D. Fixed Effects Regression Methods for Longitudinal Data; SAS Institute Inc.: Cary, NC, USA, 2005. [Google Scholar]
- Song, J.-J.; Ma, Z.; Wang, J.; Chen, L.-X.; Zhong, J.-C. Gender Differences in Hypertension. J. Cardiovasc. Transl. Res. 2020, 13, 47–54. [Google Scholar] [CrossRef]
- LIANG, K.-Y.; ZEGER, S.L. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Ahola, A.J.; Harjutsalo, V.; Thorn, L.M.; Freese, R.; Forsblom, C.; Mäkimattila, S.; Groop, P.-H. The association between macronutrient intake and the metabolic syndrome and its components in type 1 diabetes. Br. J. Nutr. 2017, 117, 450–456. [Google Scholar] [CrossRef]
- Miller, E.R., 3rd; Erlinger, T.P.; Appel, L.J. The effects of macronutrients on blood pressure and lipids: An overview of the DASH and OmniHeart trials. Curr. Atheroscler. Rep. 2006, 8, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, H.; Stamler, J.; Elliott, P.; Chan, Q.; Brown, I.J.; Carnethon, M.R.; Daviglus, M.L.; He, K.; Moag-Stahlberg, A.; Rodriguez, B.L.; et al. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension 2007, 50, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Stamler, J.; Nakagawa, H.; Elliott, P.; Ueshima, H.; Chan, Q.; Brown, I.J.; Tzoulaki, I.; Saitoh, S.; Dyer, A.R.; et al. Relationship of dietary linoleic acid to blood pressure. The International Study of Macro-Micronutrients and Blood Pressure Study [corrected]. Hypertension 2008, 52, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993, 88, 523–533. [Google Scholar] [CrossRef]
- Zhang, X.; Ritonja, J.A.; Zhou, N.; Chen, B.E.; Li, X. Omega-3 Polyunsaturated Fatty Acids Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2022, 11, e025071. [Google Scholar] [CrossRef]
- Mourad, J.-J. The evolution of systolic blood pressure as a strong predictor of cardiovascular risk and the effectiveness of fixed-dose ARB/CCB combinations in lowering levels of this preferential target. Vasc. Health Risk Manag. 2008, 4, 1315–1325. [Google Scholar] [CrossRef]
- Anderson, T.J. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can. J. Cardiol. 2006, 22 (Suppl. B), 72B–80B. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef]
- Yagi, S.; Fukuda, D.; Aihara, K.-I.; Akaike, M.; Shimabukuro, M.; Sata, M. n-3 Polyunsaturated Fatty Acids: Promising Nutrients for Preventing Cardiovascular Disease. J. Atheroscler. Thromb. 2017, 24, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Cicero, A.F.G. Omega-3 polyunsaturated fatty acids: Their potential role in blood pressure prevention and management. Heart Int. 2006, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Merkel, M.; Velez-Carrasco, W.; Hudgins, L.C.; Breslow, J.L. Compared with saturated fatty acids, dietary monounsaturated fatty acids and carbohydrates increase atherosclerosis and VLDL cholesterol levels in LDL receptor-deficient, but not apolipoprotein E-deficient, mice. Proc. Natl. Acad. Sci. USA 2001, 98, 13294–13299. [Google Scholar] [CrossRef]
- Ong, P.J.; Dean, T.S.; Hayward, C.S.; Della Monica, P.L.; Sanders, T.A.; Collins, P. Effect of fat and carbohydrate consumption on endothelial function. Lancet 1999, 354, 2134. [Google Scholar] [CrossRef]
- Hammad, S.; Pu, S.; Jones, P.J. Current Evidence Supporting the Link Between Dietary Fatty Acids and Cardiovascular Disease. Lipids 2016, 51, 507–517. [Google Scholar] [CrossRef]
- Nakamura, H.; Tsujiguchi, H.; Kambayashi, Y.; Hara, A.; Miyagi, S.; Yamada, Y.; Nguyen, T.T.T.; Shimizu, Y.; Hori, D.; Nakamura, H. Relationship between saturated fatty acid intake and hypertension and oxidative stress. Nutrition 2019, 61, 8–15. [Google Scholar] [CrossRef]
- Praagman, J.; Beulens, J.W.; Alssema, M.; Zock, P.L.; Wanders, A.J.; Sluijs, I.; van der Schouw, Y.T. The association between dietary saturated fatty acids and ischemic heart disease depends on the type and source of fatty acid in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Am. J. Clin. Nutr. 2016, 103, 356–365. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Holt, S.H.A.; Pawlak, D.B.; McMillan, J. Glycemic index and obesity. Am. J. Clin. Nutr. 2002, 76, 281S–285S. [Google Scholar] [CrossRef]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef]
- Raj, A.; Chakole, S.; Agrawal, S.; Gupta, A.; Khekade, H.; Prasad, R.; Lohakare, T.; Wanjari, M. The Impact of Menopause on Cardiovascular Aging: A Comprehensive Review of Androgen Influences. Cureus 2023, 15, e43569. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J.A.; Follmann, D.; Allender, P.S. Randomized trials of sodium reduction: An overview. Am. J. Clin. Nutr. 1997, 65, 643S–651S. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Baseline | Changes |
|---|---|---|
| Median (p25, p75) | Median (p25, p75) | |
| Age (years) | 45 (36, 53) | 7 (6, 10) |
| Systolic Blood pressure (mm Hg) | 115 (108, 123) | 3 (−7, 13) |
| Diastolic Blood pressure (mm Hg) | 71 (65, 78) | 3 (−4, 11) |
| Sleep time (hours/day) | 7.3 (6.6, 8.0) | 0 (−0.71, 0.71) |
| Physical activity (min/day) | 12.9 (3.2, 34.9) | 0 (−15.6, 11.4) |
| BMI (kg/m2) | 25.9 (23.5, 28.8) | 0.6 (−0.7, 2.0) |
| Depressive symptoms | 14 (12, 19) | 0 (−4, 4) |
| Dietary variables | ||
| Total energy (kcal/day) | 1921 (1473, 2478) | −282 (−799, 165) |
| Animal protein (% of energy) | 14.9 (11.6, 18.5) | −2.5 (−6.7, 1.6) |
| Vegetal protein (% of energy) | 8.6 (7.0, 10.5) | 1.3 (−1.1, 4.0) |
| Animal fat (% of energy) | 14.9 (11.6, 18.5) | −2.5 (−6.7, 1.6) |
| Vegetal fat (% of energy) | 8.6 (7.0, 10.5) | 1.2 (−1.0, 3.5) |
| Saturated fat (% of energy) | 8.6 (7.1, 10.4) | −0.9 (−2.8, 1.0) |
| Monounsaturated fat (% of energy) | 9.8 (8.3, 11.6) | −0.3 (−2.4, 1.7) |
| Polyunsaturated fat (% of energy) | 4.2 (3.6, 5.0) | 0.2 (−0.6, 1.2) |
| High glycemic index carbohydrate (% of energy) | 43.2 (37.1, 49.1) | 0.4 (−7.0, 7.6) |
| Low glycemic index carbohydrate (% of energy) | 17.7 (13.6, 22.9) | 1.6 (−3.5, 6.5) |
| Alcohol (g/day) | 1.0 (0.2, 4.0) | 0 (−0.9, 0.6) |
| Sodium (g/day) | 1.7 (1.3, 2.3) | −0.3 (−0.8, 0.2) |
| Macronutrients Intake | SBP | DBP | ||
|---|---|---|---|---|
| Increased | Decreased | β (IC95%) | β (IC95%) | |
| Unadjusted | Saturated fat | Vegetal Protein | 1.17 (−0.76, 3.09) | 0.46 (−1.93, 2.86) |
| Adjusted | Saturated fat | Vegetal Protein | 0.16 (−1.80, 2.11) | −0.43 (−3.00, 2.13) |
| Unadjusted | Monounsaturated fat | Vegetal Protein | 0.48 (−1.13, 2.09) | 0.51 (−1.49, 2.52) |
| Adjusted | Monounsaturated fat | Vegetal Protein | 0.02 (−1.64, 1.69) | 0.02 (−2.16, 2.20) |
| Unadjusted | Polyunsaturated fat | Vegetal Protein | −0.57 (−2.58, 1.43) | 2.91 (0.42, 5.41) * |
| Adjusted | Polyunsaturated fat | Vegetal Protein | −2.94 (−5.02, −0.86) ** | 0.79 (−1.93, 3.50) |
| Unadjusted | Saturated fat | Animal Protein | −0.31 (−1.95, 1.34) | −0.13 (−2.17, 1.90) |
| Adjusted | Saturated fat | Animal Protein | −0.86 (−2.56, 0.84) | −0.68 (−2.91, 1.54) |
| Unadjusted | Monounsaturated fat | Animal Protein | −0.27 (−2.56, 2.02) | 0.16 (−2.69, 3.00) |
| Adjusted | Monounsaturated fat | Animal Protein | 0.98 (−1.36, 3.31) | 1.24 (−1.82, 4.29) |
| Unadjusted | Polyunsaturated fat | Animal Protein | −0.17 (−2.17, 1.82) | 3.08 (0.60, 5.55)* |
| Adjusted | Polyunsaturated fat | Animal Protein | −2.68 (−4.73, −0.63) * | 0.79 (−1.90, 3.48) |
| Unadjusted | Saturated fat | LGI Carbohydrate | −2.08 (−3.13, −1.04) ** | −1.78 (−3.08, −0.49) ** |
| Adjusted | Saturated fat | LGI Carbohydrate | −0.57 (−1.65, 0.51) | −0.41 (−1.82, 1.00) |
| Unadjusted | Monounsaturated fat | LGI Carbohydrate | 0.33 (−0.96, 1.62) | −0.19 (−1.79, 1.41) |
| Adjusted | Monounsaturated fat | LGI Carbohydrate | 0.50 (−0.77, 1.78) | −0.12 (−1.79, 1.54) |
| Unadjusted | Polyunsaturated fat | LGI Carbohydrate | −1.05 (−2.84, 0.74) | 1.97 (−0.26, 4.19) |
| Adjusted | Polyunsaturated fat | LGI Carbohydrate | −2.63 (−4.40, −0.86) ** | 0.59 (−1.72, 2.91) |
| Unadjusted | Saturated fat | HGI Carbohydrate | −1.55 (−2.54, −0.56) ** | −1.79 (−3.02, −0.56) ** |
| Adjusted | Saturated fat | HGI Carbohydrate | −0.42 (−1.44, 0.59) | −0.71 (−2.04, 0.62) |
| Unadjusted | Monounsaturated fat | HGI Carbohydrate | 0.72 (−0.55, 1.98) | −0.20 (−1.77, 1.36) |
| Adjusted | Monounsaturated fat | HGI Carbohydrate | 0.61 (−0.63, 1.86) | −0.36 (−1.99, 1.27) |
| Unadjusted | Polyunsaturated fat | HGI Carbohydrate | −0.61 (−2.40, 1.19) | 2.00 (−0.23, 4.23) |
| Adjusted | Polyunsaturated fat | HGI Carbohydrate | −2.52 (−4.31, −0.74) ** | 0.33 (−2.01, 2.66) |
| Males, n = 387 | Females, n = 1060 | |||||
|---|---|---|---|---|---|---|
| Macronutrients Intake | SBP | DBP | SBP | DBP | ||
| Increased | Decreased | β (IC95%) | β (IC95%) | β (IC95%) | β (IC95%) | |
| Unadjusted | Saturated fat | Vegetal Protein | 4.14 (−0.07, 8.36) | 2.00 (−1.70, 5.71) | 0.58 (−1.58, 2.75) | 0.05 (−2.89, 2.98) |
| Adjusted | Saturated fat | Vegetal Protein | 1.87 (−2.25, 5.98) | 1.01 (−2.69, 4.70) | −0.17 (−2.41, 2.06) | −0.74 (−3.95, 2.47) |
| Unadjusted | Monounsaturated fat | Vegetal Protein | 4.02 (−0.11, 8.15) | 2.03 (−1.60, 5.67) | −0.22 (−1.97, 1.52) | 0.27 (−2.10, 2.64) |
| Adjusted | Monounsaturated fat | Vegetal Protein | 3.29 (−0.74, 7.31) | 1.83 (−1.78, 5.44) | −0.59 (−2.43, 1.25) | −0.27 (−2.91, 2.37) |
| Unadjusted | Polyunsaturated fat | Vegetal Protein | −3.14 (−7.42, 1.13) | 1.16 (−2.59, 4.92) | 0.21 (−2.06, 2.48) | 3.58 (0.50, 6.66) * |
| Adjusted | Polyunsaturated fat | Vegetal Protein | −6.57 (−10.96, 2.17) ** | −1.72 (−5.66, 2.22) | −1.80 (−4.16, 0.57) | 1.82 (−1.57, 5.21) |
| Unadjusted | Saturated fat | Animal Protein | 2.24 (−1.46, 5.94) | 1.22 (−2.03, 4.47) | −0.72 (−2.55, 1.11) | −0.51 (−2.99, 1.97) |
| Adjusted | Saturated fat | Animal Protein | 0.65 (−3.06, 4.36) | 0.70 (−2.63, 4.03) | −1.01 (−2.93, 0.91) | −1.07 (−3.82, 1.68) |
| Unadjusted | Monounsaturated fat | Animal Protein | 5.07 (−0.08, 10.21) | 0.64 (−3.88, 5.16) | −1.70 (−4.28, 0.87) | 0.34 (−3.15, 3.83) |
| Adjusted | Monounsaturated fat | Animal Protein | 6.19 (1.26, 11.12) * | 2.37 (−2.06, 6.81) | −0.50 (−3.19, 2.19) | 1.22 (−2.63, 5.07) |
| Unadjusted | Polyunsaturated fat | Animal Protein | −2.42 (−6.64, 1.81) | 1.39 (−2.32, 5.10) | 0.55 (−1.71, 2.81) | 3.72 (0.66, 6.78) * |
| Adjusted | Polyunsaturated fat | Animal Protein | −6.09 (−10.38, −1.80) ** | −1.59 (−5.45, 2.26) | −1.59 (−3.93, 0.76) | 1.81 (−1.55, 5.17) |
| Unadjusted | Saturated fat | LGI Carbohydrate | −0.75 (−3.27, 1.77) | −2.87 (−5.09, 0.64) * | −2.42 (−3.57,1.27) ** | −1.45 (−3.00, 0.10) |
| Adjusted | Saturated fat | LGI Carbohydrate | 0.81 (−1.67, 3.29) | −0.91 (−3.14, 1.32) | −1.00 (−2.21, 0.20) | −0.35 (−2.08, 1.37) |
| Unadjusted | Monounsaturated fat | LGI Carbohydrate | 1.66 (−1.56, 4.89) | −0.98 (−3.82, 1.87) | −0.04 (−1.44, 1.37) | −0.09 (−1.99, 1.81) |
| Adjusted | Monounsaturated fat | LGI Carbohydrate | 3.00 (−0.06, 6.05) | 0.32 (−2.42, 3.07) | −0.06 (−1.46, 1.34) | −0.18 (−2.19, 1.83) |
| Unadjusted | Polyunsaturated fat | LGI Carbohydrate | −4.48 (−8.40, −0.57) * | −0.60 (−4.05, 2.86) | −0.06 (−2.08, 1.96) | 2.96 (0.23, 5.69) * |
| Adjusted | Polyunsaturated fat | LGI Carbohydrate | −6.97 (−10.74, −3.19) ** | −3.10 (−6.49, 0.29) | −1.49 (−3.51, 0.53) | 1.93 (−0.97, 4.83) |
| Unadjusted | Saturated fat | HGI Carbohydrate | −0.60 (−2.95, 1.76) | −1.84 (−3.91, 0.24) | −1.82 (−2.91, −0.72) | −1.75 (−3.24, −0.27) * |
| Adjusted | Saturated fat | HGI Carbohydrate | 0.35 (−1.94, 2.64) | −0.50 (−2.56, 1.56) | −0.69 (−1.83, 0.44) | −0.85 (−2.47, 0.78) |
| Unadjusted | Monounsaturated fat | HGI Carbohydrate | 1.86 (−1.30, 5.02) | 0.02 (−2.75, 2.80) | 0.38 (−0.99, 1.75) | −0.31 (−2.16, 1.54) |
| Adjusted | Monounsaturated fat | HGI Carbohydrate | 2.60 (−0.40, 5.61) | 0.73 (−1.96, 3.43) | 0.18 (−1.20, 1.55) | −0.56 (−2.52, 1.41) |
| Unadjusted | Polyunsaturated fat | HGI Carbohydrate | −4.20 (−8.14, −0.25) * | 0.26 (−3.20, 3.73) | 0.43 (−1.59, 2.45) | 2.73 (0.00, 5.46) |
| Adjusted | Polyunsaturated fat | HGI Carbohydrate | −7.29 (−11.11, −3.47) ** | −2.67 (−6.10, 0.76) | −1.24 (−3.28, 0.79) | 1.48 (−1.44, 4.39) |
| Without Hypertension, n = 1090 | With Hypertension, n = 357 | |||||
|---|---|---|---|---|---|---|
| Macronutrients Intake | SBP | DBP | SBP | DBP | ||
| Increased | Decreased | β (IC95%) | β (IC95%) | β (IC95%) | β (IC95%) | |
| Unadjusted | Saturated fat | Vegetal Protein | −0.57 (−2.54, 1.41) | −0.34 (−3.26, 2.58) | 6.31 (1.34, 11.27) * | 1.50 (−2.47, 5.47) |
| Adjusted | Saturated fat | Vegetal Protein | −0.72 (−2.74, 1.29) | −0.66 (−3.78, 2.47) | 2.47 (−2.77, 7.72) | 0.30 (−4.09, 4.70) |
| Unadjusted | Monounsaturated fat | Vegetal Protein | −0.16 (−1.86, 1.54) | −0.30 (−2.82, 2.21) | 1.88 (−2.08, 5.85) | 2.77 (−0.40, 5.94) |
| Adjusted | Monounsaturated fat | Vegetal Protein | −0.79 (−2.54, 0.96) | −1.39 (−4.11, 1.32) | 2.56 (−1.67, 6.79) | 3.75 (0.21, 7.30) * |
| Unadjusted | Polyunsaturated fat | Vegetal Protein | −0.51 (−2.49, 1.47) | 2.44 (−0.50, 5.38) | −1.65 (−7.59, 4.29) | 3.88 (−0.86, 8.63) |
| Adjusted | Polyunsaturated fat | Vegetal Protein | −2.42 (−4.46, −0.37) * | 0.20 (−2.98, 3.37) | −5.13 (−11.58, 1.32) | 2.84 (−2.57, 8.24) |
| Unadjusted | Saturated fat | Animal Protein | −1.39 (−3.08, 0.30) | −1.17 (−3.68, 1.34) | 2.80 (−1.34, 6.95) | 1.96 (−1.34, 5.25) |
| Adjusted | Saturated fat | Animal Protein | −1.25 (−3.02, 0.51) | −1.24 (−3.98, 1.50) | 0.27 (−4.09, 4.63) | 1.40 (−2.26, 5.06) |
| Unadjusted | Monounsaturated fat | Animal Protein | −1.26 (−3.60, 1.08) | −0.87 (−4.34, 2.60) | 2.25 (−3.72, 8.22) | 3.04 (−1.71, 7.79) |
| Adjusted | Monounsaturated fat | Animal Protein | −0.41 (−2.80, 1.98) | −0.02 (−3.72, 3.69) | 5.55 (−0.67, 11.77) | 4.73 (−0.49, 9.94) |
| Unadjusted | Polyunsaturated fat | Animal Protein | −0.24 (−2.20, 1.71) | 2.70 (−0.20, 5.60) | −1.08 (−7.04, 4.89) | 3.83 (−0.91, 8.58) |
| Adjusted | Polyunsaturated fat | Animal Protein | −2.26 (−4.27, −0.24) | 0.30 (−2.82, 3.43) | −4.86 (−11.31, 1.59) | 2.79 (−2.62, 8.20) |
| Unadjusted | Saturated fat | LGI Carbohydrate | −2.40 (−3.49, −1.31) * | −2.04 (−3.65, −0.43) * | −1.21 (−3.79, 1.37) | −1.07 (−3.12, 0.97) |
| Adjusted | Saturated fat | LGI Carbohydrate | −0.67 (−1.82, 0.48) | 0.02 (−1.76, 1.80) | −0.35 (−2.96, 2.25) | −0.96 (−3.15, 1.22) |
| Unadjusted | Monounsaturated fat | LGI Carbohydrate | −0.07 (−1.45, 1.31) | −0.31 (−2.35, 1.74) | 1.93 (−1.16, 5.03) | 0.14 (−2.31, 2.59) |
| Adjusted | Monounsaturated fat | LGI Carbohydrate | −0.12 (−1.48, 1.24) | −0.61 (−2.72, 1.50) | 2.78 (−0.38, 5.95) | 0.63 (−2.02, 3.29) |
| Unadjusted | Polyunsaturated fat | LGI Carbohydrate | −0.52 (−2.28, 1.25) | 2.30 (−0.31, 4.91) | −4.02 (−9.36, 1.32) | 1.14 (−3.09, 5.38) |
| Adjusted | Polyunsaturated fat | LGI Carbohydrate | −1.90 (−3.64, −0.16) * | 0.92 (−1.77, 3.61) | −6.32 (−11.84, −0.81) * | −0.13 (−4.77, 4.50) |
| Unadjusted | Saturated fat | HGI Carbohydrate | −1.58 (−2.62, −0.54) | −2.29 (−3.83, −0.75) * | −1.58 (−4.00, 0.84) | −0.50 (−2.42, 1.41) |
| Adjusted | Saturated fat | HGI Carbohydrate | −0.25 (−1.34, 0.83) | −0.63 (−2.30, 1.05) | −1.01 (−3.44, 1.42) | −0.47 (−2.51, 1.57) |
| Unadjusted | Monounsaturated fat | HGI Carbohydrate | 0.56 (−0.79, 1.91) | −0.50 (−2.50, 1.49) | 1.65 (−1.36, 4.66) | 0.52 (−1.87, 2.90) |
| Adjusted | Monounsaturated fat | HGI Carbohydrate | 0.21 (−1.13, 1.54) | −1.14 (−3.20, 0.93) | 2.31 (−0.77, 5.39) | 0.97 (−1.61, 3.55) |
| Unadjusted | Polyunsaturated fat | HGI Carbohydrate | 0.10 (−1.67, 1.86) | 2.12 (−0.49, 4.74) | −4.03 (−9.44, 1.37) | 1.76 (−2.52, 6.04) |
| Adjusted | Polyunsaturated fat | HGI Carbohydrate | −1.60 (−3.35, 0.14) | 0.34 (−2.36, 3.05) | −6.78 (−12.40, −1.15) * | 0.49 (−4.22, 5.21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villaverde, P.; Rivera-Paredez, B.; Velázquez-Cruz, R.; Argoty-Pantoja, A.D.; Salmerón, J. Dietary Fats Substitution and Blood Pressure Levels: A Longitudinal Study in Mexican Adults. Nutrients 2025, 17, 2096. https://doi.org/10.3390/nu17132096
Villaverde P, Rivera-Paredez B, Velázquez-Cruz R, Argoty-Pantoja AD, Salmerón J. Dietary Fats Substitution and Blood Pressure Levels: A Longitudinal Study in Mexican Adults. Nutrients. 2025; 17(13):2096. https://doi.org/10.3390/nu17132096
Chicago/Turabian StyleVillaverde, Paola, Berenice Rivera-Paredez, Rafael Velázquez-Cruz, Anna D. Argoty-Pantoja, and Jorge Salmerón. 2025. "Dietary Fats Substitution and Blood Pressure Levels: A Longitudinal Study in Mexican Adults" Nutrients 17, no. 13: 2096. https://doi.org/10.3390/nu17132096
APA StyleVillaverde, P., Rivera-Paredez, B., Velázquez-Cruz, R., Argoty-Pantoja, A. D., & Salmerón, J. (2025). Dietary Fats Substitution and Blood Pressure Levels: A Longitudinal Study in Mexican Adults. Nutrients, 17(13), 2096. https://doi.org/10.3390/nu17132096





