Effects of 12 Weeks of Chromium, Phyllanthus emblica Fruit Extract, and Shilajit Supplementation on Markers of Cardiometabolic Health, Fitness, and Weight Loss in Men and Women with Risk Factors to Metabolic Syndrome Initiating an Exercise and Diet Intervention: A Randomized Double-Blind, Placebo-Controlled Trial †
Abstract
1. Introduction
2. Methods
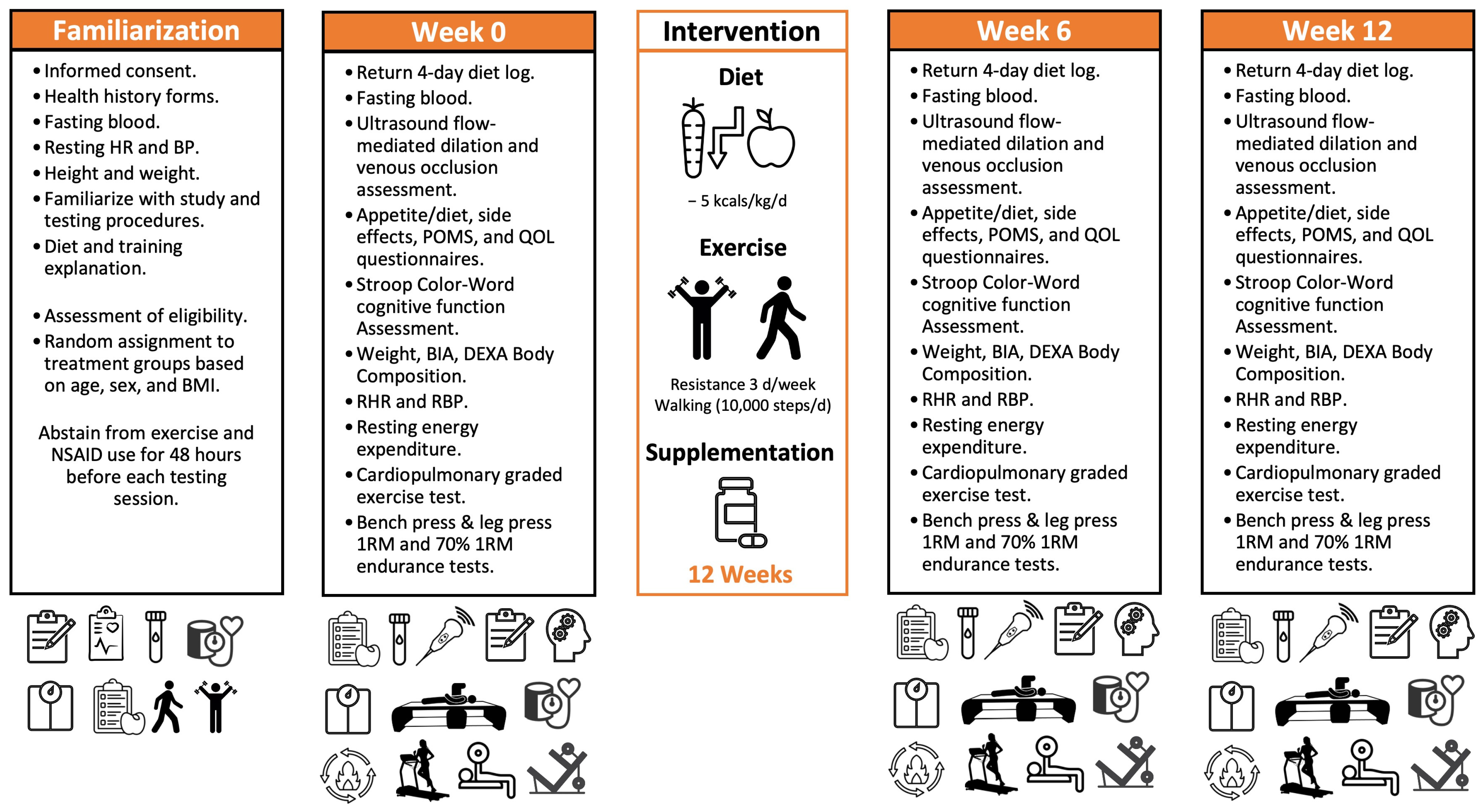
2.1. Research Design
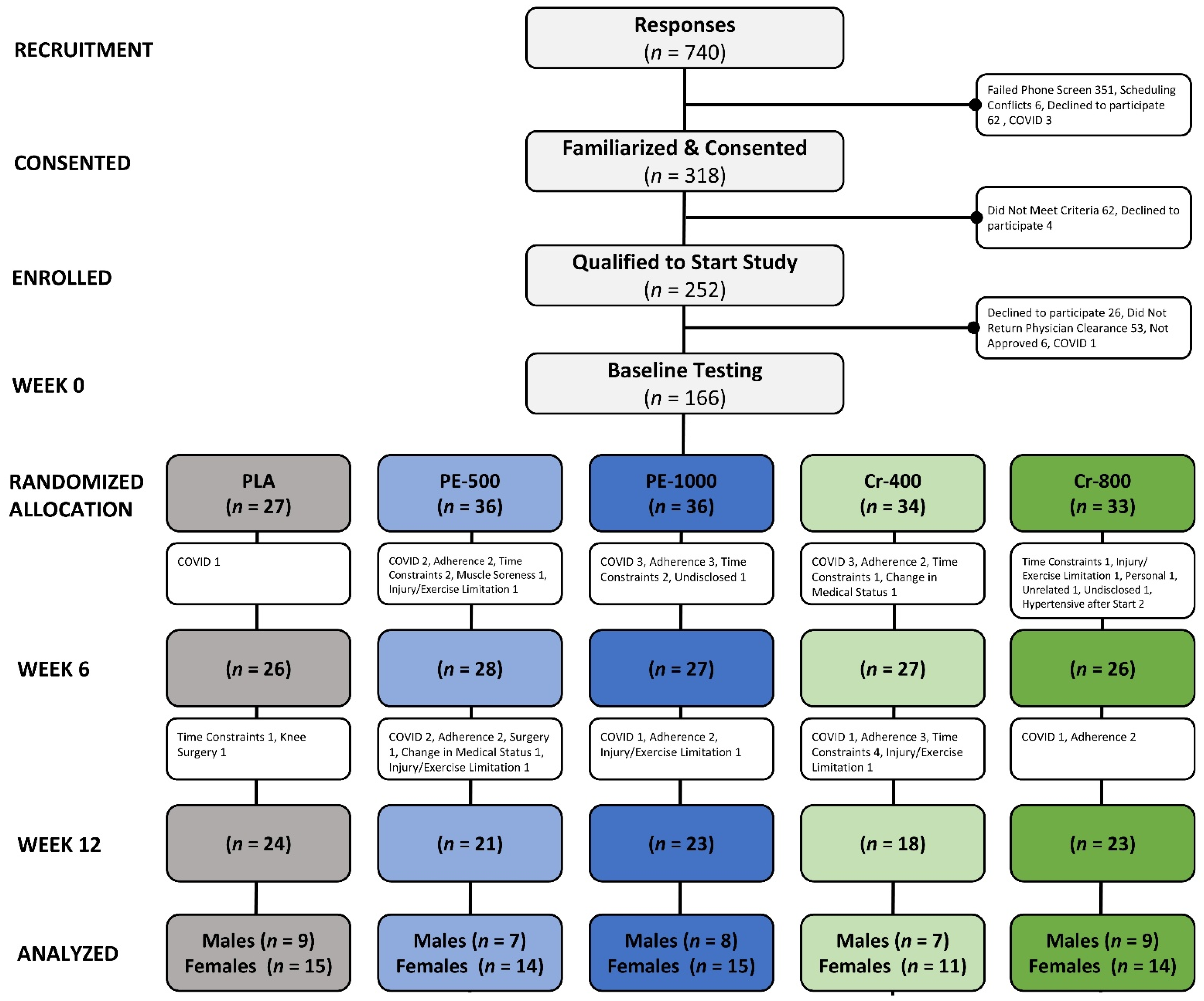
2.2. Study Participants
2.3. Familiarization
2.4. Experimental Session Testing Protocol
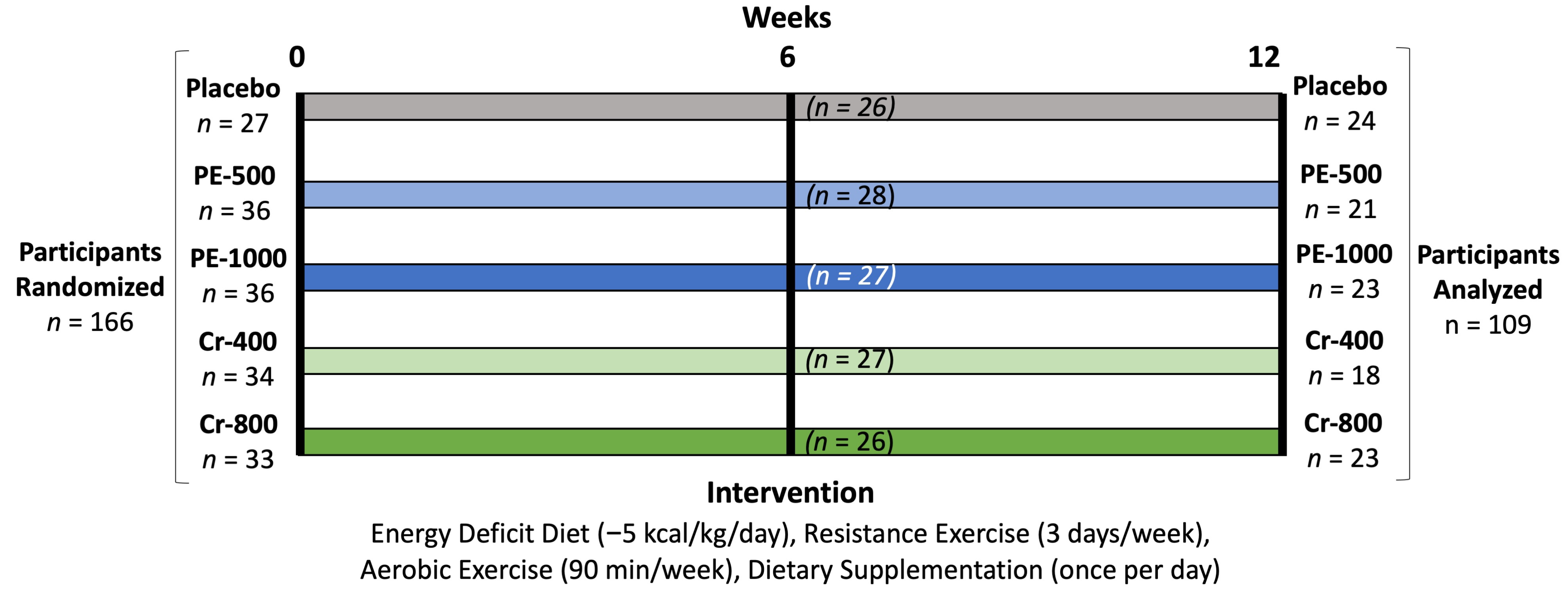
2.5. Randomization
2.6. Exercise Training
2.7. Diet Modification
2.8. Dietary Supplementation
3. Procedures
3.1. Diet Assessment
3.2. Training Volume Assessment
3.3. Resting Energy Expenditure Assessment
3.4. Body Composition, Total Body Water, and Resting Hemodynamics
3.5. Pulse Wave Velocity Assessment
3.6. Flow-Mediated Dilation Assessment
3.7. Blood Collection and Analysis
3.8. Exercise Assessment
3.9. Quality of Life
3.10. Diet Satisfaction
3.11. Side Effects
3.12. Statistical Analysis
4. Results
4.1. Participant Demographics
4.2. Energy and Macronutrient Intake
4.3. Physical Activity
4.4. Resting Energy Expenditure and Metabolism
4.5. Cardiovascular Health and Fitness Results
4.6. Muscular Strength and Endurance Results
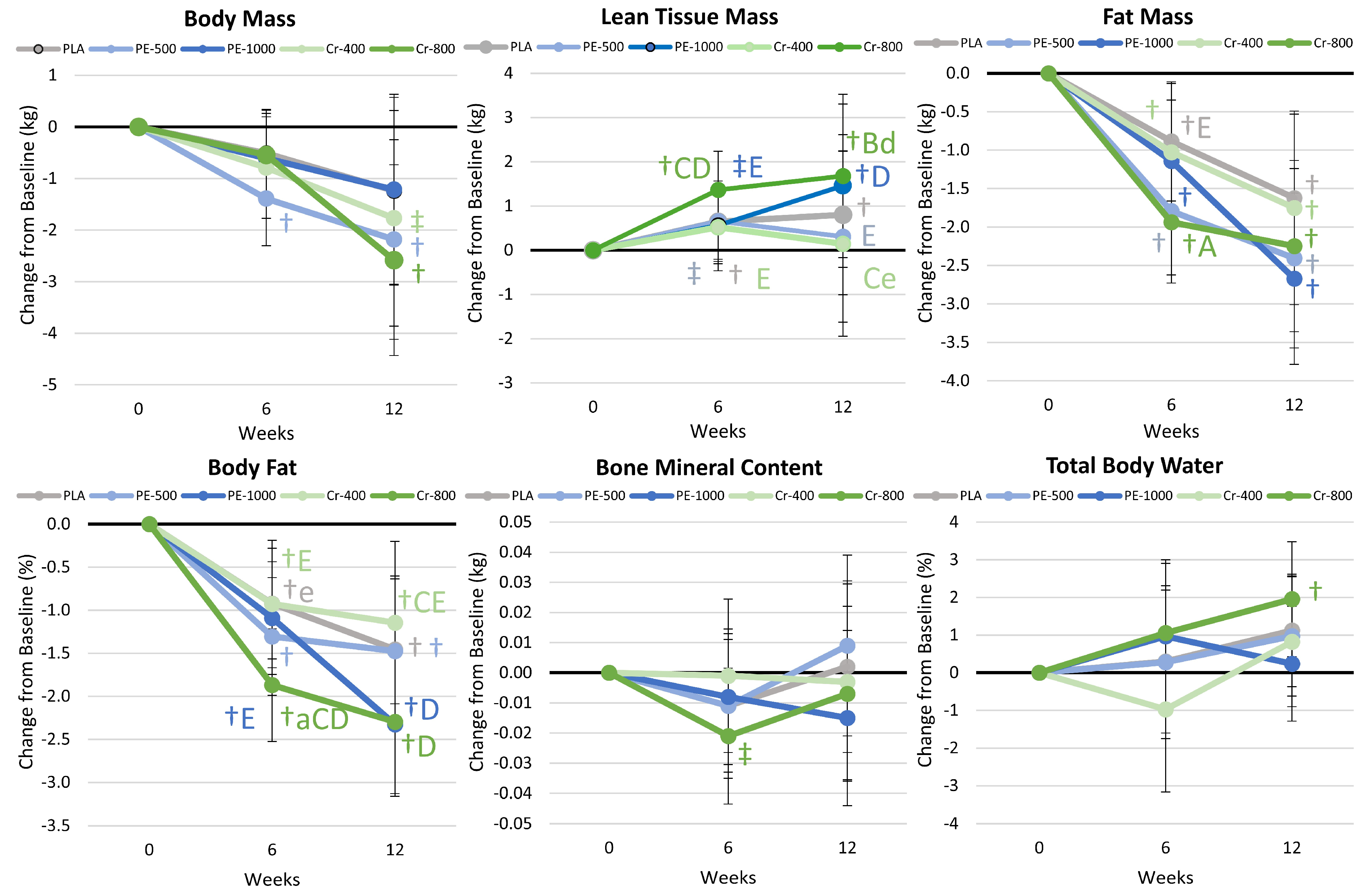
4.7. Body Composition Results
4.8. Blood Analysis
4.8.1. Whole-Blood Analysis
4.8.2. Markers of Catabolism
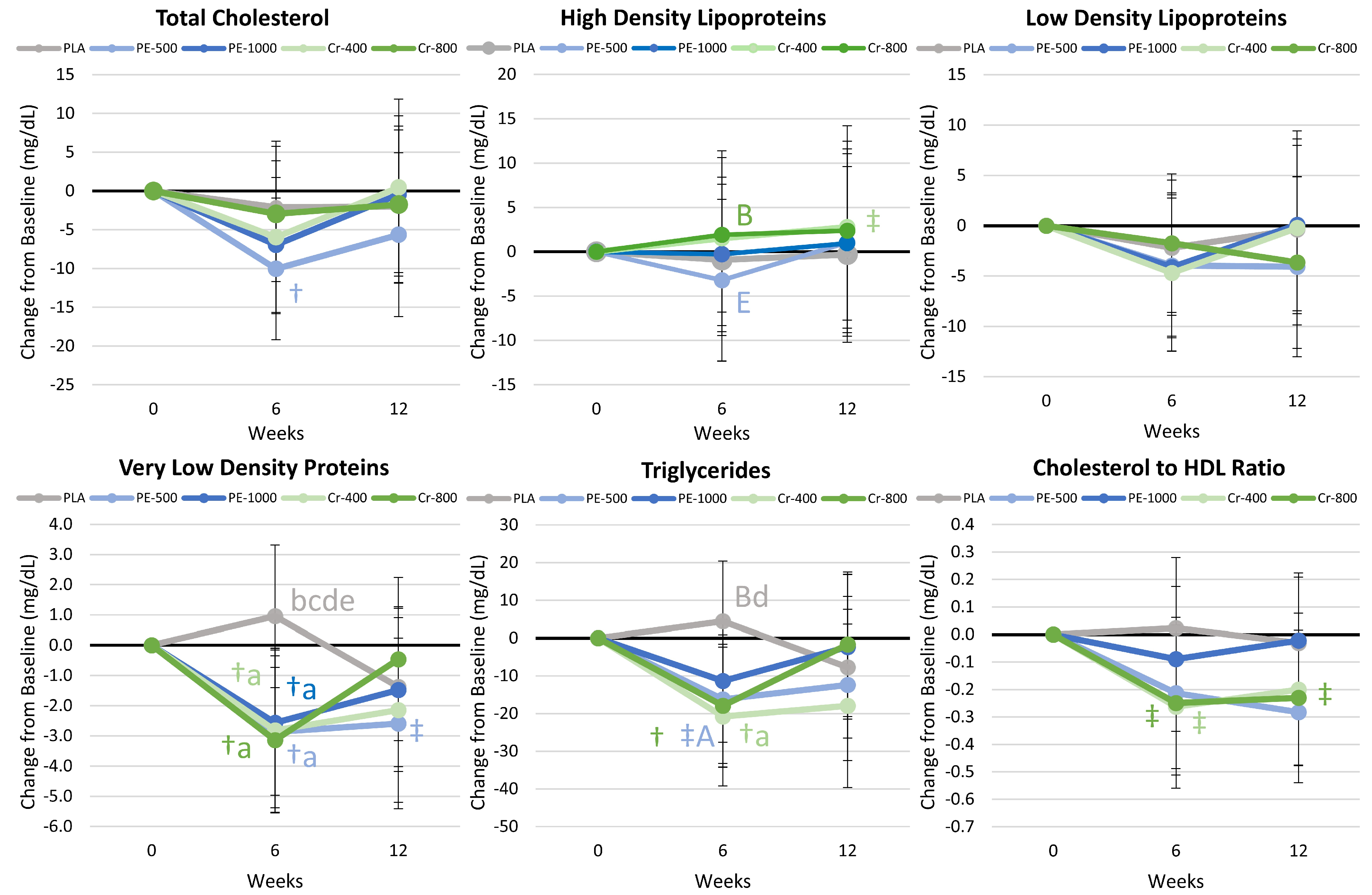
4.8.3. Blood Lipid Results
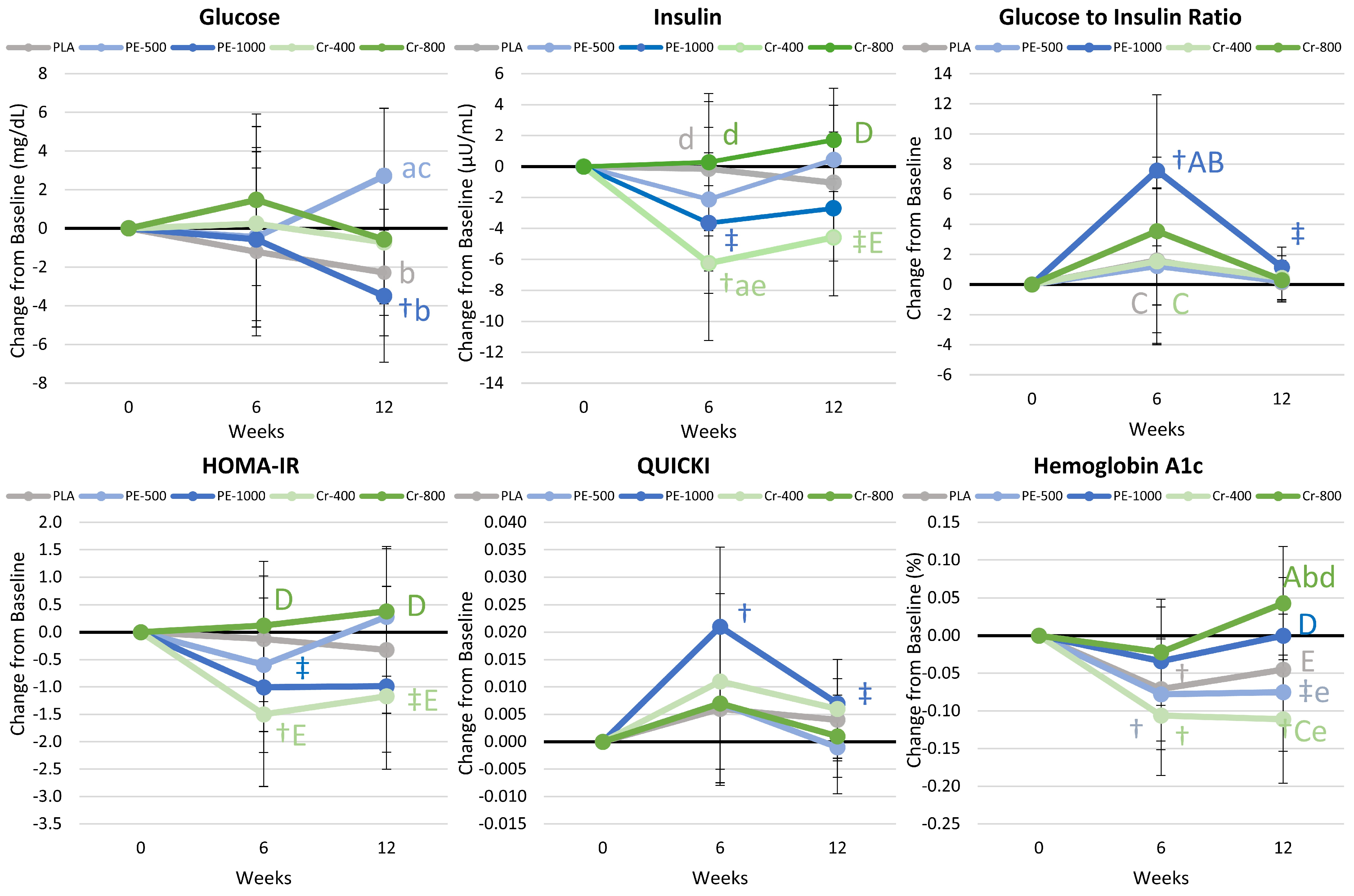
4.8.4. Glucose and Insulin Results
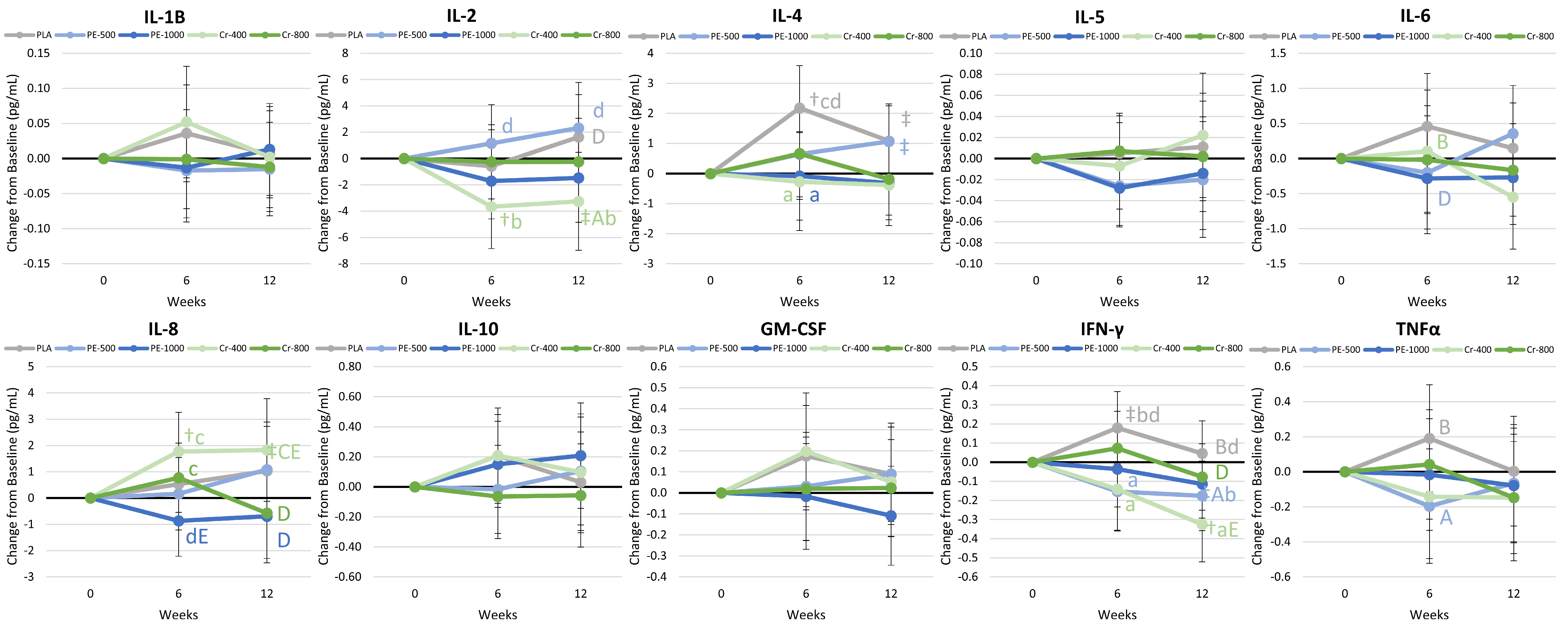
4.8.5. Cytokines and Markers of Inflammation
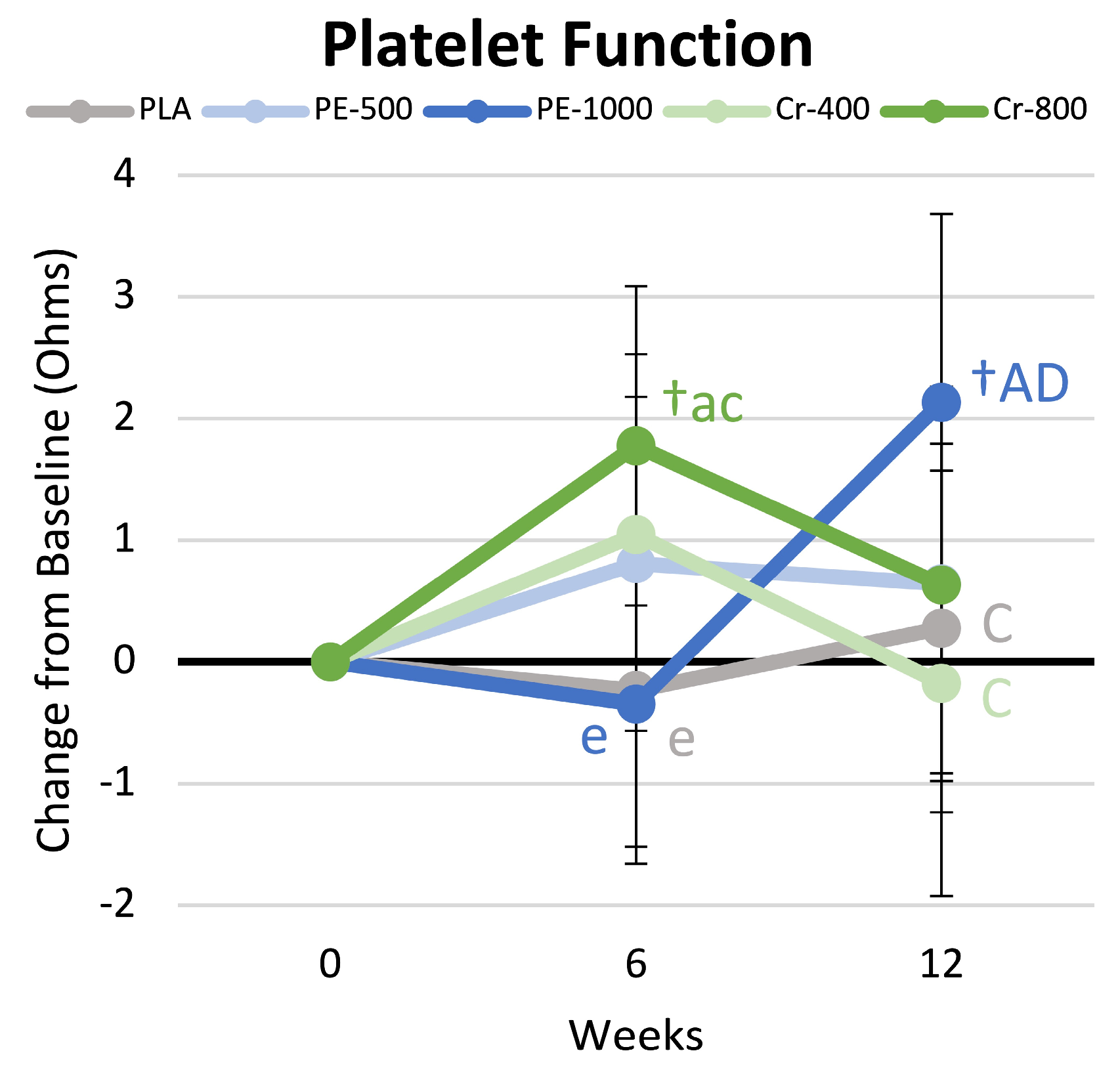
4.9. Platelet Aggregation
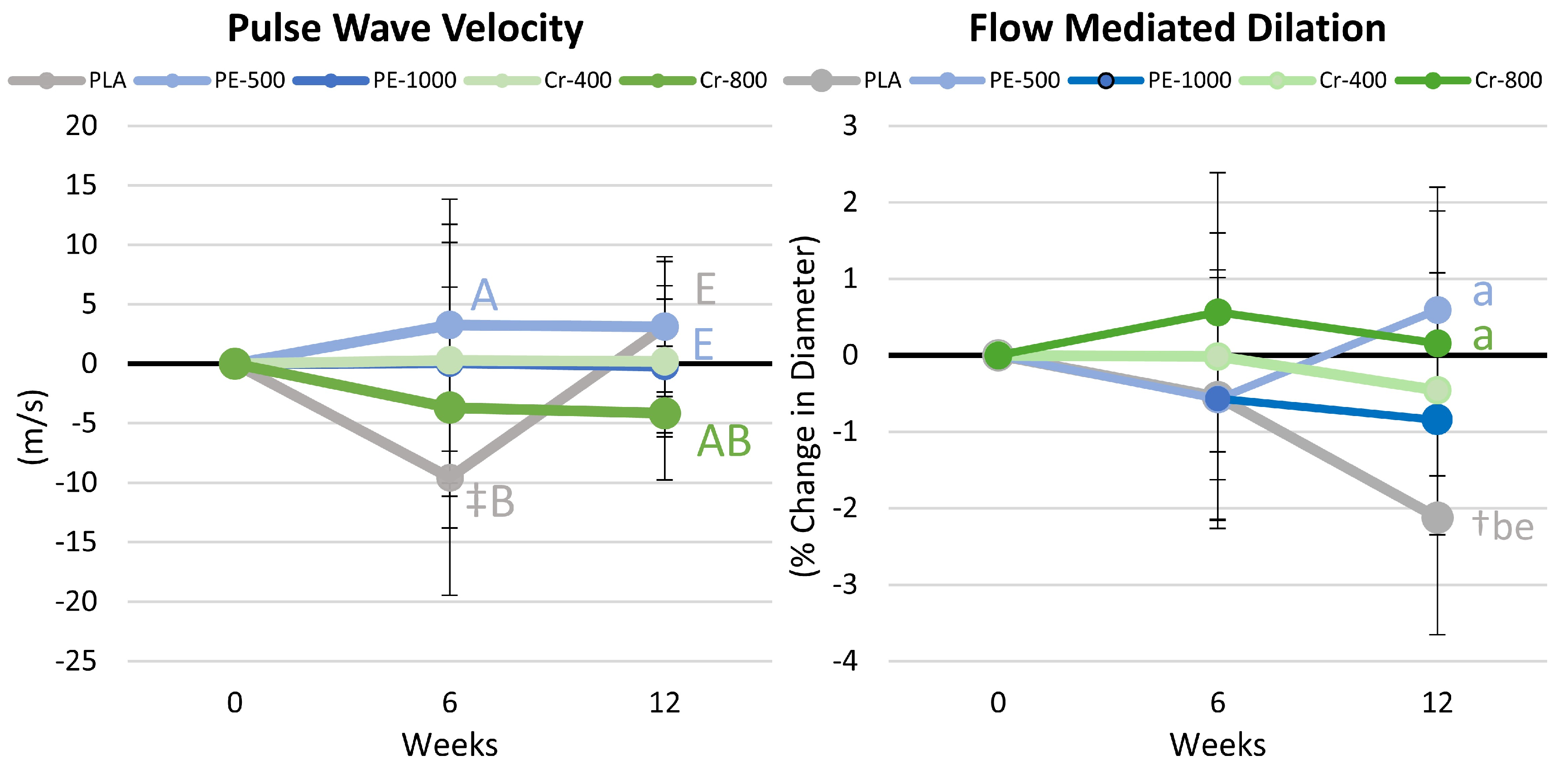
4.10. Pulse Wave and Flow-Mediated Dilation Results
4.11. Profile of Mood States Results
4.12. Quality of Life, Diet Satisfaction, and Perceptions of Side Effects
5. Discussion
5.1. Primary Outcomes
5.1.1. Endothelial Function and Platelet Aggregation
5.1.2. Glucose Homeostasis and Insulin Sensitivity
5.1.3. Blood Lipids
5.1.4. Inflammatory Markers
5.2. Secondary Outcomes
5.2.1. Body Composition
5.2.2. Training Adaptations
5.2.3. Psychometric Assessment
5.2.4. Side Effect Assessment
5.3. Limitations and Challenges
5.4. Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1RM | One-repetition maximum |
| ANOVA | Analysis of variance |
| BMC | Bone mineral content |
| BMI | Body mass index |
| CI | Confidence interval |
| CONSORT | Consolidated Standards of Reporting Trials |
| Cr | Chromium |
| Cr-400 | Chromium (400 μ/d) + 6 mg/d PE + 6 mg/d SJ |
| Cr-800 | Chromium (800 μ/d) + 12 mg/d PE + 12 mg/d SJ |
| DEXA | Dual-energy X-ray absorptiometer |
| DOAJ | Directory of open access journals |
| GIR | Glucose/insulin ratio |
| GLM | General linear model |
| GMC-SF | Granulocyte-macrophage colony-stimulating factor |
| GPXT | Cardiopulmonary exercise test |
| HDL | High-density lipoprotein |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| HRR | Heart rate reserve |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| LDL | Low-density lipoproteins |
| LSD | Least significant difference |
| LTM | Lean tissue mass |
| MDPI | Multidisciplinary Digital Publishing Institute |
| PE | Phyllanthus emblica |
| PE-1000 | Phyllanthus emblica (1000 mg/d) |
| PE-500 | Phyllanthus emblica (500 mg/d) |
| PLA | Placebo |
| POMS) | Profile of Mood States |
| QOL | Quality of life |
| QUICKI | Quantitative insulin sensitivity check index |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| SD | Standard deviation |
| SJ | Shilajit |
| TNF-α | Tumor necrosis factor-α |
| VLDL | Very-low-density lipoproteins |
References
- Power, M.; Pratley, R. Alternative and complementary treatments for metabolic syndrome. Curr. Diabetes Rep. 2011, 11, 173–178. [Google Scholar] [CrossRef]
- Lockard, B.; Earnest, C.P.; Oliver, J.; Goodenough, C.; Rasmussen, C.; Greenwood, M.; Kreider, R.B. Retrospective Analysis of Protein- and Carbohydrate-Focused Diets Combined with Exercise on Metabolic Syndrome Prevalence in Overweight and Obese Women. Metab. Syndr. Relat. Disord. 2016, 14, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Baetge, C.; Earnest, C.P.; Lockard, B.; Coletta, A.M.; Galvan, E.; Rasmussen, C.; Levers, K.; Simbo, S.Y.; Jung, Y.P.; Koozehchian, M.; et al. Efficacy of a randomized trial examining commercial weight loss programs and exercise on metabolic syndrome in overweight and obese women. Appl. Physiol. Nutr. Metab. 2017, 42, 216–227. [Google Scholar] [CrossRef]
- Maret, W. Chromium Supplementation in Human Health, Metabolic Syndrome, and Diabetes. Met. Ions Life Sci. 2019, 19, 393–412. [Google Scholar] [CrossRef]
- Prananda, A.T.; Dalimunthe, A.; Harahap, U.; Simanjuntak, Y.; Peronika, E.; Karosekali, N.E.; Hasibuan, P.A.Z.; Syahputra, R.A.; Situmorang, P.C.; Nurkolis, F. Phyllanthus emblica: A comprehensive review of its phytochemical composition and pharmacological properties. Front. Pharmacol. 2023, 14, 1288618. [Google Scholar] [CrossRef]
- Chen, T.S.; Liou, S.Y.; Chang, Y.L. Supplementation of Emblica officinalis (Amla) extract reduces oxidative stress in uremic patients. Am. J. Chin. Med. 2009, 37, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hazra, B.; Sarkar, R.; Biswas, S.; Mandal, N. Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Complement. Altern. Med. 2010, 10, 20. [Google Scholar] [CrossRef]
- Usharani, P.; Fatima, N.; Muralidhar, N. Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: A randomized, double-blind, controlled study. Diabetes Metab. Syndr. Obes. 2013, 6, 275–284. [Google Scholar] [CrossRef]
- Li, G.; Yu, Q.; Li, M.; Zhang, D.; Yu, J.; Yu, X.; Xia, C.; Lin, J.; Han, L.; Huang, H. Phyllanthus emblica fruits: A polyphenol-rich fruit with potential benefits for oral management. Food Funct. 2023, 14, 7738–7759. [Google Scholar] [CrossRef]
- Setayesh, L.; Haghighat, N.; Rasaei, N.; Rezaei, M.; Casazza, K.; Nadery, M.; Yamrali, I.; Zamani, M.; Asbaghi, O. The impact of Emblica Officinalis (Amla) on lipid profile, glucose, and C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. 2023, 17, 102729. [Google Scholar] [CrossRef]
- Krishnaveni, M.; Mirunalini, S. Chemopreventive efficacy of Phyllanthus emblica L. (amla) fruit extract on 7,12-dimethylbenz(a)anthracene induced oral carcinogenesis—A dose-response study. Environ. Toxicol. Pharmacol. 2012, 34, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Srikumar, R.; Parthasarathy, N.J.; Manikandan, S.; Narayanan, G.S.; Sheeladevi, R. Effect of Triphala on oxidative stress and on cell-mediated immune response against noise stress in rats. Mol. Cell Biochem. 2006, 283, 67–74. [Google Scholar] [CrossRef]
- Khanna, S.; Das, A.; Spieldenner, J.; Rink, C.; Roy, S. Supplementation of a standardized extract from Phyllanthus emblica improves cardiovascular risk factors and platelet aggregation in overweight/class-1 obese adults. J. Med. Food 2015, 18, 415–420. [Google Scholar] [CrossRef]
- Ansari, A.; Shahriar, M.S.; Hassan, M.M.; Das, S.R.; Rokeya, B.; Haque, M.A.; Haque, M.E.; Biswas, N.; Sarkar, T. Emblica officinalis improves glycemic status and oxidative stress in STZ induced type 2 diabetic model rats. Asian Pac. J. Trop. Med. 2014, 7, 21–25. [Google Scholar] [CrossRef]
- Wilson, E.; Rajamanickam, G.V.; Dubey, G.P.; Klose, P.; Musial, F.; Saha, F.J.; Rampp, T.; Michalsen, A.; Dobos, G.J. Review on shilajit used in traditional Indian medicine. J. Ethnopharmacol. 2011, 136, 1–9. [Google Scholar] [CrossRef]
- Jafari, M.; Forootanfar, H.; Ameri, A.; Foroutanfar, A.; Adeli-Sardou, M.; Rahimi, H.R.; Najafi, A.; Zangiabadi, N.; Shakibaie, M. Antioxidant, cytotoxic and hyperalgesia-suppressing activity of a native Shilajit obtained from Bahr Aseman mountains. Pak. J. Pharm. Sci. 2019, 32, 2167–2173. [Google Scholar] [PubMed]
- Pingali, U.; Nutalapati, C. Shilajit extract reduces oxidative stress, inflammation, and bone loss to dose-dependently preserve bone mineral density in postmenopausal women with osteopenia: A randomized, double-blind, placebo-controlled trial. Phytomedicine 2022, 105, 154334. [Google Scholar] [CrossRef]
- Stohs, S.J. Safety and efficacy of shilajit (mumie, moomiyo). Phytother. Res. 2014, 28, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Lawley, S.; Gupta, R.C.; Goad, J.T.; Canerdy, T.D.; Kalidindi, S.R. Anti-inflammatory and anti-arthritic efficacy and safety of purified shilajit in moderately arthritic dogs. J. Vet. Sci. Anim. Husb. 2013, 1, 302. [Google Scholar] [CrossRef]
- Das, A.; Datta, S.; Rhea, B.; Sinha, M.; Veeraragavan, M.; Gordillo, G.; Roy, S. The Human Skeletal Muscle Transcriptome in Response to Oral Shilajit Supplementation. J. Med. Food 2016, 19, 701–709. [Google Scholar] [CrossRef]
- Meena, H.; Pandey, H.K.; Arya, M.C.; Ahmed, Z. Shilajit: A panacea for high-altitude problems. Int. J. Ayurveda Res. 2010, 1, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Biswas, S.; Jana, U.; De, R.K.; Mukhopadhyay, S.C.; Biswas, T.K. Clinical evaluation of purified Shilajit on testosterone levels in healthy volunteers. Andrologia 2016, 48, 570–575. [Google Scholar] [CrossRef]
- Fleck, A.; Gupta, R.C.; Goad, J.T.; Lasher, M.A.; Canerdy, T.D.; Kalidindi, S.R. Anti-arthritic efficacy and safety of Crominex 3+ (Trivalent Chromium, Phyllanthus emblica Extract, and Shilajit) In moderately arthritic dogs. J. Vet. Sci. Anim. Husb. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Brahma, S.; Goyal, A.K.; Dhamodhar, P.; Kumari, M.R.; Jayashree, S.; Usha, T.; Middha, S.K. Can Polyherbal Medicine be used for the Treatment of Diabetes?—A Review of Historical Classics, Research Evidence and Current Prevention Programs. Curr. Diabetes Rev. 2024, 20, e140323214600. [Google Scholar] [CrossRef]
- Ali, A.; Ma, Y.; Reynolds, J.; Wise, J.P., Sr.; Inzucchi, S.E.; Katz, D.L. Chromium effects on glucose tolerance and insulin sensitivity in persons at risk for diabetes mellitus. Endocr. Pract. 2011, 17, 16–25. [Google Scholar] [CrossRef]
- Amato, P.; Morales, A.J.; Yen, S.S. Effects of chromium picolinate supplementation on insulin sensitivity, serum lipids, and body composition in healthy, nonobese, older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M260–M263. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Wang, Z.Q.; Zhang, X.H.; Baldor, L.C.; Russell, J.C. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle Glut-4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J. Nutr. 2002, 132, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Omani-Samani, R.; Vesali, S.; Maroufizadeh, S.; Rezaeinejad, M.; Razavi, M.; Sepidarkish, M. The Effects of Supplementation with Chromium on Insulin Resistance Indices in Women with Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Horm. Metab. Res. 2018, 50, 193–200. [Google Scholar] [CrossRef]
- Jamilian, M.; Zadeh Modarres, S.; Amiri Siavashani, M.; Karimi, M.; Mafi, A.; Ostadmohammadi, V.; Asemi, Z. The Influences of Chromium Supplementation on Glycemic Control, Markers of Cardio-Metabolic Risk, and Oxidative Stress in Infertile Polycystic ovary Syndrome Women Candidate for In vitro Fertilization: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2018, 185, 48–55. [Google Scholar] [CrossRef]
- Kim, C.W.; Kim, B.T.; Park, K.H.; Kim, K.M.; Lee, D.J.; Yang, S.W.; Joo, N.S. Effects of short-term chromium supplementation on insulin sensitivity and body composition in overweight children: Randomized, double-blind, placebo-controlled study. J. Nutr. Biochem. 2011, 22, 1030–1034. [Google Scholar] [CrossRef]
- Mark, D.A. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes: Response to Martin et al. Diabetes Care 2006, 29, 2764, author reply 2764–2765. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. The origins of western obesity: A role for animal protein? Med. Hypotheses 2000, 54, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Breithaupt, L.; Bulik, C.M.; Hamer, R.M.; La Via, M.C.; Brownley, K.A. A Double-Blind, Randomized Pilot Trial of Chromium Picolinate for Overweight Individuals with Binge-Eating Disorder: Effects on Glucose Regulation. J. Diet. Suppl. 2017, 14, 191–199. [Google Scholar] [CrossRef]
- Alkhalidi, F. A comparative study to assess the use of chromium in type 2 diabetes mellitus. J. Med. Life 2023, 16, 1178–1182. [Google Scholar] [CrossRef]
- Farrokhian, A.; Mahmoodian, M.; Bahmani, F.; Amirani, E.; Shafabakhsh, R.; Asemi, Z. The Influences of Chromium Supplementation on Metabolic Status in Patients with Type 2 Diabetes Mellitus and Coronary Heart Disease. Biol. Trace Elem. Res. 2020, 194, 313–320. [Google Scholar] [CrossRef]
- Hoeger, W.W.; Harris, C.; Long, E.M.; Hopkins, D.R. Four-week supplementation with a natural dietary compound produces favorable changes in body composition. Adv. Ther. 1998, 15, 305–314. [Google Scholar]
- Preuss, H.G.; Anderson, R.A. Chromium update: Examining recent literature 1997–1998. Curr. Opin. Clin. Nutr. Metab. Care 1998, 1, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.; Adams, B.; French, A.; Neggers, Y.; Vincent, J.B. High-dose chromium(III) supplementation has no effects on body mass and composition while altering plasma hormone and triglycerides concentrations. Biol. Trace Elem. Res. 2006, 113, 53–66. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Siders, W.A.; Penland, J.G. Chromium picolinate supplementation in women: Effects on body weight, composition, and iron status. Nutrition 2007, 23, 187–195. [Google Scholar] [CrossRef]
- Tian, H.; Guo, X.; Wang, X.; He, Z.; Sun, R.; Ge, S.; Zhang, Z. Chromium picolinate supplementation for overweight or obese adults. Cochrane Database Syst. Rev. 2013, 2013, CD010063. [Google Scholar] [CrossRef]
- Vajdi, M.; Khajeh, M.; Safaei, E.; Moeinolsadat, S.; Mousavi, S.; Seyedhosseini-Ghaheh, H.; Abbasalizad-Farhangi, M.; Askari, G. Effects of chromium supplementation on body composition in patients with type 2 diabetes: A dose-response systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2024, 81, 127338. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Taghizadeh, M.; Aghabagheri, E.; Asemi, Z.; Jafarnejad, S. A meta-analysis of the effect of chromium supplementation on anthropometric indices of subjects with overweight or obesity. Clin. Obes. 2019, 9, e12313. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, S.; Zdanowski, R.; Krzyzowska, M.; Lewicka, A.; Debski, B.; Niemcewicz, M.; Goniewicz, M. The role of Chromium III in the organism and its possible use in diabetes and obesity treatment. Ann. Agric. Environ. Med. 2014, 21, 331–335. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Escalante, G.; Campbell, B.; Kalman, D.; Antonio, J. Proceedings of the Twenty-First International Society of Sports Nutrition (ISSN) Conference and Expo. J. Int. Soc. Sports Nutr. 2024, 21 (Suppl. S1), 2374669. [Google Scholar] [CrossRef] [PubMed Central]
- Kerksick, C.; Thomas, A.; Campbell, B.; Taylor, L.; Wilborn, C.; Marcello, B.; Roberts, M.; Pfau, E.; Grimstvedt, M.; Opusunju, J.; et al. Effects of a popular exercise and weight loss program on weight loss, body composition, energy expenditure and health in obese women. Nutr. Metab. 2009, 6, 23. [Google Scholar] [CrossRef]
- Dickerson, B.; Maury, J.; Jenkins, V.; Nottingham, K.; Xing, D.; Gonzalez, D.E.; Leonard, M.; Kendra, J.; Ko, J.; Yoo, C.; et al. Effects of Supplementation with Microalgae Extract from Phaeodactylum tricornutum (Mi136) to Support Benefits from a Weight Management Intervention in Overweight Women. Nutrients 2024, 16, 990. [Google Scholar] [CrossRef]
- Teixeira, V.; Voci, S.M.; Mendes-Netto, R.S.; da Silva, D.G. The relative validity of a food record using the smartphone application MyFitnessPal. Nutr. Diet. 2018, 75, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.W.; Morgan, N.; Ward, D.; Tangney, C.; Alshurafa, N.; Van Horn, L.; Spring, B. Comparative Validity of Mostly Unprocessed and Minimally Processed Food Items Differs Among Popular Commercial Nutrition Apps Compared with a Research Food Database. J. Acad. Nutr. Diet. 2022, 122, 825–832.e821. [Google Scholar] [CrossRef]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Agreement on nutrient intake between the databases of the First National Health and Nutrition Examination Survey and the ESHA Food Processor. Am. J. Epidemiol. 2002, 156, 78–85. [Google Scholar] [CrossRef]
- Sowinski, R.; Gonzalez, D.; Xing, D.; Yoo, C.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Humphries, M.; Leonard, M.; Ko, J.; et al. Effects of Inositol-Enhanced Bonded Arginine Silicate Ingestion on Cognitive and Executive Function in Gamers. Nutrients 2021, 13, 3758. [Google Scholar] [CrossRef]
- Ko, J.; Yoo, C.; Xing, D.; Chun, J.; Gonzalez, D.E.; Dickerson, B.L.; Leonard, M.; Jenkins, V.; van der Merwe, M.; Slupsky, C.M.; et al. Effects of Human Milk Oligosaccharide 2′-Fucosyllactose Ingestion on Weight Loss and Markers of Health. Nutrients 2024, 16, 3387. [Google Scholar] [CrossRef] [PubMed]
- Matarese, L.E. Indirect calorimetry: Technical aspects. J. Am. Diet. Assoc. 1997, 97, S154–S160. [Google Scholar] [CrossRef] [PubMed]
- Feurer, I.D.; Crosby, L.O.; Mullen, J. Measured and predicted resting energy expenditure in clinically stable patients. Clin. Nutr. 1984, 3, 27–34. [Google Scholar] [CrossRef]
- Peronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport. Sci. 1991, 16, 23–29. [Google Scholar]
- Gupta, R.D.; Ramachandran, R.; Venkatesan, P.; Anoop, S.; Joseph, M.; Thomas, N. Indirect Calorimetry: From Bench to Bedside. Indian J. Endocrinol. Metab. 2017, 21, 594–599. [Google Scholar] [CrossRef]
- Lohman, T.G.; Harris, M.; Teixeira, P.J.; Weiss, L. Assessing body composition and changes in body composition. Another look at dual-energy X-ray absorptiometry. Ann. N. Y. Acad. Sci. 2000, 904, 45–54. [Google Scholar] [CrossRef]
- Klesges, R.C.; Ward, K.D.; Shelton, M.L.; Applegate, W.B.; Cantler, E.D.; Palmieri, G.M.; Harmon, K.; Davis, J. Changes in bone mineral content in male athletes. Mechanisms of action and intervention effects. JAMA 1996, 276, 226–230. [Google Scholar] [CrossRef]
- Almada, A.; Kreider, R.; Ransom, J.; Rasmussen, C. Comparison of the reliability of repeated whole body dexa scans to repeated spine and hip scans. J. Bone Miner. Res. 1999, 14, S369. [Google Scholar]
- Ji, H.; Xiong, J.; Yu, S.; Chi, C.; Bai, B.; Teliewubai, J.; Lu, Y.; Zhang, Y.; Xu, Y. Measuring the Carotid to Femoral Pulse Wave Velocity (Cf-PWV) to Evaluate Arterial Stiffness. J. Vis. Exp. 2018, 135, 57083. [Google Scholar] [CrossRef]
- Harris, R.A.; Nishiyama, S.K.; Wray, D.W.; Richardson, R.S. Ultrasound Assessment of Flow-Mediated Dilation. Hypertension 2010, 55, 1075–1085. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef]
- Atkinson, G.; Batterham, A.M. Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis 2013, 226, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Kauffman, R.P.; Castracane, V.D. Assessing insulin sensitivity.(Controlling PCOS, part 1). Contemp. OB/GYN 2003, 48, 30–39. [Google Scholar]
- Grubbs, F.E. Procedures for Detecting Outlying Observations in Samples. Technometrics 1969, 11, 1–21. [Google Scholar] [CrossRef]
- Liguori, G.; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Galbreath, M.; Campbell, B.; LaBounty, P.; Bunn, J.; Dove, J.; Harvey, T.; Hudson, G.; Gutierrez, J.L.; Levers, K.; Galvan, E.; et al. Effects of Adherence to a Higher Protein Diet on Weight Loss, Markers of Health, and Functional Capacity in Older Women Participating in a Resistance-Based Exercise Program. Nutrients 2018, 10, 1070. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Roberts, M.D.; Campbell, B.I.; Galbreath, M.M.; Taylor, L.W.; Wilborn, C.D.; Lee, A.; Dove, J.; Bunn, J.W.; Rasmussen, C.J.; et al. Differential Impact of Calcium and Vitamin D on Body Composition Changes in Post-Menopausal Women Following a Restricted Energy Diet and Exercise Program. Nutrients 2020, 12, 713. [Google Scholar] [CrossRef]
- Lockard, B.; Mardock, M.; Oliver, J.M.; Byrd, M.; Simbo, S.; Jagim, A.R.; Kresta, J.; Baetge, C.C.; Jung, Y.P.; Koozehchian, M.S.; et al. Comparison of Two Diet and Exercise Approaches on Weight Loss and Health Outcomes in Obese Women. Int. J. Environ. Res. Public. Health 2022, 19, 4877. [Google Scholar] [CrossRef]
- Haff, G.; Triplett, N.T.; National, S.; Conditioning, A. Essentials of Strength Training and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2016. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Taft, C.; Karlsson, J.; Sullivan, M. Performance of the Swedish SF-36 version 2.0. Qual. Life Res. 2004, 13, 251–256. [Google Scholar] [CrossRef]
- Çelik, D.; Çoban, Ö. Short Form Health Survey version-2.0 Turkish (SF-36v2) is an efficient outcome parameter in musculoskeletal research. Acta Orthop. Traumatol. Turc. 2016, 50, 558–561. [Google Scholar] [CrossRef]
- Grubic, T.J.; Sowinski, R.J.; Nevares, B.E.; Jenkins, V.M.; Williamson, S.L.; Reyes, A.G.; Rasmussen, C.; Greenwood, M.; Murano, P.S.; Earnest, C.P.; et al. Comparison of ingesting a food bar containing whey protein and isomalto-oligosaccharides to carbohydrate on performance and recovery from an acute bout of resistance-exercise and sprint conditioning: An open label, randomized, counterbalanced, crossover pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Sowinski, R.J.; Grubic, T.J.; Dalton, R.L.; Schlaffer, J.; Reyes-Elrod, A.G.; Jenkins, V.M.; Williamson, S.; Rasmussen, C.; Murano, P.S.; Earnest, C.P.; et al. An Examination of a Novel Weight Loss Supplement on Anthropometry and Indices of Cardiovascular Disease Risk. J. Diet. Suppl. 2021, 18, 478–506. [Google Scholar] [CrossRef] [PubMed]
- Earnest, C.P.; Roberts, B.M.; Harnish, C.R.; Kutz, J.L.; Cholewa, J.M.; Johannsen, N.M. Reporting Characteristics in Sports Nutrition. Sports 2018, 6, 139. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, E. Applications of confidence limits and effect sizes in sport research. Open Sports Sci. J. 2008, 1, 3–4. [Google Scholar] [CrossRef]
- Grabowski, B. “P < 0.05” Might Not Mean What You Think: American Statistical Association Clarifies P Values. J. Natl. Cancer Inst. 2016, 108, djw194. [Google Scholar] [CrossRef]
- Page, P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int. J. Sports Phys. Ther. 2014, 9, 726. [Google Scholar]
- Sharma, H. Statistical significance or clinical significance? A researcher’s dilemma for appropriate interpretation of research results. Saudi J. Anaesth. 2021, 15, 431–434. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Gonzalez, D.E.; Dickerson, B.L.; Johnson, S.E.; Woodruff, K.E.; Leonard, M.; Yoo, C.; Ko, J.; Xing, D.; Martinez, V.; Kendra, J.; et al. Impact of astaxanthin supplementation on markers of cardiometabolic health and tactical performance among firefighters. J. Int. Soc. Sports Nutr. 2024, 21, 2427751. [Google Scholar] [CrossRef]
- Coletta, A.M.; Sanchez, B.; O’Connor, A.; Dalton, R.; Springer, S.; Koozehchian, M.S.; Murano, P.S.; Woodman, C.R.; Rasmussen, C.; Kreider, R.B. Alignment of diet prescription to genotype does not promote greater weight loss success in women with obesity participating in an exercise and weight loss program. Obes. Sci. Pract. 2018, 4, 554–574. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Rasmussen, C.; Kerksick, C.M.; Wilborn, C.; Taylor, L.t.; Campbell, B.; Magrans-Courtney, T.; Fogt, D.; Ferreira, M.; Li, R.; et al. A carbohydrate-restricted diet during resistance training promotes more favorable changes in body composition and markers of health in obese women with and without insulin resistance. Phys. Sportsmed. 2011, 39, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Serra, M.; Beavers, K.M.; Moreillon, J.; Kresta, J.Y.; Byrd, M.; Oliver, J.M.; Gutierrez, J.; Hudson, G.; Deike, E.; et al. A structured diet and exercise program promotes favorable changes in weight loss, body composition, and weight maintenance. J. Am. Diet. Assoc. 2011, 111, 828–843. [Google Scholar] [CrossRef]
- Magrans-Courtney, T.; Wilborn, C.; Rasmussen, C.; Ferreira, M.; Greenwood, L.; Campbell, B.; Kerksick, C.M.; Nassar, E.; Li, R.; Iosia, M.; et al. Effects of diet type and supplementation of glucosamine, chondroitin, and MSM on body composition, functional status, and markers of health in women with knee osteoarthritis initiating a resistance-based exercise and weight loss program. J. Int. Soc. Sports Nutr. 2011, 8, 8. [Google Scholar] [CrossRef]
- Kang, H. The prevention and handling of the missing data. Korean J. Anesthesiol. 2013, 64, 402–406. [Google Scholar] [CrossRef]
- Quintero, M.; LeBoulluec, A. Missing Data Imputation for Ordinal Data. Int. J. Comput. Appl. 2018, 181, 10–16. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988; pp. 75–108. [Google Scholar]
- Usharani, P.; Kishan, P.; Nishat Fatima, N.F.; Kumar, C. A comparative study to evaluate the effect of highly standardised aqueous extracts of Phyllanthus emblica, Withania somnifera and their combination on endothelial dysfunction and biomarkers in patients with type II Diabetes Mellitus. Int. J. Pharm. Sci. Res. (IJPSR) 2014, 5, 2687–2697. [Google Scholar]
- Antony, B.; Benny, M.; Kaimal, T.N. A Pilot clinical study to evaluate the effect of Emblica officinalis extract (Amlamax) on markers of systemic inflammation and dyslipidemia. Indian. J. Clin. Biochem. 2008, 23, 378–381. [Google Scholar] [CrossRef]
- Imanparast, F.; Mashayekhi, F.J.; Kamankesh, F.; Rafiei, F.; Mohaghegh, P.; Alimoradian, A. Improving the endothelial dysfunction in type 2 diabetes with chromium and vitamin D3 byreducing homocysteine and oxidative stress: A randomized placebo-controlled trial. J. Trace Elem. Med. Biol. 2020, 62, 126639. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; El Masry, M.S.; Gnyawali, S.C.; Ghatak, S.; Singh, K.; Stewart, R.; Lewis, M.; Saha, A.; Gordillo, G.; Khanna, S. Skin Transcriptome of Middle-Aged Women Supplemented with Natural Herbo-mineral Shilajit Shows Induction of Microvascular and Extracellular Matrix Mechanisms. J. Am. Coll. Nutr. 2019, 38, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Pilz, N.; Heinz, V.; Ax, T.; Fesseler, L.; Patzak, A.; Bothe, T.L. Pulse Wave Velocity: Methodology, Clinical Applications, and Interplay with Heart Rate Variability. Rev. Cardiovasc. Med. 2024, 25, 266. [Google Scholar] [CrossRef]
- Yoon, I.; Han, J.H.; Jeon, H.J. Advances in Platelet-Dysfunction Diagnostic Technologies. Biomolecules 2024, 14, 714. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, Y.K.; Lee, J.; Kim, E. Could nutrient supplements provide additional glycemic control in diabetes management? A systematic review and meta-analysis of randomized controlled trials of as an add-on nutritional supplementation therapy. Arch. Pharm. Res. 2022, 45, 185–204. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Dong, Y.; Zhu, Y.; Chen, H. Chromium supplementation for adjuvant treatment of type 2 diabetes mellitus: Results from a pooled analysis. Mol. Nutr. Food Res. 2018, 62, 1700438. [Google Scholar] [CrossRef] [PubMed]
- Georgaki, M.N.; Tsokkou, S.; Keramas, A.; Papamitsou, T.; Karachrysafi, S.; Kazakis, N. Chromium supplementation and type 2 diabetes mellitus: An extensive systematic review. Environ. Geochem. Health 2024, 46, 515. [Google Scholar] [CrossRef]
- Anderson, R.A. Nutritional factors influencing the glucose/insulin system: Chromium. J. Am. Coll. Nutr. 1997, 16, 404–410. [Google Scholar] [CrossRef]
- Kooshki, F.; Tutunchi, H.; Vajdi, M.; Karimi, A.; Niazkar, H.R.; Shoorei, H.; Pourghassem Gargari, B. A Comprehensive insight into the effect of chromium supplementation on oxidative stress indices in diabetes mellitus: A systematic review. Clin. Exp. Pharmacol. Physiol. 2021, 48, 291–309. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, J.; Shrinivas, V.; Dwivedi, L.K.; Suresh, P.; Sinha, M. Shilajit: Evalution of its effects on blood chemistry of normal human subjects. Anc. Sci. Life 2003, 23, 114–119. [Google Scholar]
- Rafraf, M.; Zemestani, M.; Asghari-Jafarabadi, M. Effectiveness of chamomile tea on glycemic control and serum lipid profile in patients with type 2 diabetes. J. Endocrinol. Investig. 2015, 38, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Anila, L.; Vijayalakshmi, N.R. Flavonoids from Emblica officinalis and Mangifera indica-effectiveness for dyslipidemia. J. Ethnopharmacol. 2002, 79, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kunnen, S.; Van Eck, M. Lecithin:cholesterol acyltransferase: Old friend or foe in atherosclerosis? J. Lipid Res. 2012, 53, 1783–1799. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, M.; Shang, Y.; Dou, M.; Gao, S.; Yang, H.; Zhang, F. Effects of co-supplementation of chromium and magnesium on metabolic profiles, inflammation, and oxidative stress in impaired glucose tolerance. Diabetes Vasc. Dis. Res. 2024, 21, 14791641241228156. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, L.; Chen, B.; Xiong, Q.; Zhan, Y.; Ye, J.; Yin, Q. Effect of chromium supplementation on hs-CRP, TNF-alpha and IL-6 as risk factor for cardiovascular diseases: A meta-analysis of randomized-controlled trials. Complement. Ther. Clin. Pract. 2021, 42, 101291. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-H.; Wang, C.-W.; Lu, W.-C.; Song, T.-Y.; Wang, C.-C.R.; Antioxidant, Anti-Inflammatory Activities, and Neuroprotective Behaviors of Phyllanthus emblica L. Fruit Extracts. Agriculture 2022, 12, 588. [Google Scholar] [CrossRef]
- Carrasco-Gallardo, C.; Guzmán, L.; Maccioni, R.B. Shilajit: A natural phytocomplex with potential procognitive activity. Int. J. Alzheimers Dis. 2012, 2012, 674142. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P. Pro- and Anti-Inflammatory Prostaglandins and Cytokines in Humans: A Mini Review. Int. J. Mol. Sci. 2023, 24, 9647. [Google Scholar] [CrossRef]
- Clancy, S.P.; Clarkson, P.M.; DeCheke, M.E.; Nosaka, K.; Freedson, P.S.; Cunningham, J.J.; Valentine, B. Effects of chromium picolinate supplementation on body composition, strength, and urinary chromium loss in football players. Int. J. Sport. Nutr. 1994, 4, 142–153. [Google Scholar] [CrossRef]
- Hallmark, M.A.; Reynolds, T.H.; DeSouza, C.A.; Dotson, C.O.; Anderson, R.A.; Rogers, M.A. Effects of chromium and resistive training on muscle strength and body composition. Med. Sci. Sports Exerc. 1996, 28, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Bolonchuk, W.W.; Siders, W.A.; Milne, D.B. Chromium supplementation and resistance training: Effects on body composition, strength, and trace element status of men. Am. J. Clin. Nutr. 1996, 63, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W.; Joseph, L.J.; Anderson, R.A.; Davey, S.L.; Hinton, J.; Evans, W.J. Effects of resistive training and chromium picolinate on body composition and skeletal muscle size in older women. Int. J. Sport. Nutr. Exerc. Metab. 2002, 12, 125–135. [Google Scholar] [CrossRef]
- Opala, T.; Rzymski, P.; Pischel, I.; Wilczak, M.; Wozniak, J. Efficacy of 12 weeks supplementation of a botanical extract-based weight loss formula on body weight, body composition and blood chemistry in healthy, overweight subjects—A randomised double-blind placebo-controlled clinical trial. Eur. J. Med. Res. 2006, 11, 343–350. [Google Scholar] [PubMed]
- Diaz, M.L.; Watkins, B.A.; Li, Y.; Anderson, R.A.; Campbell, W.W. Chromium picolinate and conjugated linoleic acid do not synergistically influence diet- and exercise-induced changes in body composition and health indexes in overweight women. J. Nutr. Biochem. 2008, 19, 61–68. [Google Scholar] [CrossRef]
- Khodavirdipour, A.; Haddadi, F.; Keshavarzi, S. Chromium Supplementation; Negotiation with Diabetes Mellitus, Hyperlipidemia and Depression. J. Diabetes Metab. Disord. 2020, 19, 585–595. [Google Scholar] [CrossRef]
- Brownley, K.A.; Boettiger, C.A.; Young, L.; Cefalu, W.T. Dietary chromium supplementation for targeted treatment of diabetes patients with comorbid depression and binge eating. Med. Hypotheses 2015, 85, 45–48. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. The role of chromium in nutrition and therapeutics and as a potential toxin. Nutr. Rev. 1999, 57, 329–335. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, V.; McAngus, K.; Dickerson, B.L.; Leonard, M.; Chavez, E.; Chun, J.; Lewis, M.; Xing, D.; Gonzalez, D.E.; Yoo, C.; et al. Effects of 12 Weeks of Chromium, Phyllanthus emblica Fruit Extract, and Shilajit Supplementation on Markers of Cardiometabolic Health, Fitness, and Weight Loss in Men and Women with Risk Factors to Metabolic Syndrome Initiating an Exercise and Diet Intervention: A Randomized Double-Blind, Placebo-Controlled Trial. Nutrients 2025, 17, 2042. https://doi.org/10.3390/nu17122042
Martinez V, McAngus K, Dickerson BL, Leonard M, Chavez E, Chun J, Lewis M, Xing D, Gonzalez DE, Yoo C, et al. Effects of 12 Weeks of Chromium, Phyllanthus emblica Fruit Extract, and Shilajit Supplementation on Markers of Cardiometabolic Health, Fitness, and Weight Loss in Men and Women with Risk Factors to Metabolic Syndrome Initiating an Exercise and Diet Intervention: A Randomized Double-Blind, Placebo-Controlled Trial. Nutrients. 2025; 17(12):2042. https://doi.org/10.3390/nu17122042
Chicago/Turabian StyleMartinez, Victoria, Kay McAngus, Broderick L. Dickerson, Megan Leonard, Elena Chavez, Jisun Chun, Megan Lewis, Dante Xing, Drew E. Gonzalez, Choongsung Yoo, and et al. 2025. "Effects of 12 Weeks of Chromium, Phyllanthus emblica Fruit Extract, and Shilajit Supplementation on Markers of Cardiometabolic Health, Fitness, and Weight Loss in Men and Women with Risk Factors to Metabolic Syndrome Initiating an Exercise and Diet Intervention: A Randomized Double-Blind, Placebo-Controlled Trial" Nutrients 17, no. 12: 2042. https://doi.org/10.3390/nu17122042
APA StyleMartinez, V., McAngus, K., Dickerson, B. L., Leonard, M., Chavez, E., Chun, J., Lewis, M., Xing, D., Gonzalez, D. E., Yoo, C., Ko, J., Rhodes, H., Lee, H., Sowinski, R. J., Rasmussen, C. J., & Kreider, R. B. (2025). Effects of 12 Weeks of Chromium, Phyllanthus emblica Fruit Extract, and Shilajit Supplementation on Markers of Cardiometabolic Health, Fitness, and Weight Loss in Men and Women with Risk Factors to Metabolic Syndrome Initiating an Exercise and Diet Intervention: A Randomized Double-Blind, Placebo-Controlled Trial. Nutrients, 17(12), 2042. https://doi.org/10.3390/nu17122042







