Acute Malnutrition in Under-Five Children in KwaZulu-Natal, South Africa: Risk Factors and Implications for Dietary Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Conceptual Frameworks
2.2. Study Setting
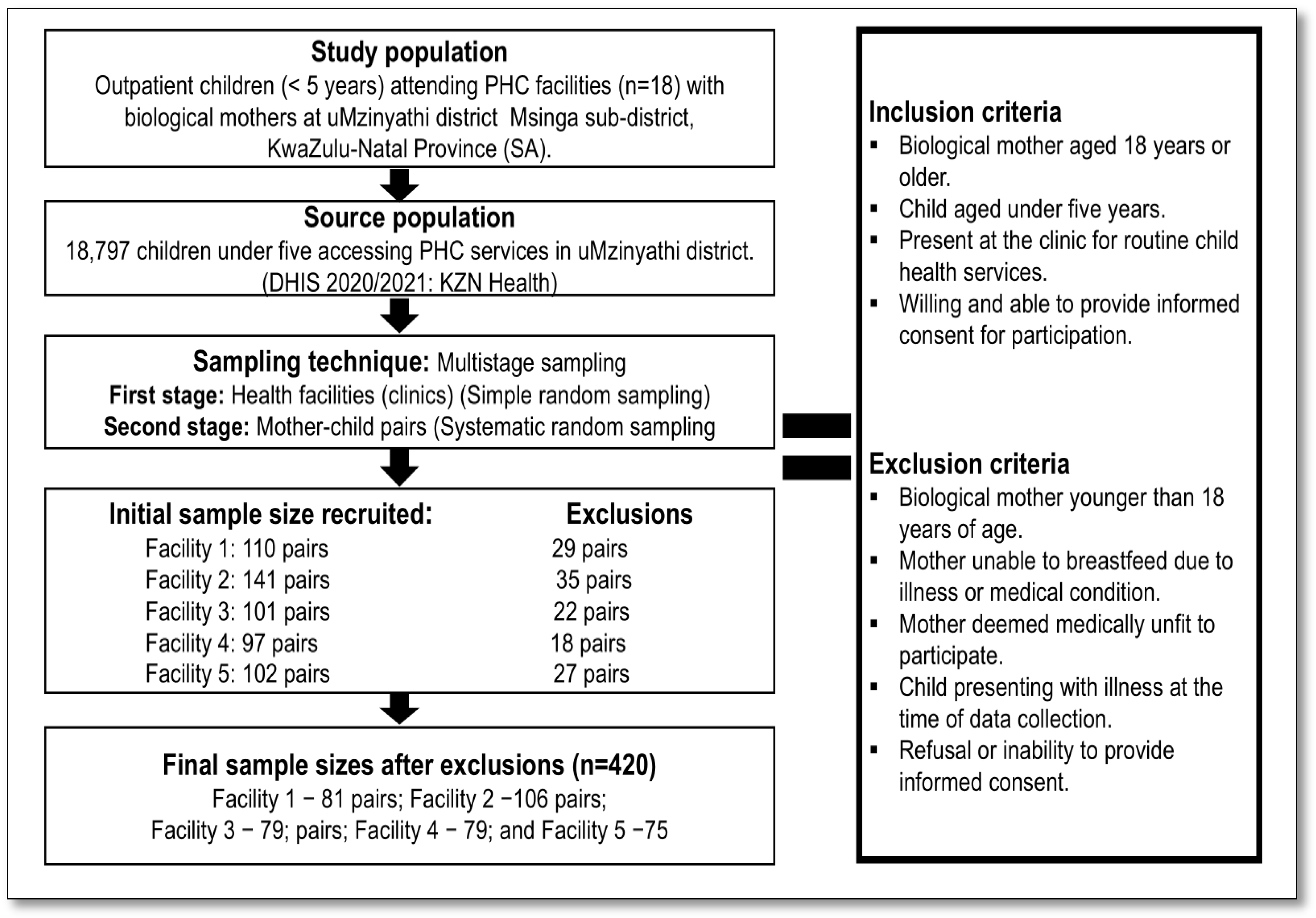
2.3. Study Population
2.4. Sample Size and Sampling Procedure
2.5. Data Collection
2.5.1. Socio-Demographics and Obstetric History of Study Participants
2.5.2. Complementary Feeding Practices and Dietary Diversity
2.5.3. Anthropometric Measurements and Nutritional Indicators of Children
2.5.4. Anthropometric Measurements and Nutritional Indicators of Mothers
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Children
3.2. Comparison of the Nutritional Indicators of Children
3.2.1. Comparison by Sex
3.2.2. Comparison of Age Groups
3.3. Characteristics of Mothers
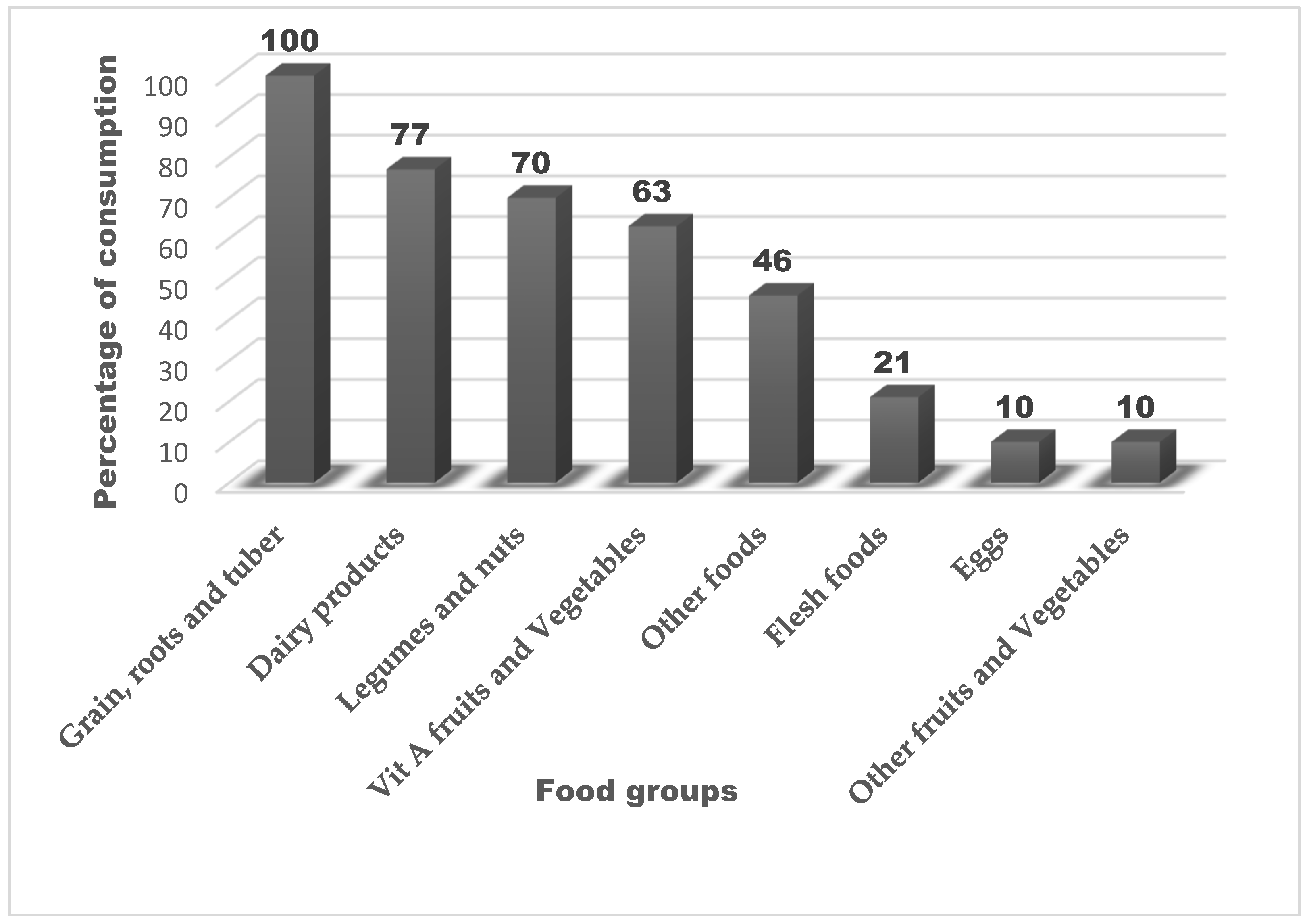
3.4. Dietary Diversity and Food Group Consumption Among Children
3.5. Multivariate Analysis of Risk Factors for Acute Malnutrition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Acute malnutrition |
| aPR | Adjusted prevalence ratio |
| BMI | Body mass index |
| CFR | Case fatality rate |
| CMAM | Community-based management of acute malnutrition |
| DDS | Dietary diversity scores |
| DHIS | District health information system |
| GLM | Generalized linear model |
| GMP | Growth monitoring and promotion |
| IYCF | Infant and young child feeding |
| INP | Integrated Nutrition Programme |
| MAD | Minimum acceptable diet |
| MAM | Moderate acute malnutrition |
| MDD | Minimum dietary diversity |
| MMF | Minimum meal frequency |
| MUACZ | Mid–upper-arm circumference z-score |
| PHC | Primary health care |
| RUFT | Ready-to-use therapeutic foods |
| SAM | Severe acute malnutrition |
| SDG | Sustainable development goal |
| WASH | Water, sanitation, and hygiene |
| WHZ | Weight-for-height z-score |
| WLZ | Weight-for-length z-score |
References
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, U.; Aryal, B.K.; Gupta, A.K.; Sapkota, S. Severe acute malnutrition and its associated factors among children under-five years: A facility-based cross-sectional study. BMC Pediatr. 2020, 20, 249. [Google Scholar] [CrossRef] [PubMed]
- Lenters, L.; Wazny, K.; Bhutta, Z.A. Management of Severe and Moderate Acute Malnutrition in Children. In Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, 3rd ed.; Black, R.E., Laxminarayan, R., Temmerman, M., Walker, N., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2016; Volume 2, Chapter 11. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines Approved by the Guidelines Review Committee. In Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- WHO. Levels and Trends in Child Malnutrition: UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2023 Edition; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240073791 (accessed on 2 June 2024).
- Annan, R.; Webb, P.; Brown, R.; Org, W. Management of Moderate Acute Malnutrition (MAM): Current Knowledge and Practice. CMAM Forum Technical Paper. 2014. Available online: https://foodaidquality.nutrition.tufts.edu/sites/default/files/publications/MAM-management-CMAM-Forum-Technical-Brief-Sept-2014.pdf (accessed on 13 May 2025).
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of Food Security and Nutrition in the World 2022: Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable. 2022. Available online: https://openknowledge.fao.org/items/c0239a36-7f34-4170-87f7-2fcc179ef064 (accessed on 12 May 2024).
- UNICEF. UNICEF Annual Report; United Nations Children’s Fund (UNICEF): New York, NY, USA, 2023; Available online: https://www.unicef.org/reports/unicef-annual-report-2023 (accessed on 7 February 2025).
- Akombi, B.J.; Agho, K.E.; Hall, J.J.; Wali, N.; Renzaho, A.M.N.; Merom, D. Stunting, Wasting and Underweight in Sub-Saharan Africa: A Systematic Review. Int. J. Environ. Res. Public Health 2017, 14, 863. [Google Scholar] [CrossRef]
- Olofin, I.; McDonald, C.M.; Ezzati, M.; Flaxman, S.; Black, R.E.; Fawzi, W.W.; Caulfield, L.E.; Danaei, G. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: A pooled analysis of ten prospective studies. PLoS ONE 2013, 8, e64636. [Google Scholar] [CrossRef]
- UNICEF. Tracking the Situation of Children During COVID-19. 2021. Available online: https://data.unicef.org/resources/rapid-situation-tracking-covid-19-socioeconomic-impacts-data-viz/ (accessed on 10 March 2024).
- Massynetal. District Health Barometer 2017/18; Health System Trust: Durban, South Africa, 2019; pp. 1–407. [Google Scholar]
- National Department of Health. Integrated Management of Children with Acute Malnutrition in South Africa: Operational Guidelines; NDoH Pretoria: Pretoria, South Africa, 2015.
- Steenkamp, L.; Lategan, R.; Raubenheimer, J. Moderate malnutrition in children aged five years and younger in South Africa: Are wasting or stunting being treated? S. Afr. J. Clin. Nutr. 2016, 29, 27–31. [Google Scholar] [CrossRef]
- Modjadji, P. Severe acute malnutrition case fatality rate. In District Health Barometer 2023/24; Ndlovu, N., Mokganya, M., Blose, N., Padarath, A., Eds.; Health Systems Trust: Durban, South Africa, 2025; pp. 63–69. Available online: https://www.hst.org.za/publications/District%20Health%20Barometers/District%20Health%20Barometer%202023_24_Chapter%201_Reproductive,%20maternal,%20newborn%20and%20child%20health.pdf (accessed on 1 May 2025).
- Mambulu-Chikankheni, F.N.; Eyles, J.; Eboreime, E.A.; Ditlopo, P. A critical appraisal of guidelines used for management of severe acute malnutrition in South Africa’s referral system. Health Res. Policy Syst. 2017, 15, 90. [Google Scholar] [CrossRef]
- Shabangu, S.V. Management of Severe Acute Malnutrition in Children Aged 6–59 Months by Professional Nurses in Primary Healthcare Facilities in the Johannesburg Health District, South Africa: A Retrospective Analysis. 2019. Available online: https://profmoosa.com/wp-content/uploads/2020/08/Shabangu_Thesis-for-examination-FINAL-20.11.2019.pdf (accessed on 17 May 2024).
- Zikhali, P.T. Social Grants and Poverty Alleviation in South Africa: Addressing Dependency Attitude and Behaviour. Gend. Behav. 2021, 19, 17854–17863. [Google Scholar]
- PMG. National School Nutrition Programme Update; with Deputy Minister; Parliamentary Monitoring Group: Cape Town, South Africa, 2023; Available online: https://pmg.org.za/committee-meeting/37835/ (accessed on 30 May 2025).
- Stewart, C.P.; Iannotti, L.; Dewey, K.G.; Michaelsen, K.F.; Onyango, A.W. Contextualising complementary feeding in a broader framework for stunting prevention. Matern. Child Nutr. 2013, 9 (Suppl. S2), 27–45. [Google Scholar] [CrossRef]
- WHO. Stunting in a Nutshell. 2015. Available online: https://www.who.int/news/item/19-11-2015-stunting-in-a-nutshell (accessed on 1 May 2025).
- UMzinyathi District Municipality. UMzinyathi District 2021 22 Final Annual Report. 2023. Available online: https://www.umzinyathi.gov.za/download/umzinyathi-district-2021-22-final-annual-report/ (accessed on 12 June 2022).
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Child Malnutrition; World Health Organization: Geneva, Switzerland, 2023; Available online: https://data.unicef.org/topic/nutrition/malnutrition/ (accessed on 12 May 2025).
- Raosoft. Sample Size Calculator. 2004. Available online: http://www.raosoft.com/samplesize.html (accessed on 14 April 2021).
- Gallo, V.; Egger, M.; McCormack, V.; Farmer, P.B.; Ioannidis, J.P.A.; Kirsch-Volders, M.; Matullo, G.; Phillips, D.H.; Schoket, B.; Stromberg, U.; et al. STrengthening the reporting of OBservational studies in Epidemiology—Molecular Epidemiology (STROBE-ME): An extension of the STROBE statement. Eur. J. Epidemiol. 2011, 26, 797–810. [Google Scholar] [CrossRef]
- Modjadji, P.; Madiba, S. The double burden of malnutrition in a rural health and demographic surveillance system site in South Africa: A study of primary schoolchildren and their mothers. BMC Public Health 2019, 19, 1087. [Google Scholar] [CrossRef]
- Modjadji, P.; Madiba, S. Childhood Undernutrition and Its Predictors in a Rural Health and Demographic Surveillance System Site in South Africa. Int. J. Environ. Res. Public Health 2019, 16, 3021. [Google Scholar] [CrossRef] [PubMed]
- Modjadji, P.; Mashishi, J. Persistent Malnutrition and Associated Factors among Children under Five Years Attending Primary Health Care Facilities in Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 7580. [Google Scholar] [CrossRef]
- WHO. Indicators for Assessing Infant and Young Child Feeding Practices (Part 1); World Health Organization: Geneva, Switzerland, 2008; Available online: https://iris.who.int/bitstream/handle/10665/43895/9789241596664_eng.pdf?sequence=1 (accessed on 13 January 2024).
- WHO. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006, 450, 76–85. [Google Scholar] [CrossRef]
- WHO. WHO Guideline for Complementary Feeding of Infants and Young Children 6–23 Months of Age; World Health Organisation: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240081864 (accessed on 12 December 2023).
- FAO. Guidelines for Measuring Household and Individual Dietary Diversity. 2011. Available online: https://www.fao.org/fileadmin/user_upload/wa_workshop/docs/FAO-guidelines-dietary-diversity2011.pdf (accessed on 13 February 2024).
- Kennedy, G.L.; Pedro, M.R.; Seghieri, C.; Nantel, G.; Brouwer, I. Dietary Diversity Score Is a Useful Indicator of Micronutrient Intake in Non-Breast-Feeding Filipino Children. J. Nutr. 2007, 137, 472–477. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- WHO. Physical Status: The Use and Interpretation of Anthropometry; WHO Technical report Series No. 854; WHO Expert Committee: Geneva, Switzerland, 1995. [Google Scholar]
- Modjadji, P. Socio-demographic Determinants of Overweight and Obesity Among Mothers of Primary School Children Living in a Rural Health and Demographic Surveillance System Site, South Africa. Open Public Health J. 2020, 13, 518–528. [Google Scholar] [CrossRef]
- Ralston, M.; Schatz, E.; Menken, J.; Gómez-Olivé, F.X.; Tollman, S. Who Benefits—Or Does Not—From South Africa’s Old Age Pension? Evidence from Characteristics of Rural Pensioners and Non-Pensioners. Int. J. Environ. Res. Public Health 2015, 13, 85. [Google Scholar] [CrossRef]
- Wabiri, N.; Chersich, M.; Zuma, K.; Blaauw, D.; Goudge, J.; Dwane, N. Equity in maternal health in South Africa: Analysis of health service access and health status in a national household survey. PLoS ONE 2013, 8, e73864. [Google Scholar] [CrossRef]
- Mihret, S.T.; Biset, G.; Nurye, N.A. Prevalence of Acute Malnutrition and Associated Factors among Children aged 6-59 months in South Wollo Zone, East Amhara, Northeast Ethiopia: A Community-based cross-sectional study. BMJ Open 2023, 13, e062582. [Google Scholar] [CrossRef] [PubMed]
- Mambulu-Chikankheni, F.N. Factors influencing the implementation of severe acute malnutrition guidelines within the healthcare referral systems of rural subdistricts in Northwest Province, South Africa. PLoS Glob. Public Health 2023, 3, e0002277. [Google Scholar] [CrossRef]
- Dunifon, R.; Kowaleski-Jones, L. The Influence of Grandparents in Single-Mother Families. J. Marriage Fam. 2007, 69, 465–481. [Google Scholar] [CrossRef]
- Harris, A.L. “I got caught up in the game”: Generational influences on contraceptive decision making in African American women. J. Am. Assoc. Nurse Pract. 2013, 25, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Mhamane, S.; Karande, Y.; Ramanathan, V. Post-partum depression: Its association with IYCF practices and effect on child growth indicators in urban slums of Mumbai, India. Clin. Epidemiol. Glob. Health 2024, 28, 101667. [Google Scholar] [CrossRef]
- Ong, K.K.; Kennedy, K.; Castañeda-Gutiérrez, E.; Forsyth, S.; Godfrey, K.M.; Koletzko, B.; Latulippe, M.E.; Ozanne, S.E.; Rueda, R.; Schoemaker, M.H.; et al. Postnatal growth in preterm infants and later health outcomes: A systematic review. Acta Paediatr. 2015, 104, 974–986. [Google Scholar] [CrossRef]
- Thomas, A.; Engelbrecht, A.L.; Slogrove, A.L. Severe acute malnutrition outcomes for children of South African compared to foreign-born parents admitted to a rural regional hospital in South Africa: A retrospective cohort study. J. Trop. Pediatr. 2022, 68, fmac097. [Google Scholar] [CrossRef]
- Modjadji, P.; Seabela, E.S.; Ntuli, B.; Madiba, S. Beliefs and Norms Influencing Initiation and Sustenance of Exclusive Breastfeeding: Experiences of Mothers in Primary Health Care Facilities in Ermelo, South Africa. Int. J. Environ. Res. Public Health 2023, 20, 1513. [Google Scholar] [CrossRef]
- Nshimyiryo, A.; Hedt-Gauthier, B.; Mutaganzwa, C.; Kirk, C.M.; Beck, K.; Ndayisaba, A.; Mubiligi, J.; Kateera, F.; El-Khatib, Z. Risk factors for stunting among children under five years: A cross-sectional population-based study in Rwanda using the 2015 Demographic and Health Survey. BMC Public Health 2019, 19, 175. [Google Scholar] [CrossRef]
- Seabela, E.S.; Modjadji, P.; Mokwena, K.E. Facilitators and barriers associated with breastfeeding among mothers attending primary healthcare facilities in Mpumalanga, South Africa. Front. Nutr. 2023, 10, 1062817. [Google Scholar] [CrossRef]
- Faber, M.; Laubscher, R.; Berti, C. Poor dietary diversity and low nutrient density of the complementary diet for 6-to 24-month-old children in urban and rural K wa Z ulu-N atal, S outh A frica. Matern. Child Nutr. 2016, 12, 528–545. [Google Scholar] [CrossRef]
- Motadi, S.A.; Zuma, M.K.; Freeland-Graves, J.H.; Gertrude Mbhenyane, X. Dietary diversity and nutritional status of children attending early childhood development centres in Vhembe District, Limpopo province, South Africa. J. Nutr. Sci. 2023, 12, e92. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.P.; Nel, J.H.; Nantel, G.; Kennedy, G.; Labadarios, D. Food variety and dietary diversity scores in children: Are they good indicators of dietary adequacy? Public Health Nutr. 2006, 9, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Paulo, H.A.; Andrew, J.; Luoga, P.; Omary, H.; Chombo, S.; Mbishi, J.V.; Addo, I.Y. Minimum dietary diversity behaviour among children aged 6 to 24 months and their determinants: Insights from 31 Sub-Saharan African (SSA) countries. BMC Nutr. 2024, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Raru, T.B.; Merga, B.T.; Mulatu, G.; Deressa, A.; Birhanu, A.; Negash, B.; Gamachu, M.; Regassa, L.D.; Ayana, G.M.; Roba, K.T. Minimum Dietary Diversity Among Children Aged 6–59 Months in East Africa Countries: A Multilevel Analysis. Int. J. Public Health 2023, 68, 1605807. [Google Scholar] [CrossRef]
- Demissie, S.; Worku, A. Magnitude and factors associated with malnutrition in children 6-59 months of age in pastoral community of Dollo Ado district, Somali region, Ethiopia. Sci. J. Public Health 2013, 1, 175–183. [Google Scholar] [CrossRef]
- Ogunlesi, T.; Ayeni, V.; Fetuga, B.; Adekanmbi, A. Severe acute malnutrition in a population of hospitalized under-five Nigerian children. Niger. Postgrad. Med. J. 2015, 22, 15–20. [Google Scholar] [CrossRef]
- Haq, I.u.; Mehmood, Z.; Khan, N.; Khan, M.N.; Israr, M.; Ali Khan, E.; Nisar, M.; Ahmad, M.I.; Ali, M. Risk factors of mid-upper arm circumference (MUAC) based child malnutrition in the Flood-affected areas of Pakistan: A cross-sectional study: Child malnutrition in flood-hit areas. Ecol. Food Nutr. 2021, 60, 491–507. [Google Scholar] [CrossRef]
- Mkhize, M.; Sibanda, M. A Review of Selected Studies on the Factors Associated with the Nutrition Status of Children Under the Age of Five Years in South Africa. Int. J. Environ. Res. Public Health 2020, 17, 7973. [Google Scholar] [CrossRef]
- NDoHetal. South Africa Health and Demographic Survey 2016 Key Indicators; Statistics South Africa, Medical Research Council and ICF: Pretoria, South Africa, 2017. [Google Scholar]
- Wambua, J.; Ali, A.; Ukwizabigira, J.B.; Kuodi, P. Prevalence and risk factors of under-five mortality due to severe acute malnutrition in Africa: A systematic review and meta-analysis. Syst. Rev. 2025, 14, 29. [Google Scholar] [CrossRef]
- Alaimo, K.; Chilton, M.; Jones, S. Food insecurity, hunger, and malnutrition. In Present Knowledge in Nutrition, 11th ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 311–326. [Google Scholar]
- Bain, L.E.; Awah, P.K.; Geraldine, N.; Kindong, N.P.; Sigal, Y.; Bernard, N.; Tanjeko, A.T. Malnutrition in Sub-Saharan Africa: Burden, causes and prospects. Pan Afr. Med. J. 2013, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.; Coll-Seck, A.M.; Banerjee, A.; Peterson, S.; Dalglish, S.L.; Ameratunga, S.; Balabanova, D.; Bhan, M.K.; Bhutta, Z.A.; Borrazzo, J.; et al. A future for the world’s children? A WHO-UNICEF-Lancet Commission. Lancet 2020, 395, 605–658. [Google Scholar] [CrossRef] [PubMed]
- Muzigaba, M.; Puoane, T.; Sanders, D. The Paradox of Undernutrition and Obesity in South Africa: A Contextual Overview of Food Quality, Access and Availability in the New Democracy. In Food Poverty and Insecurity: International Food Inequalities; Caraher, M., Coveney, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 31–41. [Google Scholar]
- Monyeki, M.A.; Awotidebe, A.; Strydom, G.L.; de Ridder, J.H.; Mamabolo, R.L.; Kemper, H.C. The challenges of underweight and overweight in South African children: Are we winning or losing the battle? A systematic review. Int. J. Environ. Res. Public Health 2015, 12, 1156–1173. [Google Scholar] [CrossRef]
- Kimani-Murage, E.W. Exploring the paradox: Double burden of malnutrition in rural South Africa. Glob. Health Action 2013, 6, 19249. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, A.E. Prevalence of overweight and obesity among primary school children in a developing country: NW-CHILD longitudinal data of 6–9-yr-old children in South Africa. BMC Obes. 2015, 2, 2. [Google Scholar] [CrossRef]
- Sharma, N.; Sanjeevi, R.R.; Balasubramanian, K.; Chahal, A.; Sharma, A.; Sidiq, M. A Systematic Review on Prevalence of Overweight and Obesity among School Children and Adolescents in Indian Population. Indian J. Endocrinol. Metab. 2024, 28, 104–116. [Google Scholar] [CrossRef]
- Ayele, B.A.; Tiruneh, S.A.; Ayele, A.A.; Zemene, M.A.; Chanie, E.S.; Hailemeskel, H.S. Prevalence and determinants of overweight/obesity among under-five children in sub-Saharan Africa: A multilevel analysis. BMC Pediatr. 2022, 22, 585. [Google Scholar] [CrossRef]
- Wells, J.C.K. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef]
- Modjadji, P.; Masilela, L.N.; Cele, L.; Mathibe, M.; Mphekgwana, P.M. Evidence of Concurrent Stunting and Obesity among Children under 2 Years from Socio-Economically Disadvantaged Backgrounds in the Era of the Integrated Nutrition Programme in South Africa. Int. J. Environ. Res. Public Health 2022, 19, 12501. [Google Scholar] [CrossRef]
- Mamabolo, R.L.; Alberts, M.; Mbenyane, G.X.; Steyn, N.P.; Nthangeni, N.G.; Delemarre-Van De Waal, H.A.; Levitt, N.S. Feeding practices and growth of infants from birth to 12 months in the central region of the Limpopo Province of South Africa. Nutrition 2004, 20, 327–333. [Google Scholar] [CrossRef]
- Mardani, R.A.D.; Wu, W.R.; Nhi, V.T.; Huang, H.C. Association of breastfeeding with undernutrition among children under 5 years of age in developing countries: A systematic review and meta-analysis. J. Nurs. Scholarsh. 2022, 54, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Fagbamigbe, A.F.; Kandala, N.B.; Uthman, O.A. Decomposing the educational inequalities in the factors associated with severe acute malnutrition among under-five children in low- and middle-income countries. BMC Public Health 2020, 20, 555. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.E.; Özaltin, E.; Canning, D. The association of maternal age with infant mortality, child anthropometric failure, diarrhoea and anaemia for first births: Evidence from 55 low- and middle-income countries. BMJ Open 2011, 1, e000226. [Google Scholar] [CrossRef] [PubMed]
- Mushaphi, L. Infant feeding practices of mothers and nutritional status of infant. S. Afr. J. Clin. Nutr. 2008, 21, 36–41. [Google Scholar]
- Modjadji, P.; Molokwane, D.; Ukegbu, P.O. Dietary Diversity and Nutritional Status of Preschool Children in North West Province, South Africa: A Cross-Sectional Study. Children 2020, 7, 174. [Google Scholar] [CrossRef]
- WHO. Minimum Diet Diversity (6–23 Months); World Health Organisation: Geneva, Switzerland, 2025; Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/7042 (accessed on 27 May 2025).
- Bantamen, G.; Belaynew, W.; Dube, J. Assessment of factors associated with malnutrition among under five years age children at Machakel Woreda, northwest Ethiopia: A case control study. J. Nutr. Food Sci. 2014, 4, 1. [Google Scholar]
- Fuchs, C.; Sultana, T.; Ahmed, T.; Iqbal Hossain, M. Factors Associated with Acute Malnutrition among Children Admitted to a Diarrhoea Treatment Facility in Bangladesh. Int. J. Pediatr. 2014, 2014, 267806. [Google Scholar] [CrossRef]
- Doherty, T.; Sanders, D.; Jackson, D.; Swanevelder, S.; Lombard, C.; Zembe, W.; Chopra, M.; Goga, A.; Colvin, M.; Fadnes, L.T. Early cessation of breastfeeding amongst women in South Africa: An area needing urgent attention to improve child health. BMC Pediatr. 2012, 12, 105. [Google Scholar] [CrossRef]
- Rippey, P.L.F.; Aravena, F.; Nyonator, J.P. Health Impacts of Early Complementary Food Introduction Between Formula-fed and Breastfed Infants. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 375–380. [Google Scholar] [CrossRef]
| Variables | Median | IQR | Minimum | Maximum |
|---|---|---|---|---|
| Age (months) | 24 | 12;24 | 12 | 60 |
| Weight (kg) | 8.3 | 6.9; 10.2 | 3.6 | 16 |
| Length/height (cm) | 73 | 65; 83 | 48 | 116 |
| WHZ/WLZ | −0.89 | −2.2; 0.41 | −6.91 | 7.99 |
| MUACZ | −1.16 | −2.1; −0.4 | −3.87 | 1.78 |
| Variables | All | Boys | Girls | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | p-Value | |
| WHZ/WLZ | 0.014 * | |||
| Normal (≥−2SD) | 252 (62) | 104 (57) | 148 (63) | |
| Acute malnutrition (<−2 SD) | 118 (29) | 54 (30) | 64 (29) | 0.415 |
| MAM (between −3 SD and −2 SD) | 64 (16) | 30 (17) | 34 (15) | 0.418 |
| SAM (<−3SD) | 54 (13) | 24 (13) | 30 (14) | 0.668 |
| Overweight/obesity (>+2SD) | 34 (8) | 23 (13) | 11 (5) | 0.004 * |
| MUACZ | 0.411 | |||
| Normal (≥−2SD) | 251 (73) | 101 (71) | 15 (75) | |
| Acute malnutrition (<−2 SD) | 93 (27) | 42 (29) | 51 (25) | 0.411 |
| MAM (between −3 SD and −2 SD) | 63 (18) | 25 (17) | 38 (19) | 0.936 |
| SAM (<−3SD) | 30 (9) | 17 (12) | 13 (6) | 0.085 |
| Variables | ≤24 Months | 24–36 Months | >36 Months | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | p-Value | |
| WHZ/WLZ | 0.001 * | |||
| Normal (≥−2SD) | 114 (64) | 87 (69) | 51 (51) | |
| Acute malnutrition (<−2 SD) | 36 (20) | 35 (28) | 47 (47) | 0.001 * |
| MAM (between −3 SD and −2 SD) | 19 (11) | 24 (19) | 21 (21) | 0.037 * |
| SAM (<−3SD) | 17 (9) | 11 (9) | 26 (26) | 0.001 * |
| Overweight/obesity (>+2SD) | 29 (16) | 4 (3) | 1 (1) | 0.001 * |
| MUACZ | 0.001 * | |||
| Normal (≥−2SD) | 99 (89) | 110 (85) | 42 (42) | |
| Acute malnutrition (<−2 SD) | 15 (13) | 20 (15) | 58 (58) | 0.001 * |
| MAM (between −3 SD and −2 SD) | 12 (10) | 15 (11) | 36 (36) | 0.001 * |
| SAM (<−3SD) | 3 (3) | 5 (4) | 22 (22) | 0.001 * |
| Variables | All n (%) | <25 Years n (%) | 25–34 Years n (%) | ≥35 Years n (%) | p-Value |
|---|---|---|---|---|---|
| Marital status | |||||
| Single | 347 (84) | 187 (97) | 143 (79) | 17 (40) | P0 = 0.001 *; P1 = 0.001 * P2 = 0.001 *; P3 = 0.001 * |
| Ever married | 68 (16) | 6 (3) | 37 (21) | 25 (60) | |
| Level of education | |||||
| No school/Primary | 11 (3) | 3 (2) | 3 (2) | 5 (12) | P0 = 0.005 * |
| Secondary | 151 (36) | 73 (38) | 63 (35) | 15 (36) | P1 = 0.130 |
| Completed grade 12. | 231 (56) | 111 (57) | 98 (54) | 22 (52) | P2 = 0.006 * |
| Post grade 12 | 22 (5) | 6 (3) | 16 (9) | 0 | P3 = 0.003 * |
| Employed | |||||
| No | 410 (98) | 191 (99) | 170 (94) | 40 (95) | P0 = 0.026 *; P1 = 0.013 * P2 = 0.091; P3 = 0.838 |
| Yes | 5 (2) | 2 (9) | 10 (6) | 2 (5) | |
| Receiving child grant | |||||
| No | 64 (15) | 29 (15) | 170 (94) | 50 (95) | P0 = 0.223; P1 = 0.473 P2 = 0.177; P3 = 0.089 |
| Yes | 351 (85) | 164 (85) | 10 (6) | 2 (5) | |
| Household head | |||||
| Spouse/partner. | 64 (15) | 6 (3) | 33 (18) | 25 (60) | P0 = 0.001 * |
| Parents | 267 (64) | 146 (76) | 105 (58) | 16 (38) | P1 = 0.001 * |
| Grandparents | 79 (19) | 39 (20) | 39 (22) | 1 (2) | P2 = 0.001 * |
| Other relatives | 5 (2) | 2 (1) | 3 (2) | 0 | P3 = 0.001 * |
| Household size | |||||
| ≤4 | 25 (6) | 4 (2) | 19 (11) | 2 (5) | P0 = 0.002 *; P1 = 0.001 * P2 = 0.317; P3 = 0.248 |
| ≥5 | 390 (94) | 189 (98) | 161 (89) | 40 (95) | |
| Household income | |||||
| <USD 280 | 391 (94) | 185 (96) | 165 (92) | 41 (98) | P0 = 0.471 |
| USD 280–USD 560 | 11 (3) | 1 (2) | 5 (3) | 1 (2) | P1 = 0.001 * |
| USD 560–USD 840 | 6 (1) | 1 (1) | 5 (3) | 0 | P2 = 0.317 |
| >USD 840 | 7 (2) | 2 (1) | 5 (2) | 0 | P3 = 0.248 |
| Dwelling place | |||||
| RDP house | 4 (1) | 3 (2) | 1 (1) | 0 | P0 = 0.002 *; P1 = 0.819; P2 = 0.053; P3 = 0.002 * |
| Shack/mud house | 74 (18) | 49 (25) | 21 (11) | 4 (10) | |
| Brick house | 337 (81) | 141 (73) | 158 (88) | 38 (90) | |
| Access to electricity | |||||
| No | 17 (4) | 10 (5) | 3 (2) | 4 (10) | P0 = 0.029 *; P1 = 0.064; P2 = 0.281; P3 = 0.009 * |
| Yes | 398 (96) | 183 (95) | 177 (98) | 38 (90) | |
| Refrigerator use | |||||
| No | 64 (15) | 34 (18) | 22 (12) | 8 (19) | P0 = 0.280; P1 = 0.145; P2 = 0.826; P3 = 0.244 |
| Yes | 351 (85) | 159 (82) | 158 (88) | 34 (81) | |
| Source of energy | |||||
| Firewood/coal | 191 (46) | 100 (52) | 69 (38) | 22 (53) | P0 = 0.013 *; P1 = 0.012 *; P2 = 0.896; P3 = 0.168 |
| Paraffin/gas | 6 (1) | 1 (1) | 5 (3) | 0 | |
| Electricity | 218 (53) | 92 (47) | 106 (59) | 20(47) | |
| Access to water | |||||
| No | 398 (96) | 187 (97) | 169 (94) | 42 (100) | P0 = 0.159; P1 = 0.165; P2 = 0.247; P3 = 0.100 |
| Yes | 17 (4) | 6 (3) | 11 (6) | 0 |
| Variables | All n (%) | <25 (G1) n (%) | 25–34 (G2) n (%) | ≥35 (G3) n (%) | p-Value |
|---|---|---|---|---|---|
| BMI (kg/m2) | |||||
| <18.5 (underweight) | 22 (5) | 6 (3) | 13 (7) | 3 (7) | P0 = 0.001 * |
| >18.5–24.99 (normal) | 286 (69) | 158 (82) | 99 (55) | 29 (69) | P1 = 0.001 * |
| 25–29.9 (overweight) | 74 (18) | 27 (14) | 41 (23) | 6 (14) | P2 = 0.008 |
| ≥30 (obesity) | 33 (8) | 2 (1) | 27 (15) | 4 (10) | P3 = 0.283 |
| Parity | |||||
| 1–2 | 350 (84) | 191 (99) | 148 (82) | 11 (26) | P0 = 0.001 *; P1 = 0.001 *; P2 = 0.001 *; P3 = 0.001 * |
| ≥3 | 65 (16) | 2 (1) | 32 (18) | 31 (74) | |
| Pregnancy full term | |||||
| No | 3 (1) | 0 | 2 (1) | 1 (2) | P0 = 0.085; P1 = 0.142; P2 = 0.032 *; P3 = 0.521 |
| Yes | 412 (99) | 193 (100) | 178 (99) | 41 (98) | |
| Obstetric complications | |||||
| No | 379 (91) | 190 (98) | 157 (88) | 30 (71) | P0 = 0.001 *; P1 = 0.001 *; P2 = 0.001 *; P3 = 0.006 |
| Yes | 36 (8) | 3 (2) | 21 (12) | 12 (29) | |
| Child breastfed | |||||
| No | 16 (3) | 6 (3) | 9 (5) | 1 (2) | P0 = 0.650; P1 = 0.353; P2: n/a; P3 = 0.628 |
| Yes | 399 (96) | 187 (97) | 17 (5) | 41 (98) | |
| Duration of breastfeeding | |||||
| <3 months | 43 (10) | 32 (17) | 9 (5) | 2 (5) | P0 = 0.001 * |
| 3–6 months | 69 (17) | 36 (18) | 27 (15) | 6 (14) | P1 = 0.001 * |
| >6 months | 242 (48) | 91 (47) | 123 (68) | 28 (67) | P2 = 0.089 |
| Continued | 61 (15) | 34 (18) | 21 (12) | 6 (14) | P3 = 0.974 |
| Mixed feeding | |||||
| No | 303 (73) | 125 (65) | 144 (80) | 34 (81) | P0 = 0.002 *; P1 = 0.001 *; P2 = 0.042 *; P3 = 0.889 |
| Yes | 112 (27) | 68 (35) | 36 (20) | 8 (19) | |
| Introduction of solid foods | |||||
| Not yet | 65 (16) | 34 (18) | 22 (12) | 9 (21) | P0 = 0.001 *; P1 = 0.001 *; P2 = 0.126; P3 = 0.285 |
| <6 months | 110 (26) | 68 (35) | 34 (19) | 8 (19) | |
| >6 months | 240 (58) | 91 (47) | 124 (69) | 25 (60) |
| Variables | All | Boys | Girls | p-Value |
| DDS | ||||
| Mean | 3.65 ± 0.7 | 3.56 ± 0.69 | 3.71 ± 0.63 | |
| Normal | 340 (82) | 158 (85) | 182 (79) | 0.030 * |
| Low | 75 (18) | 27 (15) | 48 (21) | 0.099 |
| Variables | <24 Months | 24–36 Months | ≥36 Months | p-Value |
| DDS | ||||
| Mean | 3.39 ± 0.71 | 3.79 ± 0.52 | 3.89 ± 0.58 | |
| Normal | 159 (89) | 108 (81) | 73 (71) | <0.001 * |
| Low | 20 (11) | 25 (19) | 30 (29) | <0.001 * |
| Variables | PR (95%CI) | p-Value | aPR (95%CI) | p-Value |
|---|---|---|---|---|
| Acute malnutrition (WHZ/WLZ) | ||||
| Marital status | ||||
| Single | 1 (ref) | 1 (ref) | ||
| Married | 1.28 (0.53–1.15) | 0.202 | (0.50–1.08) | 0.114 |
| Number of household members | ||||
| <5 | 1 (ref) | 1 (ref) | ||
| ≥5 | 0.65 (0.45–0.94) | 0.024 * | 0.66 (0.45–0.74) | 0.035 * |
| Full-term pregnancy | ||||
| No | 1 (ref) | 1 (ref) | ||
| Yes | 0.37 (0.33–0.42) | <0.001 * | 0.39 (0.23–0.64) | <0.001 * |
| Duration of breastfeeding | ||||
| Still breastfeeding | 1 (ref) | 1 (ref) | ||
| <3 months | 0.61 (0.38–1.03) | 0.039 * | 0.59 (0.37–0.94) | 0.028 * |
| months | 0.74 (0.52–1.05) | 0.088 | 0.77 (0.54–1.09) | 0.148 |
| >6 months | 0.79 (0.51–1.22) | 0.280 | 0.79 (0.50–1.25) | 0.302 |
| Acute malnutrition (MUACZ) | ||||
| DDS | ||||
| <4 | 1 (ref) | 1 (ref) | ||
| ≥4 | 0.46 (0.09–0.83) | 0.016 * | 0.41 (0.04–0.8378 | 0.028 * |
| Child’s age (months) | ||||
| <24 | 1 (ref) | 1 (ref) | ||
| 24–36 | 1.17 (0.63–2.18) | 0.622 | 1.34 (0.75–2.36) | 0.329 |
| ≥36 | 1.48 (0.98–1.98) | <0.001 * | 1.62 (1.15–2.10) | <0.001 * |
| Mother’s age (years) | ||||
| <25 | 1 (ref) | 1 (ref) | ||
| 25–34 | 0.63 (0.43–0.90) | 0.013 * | 0.67 (0.48–0.93) | 0.017 * |
| ≥35 | 0.67 (0.35–1.28) | 0.230 | 0.58 (0.35–0.84) | 0.043 * |
| Household income | ||||
| <USD 280 | 1 (ref) | 1 (ref) | ||
| USD 280–USD 560 | 0.00 (0.00–0.00) | <0.001 * | 0.00 (0.00–0.00) | <0.001 * |
| USD 560–USD 840 | 0.00 (0.00–0.00) | <0.001 * | 0.00 (0.00–0.00) | <0.001 * |
| >USD 840 | 0.71 (0.12–4.14) | 0.702 | 0.44 (0.07–2.75) | 0.377 |
| Mixed feeding | ||||
| No | 1 (ref) | 1 (ref) | ||
| Yes | 0.84 (0.51–1.18) | <0.001 * | 0.86 (0.55–1.18) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathosi, M.M.; Cele, L.P.; Mathibe, M.; Modjadji, P. Acute Malnutrition in Under-Five Children in KwaZulu-Natal, South Africa: Risk Factors and Implications for Dietary Quality. Nutrients 2025, 17, 2038. https://doi.org/10.3390/nu17122038
Mathosi MM, Cele LP, Mathibe M, Modjadji P. Acute Malnutrition in Under-Five Children in KwaZulu-Natal, South Africa: Risk Factors and Implications for Dietary Quality. Nutrients. 2025; 17(12):2038. https://doi.org/10.3390/nu17122038
Chicago/Turabian StyleMathosi, Meshack Mzamani, Lindiwe Priscilla Cele, Mmampedi Mathibe, and Perpetua Modjadji. 2025. "Acute Malnutrition in Under-Five Children in KwaZulu-Natal, South Africa: Risk Factors and Implications for Dietary Quality" Nutrients 17, no. 12: 2038. https://doi.org/10.3390/nu17122038
APA StyleMathosi, M. M., Cele, L. P., Mathibe, M., & Modjadji, P. (2025). Acute Malnutrition in Under-Five Children in KwaZulu-Natal, South Africa: Risk Factors and Implications for Dietary Quality. Nutrients, 17(12), 2038. https://doi.org/10.3390/nu17122038








