Influence of Dietary Calcium Intake on Skeletal Health and Body Composition in an Italian Elderly Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Ethics
2.2. Dietary Assessment
2.3. Bone Mineral Density, Body Composition, Fractures, and Laboratory Tests
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMD | Bone Mineral Density |
| FFQ | Food Frequency Questionnaire |
| PTH | Parathyroid Hormone |
| BMI | Body Mass Index |
| DXA | Dual-energy X-ray Absorptiometry |
| LS-BMD | Bone Mineral Density at Lumbar Spine |
| FN-BMD | Bone Mineral Density at Femoral Neck |
| TH-BMD | Bone Mineral Density at Total Hip |
| WB-BMD | Bone Mineral Density at Whole Body |
| FM | Fat Mass |
| LM | Lean Mass |
| 25OHD | 25-Hydroxyvitamin D |
| B-ALP | Bone Alkaline Phosphatase |
| βCTX | Type I Collagen β-Carboxy-Telopeptide |
References
- Matikainen, N.; Pekkarinen, T.; Ryhänen, E.M.; Schalin-Jäntti, C. Physiology of Calcium Homeostasis: An Overview. Endocrinol. Metab. Clin. N. Am. 2021, 50, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- Daley, D.K.; Myrie, S.B. Extra-skeletal effects of dietary calcium: Impact on the cardiovascular system, obesity, and cancer. Adv. Food. Nutr. Res. 2021, 96, 1–25. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef]
- Pattanaungkul, S.; Riggs, B.L.; Yergey, A.L.; Vieira, N.E.; O’Fallon, W.M.; Khosla, S. Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women: Evidence for age-related intestinal resistance to 1,25(OH)2D action. J. Clin. Endocrinol. Metab. 2000, 85, 4023–4027. [Google Scholar] [CrossRef] [PubMed]
- Nordin, B.E.; Need, A.G.; Morris, H.A.; O’Loughlin, P.D.; Horowitz, M. Effect of age on calcium absorption in postmenopausal women. Am. J. Clin. Nutr. 2004, 80, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Baron, J.A.; Kanis, J.A.; Orav, E.J.; Staehelin, H.B.; Kiel, D.P.; Burckhardt, P.; Henschkowski, J.; Spiegelman, D.; et al. Milk intake and risk of hip fracture in men and women: A meta-analysis of prospective cohort studies. J. Bone Miner. Res. 2011, 26, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.P.; Benoit, V.; Payen, F.; Kraenzlin, M. Consumption of yogurts fortified in vitamin D and calcium reduces serum parathyroid hormone and markers of bone resorption: A double-blind randomized controlled trial in institutionalized elderly women. J. Clin. Endocrinol. Metab. 2013, 98, 2915–2921. [Google Scholar] [CrossRef]
- Seo, M.H.; Kim, M.K.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Lee, W.Y.; Baek, K.H.; Song, K.H.; Kang, M.I.; Oh, K.W. The association between daily calcium intake and sarcopenia in older, non-obese Korean adults: The fourth Korea National Health and Nutrition Examination Survey (KNHANES IV) 2009. Endocr. J. 2013, 60, 679–686. [Google Scholar] [CrossRef]
- Gehlert, S.; Bloch, W.; Suhr, F. Ca2+-dependent regulations and signaling in skeletal muscle: From electro-mechanical coupling to adaptation. Int. J. Mol. Sci. 2015, 16, 1066–1095. [Google Scholar] [CrossRef]
- Torres-Costoso, A.; Martínez-Vizcaíno, V.; Fernández-Rodríguez, R.; Sequí-Dominguez, I.; Reina-Gutiérrez, S.; Núñez de Arenas-Arroyo, S.; Garrido-Miguel, M. Dietary Calcium Intake and Fat Mass in Spanish Young Adults: The Role of Muscle Strength. Nutrients 2021, 13, 4498. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Lappe, J.; O’Brien, K.O.; Wang, D.D.; Sahni, S.; Weaver, C.M. Dairy intake and bone health across the lifespan: A systematic review and expert narrative. Crit. Rev. Food. Sci. Nutr. 2021, 61, 3661–3707. [Google Scholar] [CrossRef] [PubMed]
- Kerstetter, J.E.; O’Brien, K.O.; Insogna, K.L. Low protein intake: The impact on calcium and bone homeostasis in humans. J. Nutr. 2003, 133, 855S–861S. [Google Scholar] [CrossRef] [PubMed]
- Ramsubeik, K.; Keuler, N.S.; Davis, L.A.; Hansen, K.E. Factors associated with calcium absorption in postmenopausal women: A post hoc analysis of dual-isotope studies. J. Acad. Nutr. Diet. 2014, 114, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.P.; Li, K.J.; Shi, H.Y.; He, J.J.; Li, H. Habitual dietary calcium intakes and calcium metabolism in healthy adults Chinese: A systematic review and meta-analysis. Asia Pac. J. Clin. Nutr. 2016, 25, 776–784. [Google Scholar] [CrossRef]
- Gonnelli, S.; Caffarelli, C.; Tanzilli, L.; Alessi, C.; Tomai Pitinca, M.D.; Rossi, S.; Campagna, M.S.; Nuti, R. The associations of body composition and fat distribution with bone mineral density in elderly Italian men and women. J. Clin. Densitom. 2013, 16, 168–177. [Google Scholar] [CrossRef]
- Montomoli, M.; Gonnelli, S.; Giacchi, M.; Mattei, R.; Cuda, C.; Rossi, S.; Gennari, C. Validation of a food frequency questionnaire for nutritional calcium intake assessment in Italian women. Eur. J. Clin. Nutr. 2002, 56, 21–30. [Google Scholar] [CrossRef]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Arcella, D.; Turrini, A.; Leclercq, C.; INRAN-SCAI 2005-6 Study Group. The third Italian National Food Consumption Survey, INRAN-SCAI 2005–06–part 1: Nutrient intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 922–932. [Google Scholar] [CrossRef]
- Vannucci, L.; Masi, L.; Gronchi, G.; Fossi, C.; Carossino, A.M.; Brandi, M.L. Calcium intake, bone mineral density, and fragility fractures: Evidence from an Italian outpatient population. Arch. Osteoporos. 2017, 12, 40. [Google Scholar] [CrossRef]
- Cairoli, E.; Aresta, C.; Giovanelli, L.; Eller-Vainicher, C.; Migliaccio, S.; Giannini, S.; Giusti, A.; Marcocci, C.; Gonnelli, S.; Isaia, G.C.; et al. Dietary calcium intake in a cohort of individuals evaluated for low bone mineral density: A multicenter Italian study. Aging. Clin. Exp. Res. 2021, 33, 3223–3235. [Google Scholar] [CrossRef]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Adami, S.; Bertoldo, F.; Diacinti, D.; Gatti, D.; Giannini, S.; Giusti, A.; Malavolta, N.; Minisola, S.; Osella, G.; et al. Guidelines for the diagnosis, prevention and management of osteoporosis. Reumatismo 2016, 68, 1–39. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, J.; Zhu, X.; Wang, L.; Gao, P.; Shu, G.; Jiang, Q.; Wang, S. Anti-Obesity Effects of Dietary Calcium: The Evidence and Possible Mechanisms. Int. J. Mol. Sci. 2019, 20, 3072. [Google Scholar] [CrossRef]
- Shlisky, J.; Mandlik, R.; Askari, S.; Abrams, S.; Belizan, J.M.; Bourassa, M.W.; Cormick, G.; Driller-Colangelo, A.; Gomes, F.; Khadilkar, A.; et al. Calcium deficiency worldwide: Prevalence of inadequate intakes and associated health outcomes. Ann. N. Y. Acad. Sci. 2022, 1512, 10–28. [Google Scholar] [CrossRef]
- Bredariol, A.N.M.; Rossato, L.T.; de Branco, F.M.S.; Nahas, P.C.; Orsatti, F.L.; de Oliveira, E.P. Calcium intake is inversely associated with body fat in postmenopausal women. Calcium intake is inversely associated with body fat in postmenopausal women. Clin. Nutr. ESPEN 2020, 39, 206–209. [Google Scholar] [CrossRef]
- Bendsen, N.T.; Hother, A.L.; Jensen, S.K.; Lorenzen, J.K.; Astrup, A. Effect of dairy calcium on fecal fat excretion: A randomized crossover trial. Int. J. Obes. 2008, 32, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Ciapponi, A.; Harbron, J.; Perez, S.M.; Vazquez, P.; Rivo, J.; Metzendorf, M.I.; Althabe, F.; Belizán, J.M. Calcium supplementation for people with overweight or obesity. Cochrane Database Syst. Rev. 2024, 5, CD012268. [Google Scholar] [CrossRef] [PubMed]
- Van Loan, M.D.; Keim, N.L.; Adams, S.H.; Souza, E.; Woodhouse, L.R.; Thomas, A.; Witbracht, M.; Gertz, E.R.; Piccolo, B.; Bremer, A.A.; et al. Dairy Foods in a Moderate Energy Restricted Diet Do Not Enhance Central Fat, Weight, and Intra-Abdominal Adipose Tissue Losses nor Reduce Adipocyte Size or Inflammatory Markers in Overweight and Obese Adults: A Controlled Feeding Study. J. Obes. 2011, 2011, 989657. [Google Scholar] [CrossRef]
- Jin, X.; Jin, X.; Guan, W.; Tang, M. Dietary Calcium-to-Phosphorous Ratio, Metabolic Risk Factors and Lipid Accumulation Product, Skeletal Muscle Mass, and Visceral Fat Area Among Healthy Young Individuals. Int. J. Sport. Nutr. Exerc. Metab. 2024, 35, 43–50. [Google Scholar] [CrossRef]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11.e3. [Google Scholar] [CrossRef]
- Kirk, B.; Prokopidis, K.; Duque, G. Nutrients to mitigate osteosarcopenia: The role of protein, vitamin D and calcium. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Choi, S.H.; Lim, S.; Moon, J.H.; Kim, J.H.; Kim, S.W.; Jang, H.C.; Shin, C.S. Interactions between dietary calcium intake and bone mineral density or bone geometry in a low calcium intake population (KNHANES IV 2008-2010). J. Clin. Endocrinol. Metab. 2014, 99, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Kiel, D.P.; Dawson-Hughes, B.; Orav, J.E.; Li, R.; Spiegelman, D.; Dietrich, T.; Willett, W.C. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J. Bone Miner. Res. 2009, 24, 935–942. [Google Scholar] [CrossRef]
- Tai, V.; Leung, W.; Grey, A.; Reid, I.R.; Bolland, M.J. Calcium intake and bone mineral density: Systematic review and meta-analysis. BMJ 2015, 351, h4183. [Google Scholar] [CrossRef]
- Anderson, J.J.; Roggenkamp, K.J.; Suchindran, C.M. Calcium intakes and femoral and lumbar bone density of elderly U.S. men and women: National Health and Nutrition Examination Survey 2005–2006 analysis. J. Clin. Endocrinol. Metab. 2012, 97, 4531–4539. [Google Scholar] [CrossRef]
- Cianferotti, L.; Bifolco, G.; Caffarelli, C.; Mazziotti, G.; Migliaccio, S.; Napoli, N.; Ruggiero, C.; Cipriani, C. Nutrition, Vitamin D, and Calcium in Elderly Patients before and after a Hip Fracture and Their Impact on the Musculoskeletal System: A Narrative Review. Nutrients 2024, 16, 1773. [Google Scholar] [CrossRef]
- Iuliano, S.; Poon, S.; Robbins, J.; Bui, M.; Wang, X.; De Groot, L.; Van Loan, M.; Zadeh, A.G.; Nguyen, T.; Seeman, E. Effect of dietary sources of calcium and protein on hip fractures and falls in older adults in residential care: Cluster randomised controlled trial. BMJ 2021, 375, n2364. [Google Scholar] [CrossRef]
- Bolland, M.J.; Leung, W.; Tai, V.; Bastin, S.; Gamble, G.D.; Grey, A.; Reid, I.R. Calcium intake and risk of fracture: Systematic review. BMJ 2015, 351, h4580. [Google Scholar] [CrossRef]
- Zhao, J.G.; Zeng, X.T.; Wang, J.; Liu, L. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA 2017, 318, 2466–2482. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Meyer, H.E.; Fung, T.T.; Bischoff-Ferrari, H.A.; Willett, W.C. Milk and other dairy foods and risk of hip fracture in men and women. Osteoporos. Int. 2018, 29, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Hu, F.B.; Li, Y.; Cabral, H.J.; Das, S.K.; Deeney, J.T.; Zhou, X.; Paik, J.M.; Moore, L.L. Types of dairy foods and risk of fragility fracture in the prospective Nurses’ Health Study cohort. Am. J. Clin. Nutr. 2023, 118, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
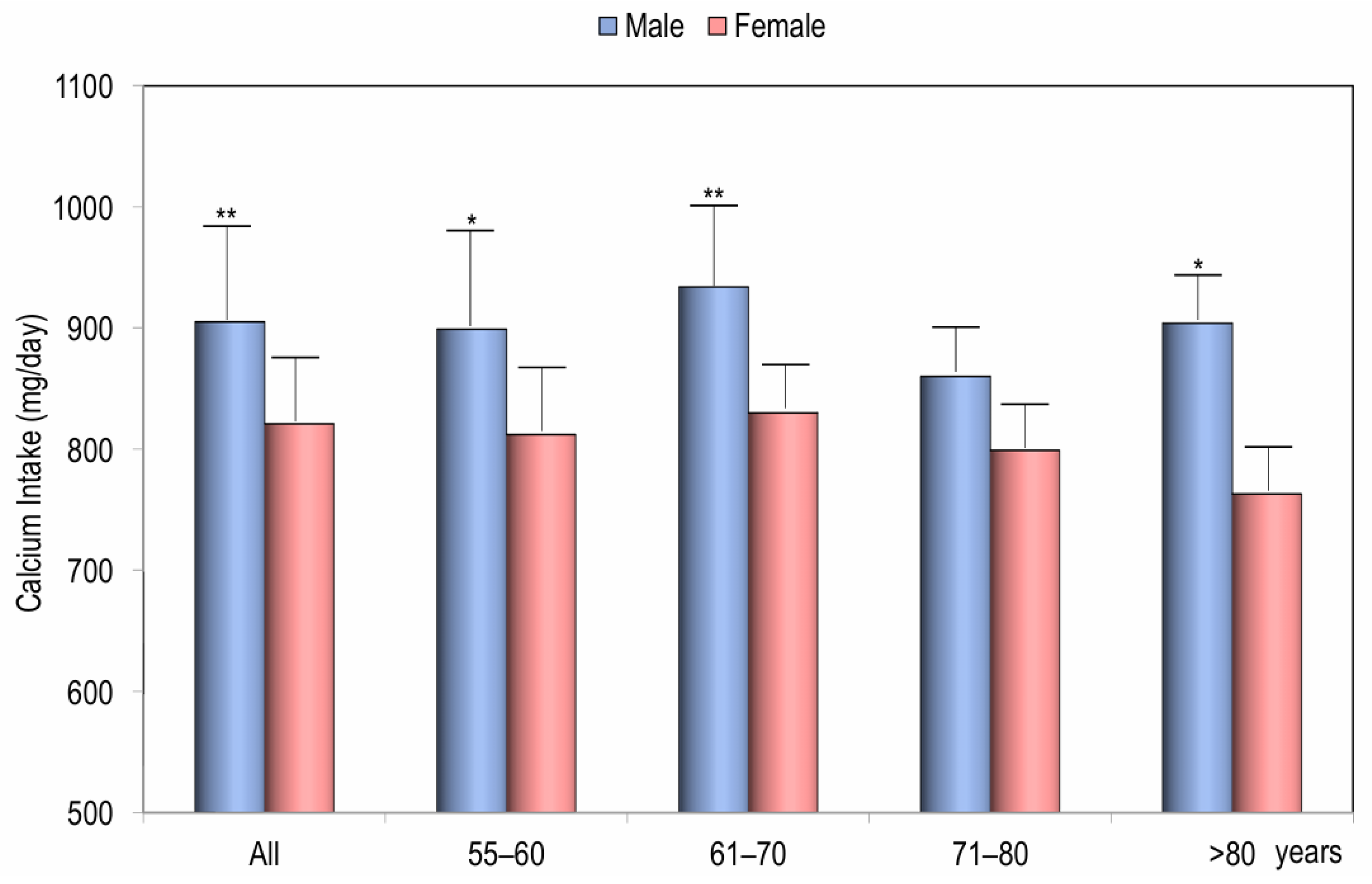



| Variable | Women (n = 939) | Men (n = 173) |
|---|---|---|
| Age (yrs) | 64.4 ± 6.4 | 65.1 ± 6.1 |
| Weight (Kg) | 65.6 ± 11.1 | 77.6 ± 11.0 *** |
| Height (cm) | 160.5 ± 5.9 | 171.2 ± 6.5 *** |
| BMI (Kg/m2) | 25.5 ± 4.2 | 26.4 ± 3.4 *** |
| Menopause Age (yrs) | 49.5 ± 5.3 | - |
| Creatinine (mg/dL) | 0.89 ± 0.20 | 1.01 ± 0.26 * |
| Albumin (g/dL) | 3.45 ± 0.37 | 3.51 ± 0.35 |
| Calcium (mg/dL) | 9.31 ± 0.51 | 9.12 ± 0.49 * |
| Phosphate (mg/dL) | 3.47 ± 0.52 | 3.13 ± 0.58 * |
| ALP (UI/L) | 82.61 ± 42.85 | 81.84 ± 34.65 |
| 25OHD (ng/mL) | 23.47 ± 17.77 | 25.69 ± 13.62 * |
| PTH (pg/mL) | 27.86 ± 14.86 | 24.60 ± 19.03 * |
| B-ALP (µg/L) | 11.62 ± 6.25 | 9.87 ± 4.30 ** |
| β-CTX (ng/mL) | 0.613 ± 0.276 | 0.511 ± 0.289 ** |
| LS-BMD (g/cm2) | 0.986 ± 0.163 | 1.155 ± 0.187 *** |
| FN-BMD (g/cm2) | 0.817 ± 0.116 | 0.904 ± 0.128 *** |
| TH-BMD (g/cm2) | 0.881 ± 0.123 | 0.994 ± 0.142 *** |
| WB-BMD (g/cm2) | 1.034 ± 0.106 | 1.153 ± 0.097 *** |
| Calcium Intake (mg/day) in Males | Calcium Intake (mg/day) in Females | |
|---|---|---|
| 25OHD (ng/mL) | 0.06 | 0.12 ** |
| PTH (pg/mL) | −0.01 | −0.09 * |
| B-ALP (µg/L) | −0.08 | −0.05 |
| β-CTX (ng/mL) | −0.24 * | −0.08 * |
| Predictor Variable | b | 95% CI | p | |
|---|---|---|---|---|
| Males | ||||
| LS-BMD R2adj = 0.16 | ||||
| BMI | 0.021 | 0.011;0.031 | 0.001 | |
| FN-BMD R2adj = 0.14 | ||||
| Calcium Intake | 0.001 | 0.000;0.003 | 0.004 | |
| BMI | 0.010 | 0.003;0.017 | 0.017 | |
| TH-BMD R2adj = 0.20 | ||||
| BMI | 0.015 | 0.008;0.023 | 0.023 | |
| Calcium Intake | 0.002 | 0.001;0.003 | 0.001 | |
| WB-BMD R2adj = 0.21 | ||||
| BMI | 0.011 | 0.006;0.016 | 0.001 | |
| Calcium Intake | 0.001 | −0.001;0.002 | 0.011 | |
| Females | ||||
| LS-BMD R2adj = 0.16 | ||||
| BMI | 0.010 | 0.008;0.013 | 0.001 | |
| Age | −0.006 | −0.008;−0.005 | 0.001 | |
| History of Fracture | −0.024 | −0.008;−0.005 | 0.049 | |
| FN-BMD R2adj = 0.14 | ||||
| Age | −0.006 | −0.007;−0.005 | 0.001 | |
| BMI | 0.008 | 0.006;0.010 | 0.001 | |
| TH-BMD R2adj = 0.20 | ||||
| BMI | 0.013 | 0.011;0.014 | 0.001 | |
| Age | −0.005 | −0.006;−0.004 | 0.001 | |
| B-ALP | −0.002 | −0.003;−0.001 | 0.004 | |
| WB-BMD R2adj = 0.21 | ||||
| BMI | 0.010 | 0.008;0.011 | 0.001 | |
| Age | −0.005 | −0.006;−0.004 | 0.001 | |
| B-ALP | −0.002 | −0.003;−0.001 | 0.001 | |
| History of Fracture | −0.024 | −0.040;−0.009 | 0.002 | |
| Calcium Intake | 0.010 | 0.007;0.018 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caffarelli, C.; Al Refaie, A.; Mondillo, C.; Cavati, G.; Lora, A.; Gennari, L.; Nuti, R.; Gonnelli, S. Influence of Dietary Calcium Intake on Skeletal Health and Body Composition in an Italian Elderly Population. Nutrients 2025, 17, 2073. https://doi.org/10.3390/nu17132073
Caffarelli C, Al Refaie A, Mondillo C, Cavati G, Lora A, Gennari L, Nuti R, Gonnelli S. Influence of Dietary Calcium Intake on Skeletal Health and Body Composition in an Italian Elderly Population. Nutrients. 2025; 17(13):2073. https://doi.org/10.3390/nu17132073
Chicago/Turabian StyleCaffarelli, Carla, Antonella Al Refaie, Caterina Mondillo, Guido Cavati, Anna Lora, Luigi Gennari, Ranuccio Nuti, and Stefano Gonnelli. 2025. "Influence of Dietary Calcium Intake on Skeletal Health and Body Composition in an Italian Elderly Population" Nutrients 17, no. 13: 2073. https://doi.org/10.3390/nu17132073
APA StyleCaffarelli, C., Al Refaie, A., Mondillo, C., Cavati, G., Lora, A., Gennari, L., Nuti, R., & Gonnelli, S. (2025). Influence of Dietary Calcium Intake on Skeletal Health and Body Composition in an Italian Elderly Population. Nutrients, 17(13), 2073. https://doi.org/10.3390/nu17132073








