Pretreatment with Citrus reticulata ‘Chachi’ Polysaccharide Alleviates Alcohol-Induced Gastric Ulcer by Inhibiting NLRP3/ASC/Caspase-1 and Nrf2/HO-1 Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Extraction and Purification of CRP
2.3. Structural Characterization of CRP
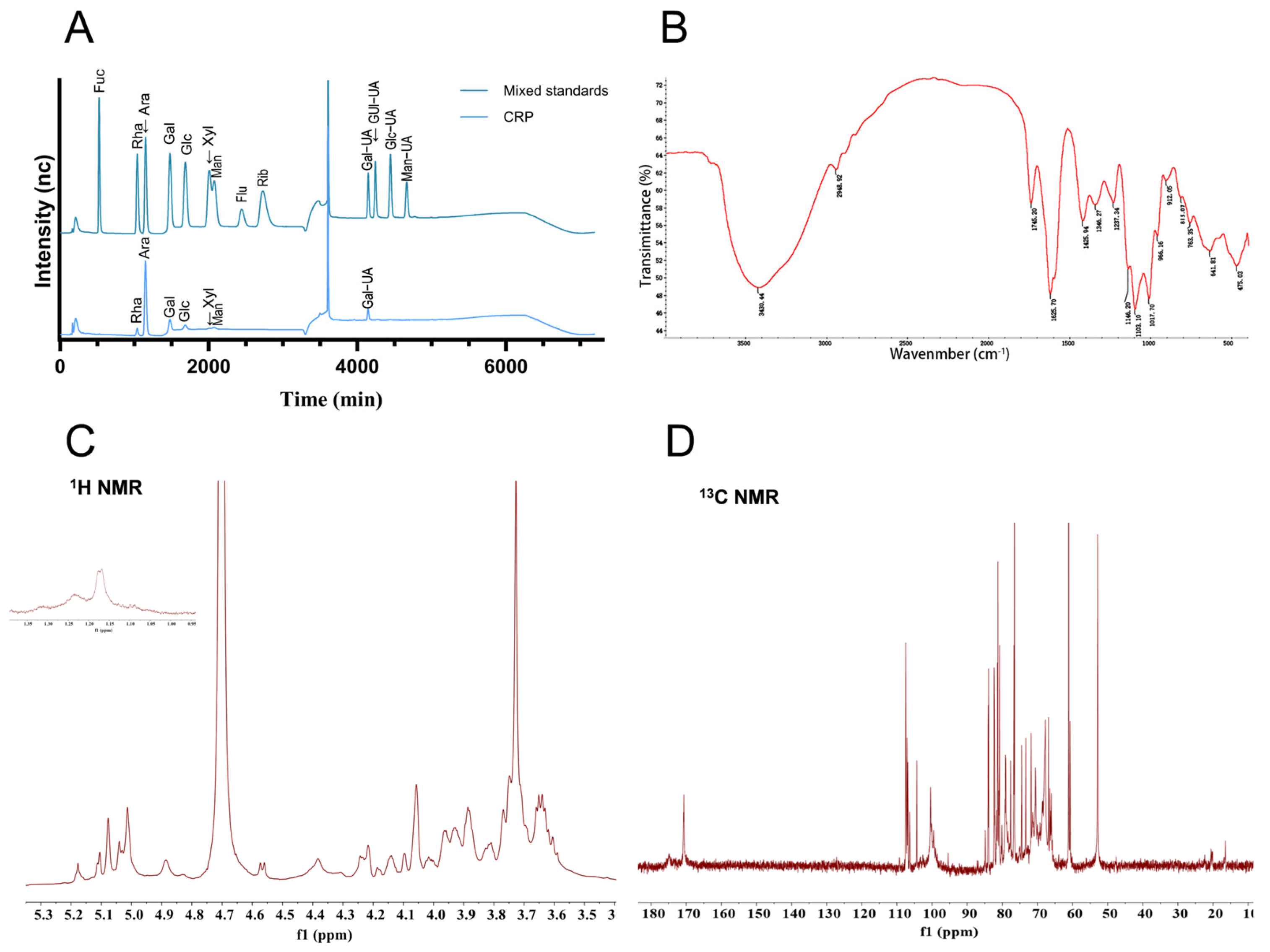
2.3.1. Chemical Composition Identification
2.3.2. Fourier-Transform Infrared (FT-IR) Analysis
2.3.3. Monosaccharide Composition Analysis
2.3.4. Nuclear Magnetic Resonance (NMR) Analysis
2.4. Cell Culture
2.5. CCK-8 Assay
2.6. Immunofluorescence (IF) Staining
2.7. Annexin V-FITC-Based Apoptosis Assay for Detecting Cell Apoptosis
2.8. Wound Healing Assay
2.9. Animal Grouping, Model Establishment, and Drug Administration
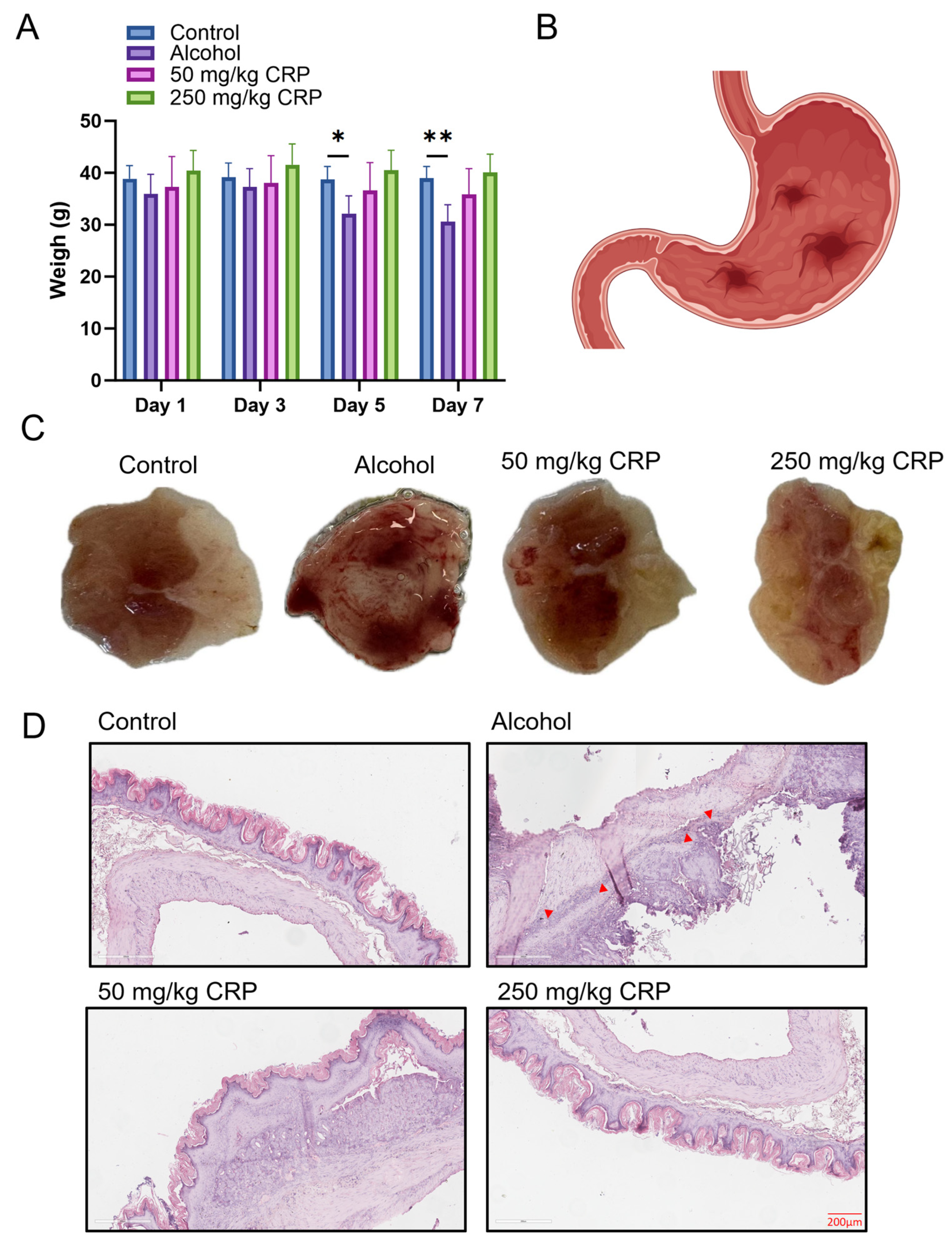
2.10. Evaluation of Gastric Ulcer Degree
2.11. Western Blot Analysis
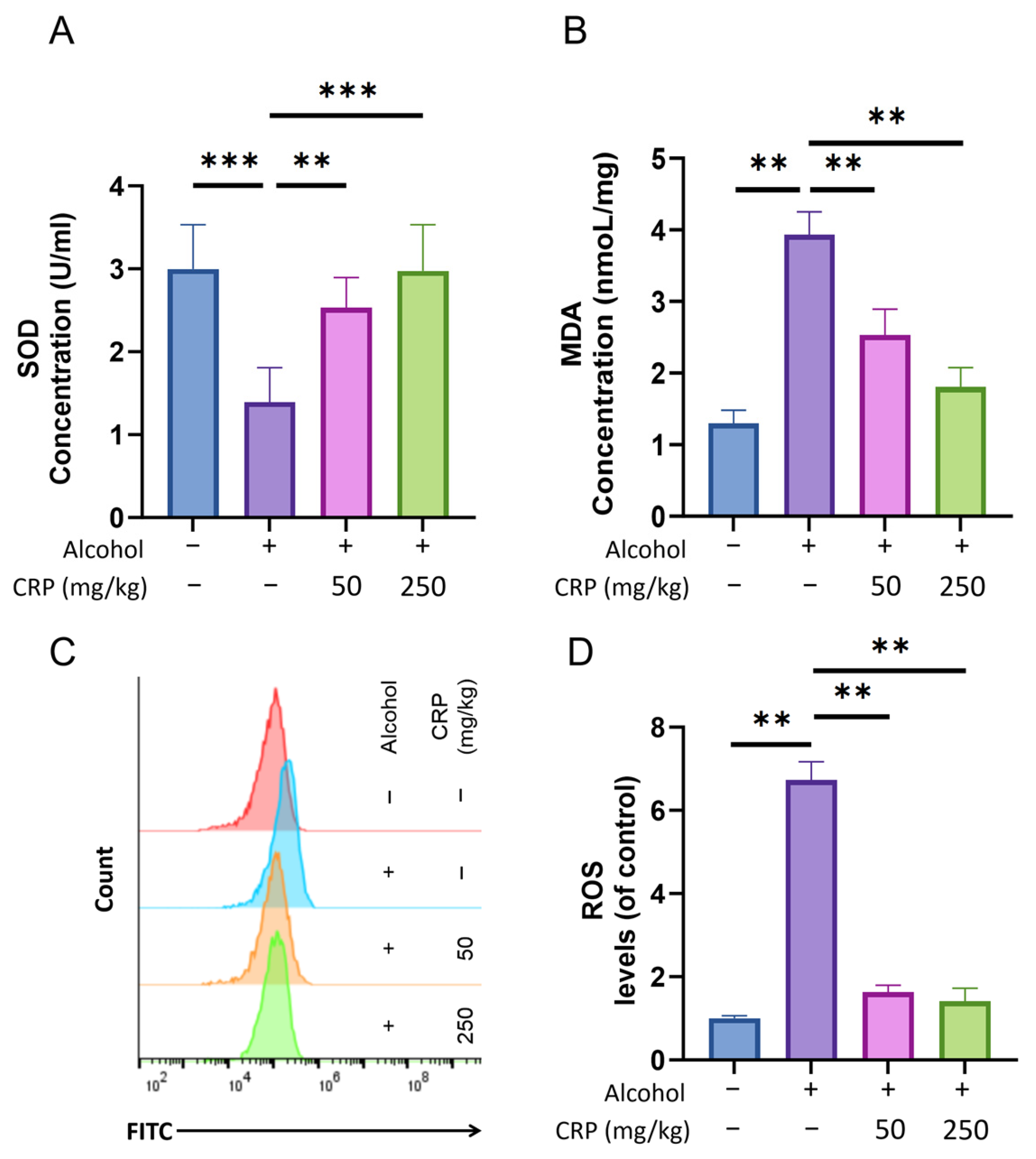
2.12. Assessment of the Oxidative Stress Index
2.13. Hematoxylin and Eosin (H&E) Staining
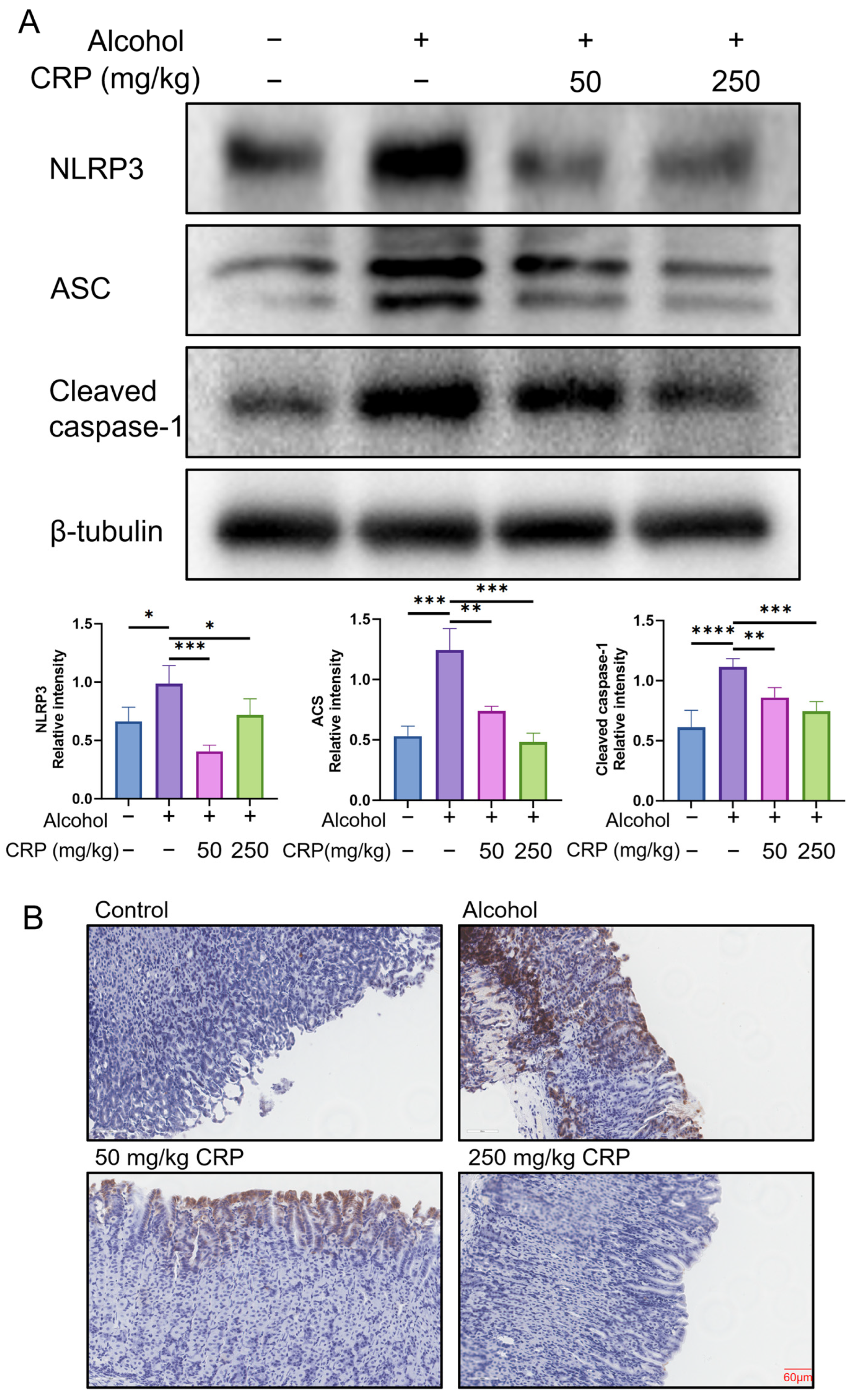
2.14. Immunohistochemistry (IHC) Staining
2.15. qRT-PCR Analysis
2.16. Statistical Analysis
3. Results
3.1. Extraction, Purification, and Preliminary Characterization of CRP
3.2. CRP Protected Against Alcohol-Induced Loss of Body Weight and Gastric Mucosal Damage in BALB/c Mice
3.3. CRP Alleviated Gastric Inflammatory Damages in Alcohol-Induced GU Mice
3.4. CRP Inhibited NLRP3 Inflammasome Activation in Alcohol-Induced GU Mice
3.5. CRP Decreased Lipid Peroxidation Level, SOD Activity, and the Production of ROS in Alcohol-Induced GU Mice
3.6. CRP Improved Gastric Mucosal Oxidative Damage by Activating Nrf2 Signaling Pathway
3.7. CRP Stimulated the Expression of ZO-1, Occludin, and Claudin5 in the Gastric Mucosa of Alcohol-Induced Mice
3.8. CRP Improves Survival in Alcohol-Induced GES-1 Cells Injury
3.9. CRP Inhibited the NLRP3 Inflammasome Activation of GES-1 Cells
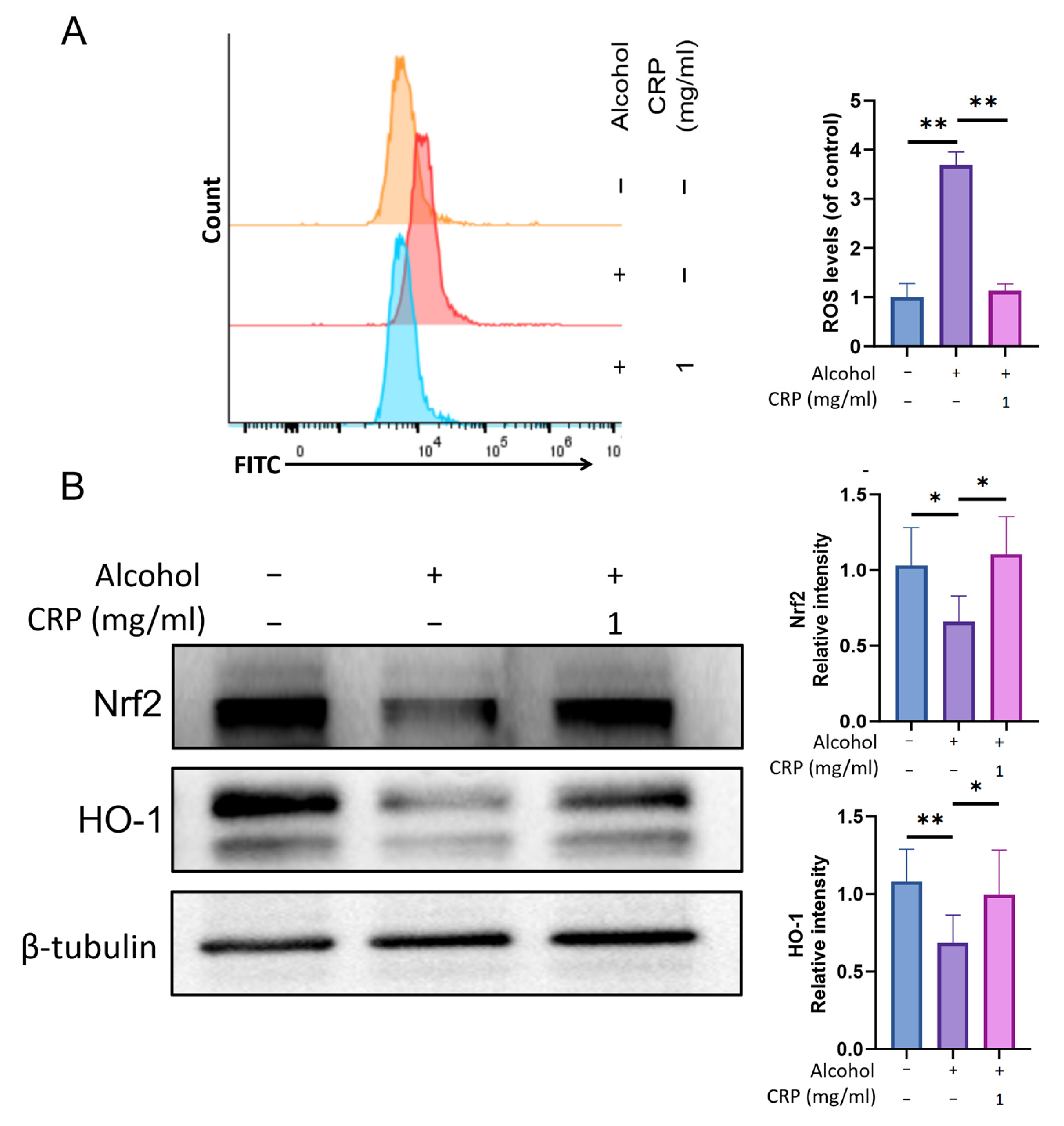
3.10. CRP Ameliorates Alcohol-Induced Oxidative Stress in GES-1 Cells Through Modulating Nrf2/HO-1 Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRP | Citrus reticulata ‘Chachi’ polysaccharide |
| GU | Gastric ulcer |
| ROS | Reactive oxygen species |
| HO-1 | Heme oxygenase-1 |
| H&E | Hematoxylin and eosin |
| SOD | Superoxide dismutase |
| SD | Standard deviation |
| TNF-α | Tumor Necrosis Factor-α |
| TJs | Tight junctions |
| TFA | Trifluoroacetic acid |
| D2O | Deuterium oxide |
| FT-IR | Fourier-transform infrared |
| FBS | Fetal bovine serum |
| NMR | Nuclear magnetic resonance |
| Ara | Arabinose |
| Gal | Galactose |
| GalA | Galacturonic acid |
| IHC | Immunohistochemistry |
| IF | Immunofluorescence |
References
- Gong, H.; Zhao, N.; Zhu, C.; Luo, L.; Liu, S. Treatment of gastric ulcer, traditional Chinese medicine may be a better choice. J. Ethnopharmacol. 2024, 324, 117793. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, Y.; Huang, T.; Huang, D.; Liu, L.; Shen, C.; Jiang, C.; Wang, Z.; Chen, H.; Liang, P.; et al. Licorice flavonoid alleviates gastric ulcers by producing changes in gut microbiota and promoting mucus cell regeneration. Biomed. Pharmacother. 2023, 169, 115868. [Google Scholar] [CrossRef]
- Ye, H.Y.; Shang, Z.Z.; Zhang, F.Y.; Zha, X.Q.; Li, Q.M.; Luo, J.P. Dendrobium huoshanense stem polysaccharide ameliorates alcohol-induced gastric ulcer in rats through Nrf2-mediated strengthening of gastric mucosal barrier. Int. J. Biol. Macromol. 2023, 236, 124001. [Google Scholar] [CrossRef]
- Wu, Y.; Murray, G.K.; Byrne, E.M.; Sidorenko, J.; Visscher, P.M.; Wray, N.R. GWAS of peptic ulcer disease implicates Helicobacter pylori infection, other gastrointestinal disorders and depression. Nat. Commun. 2021, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, D.; Wang, Q.; Zhou, A. Gastroprotective Effects of the Aqueous Extract of Finger Citron Pickled Products against Ethanol-Induced Gastric Damage: In Vitro and In Vivo Studies. Foods 2023, 12, 2355. [Google Scholar] [CrossRef]
- Chey, W.D.; Howden, C.W.; Moss, S.F.; Morgan, D.R.; Greer, K.B.; Grover, S.; Shah, S.C. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2024, 119, 1730–1753. [Google Scholar] [CrossRef]
- Hossen, M.A.; Reza, A.; Ahmed, A.; Islam, M.K.; Jahan, I.; Hossain, R.; Khan, M.F.; Maruf, M.; Haque, M.A.; Rahman, M.A. Pretreatment of Blumea lacera leaves ameliorate acute ulcer and oxidative stress in ethanol-induced Long-Evan rat: A combined experimental and chemico-biological interaction. Biomed. Pharmacother. 2021, 135, 111211. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chen, J.; Ruan, X.; Sun, Y.; Zhang, K.; Wang, X.; Li, X.; Gill, D.; Burgess, S.; Giovannucci, E.; et al. Smoking, alcohol consumption, and 24 gastrointestinal diseases: Mendelian randomization analysis. eLife 2023, 12, e84051. [Google Scholar] [CrossRef] [PubMed]
- El-Naga, R.N. Apocynin protects against ethanol-induced gastric ulcer in rats by attenuating the upregulation of NADPH oxidases 1 and 4. Chem.-Biol. Interact. 2015, 242, 317–326. [Google Scholar] [CrossRef]
- Badr, A.M.; El-Orabi, N.F.; Mahran, Y.F.; Badr, A.M.; Bayoumy, N.M.; Hagar, H.; Elmongy, E.I.; Atawia, R.T. In vivo and In silico evidence of the protective properties of carvacrol against experimentally-induced gastric ulcer: Implication of antioxidant, anti-inflammatory, and antiapoptotic mechanisms. Chem.-Biol. Interact. 2023, 382, 110649. [Google Scholar] [CrossRef]
- Sayed, A.H.; Mahmoud, N.S.; Mohawed, O.; Ahmed, H.H. Combined effect of pantoprazole and mesenchymal stem cells on experimentally induced gastric ulcer: Implication of oxidative stress, inflammation and apoptosis pathways. Inflammopharmacology 2024, 32, 1961–1982. [Google Scholar] [CrossRef] [PubMed]
- Gommers, L.; Hoenderop, J.; de Baaij, J. Mechanisms of proton pump inhibitor-induced hypomagnesemia. Acta Physiol. 2022, 235, e13846. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Ma, H.; Zhou, J.; Chen, L.; Chen, X.; Wang, J.; Jiang, Z.; Zhang, T. Quality evaluation of Citri Reticulatae Pericarpium by supercritical fluid chromatography with chemical pattern recognition. J. Pharm. Biomed. Anal. 2025, 262, 116902. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Wu, S.; Liu, Y.; Chen, J.; Li, T.; Su, D. Microcapsule Preparation and Properties of Flavonoid Extract from Immature Citrus reticulata ‘Chachiensis’ Peel. Foods 2024, 13, 3096. [Google Scholar] [CrossRef]
- Chen, G.; Xie, X.; Peng, F.; Wang, T.; Chen, J.; Li, G.; Liu, J.; Peng, C. Protective effect of the combination of essential oil from patchouli and tangerine peel against gastric ulcer in rats. J. Ethnopharmacol. 2022, 282, 114645. [Google Scholar] [CrossRef] [PubMed]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Protective effects of orange (Citrus sinensis L.) peel aqueous extract and hesperidin on oxidative stress and peptic ulcer induced by alcohol in rat. Lipids Health Dis. 2017, 16, 152. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, D.; Fu, W.; Sun, L.; Li, Y.; Yu, P.; Yu, X.; Zhou, Y.; Xu, H. Protective effects of polysaccharides from Panax ginseng on acute gastric ulcers induced by ethanol in rats. Food Funct. 2021, 12, 2741–2749. [Google Scholar] [CrossRef]
- Hou, C.; Liu, L.; Ren, J.; Huang, M.; Yuan, E. Structural characterization of two Hericium erinaceus polysaccharides and their protective effects on the alcohol-induced gastric mucosal injury. Food Chem. 2022, 375, 131896. [Google Scholar] [CrossRef]
- Fan, Y.; Hong, R.; Sun, X.; Luo, Q.; Wei, H.; Chen, Y.; Zhang, Z.; Zhou, X.; Wan, J. Gastric acid-responsive deformable sodium alginate/Bletilla striata polysaccharide in situ gel for the protection and treatment of alcohol-induced peptic ulcers. Int. J. Biol. Macromol. 2024, 258, 128815. [Google Scholar] [CrossRef]
- Guo, M.; Yu, H.; Meng, M.; Wang, C. Research on the structural characteristics of a novel Chinese Iron Yam polysaccharide and its gastroprotection mechanism against ethanol-induced gastric mucosal lesion in a BALB/c mouse model. Food Funct. 2020, 11, 6054–6065. [Google Scholar] [CrossRef]
- Wu, L.; Lin, L.; Yu, M.; Li, H.; Dang, Y.; Liang, H.; Chen, G.; Muhetaer, H.; Zheng, G.; Li, J.; et al. Antitumor Activity of USP7 Inhibitor GNE-6776 in Non-Small Cell Lung Cancer Involves Regulation of Epithelial-Mesenchymal Transition, Cell Cycle, Wnt/beta-Catenin, and PI3K/AKT/mTOR Pathways. Pharmaceuticals 2025, 18, 245. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, M.; Liang, H.; Lin, L.; Li, H.; Chen, G.; Muhetaer, H.; Li, J.; Wu, B.; Jia, X.; et al. SJB2-043, a USP1 Inhibitor, Suppresses A549 Cell Proliferation, Migration, and EMT via Modulation of PI3K/AKT/mTOR, MAPK, and Wnt Signaling Pathways. Curr. Issues Mol. Biol. 2025, 47, 155. [Google Scholar] [CrossRef] [PubMed]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kuhl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Boby, N.; Abbas, M.A.; Lee, E.B.; Im, Z.E.; Hsu, W.H.; Park, S.C. Protective Effect of Pyrus ussuriensis Maxim. Extract against Ethanol-Induced Gastritis in Rats. Antioxidants 2021, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Teka, N.; Alminderej, F.M.; Souid, G.; El-Ghoul, Y.; Le Cerf, D.; Majdoub, H. Characterization of Polysaccharides Sequentially Extracted from Allium roseum Leaves and Their Hepatoprotective Effects against Cadmium Induced Toxicity in Mouse Liver. Antioxidants 2022, 11, 1866. [Google Scholar] [CrossRef]
- Mohammed, H.S.; Elariny, H.A.; Seif-Eldein, N.A.; Mahgoub, S.; El-Said, N.T.; Abu, E.W.S.; Taha, E.F. Investigating the involvement of the NLRP3/ASC/caspase-1 and NF-kappab/MAPK pathways in the pathogenesis of gouty arthritis: Insights from irradiated and non-irradiated Trifolium alexandrium L. extracts and some metabolites. J. Ethnopharmacol. 2024, 334, 118566. [Google Scholar] [CrossRef]
- Paramel, V.G.; Folkersen, L.; Strawbridge, R.J.; Halvorsen, B.; Yndestad, A.; Ranheim, T.; Krohg-Sorensen, K.; Skjelland, M.; Espevik, T.; Aukrust, P.; et al. NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J. Am. Heart Assoc. 2016, 5, e3031. [Google Scholar]
- Li, Y.; Pan, H.; Liu, J.X.; Li, T.; Liu, S.; Shi, W.; Sun, C.; Fan, M.; Xue, L.; Wang, Y.; et al. l-Arabinose Inhibits Colitis by Modulating Gut Microbiota in Mice. J. Agric. Food Chem. 2019, 67, 13299–13306. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, Y.A.; Kim, H.Y.; Okamoto, T.; Sei, Y. Protective effect of the Chinese prescription Kangen-karyu against high glucose-induced oxidative stress in LLC-PK1 cells. J. Ethnopharmacol. 2007, 109, 113–120. [Google Scholar] [CrossRef]
- Fehér, C. Novel approaches for biotechnological production and application of L-arabinose. J. Carbohydr. Chem. 2018, 37, 251–284. [Google Scholar] [CrossRef]
- Song, Y.B.; Kim, B.; Choi, M.J.; Song, Y.O.; Cho, E.J. Protective effect of arabinose and sugar beet pulp against high glucose-induced oxidative stress in LLC-PK1 cells. Food Chem. 2012, 134, 189–194. [Google Scholar] [CrossRef]
- Gao, Q.; Chen, F.; Zhang, L.; Wei, A.; Wang, Y.; Wu, Z.; Cao, W. Inhibition of DNA methyltransferase aberrations reinstates antioxidant aging suppressors and ameliorates renal aging. Aging Cell 2022, 21, e13526. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, H.M.; Qin, G.Y. Structure characterization and antioxidant activity of polysaccharides from Chinese quince seed meal. Food Chem. 2017, 234, 314–322. [Google Scholar] [CrossRef]
- Harsha, M.R.; Chandra, P.S.; Dharmesh, S.M. Modified pectic polysaccharide from turmeric (Curcuma longa): A potent dietary component against gastric ulcer. Carbohydr. Polym. 2016, 138, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Li, M.; Huang, R.; Wu, X.; Sun, Y.; Xu, Z. Structural features and anti-inflammatory properties of pectic polysaccharides: A review. Trends Food Sci. Technol. 2021, 107, 284–298. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Meng, X.; Liu, J.; Kang, J.; Wang, M.; Guan, Z.; Tian, D.; Chen, X. Lamivudine protects mice from gastric ulcer by activating PGK1 to suppress ferroptosis. Biochem. Pharmacol. 2024, 227, 116440. [Google Scholar] [CrossRef]
- Ali, D.E.; El-Shiekh, R.A.; El, S.M.; Khalifa, A.A.; Elblehi, S.S.; Elsokkary, N.H.; Ali, M.A. In vivo anti-gastric ulcer activity of 7-O-methyl aromadendrin and sakuranetin via mitigating inflammatory and oxidative stress trails. J. Ethnopharmacol. 2024, 335, 118617. [Google Scholar] [CrossRef]
- Wang, Y.; Mou, C.; Huang, L.; Su, J.; You, L.; Zhang, J.; He, Z.; Hu, Y.; Htwe, K.M.; Lee, S.G.; et al. The ethanolic extract of Rhaphidophora peepla prevents inflammation by inhibiting the activation of Syk/AKT/NF-kappaB and TAK1/MAPK/AP-1. Phytomedicine 2025, 136, 156339. [Google Scholar] [CrossRef]
- Fahim, M.; Rafiee, Z.A.; Shoureshi, P.; Ghadimi, K.; Cheshmavar, M.; Sheikhinia, N.; Afzali, M. Alcohol and multiple sclerosis: An immune system-based review. Int. J. Physiol. Pathophysiol. Pharmacol. 2020, 12, 58–69. [Google Scholar]
- Gundogdu, A.C.; Ozbayer, C.; Kar, F. Boric Acid Alleviates Gastric Ulcer by Regulating Oxidative Stress and Inflammation-Related Multiple Signaling Pathways. Biol. Trace Elem. Res. 2024, 202, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Guo, Y.L.; Chen, Y.R.; Zhang, L.Y.; Wang, Z.C.; Zhang, T.; Wang, B. A potential drug combination of omeprazole and patchouli alcohol significantly normalizes oxidative stress and inflammatory responses against gastric ulcer in ethanol-induced rat model. Int. Immunopharmacol. 2020, 85, 106660. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, E.; Guerra, G.; Andrade, A.; Fernandes, J.M.; Da, S.V.; De Aragao, T.E.; De Araujo, A.A.; de Araujo, J.R.; Zucolotto, S.M. Gastric Ulcer Healing Property of Bryophyllum pinnatum Leaf Extract in Chronic Model In Vivo and Gastroprotective Activity of Its Major Flavonoid. Front. Pharmacol. 2021, 12, 744192. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, W.; Lu, L.; Zheng, J.; Hu, X.; Yu, Z.; Zhu, L. Study on the antiulcer effects of Veronicastrum axillare on gastric ulcer in rats induced by ethanol based on tumor necrosis factor-alpha (TNF-alpha) and endothelin-1 (ET-1). Asian Pac. J. Trop. Biomed. 2013, 3, 925–930. [Google Scholar] [CrossRef]
- Lee, S.M. Anti-inflammatory effects of ginsenosides Rg5, Rz1, and Rk1: Inhibition of TNF-alpha-induced NF-kappaB, COX-2, and iNOS transcriptional expression. Phytother. Res. 2014, 28, 1893–1896. [Google Scholar] [CrossRef]
- Kim, T.S.; Ikeuchi, T.; Theofilou, V.I.; Williams, D.W.; Greenwell-Wild, T.; June, A.; Adade, E.E.; Li, L.; Abusleme, L.; Dutzan, N.; et al. Epithelial-derived interleukin-23 promotes oral mucosal immunopathology. Immunity 2024, 57, 859–875. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar]
- Gao, H.L.; Yang, Y.; Tian, H.; Xu, S.L.; Li, B.W.; Fu, L.Y.; Liu, K.L.; Shi, X.L.; Kang, Y.M.; Yu, X.J. Puerarin Alleviates Blood Pressure via Inhibition of ROS/TLR4/NLRP3 Inflammasome Signaling Pathway in the Hypothalamic Paraventricular Nucleus of Salt-Induced Prehypertensive Rats. Nutrients 2024, 16, 2580. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Fan, P.; Xie, S.; Zhang, Z.; Yuan, Q.; He, J.; Zhang, J.; Liu, X.; Liu, X.; Xu, L. Dendrobium officinale flos water extract ameliorates ethanol-induced acute gastric mucosal injury via inhibiting oxidative stress and inflammation. J. Sci. Food Agric. 2024, 104, 8593–8603. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, H.; Jin, Y.; Zhang, M.; Zhao, Q.; Li, H.; Wang, S.; Lu, Y.; Chen, S.; Du, H.; et al. The active components and potential mechanisms of Wuji Wan in the treatment of ethanol-induced gastric ulcer: An integrated metabolomics, network pharmacology and experimental validation. J. Ethnopharmacol. 2024, 326, 117901. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Xue, H.; Li, Y.; Liu, J.; Lou, Y.; Li, S.; Wang, Y.; Lu, J.; Chen, X. Potential Mechanisms and Effects of Dai Bai Jie Ethanol Extract in Preventing Acute Alcoholic Liver Injury. Curr. Issues Mol. Biol. 2025, 47, 3. [Google Scholar] [CrossRef]
- Bu, J.; Liu, Y.; Zhang, R.; Lin, S.; Zhuang, J.; Sun, L.; Zhang, L.; He, H.; Zong, R.; Wu, Y.; et al. Potential New Target for Dry Eye Disease-Oxidative Stress. Antioxidants 2024, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Selim, H.M.; Negm, W.A.; Hawwal, M.F.; Hussein, I.A.; Elekhnawy, E.; Ulber, R.; Zayed, A. Fucoidan mitigates gastric ulcer injury through managing inflammation, oxidative stress, and NLRP3-mediated pyroptosis. Int. Immunopharmacol. 2023, 120, 110335. [Google Scholar] [CrossRef]
- Duan, Z.; Yu, S.; Wang, S.; Deng, H.; Guo, L.; Yang, H.; Xie, H. Protective Effects of Piperine on Ethanol-Induced Gastric Mucosa Injury by Oxidative Stress Inhibition. Nutrients 2022, 14, 4744. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Y.; Zhou, L.; Lin, F.; Wan, M.; Gan, A.; Wu, B.; Yan, T.; Jia, Y. Costunolide ameliorates MNNG-induced chronic atrophic gastritis through inhibiting oxidative stress and DNA damage via activation of Nrf2. Phytomedicine 2024, 130, 155581. [Google Scholar] [CrossRef]
- Xu, S.; Sun, X.; Wu, J.; Li, K.; Li, X.; Zhang, Y.; Gao, X.J. TBBPA causes inflammation and cell death via the ROS/NF-kappaB pathway in the gastric mucosa. Ecotoxicol. Environ. Saf. 2023, 262, 115320. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, L.; Hu, Z.; Kong, S.; Zhang, Z.; Li, G. Optimized preparation of gastric acid-response sulfhydryl functionalized chitosan/alginate/tilapia peptide hydrogel and its protective effects on alcohol-induced liver and brain injury. Rsc Adv. 2021, 11, 34544–34557. [Google Scholar] [CrossRef]
- Jeong, M.; Kwon, H.; Kim, Y.; Jin, H.; Choi, G.; Hyun, K. Erigeron annuus Extract Improves DNCB-Induced Atopic Dermatitis in a Mouse Model via the Nrf2/HO-1 Pathway. Nutrients 2024, 16, 451. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, S.; Patinen, T.; Jawahar Deen, A.; Pitkänen, S.; Härkönen, J.; Kansanen, E.; Küblbeck, J.; Levonen, A. The KEAP1-NRF2 pathway: Targets for therapy and role in cancer. Redox Biol. 2023, 63, 102726. [Google Scholar] [CrossRef]
- O’Rourke, S.A.; Shanley, L.C.; Dunne, A. The Nrf2-HO-1 system and inflammaging. Front. Immunol. 2024, 15, 1457010. [Google Scholar] [CrossRef]
- Niu, Z.; Li, X.; Yang, X.; Sun, Z. Protective effects of sinomenine against dextran sulfate sodium-induced ulcerative colitis in rats via alteration of HO-1/Nrf2 and inflammatory pathway. Inflammopharmacology 2024, 32, 2007–2022. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Saad, M.A.; El-Sahar, A.E.; Al-Shorbagy, M.Y. Mechanistic perspective of morin protection against ketoprofen-induced gastric mucosal injury: Targeting HMGB1/RAGE/NF-kappaB, DJ-1/Nrf2/HO-1 and PI3K/mTOR pathways. Arch. Biochem. Biophys. 2020, 693, 108552. [Google Scholar] [CrossRef]
- Jia, X.; He, Y.; Li, L.; Xu, D. Pharmacological targeting of gastric mucosal barrier with traditional Chinese medications for repairing gastric mucosal injury. Front. Pharmacol. 2023, 14, 1091530. [Google Scholar] [CrossRef] [PubMed]
- Ham, M.; Kaunitz, J.D. Gastroduodenal defense. Curr. Opin. Gastroenterol. 2007, 23, 607–616. [Google Scholar] [CrossRef]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a29314. [Google Scholar] [CrossRef]
- Chen, T.; Bao, S.; Chen, J.; Zhang, J.; Wei, H.; Hu, X.; Liang, Y.; Li, J.; Yan, S. Xiaojianzhong decoction attenuates aspirin-induced gastric mucosal injury via the PI3K/AKT/mTOR/ULK1 and AMPK/ULK1 pathways. Pharm. Biol. 2023, 61, 1234–1248. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 327745. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Liang, Y.; Wu, L.; Lin, L.; Yao, Y.; Deng, J.; Xu, J.; Li, H.; Gao, F.; Xing, W.; et al. Pretreatment with Citrus reticulata ‘Chachi’ Polysaccharide Alleviates Alcohol-Induced Gastric Ulcer by Inhibiting NLRP3/ASC/Caspase-1 and Nrf2/HO-1 Signaling Pathways. Nutrients 2025, 17, 2062. https://doi.org/10.3390/nu17132062
Liang H, Liang Y, Wu L, Lin L, Yao Y, Deng J, Xu J, Li H, Gao F, Xing W, et al. Pretreatment with Citrus reticulata ‘Chachi’ Polysaccharide Alleviates Alcohol-Induced Gastric Ulcer by Inhibiting NLRP3/ASC/Caspase-1 and Nrf2/HO-1 Signaling Pathways. Nutrients. 2025; 17(13):2062. https://doi.org/10.3390/nu17132062
Chicago/Turabian StyleLiang, Huosheng, Yiyao Liang, Lipeng Wu, Long Lin, Yunan Yao, Jinji Deng, Jiepei Xu, Huajian Li, Fangfang Gao, Wenlong Xing, and et al. 2025. "Pretreatment with Citrus reticulata ‘Chachi’ Polysaccharide Alleviates Alcohol-Induced Gastric Ulcer by Inhibiting NLRP3/ASC/Caspase-1 and Nrf2/HO-1 Signaling Pathways" Nutrients 17, no. 13: 2062. https://doi.org/10.3390/nu17132062
APA StyleLiang, H., Liang, Y., Wu, L., Lin, L., Yao, Y., Deng, J., Xu, J., Li, H., Gao, F., Xing, W., Yu, M., Jia, X., Wei, M., Li, C., & Zheng, G. (2025). Pretreatment with Citrus reticulata ‘Chachi’ Polysaccharide Alleviates Alcohol-Induced Gastric Ulcer by Inhibiting NLRP3/ASC/Caspase-1 and Nrf2/HO-1 Signaling Pathways. Nutrients, 17(13), 2062. https://doi.org/10.3390/nu17132062






