Dos and Don’ts in Kidney Nutrition: Practical Considerations of a Panel of Experts on Protein Restriction and Plant-Based Diets for Patients Living with Chronic Kidney Disease
Abstract
1. Introduction
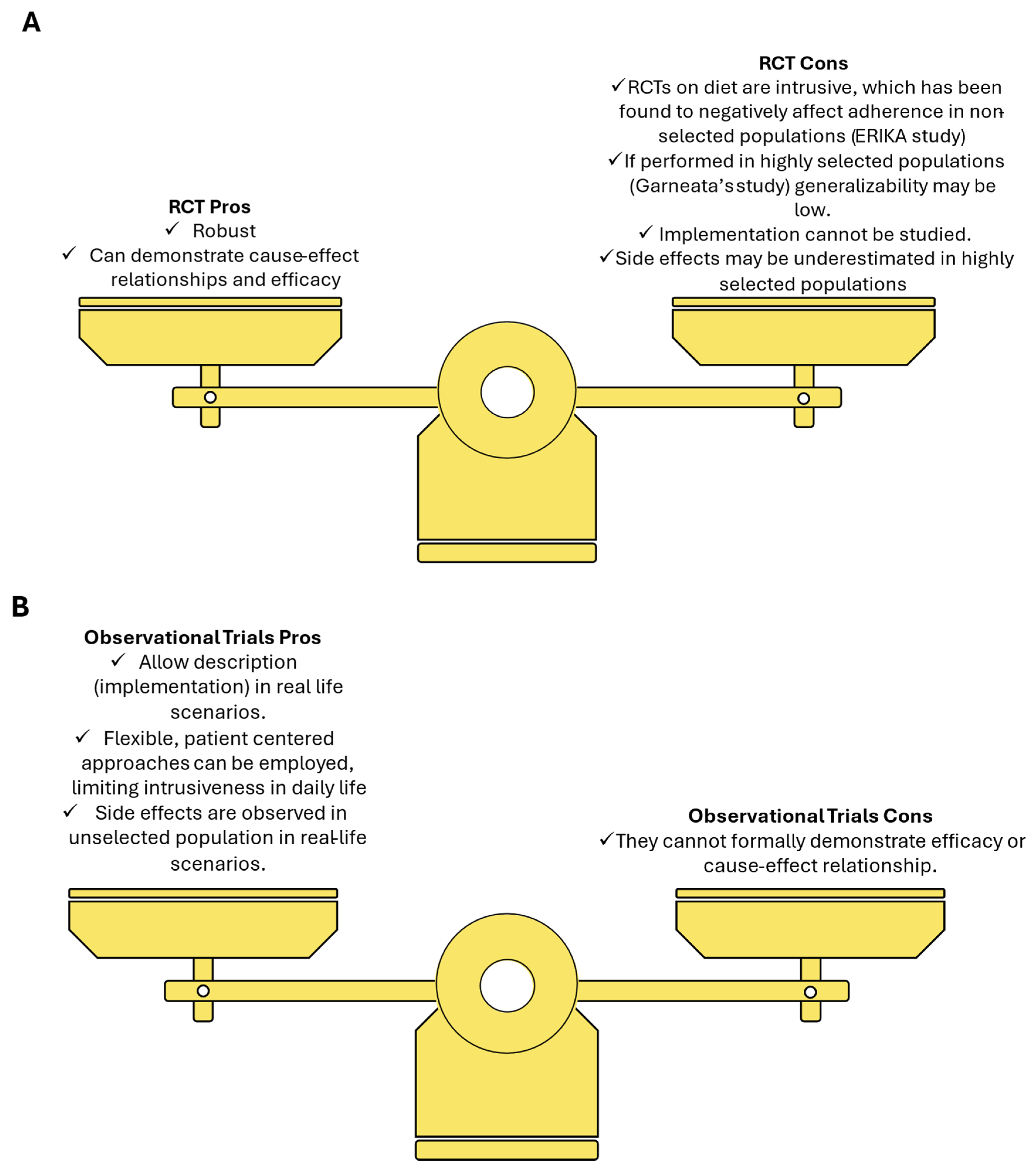
2. General Issues
3. The Animal vs. Vegetal Protein Conundrum
| Diet | Composition | Expected Benefits in CKD | |
|---|---|---|---|
| Mediterranean diet [30] | Predominance of vegetal and fish protein versus meat protein. | Reduced cardiovascular risk Lower mortality risk Reduced phosphate load Reduced infalmmation and oxydative stress Better microbiota regulation Lower CKD progression | |
| Plant-dominant Low-protein diet (PLADO) [31] | 0.6–0.8 g of proteins/kg/day, >50% plant-based sources. | Reduced acid load | |
| Okinawan diet [32] | Low caloric intake, predominance of sweet potatoes, green-leafy or yellow-root vegetables, and soy. | ||
| Dietary Approaches to Stop Hypertension diet (DASH) [33,34] | High in fruits, vegetables, and low-fat dairy products, with reduced saturated and total fat. | Lower blood pressure | |
| Flexitarian [35] | Predominantly vegetarian, with occasional inclusion of animal products. | ||
| New Nordic renal diet [36] | Predominant plant-based food, 0.8 g of proteins/kg/day, <5 g/day of sodium chloride. | Reduced proteinuria Reduced body weight Lower blood pressure | |
| Ketogenic diet [37,38] | Predominance of fat (>70%), proteins 6–20%, carbohydrates < 10%. | Improved glycemic control Weight loss Lower blood pressure Reduced inflammation Slower CKD progression Possible benefit in polycistic kidney disease | |
| Western diet [39] | Highly caloric, processed and refined foods, high content of sugars, salt, and fat and protein from red meat. | None: Increased proteinuria Faster CKD progression Increase inflammation and oxydative stress Intestinal dysbiosis Increased acid load Increased potassium and phosphorus load Increased salt load Increased blood pressure | |
4. Should We Worry About Potassium?
5. Phosphorus and the Diet
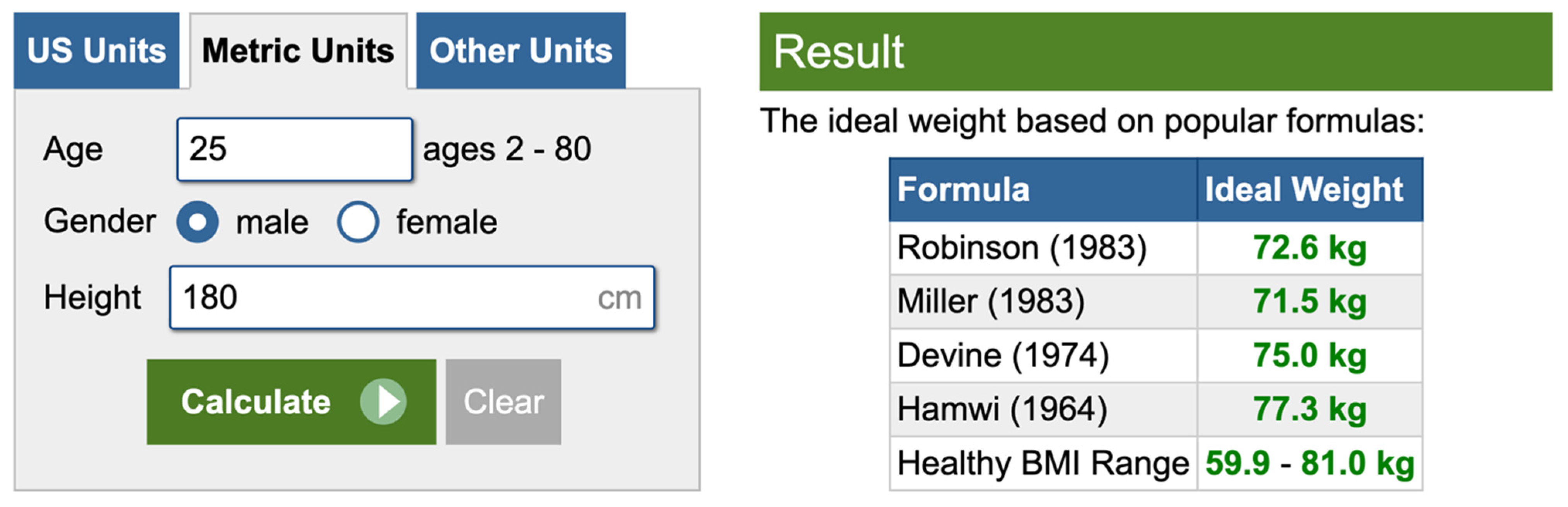
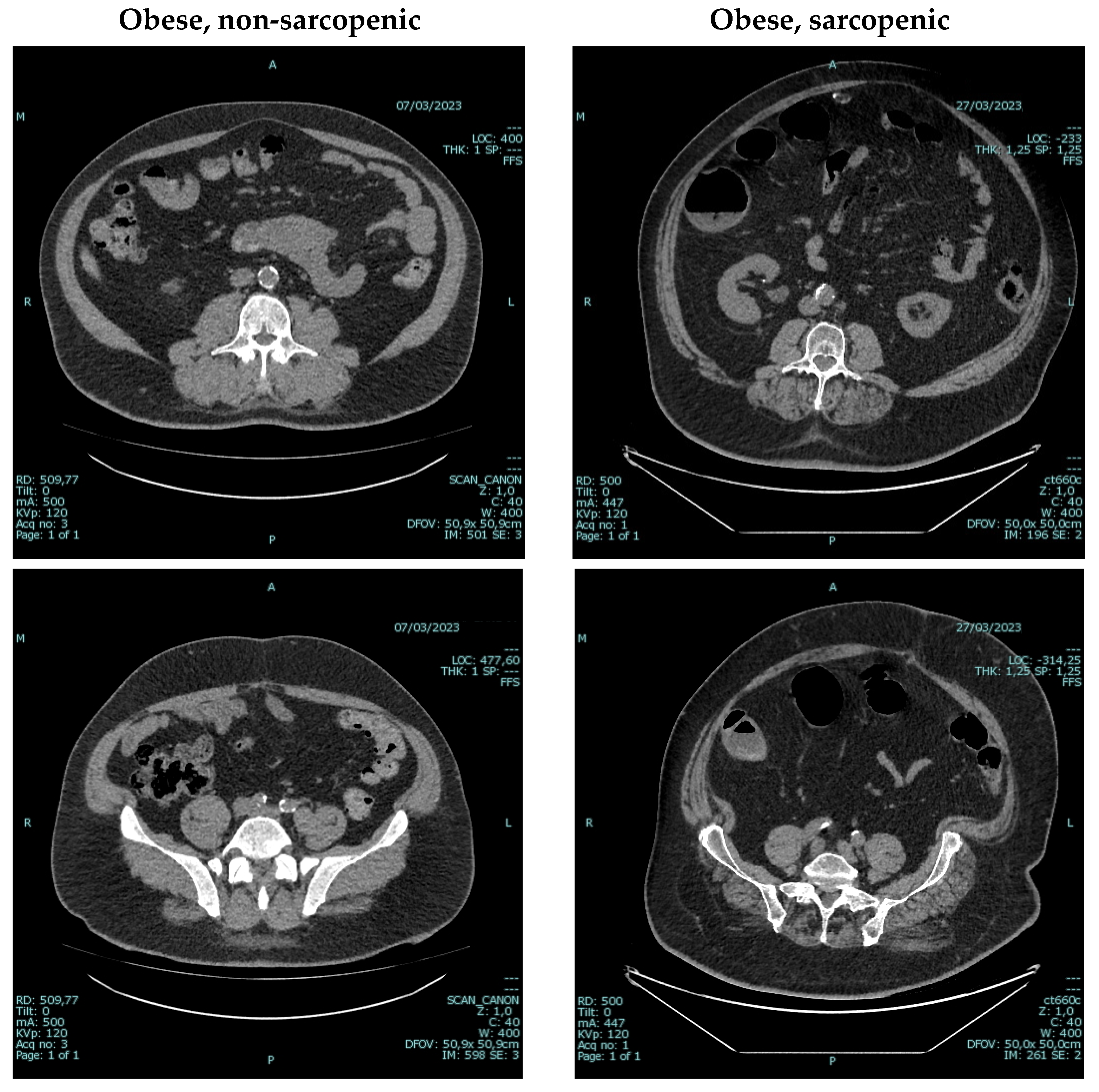
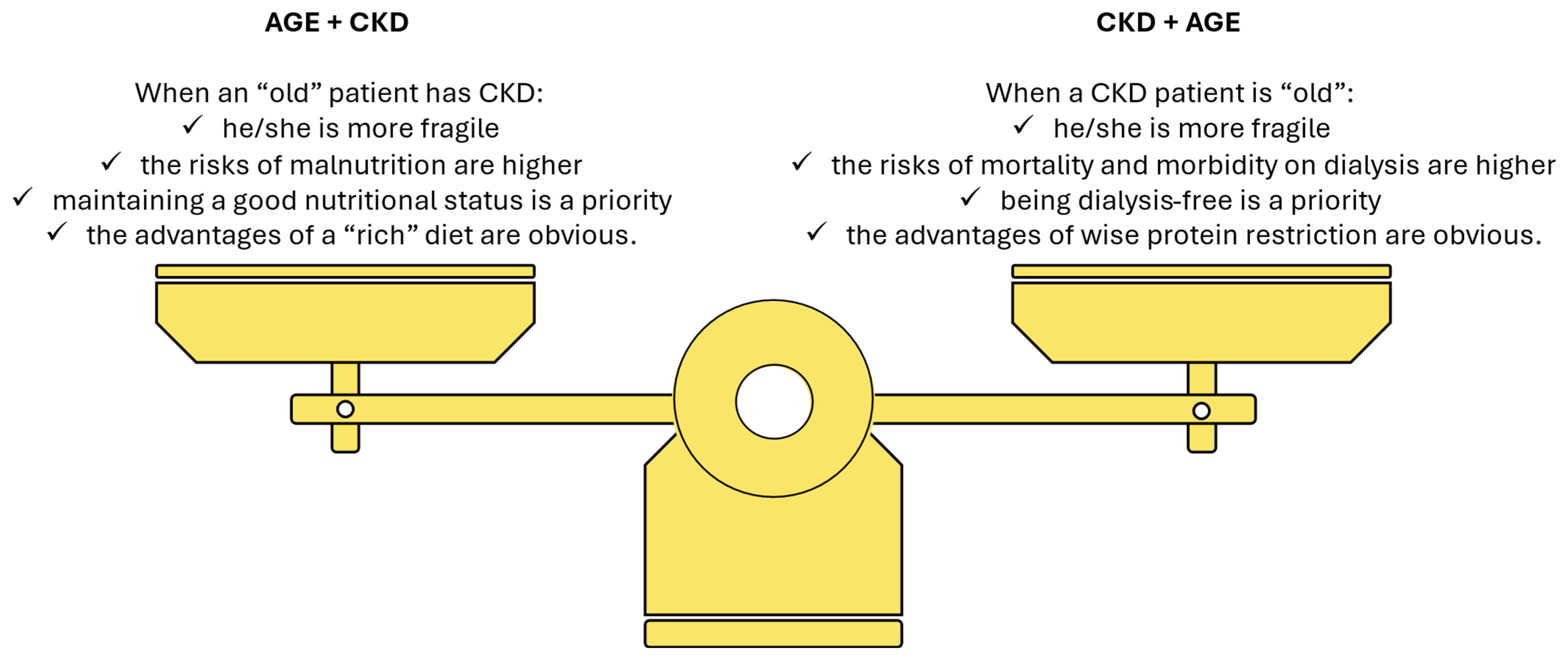
6. An Underestimated Problem: Establishing the “Ideal” Body Weight
7. Energy Intake and the Risk of Protein Energy Wasting, the Boogeyman of Dietary Management of CKD
8. Very Low Protein Diets Plus Amino Acid and Ketoacid Supplements
9. Avoiding Problems: The Ultra-Processed Food Issue
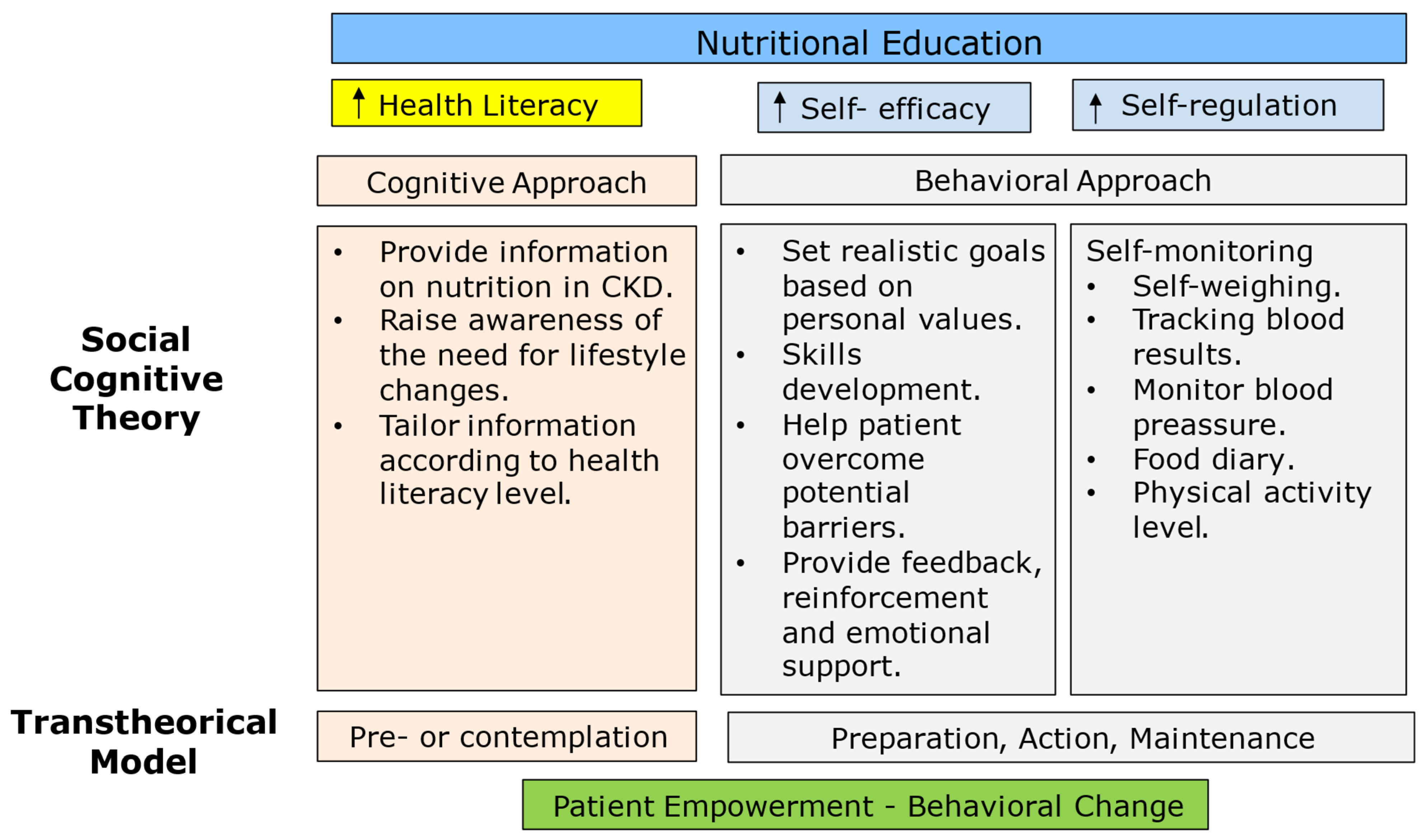
10. Education and Counseling as a Key to Success
11. Control Strategy
12. The Dark Side of Dietary Management of CKD: What We Do Not Know
13. Final Considerations and Need for Research
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Addis, T.; Lew, W. Diet and Death in Acute Uremia. J. Clin. Investig. 1939, 18, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Woodrow, G. Con: The role of diet for people with advanced Stage 5 CKD. Nephrol. Dial. Transplant. 2018, 33, 380–384. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Di Iorio, B.R.; Chatrenet, A.; D’Alessandro, C.; Nazha, M.; Capizzi, I.; Vigotti, F.N.; Fois, A.; Maxia, S.; Saulnier, P.; et al. Dietary satisfaction and quality of life in chronic kidney disease patients on low-protein diets: A multicentre study with long-term outcome data (TOrino-Pisa study). Nephrol. Dial. Transplant. 2020, 35, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Fois, A.; Torreggiani, M.; Trabace, T.; Chatrenet, A.; Longhitano, E.; Maze, B.; Lippi, F.; Vigreux, J.; Beaumont, C.; Moio, M.R.; et al. Quality of Life in CKD Patients on Low-Protein Diets in a Multiple-Choice Diet System. Comparison between a French and an Italian Experience. Nutrients 2021, 13, 1354. [Google Scholar] [CrossRef]
- Neale, E.P.; Rosario, V.D.; Probst, Y.; Beck, E.; Tran, T.B.; Lambert, K. Lifestyle Interventions, Kidney Disease Progression, and Quality of Life: A Systematic Review and Meta-analysis. Kidney Med. 2023, 5, 100643. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach; Updated October 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html#h.hnedbo8gqjqk (accessed on 9 December 2024).
- Locatelli, F.; Alberti, D.; Graziani, G.; Buccianti, G.; Redaelli, B.; Giangrande, A. Prospective, randomised, multicentre trial of effect of protein restriction on progression of chronic renal insufficiency. Northern Italian Cooperative Study Group. Lancet 1991, 337, 1299–1304. [Google Scholar] [CrossRef]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef]
- Bellizzi, V.; Signoriello, S.; Minutolo, R.; Di Iorio, B.; Nazzaro, P.; Garofalo, C.; Calella, P.; Chiodini, P.; De Nicola, L.; ERIKA Study Group Investigators of the Italian Society of Nephrology-Conservative Therapy of CKD Work Group. No additional benefit of prescribing a very low-protein diet in patients with advanced chronic kidney disease under regular nephrology care: A pragmatic, randomized, controlled trial. Am. J. Clin. Nutr. 2022, 115, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- De Mauri, A.; Carrera, D.; Vidali, M.; Bagnati, M.; Rolla, R.; Riso, S.; Torreggiani, M.; Chiarinotti, D. Compliance, Adherence and Concordance Differently Predict the Improvement of Uremic and Microbial Toxins in Chronic Kidney Disease on Low Protein Diet. Nutrients 2022, 14, 487. [Google Scholar] [CrossRef]
- Torreggiani, M.; Wang, A.Y.; Fois, A.; Piccoli, G.B. Personalized Low-Protein Diet Prescription in CKD Population: Merging Evidence From Randomized Trials With Observational Data. Semin. Nephrol. 2023, 43, 151402. [Google Scholar] [CrossRef]
- Fois, A.; Chatrenet, A.; Cataldo, E.; Lippi, F.; Kaniassi, A.; Vigreux, J.; Froger, L.; Mongilardi, E.; Capizzi, I.; Biolcati, M.; et al. Moderate Protein Restriction in Advanced CKD: A Feasible Option in An Elderly, High-Comorbidity Population. A Stepwise Multiple-Choice System Approach. Nutrients 2018, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.; Hodson, E.M.; Fouque, D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst. Rev. 2020, 10, CD001892. [Google Scholar] [CrossRef]
- Zoccali, C.; Tripepi, G. Clinical trial emulation in nephrology. J. Nephrol. 2024, 38, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, D.J.; Sibbald, B. Understanding controlled trials. What is a patient preference trial? BMJ 1998, 316, 360. [Google Scholar] [CrossRef]
- Kolff, W.J. Forced high caloric, low protein diet and the treatment of uremia. Am. J. Med. 1952, 12, 667–679. [Google Scholar] [CrossRef]
- Monasterio, G.; Giovannetti, S.; Maggiore, Q. The place of the low protein diet in the treatment of chronic uraemia. Panminerva. Med. 1965, 7, 479–484. [Google Scholar]
- Salteri, F.; Pittaluga, F. Dietetic products, treatment of chronic uremia, and the Giordano-Giovannetti low protein diet. Am. J. Clin. Nutr. 1968, 21, 590–591. [Google Scholar] [CrossRef]
- Sorensen, M.K.; Kopple, J.D. Assessment of adherence to protein-restricted diets during conservative management of uremia. Am. J. Clin. Nutr. 1968, 21, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Rosman, J.B.; Langer, K.; Brandl, M.; Piers-Becht, T.P.; van der Hem, G.K.; ter Wee, P.M.; Donker, A.J. Protein-restricted diets in chronic renal failure: A four year follow-up shows limited indications. Kidney Int. Suppl. 1989, 27, S96–S102. [Google Scholar]
- K/DOQI, National Kidney Foundation. Clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2000, 35, S17–S104. [Google Scholar] [CrossRef]
- Barsotti, G.; Morelli, E.; Cupisti, A.; Meola, M.; Dani, L.; Giovannetti, S. A low-nitrogen low-phosphorus Vegan diet for patients with chronic renal failure. Nephron 1996, 74, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Heo, G.Y.; Koh, H.B.; Kim, H.J.; Kim, K.W.; Jung, C.Y.; Kim, H.W.; Chang, T.I.; Park, J.T.; Yoo, T.H.; Kang, S.W.; et al. Association of Plant Protein Intake With Risk of Incident CKD: A UK Biobank Study. Am. J. Kidney Dis. 2023, 82, 687–697. [Google Scholar] [CrossRef]
- Carrero, J.J.; Gonzalez-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Chauveau, P.; Aparicio, M.; Bellizzi, V.; Campbell, K.; Hong, X.; Johansson, L.; Kolko, A.; Molina, P.; Sezer, S.; Wanner, C.; et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 725–735. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef]
- Willcox, D.C.; Scapagnini, G.; Willcox, B.J. Healthy aging diets other than the Mediterranean: A focus on the Okinawan diet. Mech. Ageing Dev. 2014, 136, 148–162. [Google Scholar] [CrossRef]
- Raphael, K.L. The Dietary Approaches to Stop Hypertension (DASH) diet in chronic kidney disease: Should we embrace it? Kidney Int. 2019, 95, 1296–1298. [Google Scholar] [CrossRef]
- Banerjee, T.; Crews, D.C.; Tuot, D.S.; Pavkov, M.E.; Burrows, N.R.; Stack, A.G.; Saran, R.; Bragg-Gresham, J.; Powe, N.R.; Centers for Disease, C.; et al. Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kidney Int. 2019, 95, 1433–1442. [Google Scholar] [CrossRef]
- Marrone, G.; Di Lauro, M.; Cornali, K.; Masci, C.; Vanni, G.; Vita, C.; Noce, A. Sustainability and role of plant-based diets in chronic kidney disease prevention and treatment. Front. Pharmacol. 2025, 16, 1562409. [Google Scholar] [CrossRef] [PubMed]
- Misella Hansen, N.; Kamper, A.L.; Rix, M.; Feldt-Rasmussen, B.; Leipziger, J.; Sorensen, M.V.; Berg, P.; Astrup, A.; Salomo, L. Health effects of the New Nordic Renal Diet in patients with stage 3 and 4 chronic kidney disease, compared with habitual diet: A randomized trial. Am. J. Clin. Nutr. 2023, 118, 1042–1054. [Google Scholar] [CrossRef]
- Weimbs, T.; Saville, J.; Kalantar-Zadeh, K. Ketogenic metabolic therapy for chronic kidney disease—The pro part. Clin. Kidney J. 2024, 17, sfad273. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Shi, R.; Patel, J. Risks of the ketogenic diet in CKD—The con part. Clin. Kidney J. 2024, 17, sfad274. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, A. The Western-style diet: A major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol. Renal. Physiol. 2011, 301, F919–F931. [Google Scholar] [CrossRef]
- Costa, D.; Patella, G.; Provenzano, M.; Ielapi, N.; Faga, T.; Zicarelli, M.; Arturi, F.; Coppolino, G.; Bolignano, D.; De Sarro, G.; et al. Hyperkalemia in CKD: An overview of available therapeutic strategies. Front. Med. 2023, 10, 1178140. [Google Scholar] [CrossRef]
- Collins, A.J.; Pitt, B.; Reaven, N.; Funk, S.; McGaughey, K.; Wilson, D.; Bushinsky, D.A. Association of Serum Potassium with All-Cause Mortality in Patients with and without Heart Failure, Chronic Kidney Disease, and/or Diabetes. Am. J. Nephrol. 2017, 46, 213–221. [Google Scholar] [CrossRef]
- Ceccanti, C.; Guidi, L.; D’Alessandro, C.; Cupisti, A. Potassium Bioaccessibility in Uncooked and Cooked Plant Foods: Results from a Static In Vitro Digestion Methodology. Toxins 2022, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Munoz-Maldonado, Y.; Simoni, J.; Wesson, D.E. Treatment of Chronic Kidney Disease-Related Metabolic Acidosis With Fruits and Vegetables Compared to NaHCO(3) Yields More and Better Overall Health Outcomes and at Comparable Five-Year Cost. J. Ren. Nutr. 2021, 31, 239–247. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Wilson Jones, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2021, 13, 2534. [Google Scholar] [CrossRef]
- Cigarran Guldris, S.; Latorre Catala, J.A.; Sanjurjo Amado, A.; Menendez Granados, N.; Pineiro Varela, E. Fibre Intake in Chronic Kidney Disease: What Fibre Should We Recommend? Nutrients 2022, 14, 4419. [Google Scholar] [CrossRef]
- Sherman, R.A.; Mehta, O. Phosphorus and potassium content of enhanced meat and poultry products: Implications for patients who receive dialysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Picard, K.; Griffiths, M.; Mager, D.R.; Richard, C. Handouts for Low-Potassium Diets Disproportionately Restrict Fruits and Vegetables. J. Ren. Nutr. 2021, 31, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Allon, M.; Dansby, L.; Shanklin, N. Glucose modulation of the disposal of an acute potassium load in patients with end-stage renal disease. Am. J. Med. 1993, 94, 475–482. [Google Scholar] [CrossRef]
- Carlisle, E.J.; Donnelly, S.M.; Ethier, J.H.; Quaggin, S.E.; Kaiser, U.B.; Vasuvattakul, S.; Kamel, K.S.; Halperin, M.L. Modulation of the secretion of potassium by accompanying anions in humans. Kidney Int. 1991, 39, 1206–1212. [Google Scholar] [CrossRef]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef]
- Cecchini, V.; Sabatino, A.; Contzen, B.; Avesani, C.M. Food additives containing potassium, phosphorus, and sodium in ultra-processed foods: Potential harms to individuals with chronic kidney disease. Eur. J. Clin. Nutr. 2025. [Google Scholar] [CrossRef]
- Ramos, C.I.; Gonzalez-Ortiz, A.; Espinosa-Cuevas, A.; Avesani, C.M.; Carrero, J.J.; Cuppari, L. Does dietary potassium intake associate with hyperkalemia in patients with chronic kidney disease? Nephrol. Dial. Transplant. 2021, 36, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Zhang, Z.; Carriquiry, A.L.; Gunn, J.P.; Kuklina, E.V.; Saydah, S.H.; Yang, Q.; Moshfegh, A.J. Sodium and potassium intakes among US adults: NHANES 2003–2008. Am. J. Clin. Nutr. 2012, 96, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Reddin, C.; Ferguson, J.; Murphy, R.; Clarke, A.; Judge, C.; Griffith, V.; Alvarez, A.; Smyth, A.; Mente, A.; Yusuf, S.; et al. Global mean potassium intake: A systematic review and Bayesian meta-analysis. Eur. J. Nutr. 2023, 62, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, L.; Garofalo, C.; Borrelli, S.; Minutolo, R. Recommendations on nutritional intake of potassium in CKD: It’s now time to be more flexible! Kidney Int. 2022, 102, 700–703. [Google Scholar] [CrossRef]
- Borrelli, S.; De Nicola, L.; Minutolo, R.; Conte, G.; Chiodini, P.; Cupisti, A.; Santoro, D.; Calabrese, V.; Giannese, D.; Garofalo, C.; et al. Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care. Nutrients 2021, 13, 942. [Google Scholar] [CrossRef]
- Brezic, N.; Milojevic, I.; Hassan, A.; Swanson, K.; Bhavsar, T. Sodium Polystyrene Sulfonate-Induced Massive Bowel Necrosis With Distant Extraintestinal Crystal Deposition: A Case Report and Review of the Literature. Cureus 2024, 16, e71523. [Google Scholar] [CrossRef]
- Sterns, R.H.; Grieff, M.; Bernstein, P.L. Treatment of hyperkalemia: Something old, something new. Kidney Int. 2016, 89, 546–554. [Google Scholar] [CrossRef]
- Avesani, C.M.; Heimburger, O.; Rubin, C.; Sallstrom, T.; Faxen-Irving, G.; Lindholm, B.; Stenvinkel, P. Plant-based diet in hyperkalemic chronic kidney disease patients receiving sodium zirconium cyclosilicate: A feasibility clinical trial. Am. J. Clin. Nutr. 2024, 120, 719–726. [Google Scholar] [CrossRef]
- Agarwal, R.; Joseph, A.; Anker, S.D.; Filippatos, G.; Rossing, P.; Ruilope, L.M.; Pitt, B.; Kolkhof, P.; Scott, C.; Lawatscheck, R.; et al. Hyperkalemia Risk with Finerenone: Results from the FIDELIO-DKD Trial. J. Am. Soc. Nephrol. 2022, 33, 225–237. [Google Scholar] [CrossRef]
- Cupisti, A.; Giannese, D.; Moriconi, D.; D’Alessandro, C.; Torreggiani, M.; Piccoli, G.B. Nephroprotection by SGLT2i in CKD Patients: May It Be Modulated by Low-Protein Plant-Based Diets? Front. Med. 2020, 7, 622593. [Google Scholar] [CrossRef]
- Zaimi, M.; Grapsa, E. Current therapeutic approach of chronic kidney disease-mineral and bone disorder. Ther. Apher. Dial. 2024, 28, 671–689. [Google Scholar] [CrossRef] [PubMed]
- Magagnoli, L.; Cozzolino, M.; Caskey, F.J.; Evans, M.; Torino, C.; Porto, G.; Szymczak, M.; Krajewska, M.; Drechsler, C.; Stenvinkel, P.; et al. Association between CKD-MBD and mortality in older patients with advanced CKD-results from the EQUAL study. Nephrol. Dial. Transplant. 2023, 38, 2562–2575. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Mathew, S. The roles of the skeleton and phosphorus in the CKD mineral bone disorder. Adv. Chronic Kidney Dis. 2011, 18, 98–104. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Piccoli, G.B.; Cupisti, A. The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Karp, H.; Ekholm, P.; Kemi, V.; Hirvonen, T.; Lamberg-Allardt, C. Differences among total and in vitro digestible phosphorus content of meat and milk products. J. Ren. Nutr. 2012, 22, 344–349. [Google Scholar] [CrossRef]
- Karp, H.; Ekholm, P.; Kemi, V.; Itkonen, S.; Hirvonen, T.; Narkki, S.; Lamberg-Allardt, C. Differences among total and in vitro digestible phosphorus content of plant foods and beverages. J. Ren. Nutr. 2012, 22, 416–422. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J. Bioavailable dietary phosphate, a mediator of cardiovascular disease, may be decreased with plant-based diets, phosphate binders, niacin, and avoidance of phosphate additives. Nutrition 2014, 30, 739–747. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Kuro, O.M.; Moe, O.W. FGF23-alphaKlotho as a paradigm for a kidney-bone network. Bone 2017, 100, 4–18. [Google Scholar] [CrossRef]
- Bellizzi, V.; Annunziata, G.; Albanese, A.; D’Alessandro, C.; Garofalo, C.; Foletto, M.; Barrea, L.; Cupisti, A.; Zoccali, C.; De Nicola, L. Approaches to patients with obesity and CKD: Focus on nutrition and surgery. Clin. Kidney J. 2024, 17, 51–64. [Google Scholar] [CrossRef]
- Lemmens, H.J.; Brodsky, J.B.; Bernstein, D.P. Estimating ideal body weight--a new formula. Obes. Surg. 2005, 15, 1082–1083. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.M.; Thomas, D.M.; Blackburn, G.L.; Heymsfield, S.B. Universal equation for estimating ideal body weight and body weight at any BMI. Am. J. Clin. Nutr. 2016, 103, 1197–1203. [Google Scholar] [CrossRef]
- Robinson, J.D.; Lupkiewicz, S.M.; Palenik, L.; Lopez, L.M.; Ariet, M. Determination of ideal body weight for drug dosage calculations. Am. J. Hosp. Pharm. 1983, 40, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- McCarron, M.M.; Devine, B.J. Clinical Pharmacy: Case Studies:Case Number 25 Gentamicin Therapy. Drug Intell. Clin. Pharm. 1974, 8, 650–655. [Google Scholar] [CrossRef]
- Pai, M.P.; Paloucek, F.P. The origin of the “ideal” body weight equations. Ann. Pharmacother. 2000, 34, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Hamwi, G.J. Therapy: Changing dietary concepts. Diabetes Mellit. Diagn. Treat. 1964, 1, 73–78. [Google Scholar]
- Miller, D.R. Determining ideal body weight (and mass). Am. J. Hosp. Pharm. 1983, 40, 1622. [Google Scholar]
- Nahler, G. Lorentz-formula. In Dictionary of Pharmaceutical Medicine; Springer: Vienna, Austria, 2009; p. 107. [Google Scholar]
- Karkeck, J. Adjusted body weight for obesity. Am. Diet. Assoc. Ren. Pract. Group Newsl. 1984, 3, S94. [Google Scholar]
- Tolonen, A.; Pakarinen, T.; Sassi, A.; Kytta, J.; Cancino, W.; Rinta-Kiikka, I.; Pertuz, S.; Arponen, O. Methodology, clinical applications, and future directions of body composition analysis using computed tomography (CT) images: A review. Eur. J. Radiol. 2021, 145, 109943. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Gitto, S.; Ruffo, G.; Guarino, S.; Del Grande, F.; Sconfienza, L.M. Sarcopenia: Imaging assessment and clinical application. Abdom. Radiol. 2022, 47, 3205–3216. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Benigno, G.; Di Mario, F.; Avesani, C.M.; Fiaccadori, E. Low skeletal muscle mass by computerized tomography is associated with increased mortality risk in end-stage kidney disease patients on hemodialysis. J. Nephrol. 2022, 35, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Lippi, F.; Fois, A.; Gendrot, L.; Nielsen, L.; Vigreux, J.; Chatrenet, A.; D’Alessandro, C.; Cabiddu, G.; Cupisti, A. Intradialytic Nutrition and Hemodialysis Prescriptions: A Personalized Stepwise Approach. Nutrients 2020, 12, 785. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Ceglia, L. Alkaline diets favor lean tissue mass in older adults. Am. J. Clin. Nutr. 2008, 87, 662–665. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J.; Woo, J. Association Between Estimated Net Endogenous Acid Production and Subsequent Decline in Muscle Mass Over Four Years in Ambulatory Older Chinese People in Hong Kong: A Prospective Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 905–911. [Google Scholar] [CrossRef]
- Welch, A.A.; MacGregor, A.J.; Skinner, J.; Spector, T.D.; Moayyeri, A.; Cassidy, A. A higher alkaline dietary load is associated with greater indexes of skeletal muscle mass in women. Osteoporos. Int. 2013, 24, 1899–1908. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects 2024: Summary of Results; UN DESA/POP/2024/TR/NO. 9; United Nations: New York, NY, USA, 2024. [Google Scholar]
- The Lancet Gastroenterology & Hepatology. Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021, 6, 411. [Google Scholar] [CrossRef]
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, M.; Fois, A.; Moio, M.R.; Chatrenet, A.; Maze, B.; Lippi, F.; Vigreux, J.; Beaumont, C.; Santagati, G.; Paulin, N.; et al. Spontaneously Low Protein Intake in Elderly CKD Patients: Myth or Reality? Analysis of Baseline Protein Intake in a Large Cohort of Patients with Advanced CKD. Nutrients 2021, 13, 4371. [Google Scholar] [CrossRef]
- Moore, L.W.; Byham-Gray, L.D.; Scott Parrott, J.; Rigassio-Radler, D.; Mandayam, S.; Jones, S.L.; Mitch, W.E.; Osama Gaber, A. The mean dietary protein intake at different stages of chronic kidney disease is higher than current guidelines. Kidney Int. 2013, 83, 724–732. [Google Scholar] [CrossRef]
- Boenink, R.; Bonthuis, M.; Boerstra, B.A.; Astley, M.E.; Montez de Sousa, I.R.; Helve, J.; Komissarov, K.S.; Comas, J.; Radunovic, D.; Buchwinkler, L.; et al. The ERA Registry Annual Report 2022: Epidemiology of Kidney Replacement Therapy in Europe, with a focus on sex comparisons. Clin. Kidney J. 2025, 18, sfae405. [Google Scholar] [CrossRef]
- Kurella, M.; Covinsky, K.E.; Collins, A.J.; Chertow, G.M. Octogenarians and nonagenarians starting dialysis in the United States. Ann. Intern. Med. 2007, 146, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Viswanathan, G.; Weiner, D.E. Chronic kidney disease and end-stage renal disease in the elderly population: Current prevalence, future projections, and clinical significance. Adv. Chronic. Kidney Dis. 2010, 17, 293–301. [Google Scholar] [CrossRef] [PubMed]
- United States Renal Data System. 2024 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2024.
- Kazes, I.; Solignac, J.; Lassalle, M.; Mercadal, L.; Couchoud, C. Twenty years of the French Renal Epidemiology and Information Network. Clin. Kidney J. 2023, 17, sfad240. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cederholm, T.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Cuerda, C.; Cupisti, A.; Sabatino, A.; Schneider, S.; Torreggiani, M.; et al. Nutritional status and the risk of malnutrition in older adults with chronic kidney disease—implications for low protein intake and nutritional care: A critical review endorsed by ERN-ERA and ESPEN. Clin. Nutr. 2023, 42, 443–457. [Google Scholar] [CrossRef]
- Claire Baseley. The Truth About Almond Milk. Available online: https://www.clairebaseley.co.uk/2016/10/27/the-truth-about-almond-milk/?utm_source=chatgpt.com (accessed on 25 April 2025).
- Khor, B.H.; Sumida, K.; Scholes-Robertson, N.; Chan, M.; Lambert, K.; Kramer, H.; Lui, S.F.; Wang, A.Y. Nutrition Education Models for Patients With Chronic Kidney Disease. Semin. Nephrol. 2023, 43, 151404. [Google Scholar] [CrossRef]
- Brunori, G.; Viola, B.F.; Parrinello, G.; De Biase, V.; Como, G.; Franco, V.; Garibotto, G.; Zubani, R.; Cancarini, G.C. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: A prospective randomized multicenter controlled study. Am. J. Kidney Dis. 2007, 49, 569–580. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef]
- Levey, A.S.; Greene, T.; Beck, G.J.; Caggiula, A.W.; Kusek, J.W.; Hunsicker, L.G.; Klahr, S. Dietary protein restriction and the progression of chronic renal disease: What have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. J. Am. Soc. Nephrol. 1999, 10, 2426–2439. [Google Scholar] [CrossRef] [PubMed]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Koppe, L.; Cassani de Oliveira, M.; Fouque, D. Ketoacid Analogues Supplementation in Chronic Kidney Disease and Future Perspectives. Nutrients 2019, 11, 2071. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, W.; Parrish, N.; Sud, K.; Grandinetti, A.; Castelino, R. Medication Adherence Among Patients With Kidney Disease: An Umbrella Review. Adv. Kidney Dis. Health 2024, 31, 68–83. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Piccoli, G.B.; Calella, P.; Brunori, G.; Pasticci, F.; Egidi, M.F.; Capizzi, I.; Bellizzi, V.; Cupisti, A. “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016, 17, 102. [Google Scholar] [CrossRef]
- Malone, S.; Tynan, C.; McKechnie, S. Unconventional luxury: The reappropriation of time and substance. J. Bus. Res. 2023, 163, 113939. [Google Scholar] [CrossRef]
- Wang, L.; Martinez Steele, E.; Du, M.; Pomeranz, J.L.; O’Connor, L.E.; Herrick, K.A.; Luo, H.; Zhang, X.; Mozaffarian, D.; Zhang, F.F. Trends in Consumption of Ultraprocessed Foods Among US Youths Aged 2–19 Years, 1999-2018. JAMA 2021, 326, 519–530. [Google Scholar] [CrossRef]
- Madruga, M.; Martinez Steele, E.; Reynolds, C.; Levy, R.B.; Rauber, F. Trends in food consumption according to the degree of food processing among the UK population over 11 years. Br. J. Nutr. 2023, 130, 476–483. [Google Scholar] [CrossRef]
- Juul, F.; Parekh, N.; Martinez-Steele, E.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022, 115, 211–221. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14 (Suppl. S2), 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ver Ploeg, M.; Breneman, V.; Farrigan, T.; Hamrick, K.; Hopkins, D.; Kaufman, P.; Lin, B.-H.; Nord, M.; Smith, T.; Williams, R.; et al. Access to Affordable and Nutritious Food: Measuring and Understanding Food Deserts and Their Consequences: Report to Congress; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 2009.
- Suarez, J.J.; Isakova, T.; Anderson, C.A.; Boulware, L.E.; Wolf, M.; Scialla, J.J. Food Access, Chronic Kidney Disease, and Hypertension in the U.S. Am. J. Prev. Med. 2015, 49, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.L.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Cordova, R.; Viallon, V.; Fontvieille, E.; Peruchet-Noray, L.; Jansana, A.; Wagner, K.H.; Kyro, C.; Tjonneland, A.; Katzke, V.; Bajracharya, R.; et al. Consumption of ultra-processed foods and risk of multimorbidity of cancer and cardiometabolic diseases: A multinational cohort study. Lancet. Reg. Health Eur. 2023, 35, 100771. [Google Scholar] [CrossRef] [PubMed]
- Dicken, S.J.; Batterham, R.L. Ultra-processed Food and Obesity: What Is the Evidence? Curr. Nutr. Rep. 2024, 13, 23–38. [Google Scholar] [CrossRef]
- Oste, M.C.J.; Duan, M.J.; Gomes-Neto, A.W.; Vinke, P.C.; Carrero, J.J.; Avesani, C.; Cai, Q.; Dekker, L.H.; Navis, G.J.; Bakker, S.J.L.; et al. Ultra-processed foods and risk of all-cause mortality in renal transplant recipients. Am. J. Clin. Nutr. 2022, 115, 1646–1657. [Google Scholar] [CrossRef]
- Du, S.; Kim, H.; Crews, D.C.; White, K.; Rebholz, C.M. Association Between Ultraprocessed Food Consumption and Risk of Incident CKD: A Prospective Cohort Study. Am. J. Kidney Dis. 2022, 80, 589–598. [Google Scholar] [CrossRef]
- Kityo, A.; Lee, S.A. The Intake of Ultra-Processed Foods and Prevalence of Chronic Kidney Disease: The Health Examinees Study. Nutrients 2022, 14, 3548. [Google Scholar] [CrossRef]
- Montero-Salazar, H.; Guallar-Castillon, P.; Banegas, J.R.; Akesson, A.; Rey-Garcia, J.; Rodriguez-Artalejo, F.; Donat-Vargas, C. Food consumption based on the nutrient profile system underlying the Nutri-Score and renal function in older adults. Clin. Nutr. 2022, 41, 1541–1548. [Google Scholar] [CrossRef]
- Gu, Y.; Li, H.; Ma, H.; Zhang, S.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Zhang, T.; Wang, X.; et al. Consumption of ultraprocessed food and development of chronic kidney disease: The Tianjin Chronic Low-Grade Systemic Inflammation and Health and UK Biobank Cohort Studies. Am. J. Clin. Nutr. 2023, 117, 373–382. [Google Scholar] [CrossRef]
- Cai, Q.; Duan, M.J.; Dekker, L.H.; Carrero, J.J.; Avesani, C.M.; Bakker, S.J.L.; de Borst, M.H.; Navis, G.J. Ultraprocessed food consumption and kidney function decline in a population-based cohort in the Netherlands. Am. J. Clin. Nutr. 2022, 116, 263–273. [Google Scholar] [CrossRef]
- Avesani, C.M.; Cuppari, L.; Nerbass, F.B.; Lindholm, B.; Stenvinkel, P. Ultraprocessed foods and chronic kidney disease-double trouble. Clin. Kidney J. 2023, 16, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, M.; Fois, A.; Lippi, F.; Attini, R.; Longhitano, E.; Matarazzo, I.; Masturzo, B.; Cabiddu, G.; Versino, E.; Piccoli, G.B. Plant-based diets for CKD patients: Fascinating, trendy, but feasible? A green nephrology perspective. Clin. Kidney J. 2023, 16, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.L. Demineralization of a wide variety of foods for the renal patient. J. Ren. Nutr. 2001, 11, 90–96. [Google Scholar] [CrossRef]
- Orozco-Guillien, A.O.; Munoz-Manrique, C.; Reyes-Lopez, M.A.; Perichat-Perera, O.; Miranda-Araujo, O.; D’Alessandro, C.; Piccoli, G.B. Quality or Quantity of Proteins in the Diet for CKD Patients: Does “Junk Food” Make a Difference? Lessons from a High-Risk Pregnancy. Kidney Blood Press. Res. 2021, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Avesani, C.M.; Cecchini, V.; Heimbürger, O.; Sallstrom, T.; Rubin, C.; Canella, D.; Lindholm, B.; Stenvinkel, P. #2481 Reduction in ultra-processed food by providing medically tailored healthy food baskets to patients with CKD: The HELPFUL trial. Nephrol. Dial. Transplant. 2024, 39, gfae069-0706. [Google Scholar] [CrossRef]
- Paterick, T.E.; Patel, N.; Tajik, A.J.; Chandrasekaran, K. Improving health outcomes through patient education and partnerships with patients. Bayl. Univ. Med. Cent. Proc. 2017, 30, 112–113. [Google Scholar] [CrossRef]
- Butler, R.; Monsalve, M.; Thomas, G.W.; Herman, T.; Segre, A.M.; Polgreen, P.M.; Suneja, M. Estimating Time Physicians and Other Health Care Workers Spend with Patients in an Intensive Care Unit Using a Sensor Network. Am. J. Med. 2018, 131, 972.e9–972.e15. [Google Scholar] [CrossRef]
- Ladin, K.; Rossi, A. Person-Centered Kidney Education: The Path Forward. Kidney Med. 2020, 2, 511–513. [Google Scholar] [CrossRef]
- Narva, A.S.; Norton, J.M.; Boulware, L.E. Educating Patients about CKD: The Path to Self-Management and Patient-Centered Care. Clin. J. Am. Soc. Nephrol. 2016, 11, 694–703. [Google Scholar] [CrossRef]
- Kurella Tamura, M.; Li, S.; Chen, S.C.; Cavanaugh, K.L.; Whaley-Connell, A.T.; McCullough, P.A.; Mehrotra, R.L. Educational programs improve the preparation for dialysis and survival of patients with chronic kidney disease. Kidney Int. 2014, 85, 686–692. [Google Scholar] [CrossRef]
- Padial, M.; Taylor, A.; Sabatino, A.; Piccoli, G.B.; Avesani, C.M. From ultra-processed foods towards healthy eating for CKD patients: A proposal of educational infographics. J. Nephrol. 2024, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Nazha, M.; Capizzi, I.; Vigotti, F.N.; Scognamiglio, S.; Consiglio, V.; Mongilardi, E.; Bilocati, M.; Avagnina, P.; Versino, E. Diet as a system: An observational study investigating a multi-choice system of moderately restricted low-protein diets. BMC Nephrol. 2016, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- European Renal Association (ERA). Leaflets for Patients. Available online: https://www.era-online.org/cookbook/materials/ (accessed on 25 March 2025).
- Avesani, C.M. Eating healthy is tasty: A message of enjoyment for kidney patients and health care providers. J. Nephrol. 2023, 36, 2413–2416. [Google Scholar] [CrossRef]
- Pan, Y.; Guo, L.L.; Jin, H.M. Low-protein diet for diabetic nephropathy: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 660–666. [Google Scholar] [CrossRef]
- Nezu, U.; Kamiyama, H.; Kondo, Y.; Sakuma, M.; Morimoto, T.; Ueda, S. Effect of low-protein diet on kidney function in diabetic nephropathy: Meta-analysis of randomised controlled trials. BMJ Open 2013, 3, e002934. [Google Scholar] [CrossRef]
- Zhu, H.G.; Jiang, Z.S.; Gong, P.Y.; Zhang, D.M.; Zou, Z.W.; Qian, Z.; Ma, H.M.; Guo, Z.G.; Zhao, J.Y.; Dong, J.J.; et al. Efficacy of low-protein diet for diabetic nephropathy: A systematic review of randomized controlled trials. Lipids Health Dis. 2018, 17, 141. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Lockwood, M.B.; Rhee, C.M.; Tantisattamo, E.; Andreoli, S.; Balducci, A.; Laffin, P.; Harris, T.; Knight, R.; Kumaraswami, L.; et al. Patient-centred approaches for the management of unpleasant symptoms in kidney disease. Nat. Rev. Nephrol. 2022, 18, 185–198. [Google Scholar] [CrossRef]
- Castro, M.C.M. Conservative management for patients with chronic kidney disease refusing dialysis. J. Bras. Nefrol. 2019, 41, 95–102. [Google Scholar] [CrossRef]

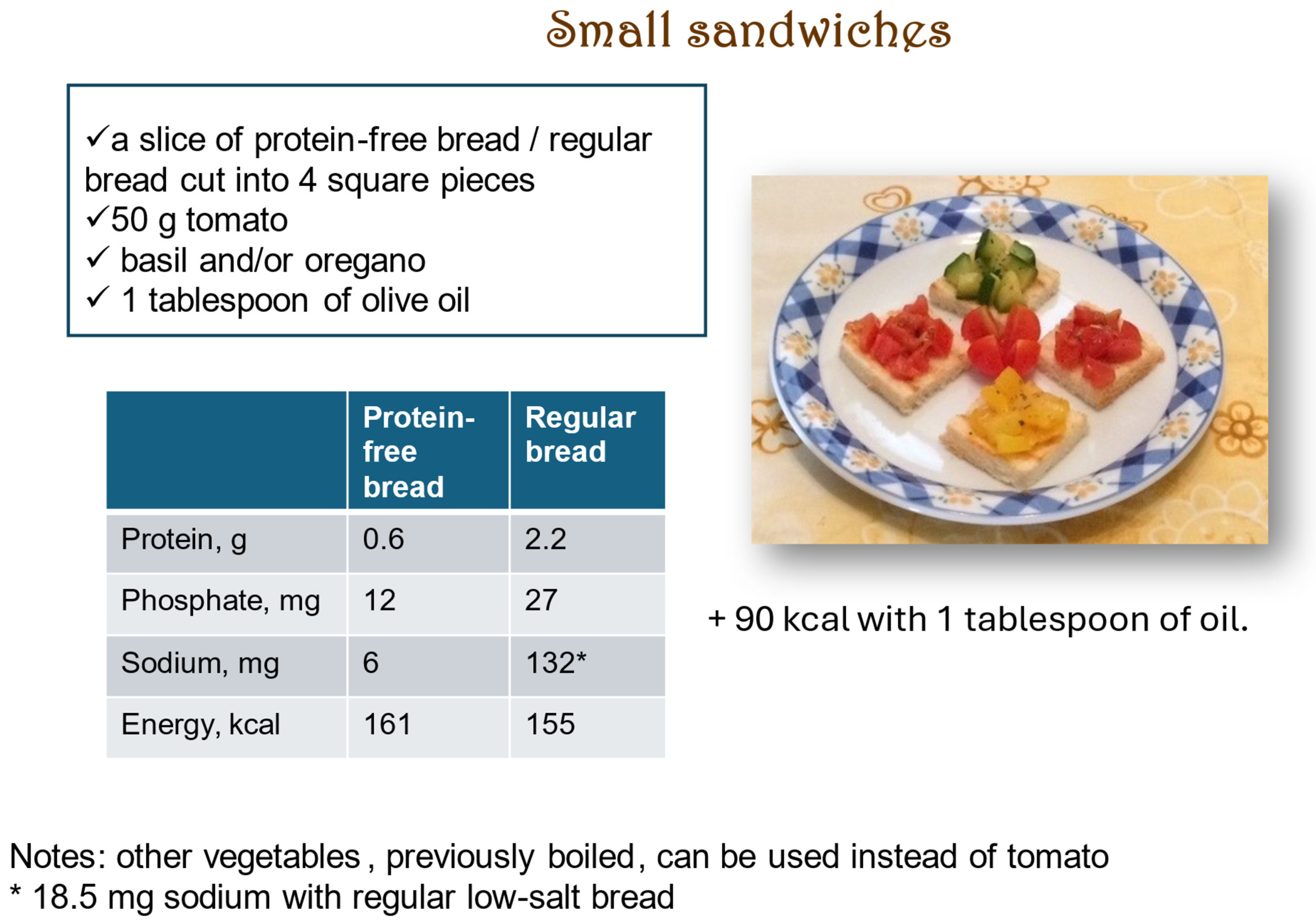
| Actual Weight (kg) | Ideal Body Weight (Lorentz [79]) (kg) | Ideal Body Weight (Hamwi [77]) (kg) | |
|---|---|---|---|
| Woman: 62 years old; actual weight, 62 kg; height, 163 cm; BMI, 23.3 kg/m2 | |||
| 62 | 57 | 55 | |
| Energy (30 kcal/kg/day) | 1860 | 1710 | 1650 |
| Protein (0.6 g/kg/day) | 37.2 | 34.2 | 33.0 |
| Man: 91 years old, actual weight, 80 kg; height, 175 cm; BMI: 26.1 kg/m2 | |||
| 80 | 69 | 72 | |
| Energy (30 kcal/kg/day) | 2400 | 2070 | 2160 |
| Protein (0.6 g/kg/day) | 48.0 | 41.4 | 43.2 |
| Modality | Options | Topics Do Discuss |
|---|---|---|
| Setting | One-to-one Group Patient and family | Kidney physiology/pathology, treatment of CKD Medication management/adherence Nutrition and kidney diseases Pharmacological and medical protocols Nutritional counseling/dietician’s advice Lifestyle modification (e.g., weight loss, smoking) Exercise/program/information/participation Fresh food vs. ultra-processed food Self-management skills |
| Delivery technique | Face-to-face: slide, lectures, counseling, interviews Remote: e-learning, messages, social media, phone calls Written material: leaflets, books Other: medication charts, review of patient’s dietary journal | |
| Teaching method | Didactic Goal setting, dictated Goal setting, negotiated: self-management Situational problem solving: practical skills Other: support group discussions Workshops |
| Supermarket Guide | How to Buy, How to Choose Food [137] |
|---|---|
| Videos | Explaining food choices according to degree of food processing [137]. |
| Personal adaptions | Food recipes adapted to the cultural background, identifying kidney-friendly options [109]. |
| Cooking lessons | Workshops with professional cooks [141]. |
| Group discussions | Identifying barriers and facilitators; involve expert patients. |
| Involve patient’s organizations | Establishing priorities and expectations. |
| Be the example | Discussing how you follow the recommendations patients are given. |
| Dos ✓ | Don’ts ✗ |
|---|---|
| Animal vs. vegetal proteins | |
| Prescribe a plant-based diet in all CKD stages; regularly check nutritional status; periodically check for intake of all essential amino acids in the same meal (ideally) or at least in the same day, in particular in vegan diets. Consider occasionally including unrestricted meals. Consider supplementation with essential ketoacids and amino acids in vegan diets, both for simplifying management (0.6 g/kg/day diets) and making it possible to use VLPDs. | Do not limit prescription for fear of PEW. Do not prescribe a plant-based (vegan) or a VLPD without a detailed monitoring plan. |
| Potassium | |
| Prescribe plant-based diets in all CKD stages with regular checks of potassium and bicarbonate levels. Add potassium binders, provided that food sources are controlled (readily absorbed potassium salts are ubiquitous additives in ultra-processed food; they are not necessarily disclosed on food labels). | Do not limit prescription of plant-based diets in the case of high potassium levels, or fear of causing them. Do not prescribe a plant-based (vegan) or a VLPD without preparing a strict monitoring plan. |
| Phosphorus | |
| Keep in mind that a plant-based diet is a source of phosphorus whose bioavailability is lower than animal-based diets. Beware of ultra-processed foods, as readily absorbed phosphate salts are ubiquitous additives in ultra-processed food and they are not necessarily disclosed on food labels. | Do not limit prescription of plant-based diets in the case of high phosphate levels, or fear of causing them. |
| Ideal body weight | |
| Consider each person living with CKD’s body composition, weight trajectory and perspectives when approaching the issue of real or ideal body weight. Consider real body weight at least below a BMI of 30 kg/m2. Evaluate obese people living with CKD individually and review prescriptions in case of modifications of body weight. | Do not use a formula to calculate ideal or adjusted body weight without a previous critical appraisal of the individual person living with CKD. |
| Energy intake and the risk of protein energy wasting | |
| When starting nutritional management of people living with CKD, always start from energy intake, modulating it according to the person’s nutritional status. A plant-based diet is compatible with high energy intake, and if necessary, can be combined with energy-rich low-protein supplements. | In people living with CKD with evidence of PEW, do not restrict protein intake, mainly from vegetable sources, at least until energy needs are met. Avoid a high-protein intake to prevent protein hypercatabolism, potentially leading to acidosis, hyperkalemia, hyperphosphatemia and intestinal dysbiosis. |
| Supplemented very low protein diets | |
| Prescribe VLPDs to both young and elderly people living with CKD. They are in essence plant-based. Especially in people living with CKD on unrestricted baseline diets, proceed stepwise, both with quality changes (from omnivorous to vegan) and protein intake. | Do not prescribe a severe protein restriction without supplementation and without close monitoring. Do not prescribe sVLPD in malnourished people living with CKD, especially if the etiology of PEW is uncertain. Do not use the same doses when supplementation is an add-on treatment in moderately protein-restricted diets. Consider a lower pill burden, instead. |
| Ultra-processed foods | |
| Pay attention to the quality of food and minimize the use of ultra-processed foods. | Do not reduce plant-based food, for example, in the case of hyperkalemia, until you have excluded regular use of UPFs and educated people living with CKD to limit their use. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torreggiani, M.; Avesani, C.M.; Contzen, B.; Cupisti, A.; Czaja-Stolc, S.; D’Alessandro, C.; Garneata, L.; Gutiérrez, A.; Lippi, F.; Mocanu, C.A.; et al. Dos and Don’ts in Kidney Nutrition: Practical Considerations of a Panel of Experts on Protein Restriction and Plant-Based Diets for Patients Living with Chronic Kidney Disease. Nutrients 2025, 17, 2002. https://doi.org/10.3390/nu17122002
Torreggiani M, Avesani CM, Contzen B, Cupisti A, Czaja-Stolc S, D’Alessandro C, Garneata L, Gutiérrez A, Lippi F, Mocanu CA, et al. Dos and Don’ts in Kidney Nutrition: Practical Considerations of a Panel of Experts on Protein Restriction and Plant-Based Diets for Patients Living with Chronic Kidney Disease. Nutrients. 2025; 17(12):2002. https://doi.org/10.3390/nu17122002
Chicago/Turabian StyleTorreggiani, Massimo, Carla Maria Avesani, Barbara Contzen, Adamasco Cupisti, Sylwia Czaja-Stolc, Claudia D’Alessandro, Liliana Garneata, Abril Gutiérrez, Françoise Lippi, Carmen Antonia Mocanu, and et al. 2025. "Dos and Don’ts in Kidney Nutrition: Practical Considerations of a Panel of Experts on Protein Restriction and Plant-Based Diets for Patients Living with Chronic Kidney Disease" Nutrients 17, no. 12: 2002. https://doi.org/10.3390/nu17122002
APA StyleTorreggiani, M., Avesani, C. M., Contzen, B., Cupisti, A., Czaja-Stolc, S., D’Alessandro, C., Garneata, L., Gutiérrez, A., Lippi, F., Mocanu, C. A., Sabatino, A., & Piccoli, G. B., on behalf of the European Renal Nutrition (ERN) Working Group of the European Renal Association (ERA). (2025). Dos and Don’ts in Kidney Nutrition: Practical Considerations of a Panel of Experts on Protein Restriction and Plant-Based Diets for Patients Living with Chronic Kidney Disease. Nutrients, 17(12), 2002. https://doi.org/10.3390/nu17122002









