Abstract
Background/Objectives:Arterial hypertension, increased carotid intima-media thickness (cIMT), and arterial stiffness (AS) are recognized predictors of cardiovascular disease (CVD). Emerging evidence suggests that vascular remodeling may precede the full development of hypertension. Furthermore, body mass index (BMI), fat mass percentage (FM%), and visceral adipose tissue (VAT), are significant risk factors for cardiovascular events. Conversely, adherence to the Mediterranean diet is associated with reduced cardiovascular risk due to its beneficial effects on lipid metabolism, inflammation, and vascular health. Methods: This observational study explored the association between nutritional care and cardiovascular risk in 55 Italian adults (27 women, 28 men) consecutively evaluated at the Section of Clinical Nutrition and Nutrigenomics, University of Rome “Tor Vergata”, in 2024. Nutritional and vascular assessments, including BMI, waist-to-hip ratio (WHR), BIA, DXA, lab tests, blood pressure (BP), pulse wave velocity (PWV), and cIMT, were recorded. Spearman’s rank correlation coefficient was used to evaluate the relationship between vascular and nutritional parameters. Wilcoxon rank sum test; Fisher’s exact test; and Pearson’s Chi-squared test were performed for statistical analysis. Participants were divided into two groups based on cIMT > 0.90 mm and ≤0.90 mm. Results: Significant correlations emerged between higher MEDAS scores and BMI (r = −0.53, p < 0.01), FM% (r = −0.49, p < 0.01), VAT (r = −0.63, p < 0.01), and cIMT (r = −0.88, p < 0.01). Higher WHR and VAT were associated with increased brachial and central BP and PWV. Notable dietary differences were significantly higher between cIMT groups. Total cholesterol/C-HDL, C-LDL/C-HDL, the Atherogenic Index of Plasma, and the HOMA Index differed significantly between groups. Significant differences were also observed in the left ventricular diastolic function (p = 0.04), LVM/BSA, and LVM/h2.7 in individuals with subclinical atherosclerosis (p < 0.05). Conclusions: These innovative findings underline the importance of multidisciplinary approaches to prevent CVD and suggest long-term benefits of Mediterranean diet adherence on vascular health.
1. Introduction
Cardiovascular disease (CVD) remains a leading cause of morbidity and mortality worldwide, with carotid atherosclerosis being a prevalent condition that significantly contributes to its overall burden [1]. Atherosclerosis plays a pivotal role in the onset of cardiovascular events by promoting plaque formation, vascular inflammation, and arterial narrowing, ultimately leading to ischemic complications such as stroke and myocardial infarction [2]. Among the recognized predictors of CVD [3], high blood pressure (BP) [4], increased carotid intima-media thickness (cIMT) [5], and arterial stiffness (AS) [6] are key indicators of vascular dysfunction and heightened cardiovascular risk. Notably, emerging evidence suggests that vascular stiffening may develop before the full manifestation of hypertension, indicating that early arterial changes could serve as an initial trigger in the progression toward CVD [7].
Additionally, body composition, particularly fat mass, has been increasingly recognized as a critical factor influencing vascular health [8]. Body mass index (BMI) [9], fat mass (FM) [10], and visceral adipose tissue (VAT) [11] are significant risk factors for cardiovascular events. Excess adiposity contributes to systemic inflammation, oxidative stress, and metabolic dysregulation, all of which accelerate atherosclerotic processes and compromise arterial function, further reinforcing the complex interplay between body composition and cardiovascular risk [12].
Diet plays a crucial role in modulating the risk of atherosclerosis by influencing metabolic pathways, systemic inflammation, oxidative stress, and body composition [13]. Specific nutrients contribute to the prevention of chronic degenerative diseases by exerting beneficial effects on lipid metabolism, inflammation, and vascular health [14,15,16]. Unhealthy dietary patterns, characterized by high consumption of saturated fats, refined sugars, and processed foods, promote endothelial dysfunction and lipid accumulation, increasing the risk of atherosclerosis [17]. Conversely, the Mediterranean diet (MedDiet) is associated with improved lipid profiles, reduced inflammation, and enhanced vascular function, thereby lowering the risk of atherosclerosis [18]. The MedDiet is distinguished by a high intake of fresh fruits and vegetables, whole grains, olive oil, and lean protein sources such as fish and poultry, along with moderate consumption of dairy products, particularly yogurt and cheese. It also emphasizes the use of herbs and spices like basil, oregano, and garlic, as well as nutrient-dense nuts and seeds, including almonds and sunflower seeds [19]. Moreover, adherence to the MedDiet has been linked to a significant increase in High-Density Lipoprotein Cholesterol (C-HDL) and upregulation of Apolipoprotein E (APOE) expression, particularly in females, further supporting its cardioprotective effects [20].
Given the role of body composition and the MedDiet in carotid atherosclerosis, this observational study aims to investigate the influence of individual dietary components and specific body composition parameters on different indices of atherosclerosis, with the purpose of identifying potential predictive parameters in the development of the disease.
2. Materials and Methods
2.1. Study Design
This observational study was conducted between September and December 2024 at the Section of Clinical Nutrition and Nutrigenomics, University of Rome “Tor Vergata”. A total of 55 subjects enrolled in the study gave their consent by reading and signing the informed consent form, in accordance with the Helsinki Declaration of 1975, as revised in 2013. The trial received approval from the Ethics Committee of the Calabria Region Central Area Section (register protocol no. 97, 20 April 2023).
Eligibility criteria included males and females aged 18 to 65 years with a BMI > 19 kg/m2. We included individuals with or without arterial hypertension under stable treatment (at least two years), without substantial changes in dietary habit over the past 12 months. Exclusion criteria comprised pregnancy and feeding, BMI exceeding 40 kg/m2, being a current smoker, acute illnesses, autoimmune and intestinal disorders, HIV/AIDS, and neoplastic diseases. Participants receiving statin therapy were excluded due to the potential influence on the lipid level and vascular parameters. Patients with a recent diagnosis of arterial hypertension were excluded from the study, as the absence of a stable pharmacological treatment and the lack of assessment of hypertension-mediated organ damage could have influenced the study outcomes, introducing potential confounding factors. Individuals with recent known coronary artery disease or a history of major cardiovascular events—including myocardial infarction or stroke—were also excluded from the study.
All patients underwent an assessment of their pathological history, dietary habits, nutritional status, body composition, and complete cardiovascular evaluation.
2.2. Dietary Habits and MedDiet Adherence
The subject’s dietary intake was evaluated using a 24-h recall, while a food frequency questionnaire (FFQ) was administered to determine the weekly consumption patterns of various foods [21]. The FFQ assessed the intake frequency of 36 commonly consumed foods in Italy, along with their portion sizes. Compliance rates were calculated for each food item.
Adherence to the MedDiet was assessed using the validated 14-item Mediterranean Diet Adherence Screener (MEDAS), which assigns a score ranging from 0 to 14 points (Table A1, Appendix A.1) [22]. Based on the MEDAS scores, subjects were classified into the following three adherence categories: low (0–5), medium (6–9), and high (≥10).
2.3. Evaluation of Body Composition
Weight, height, and waist circumference were measured following standard protocols [23].
BMI was calculated as body weight (kg) divided by height squared (m2) [24]. The waist-to-height ratio (WHR) was determined using a cutoff value of 0.5 [25]. The body adiposity index (BAI) was calculated using the following formula [26]:
BAI = 100 × (hip circumference (m)/(height (m))1.5) − 18
Bioelectrical impedance analysis (BIA) was performed using a phase-sensitive system (BIA 101S, Akern/RJL Systems, Florence, Italy) at a frequency of 50 kHz to measure resistance, reactance, impedance, phase angle (PhA), and body cell mass (BCM) following standard procedures [27]. Total body water (TBW) (%) was estimated using the equation proposed by De Lorenzo et al. [28].
Body composition was analyzed at baseline using Dual-Energy X-Ray Absorptiometry (DXA) (Primus, X-ray densitometer; software version 1.2.2, Osteosys Co., Ltd., Gurogu, Seoul, Republic of Korea), following previously described procedures [29]. This assessment included soft tissue evaluation, FM, total body lean mass (LM), bone tissue, total body bone mass (TBB), and VAT [29].
The intramuscular adipose tissue (IMAT) was calculated using the equations proposed by Bauer et al. [30], which are as follows:
For women, Log (IMAT) = −2.21 + (0.12 × FM) − (0.0013 × FM2)
For men, Log (IMAT) = −2.05 + (0.12 × FM) − (0.0013 × FM2)
2.4. Cardiovascular Evaluation
Systolic and diastolic brachial office blood pressure (BP), as well as central (aortic) BP, were assessed non-invasively using a cuff-based oscillometric device (Mobil-O-Graph PWA Monitor, I.E.M. GmbH, Stolberg, Germany), following the European Society of Hypertension guidelines [31]. Central BP was recorded 30 s after brachial BP, and heart rate (HR) was measured simultaneously. Cuff sizes were adapted to participants’ individual arm circumference to ensure accurate readings [31].
Pulse wave velocity (PWV), the gold standard to assess arterial stiffness (AS) in clinical practice, was derived from the time interval between the estimated forward and reflected pressure waves, and has been shown to correlate well with invasive measurements [32].
As AS rises, the velocity of both forward and reflected pressure waves increases, leading to an earlier return of the reflected wave in the central aorta. This earlier arrival enhances central systolic BP during late systole, thereby increasing the Augmentation Index (AI@75) [33]. AI@75 was determined as the ratio between augmentation pressure and pulse pressure (PP), and the values were standardized to a heart rate of 75 beats per minute [34].
All participants underwent a color Doppler ultrasound examination of the supra-aortic trunks (TSA) MyLab™ Seven ultrasound system (Esaote S.p.A., Genoa, Italy). cIMT was measured bilaterally in the common carotid artery at approximately 1 cm proximal to the carotid bifurcation, in accordance with current clinical guidelines [31]. Measurements were taken on the far wall during end-diastole and averaged over multiple cardiac cycles to ensure accuracy.
Individuals underwent transthoracic echocardiography (MyLab™ Seven ultrasound system (Esaote S.p.A., Genoa, Italy)) in the left lateral decubitus position. A single experienced operator performed the exams according to standard guidelines [35]. Measurements including the left atrium diameter, left ventricular (LV) internal dimensions, interventricular septal thickness in diastole (IVSd), posterior wall thickness in diastole (PWTd), and ejection fraction (calculated using the Teichholz method) were assessed. LV geometry was classified into four patterns [36]. Diastolic function was evaluated using Doppler echocardiography and tissue Doppler imaging (TDI), by analyzing the ratio of early (E) to late (A) ventricular filling velocities (E/A), deceleration time, isovolumetric relaxation time (IVRT), and the ratio of early mitral inflow velocity to early diastolic mitral annular velocity (E/e′).
LV mass (LVM) was calculated as follows: LVM = 0.8 × 1.04 × [(IVS + LVEDD + PW)3 − LVEDD3] + 0.6 g. LVM obtained via echocardiography was normalized to the body surface area (BSA) following the recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging (ASE/EACVI) [37].
In accordance with EACVI guidelines, LVM was also indexed to height raised to the power of 2.7 [38]. Left ventricular hypertrophy (LVH) was defined by LVM/BSA values exceeding 115 g/m2 for men and 95 g/m2 for women, or by LVM/height2.7 values greater than 50 g/m2.7 for men and 47 g/m2.7 for women.
After an overnight fast (≥8 h), blood samples were collected at baseline and after 8 weeks to assess fasting glucose, insulin, creatinine, and liver enzymes (Glutamic Oxalo-Acetic Transaminase (GOT), Glutamate Pyruvate Transaminase (GPT) and the lipid profile as total cholesterol (C-TOT), C-HDL, Low-Density Lipoprotein Cholesterol (C-LDL)—calculated using the Friedewald equation [39]—and triglycerides (TG).
The Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using fasting glucose and insulin levels, according to the following formula: [fasting glucose (mg/dL) × 0.0555 × fasting serum insulin (µIU/mL)]/22 [40].
Moreover, the Atherogenic Index of Plasma (AIP), a reliable marker for assessing the risk of atherosclerosis and CVD, calculated as the logarithm of the ratio between plasma TG and C-HDL levels, was considered [41].
Furthermore, we analyzed Lipoprotein(a) (Lp(a)), which has gained increasing recognition as an independent risk factor for atherosclerotic CVD, due to its pro-inflammatory and pro-thrombotic properties that significantly enhance its atherogenic potential—estimated to be 5 to 6 times greater than that of C-LDL particles on a molar basis. Lp(a) levels vary widely among individuals, ranging from 0.2 to 750 nmol/L (0.1 to 300 mg/dL); however, no universally accepted cutoff exists to define elevated Lp(a), although values above 30 mg/dL have been associated with increased cardiovascular risk [42].
2.5. Statistical Analysis
Demographic, pharmacological, anthropometric, and clinical characteristics of patients were reported. No formal sample size calculation was performed, as this was an observational study aimed at exploring associations rather than testing predefined hypotheses.
Descriptive statistics were conducted for the above characteristics. Continuous variables were summarized using the mean and standard deviation (SD), while categorical variables were presented as frequency and percentage. All data were stratified by cIMT ≤ 0.9 mm and cIMT > 0.9 mm, and the appropriate statistical tests were employed to evaluate significant differences between the groups (including Pearson’s Chi-squared test, Kruskal–Wallis rank sum test, and Fisher’s exact test).
Given the non-linear relationships observed between most of the variables, Spearman’s rank correlation was chosen, as it effectively captures monotonic associations regardless of the linearity of the data. Spearman’s correlation coefficient ® ranges from −1 to 1, where absolute values of 0.70 and above indicate a strong correlation, absolute values between 0.40 and 0.70 suggest a moderate correlation, and absolute values below 0.40 represent a weak correlation.
The level of significance was 0.05 and all analyses were explorative and without adjustment of p-values. All statistical analyses were performed using R software (version 4.4.2, R Foundation for Statistical Computing, Vienna, Austria).
3. Results
The study population included 55 individuals, with a mean age of 52.00 ± 12.00 years and a nearly equal sex distribution (52.7% female). The mean BMI was 27.30 ± 4.70 kg/m2. Out of these patients, twenty had arterial hypertension, and among them, three had dyslipidemia without any treatment. There were no other relevant medical conditions in the patients’ medical history, and they were not taking any medications other than anti-hypertensives (Angiotensin II Receptor Blockers and/or Calcium Channel Blockers). All of these demographic, pharmacological, and anthropometric parameters are summarized in Table 1.

Table 1.
Demographic, pharmacological, and anthropometric parameters.
The average FM% was 28.00 ± 9.00%, with a corresponding Fat-Free Mass (FFM) of 55.00 ± 13.00 kg. Handgrip strength averaged 35.00 ± 11.00 kg, and the PhA was 5.89 ± 0.88°. The VAT-to-subcutaneous fat (SAT) ratio (VAT/SAT) was 0.34 ± 0.17.
Metabolic parameters, including fasting glucose and cardiovascular indices, were within standard ranges. However, it has to be underlined that the total cholesterol levels were at the upper limit according to the latest ESC cardiovascular prevention [43], with a mean total cholesterol of 213 ± 43.00 mg/dL and LDL cholesterol of 131.00 ± 33.00 mg/dL.
The BP and AS parameters were within normal ranges according to the latest ESC Guidelines. AS, measured by PWV, averaged 7.87 ± 1.02 m/s, and Systolic and Diastolic BP measured 130.00 ± 16.00 mmHg and 84.00 ± 10.00 mmHg, respectively [31].
Finally, the mean cIMT was within the normal range, averaging 0.80 mm. All of these parameters are reported in Table A2 (Appendix A).
According to the latest 2023 guidelines, asymptomatic intimal-medial thickening is considered a sign of organ damage if the cIMT measured by ultrasound exceeds 0.90 mm [31]. Therefore, by dividing the study population into two groups, we observed that 32 individuals had normal cIMT (≤0.90 mm) and 23 individuals had increased cIMT > 0.90 mm.
Key cardiovascular risk markers, including C-TOT/C-HDL, C-LDL/C-HDL and Atherogenic Index of Plasma (AIP), were significantly different between groups (p = 0.03; 0.04; 0.02, respectively). There were no statistical differences between the groups’ C-LDL and TG levels. The HOMA Index was statistically significantly higher in the cIMT > 0.90 mm group compared to the other group.
A comparison of cardiometabolic risk parameters between the groups is represented in Figure 1.
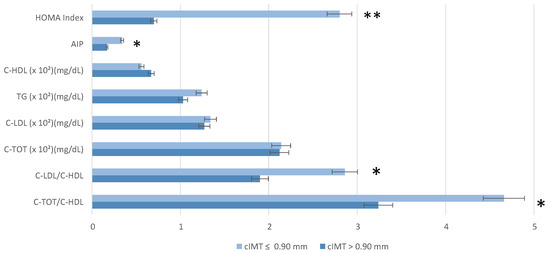
Figure 1.
Cardiometabolic risk parameters (lipid profile and (Homeostasis Model Assessment index) stratified by carotid intima-media thickness (cIMT). Wilcoxon rank sum test; Fisher’s exact test; and Pearson’s Chi-squared test were performed for statistical analysis. p < 0.05 *; p = 0.01 **. Abbreviation: AIP, Atherogenic Index of Plasma; C-HDL, Cholesterol—high-density lipoporotein; C-LDL, Cholesterol—low-density lipoporotein; C-TOT, Total Cholesterol; C-TOT/C-HDL, Total Cholesterol/high-density lipoporotein; C-LDL/C-HDL, Cholesterol—low-density lipoporotein/Cholesterol—high-density lipoporotein.
Additionally, the MEDAS score was significantly higher in individuals with lower cIMT (p = 0.03), supporting the association between MedDiet adherence and lower subclinical atherosclerosis risk [44]. The MEDAS responses between the groups are reported in Table 2 and Figure 2.

Table 2.
MEDAS questionnaire.
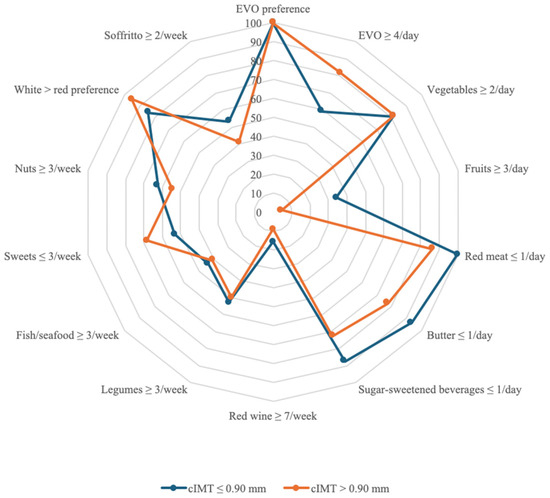
Figure 2.
Answers and scoring of the MEDAS. The “YES” answer to the MEDAS items is compared between the sample with cIMT ≤ 0.90 mm and the group with cIMT > 0.90 mm. The radar chart shows for each MEDAS score item the affirmative responses of the sample with cIMT ≤ 0.90 mm compared to the group with cIMT > 0.90 mm with separate axes starting from the center of the draft (0% “YES” responses) and at the end of the last circle (100% “YES” responses). Values are in percentages. Abbreviations: EVO, extra virgin olive oil; cIMT, carotid intima-media thickness.
Notable dietary differences included fresh cheese consumption, which was significantly higher in the lower cIMT group (p = 0.03 monthly). Regarding red meat consumption, a statistically significant difference was found (p = 0.03). Similarly, the intake of sausage and processed meat products was significantly different between groups (p = 0.03). Lastly, extra virgin olive oil (EVO) consumption was also significantly different (p < 0.01), with higher adherence to EVO intake in the cIMT ≤ 0.90 mm group.
Findings regarding BP and AS highlight trends rather than statistically significant differences (Figure 3).
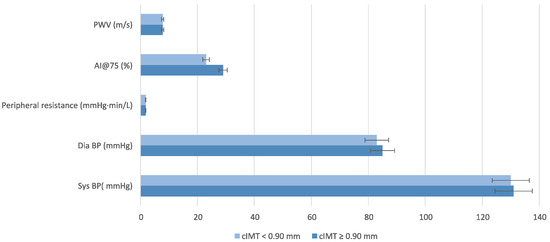
Figure 3.
Blood Pressure Parameters stratified by carotid intima-media thickness (cIMT). Wilcoxon rank sum test; Fisher’s exact test; Pearson’s Chi-squared test was performed for statistical analysis. Abbreviation: AI@75, Augmentation Index; cIMT, intima-media thickness; PWV, pulse wave velocity.
There were no significant differences between the two groups in terms of FM, FM%, FFM, or FFM% (Figure 4A). No significant differences were found in the absolute LVM between groups (p = 0.32); however, when indexed to body surface area (LVM/BSA), a significant difference was observed (p = 0.04), with lower values in the cIMT ≤ 0.90 mm group compared to the cIMT > 0.90 mm group. Additionally, when normalized by height2.7 (LV MASS/h2.7), the cMT > 0.90 mm group had significantly higher values (p < 0.01), (Figure 4B).
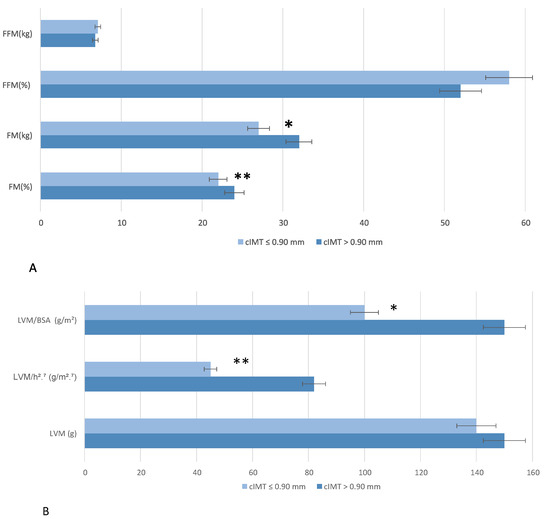
Figure 4.
Bioimpedentiometric (A) and ecocardiographic (B) parameters stratified by carotid intima-media thickness (cIMT). Wilcoxon rank sum test; Fisher’s exact test; Pearson’s Chi-squared test was performed for statistical analysis. p < 0.5 *; p < 0.01 **. Abbreviation: BSA, body surface area; FFM, Fat-Free Mass; FM, fat mass; LVM, left ventricular mass.
Finally, significant differences were observed in LV diastolic function (p = 0.04) (66.7% of individuals had I grade diastolic disfunction in the cIMT > 0.9 mm and 35.5% of individuals had I grade diastolic disfunction in the cIMT ≤ 0.9 mm).
Spearman’s rank correlation coefficient was used to evaluate the relationship between variables. First, a strong inverse correlation was observed between the MEDAS score and carotid cIMT (r = −0.88, p < 0.001).
Moreover, a significant negative correlation was observed between weekly legume consumption and Lp(a) levels (r = −0.83, p < 0.001). Additionally, weekly legume consumption was positively correlated with FT3 (free-Triiodothyronine) levels (r = 0.72, p < 0.01). A negative correlation was found between MEDAS score and BMI (r = −0.53, p < 0.001), as well as VAT (r = −0.63, p < 0.001). Higher MEDAS scores were significantly associated with lower BAI (r = −0.43, p = 0.001), lower FM% (r = −0.49, p < 0.001), and lower FM (kg) (r = −0.50, p < 0.001). Conversely, higher adherence to the MEDAS score correlated positively with FFM% (r = 0.44, p < 0.001).
A strong positive correlation was observed between BCM and FFM (r = 0.92, p < 0.001). Similarly, IMAT and FM were significantly correlated (r = 0.92, p < 0.001), indicating that higher IMAT is associated with increased FM levels. Conversely, FM% was strongly and inversely correlated with FFM% (r = −0.92, p < 0.001) and BCM% (r = −0.89, p < 0.001). Regarding muscle strength, FFM (kg) was positively correlated with maximal handgrip strength (r = 0.74, p < 0.001).
Furthermore, VAT showed a positive correlation with central diastolic BP (r = 0.30, p = 0.03), systolic BP (Sys) (r = 0.36, p <0.01), and central systolic BP (c-Sys) (r = 0.37, p < 0.01). Moreover, VAT was strongly correlated with PWV (r = 0.63, p < 0.001).
Moreover, WHR was positively correlated with PWV (r = 0.33, p = 0.01), central diastolic (c-dia) BP (r = 0.62, p < 0.001), systolic BP (r = 0.58, p < 0.001), and c-Sys BP (r = 0.48, p < 0.01).
All correlations are summarized in Figure 5.
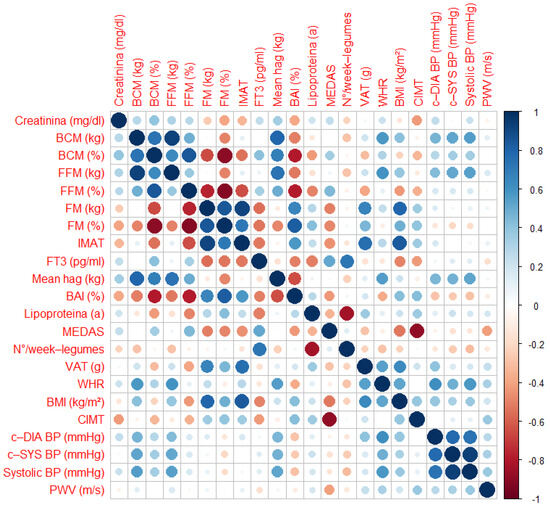
Figure 5.
Matrix correlations. Abbreviations: BAI, Body Adiposity Index; BCM, Body Cell Mass; BMI, Body Mass Index; c-DIA BP, Central Diastolic Blood Pressure; c-SYS BP, Central Systolic Blood Pressure; CIMT, Carotid Intima-Media Thickness; FFM, Fat-Free Mass; FM, Fat Mass; FT3, Triiodothyronine; IMAT, Intramuscular Adipose Tissue; MEDAS, Mediterranean Diet Adherence Screener; PWV, Pulse Wave Velocity; Systolic BP, Systolic Blood Pressure; VAT, Visceral Adipose Tissue; WHR, Waist-to-Height ratio.
4. Discussion
The results of this study provide further evidence on the protective role of adherence to the MedDiet in the prevention of CVD. Numerous epidemiological and clinical studies have shown that high adherence to the MedDiet is associated with a reduction in the incidence of cardiovascular events, including myocardial infarction and stroke [45,46,47]. This protective effect is attributable to multiple mechanisms, including reducing oxidative stress, modulating inflammation, and improving lipid profiles [48,49]. Indeed, previous and recent studies deeply investigated the connection between the Mediterranean diet and vascular aging [50,51].
In particular, our study’s novelty lies in its focus on a well-characterized Italian sample with a long-standing, stable adherence to the Mediterranean lifestyle. This was objectively confirmed through both the MEDAS and CCF scores. Such dietary stability may offer a more accurate snapshot of the cardiovascular system’s response to long-term lifestyle exposure. We believe that this context enhances the clinical relevance of our findings by suggesting that simple dietary screening tools may help to identify individuals who would benefit from targeted cardiovascular assessments and personalized preventive strategies. Specifically, according to our knowledge, our data showed a significant inverse association between the MEDAS score and cIMT (r = −0.88, p < 0.001), an indicator of subclinical atherosclerosis and an early marker of vascular damage [31]. This result suggests that greater adherence to the MedDiet is associated with decreased progression of subclinical atherosclerosis, strengthening the evidence that a diet rich in foods typical of the Mediterranean model may exert a protective role on vascular health, potentially mediated by reducing systemic inflammatory status and improving lipid metabolism [18,52,53].
While more extensive dietary instruments may capture a broader range of food items, shorter tools, such as ours, have demonstrated adequate validity when tailored to the cultural and dietary context of the population studied. Therefore, we believe that the combination of the culturally adapted FFQ and the MEDAS score provides a robust and pragmatic estimate of dietary habits in our cohort [21,22].
Analysis of dietary habits showed significant differences between the groups divided according to cIMT. Consumption of fresh cheese was significantly higher in the group with cIMT ≤ 0.90 mm than in the group with cIMT > 0.90 mm (p = 0.03), suggesting a possible role of the type of dairy products consumed in modulating cardiovascular risk [54]. Similarly, red meat consumption showed a statistically significant difference between groups (p = 0.03), with higher intake in subjects with cIMT > 0.90 mm, consistent with previous studies that have associated high red meat intake with increased atherosclerotic risk [55,56]. Consumption of sausages and processed meat products was also significantly different between groups (p = 0.04), indicating the potential negative contribution of these foods on vascular health [57,58]. Moreover, EVO consumption was significantly higher in the group with cIMT ≤ 0.90 mm (p < 0.01), supporting the hypothesis that regular intake of EVO is associated with beneficial effects on the cardiovascular system due to its antioxidant and anti-inflammatory properties [59,60,61]. Furthermore, a significant negative correlation was observed between weekly legume consumption and Lp (a) levels (r = −0.83, p < 0.001), suggesting a potential hypolipidemic effect associated with legume intake [62]. Considering that Lp(a) is a known cardiovascular risk factor for which there are currently no specific pharmacological therapies, these data indicate that targeted dietary strategies could be an effective option to reduce atherosclerotic risk through independent and adjuvant mechanisms to conventional pharmacotherapy [63].
Our results are consistent with a study on peritoneal dialysis patients, which demonstrated that soy isoflavones—bioactive compounds found in legumes—significantly reduced Lp(a) levels, supporting the potential lipid-lowering effects of legume components [64].
To the best of our knowledge, our study reports, for the first time, that the mean MEDAS score achieved by patients with cIMT ≤ 0.90 mm was significantly higher than the group with cIMT > 0.90 mm, confirming the protective role of the MedDiet [44].
Remarkably, FM% was significantly higher in the group with cIMT > 0.90 mm (32%) than in the group with cIMT ≤ 0.90 mm (27%), suggesting a potential correlation between the increased adipose component and progressive deterioration of vascular function [65]. This association could reflect the pro-atherogenic role of adipose tissue, which, through production of pro-inflammatory cytokines and insulin resistance, contributes to AS and the progression of atherosclerosis [66,67,68]. Although this difference did not reach statistical significance, the observed trend in the slightly increase of FM (Kg) could indicate a role of the adipose compartment in impaired vascular health. Previous studies have shown that fat accumulation, particularly at the visceral level, is associated with increased cardiovascular risk, mediated by increased chronic inflammatory status and alterations in lipid and glucose metabolism [69,70].
Our data show that increased VAT is associated with higher BP values, both brachial Sys (r = 0.36, p < 0.01) and, especially, central sys-diastolic (r = 0.37, p < 0.01, r = 0.30, p = 0. 03; respectively), considering the latter to be an important early marker of vascular damage [31]. This report emphasizes how fat accumulation in the abdominal region can negatively affect vascular function, contributing to increased BP, consistent with previous studies [71]. In addition, we were the first to demonstrate a strong correlation between VAT and PWV (r = 0.63, p < 0.001), a key indicator of AS. These findings suggest that VAT excess not only increases arterial pressure, but also affects arterial elasticity, leading to increased stiffness and reduced vascular adaptability [72]. While previous studies have highlighted the association between VAT accumulation and elevated BP, data directly linking VAT to arterial stiffness have been lacking [73]. This underscores both the novelty and the clinical relevance of our findings. Similarly, WHR, a simple but effective anthropometric measure, was found to be positively correlated with PWV (r = 0.33, p = 0.01), brachial Sys (r = 0.58, p < 0.001), and central systolic–diastolic (r = 0.48, p < 0.01; r = 0.62, p < 0.001, respectively) BP values.
It is important to emphasize that composite indices of inflammation and cardiovascular risk (C-TOT/C-HDL, C-LDC-L/HDL, and AIP) were found to be more sensitive and predictive than isolated values of C-LDL and TG that were not significant. This underscores the importance of a more integrated assessment of the individual cardiovascular risk profile, which considers not only traditional lipid parameters, but also combined markers such as C-TOT/C-HDL, C-LDC-L/HDL, and AIP [74,75]. Particularly, the most recent 2019 guidelines on dyslipidaemia emphasize that lipid-lowering therapy should not be initiated based solely on absolute cholesterol values, but rather on a comprehensive evaluation of the individual’s overall cardiovascular risk [76].
In addition, the HOMA Index, indicative of insulin resistance, was significantly higher in the group with cIMT > 0.90 mm, highlighting a possible metabolic component associated with increased mid-intimal thickness [77]. Indeed, improving insulin sensitivity before the onset of type 2 diabetes mellitus may help to prevent or slow down the development and progression of atherosclerosis [78].
Regarding cardiac remodeling, LVM is indicated for body surface area (LVM/BSA, p = 0.04), and, especially, when indexing LVM values to an elevated height at 2.7 (LVM/h2.7, p < 0.01), a statistically significant difference was found between the two groups. Our data, in line with data in the literature [79], suggest an initial process of cardiac remodeling in subjects with signs of subclinical atherosclerosis, even in the absence of obvious changes in absolute LVM. Moreover, findings regarding BP and AS highlight trends rather than statistically significant differences, suggesting that while individuals with higher cIMT may have slightly elevated BP and altered vascular parameters, the differences require further investigation with a larger sample size.
Overall, the data obtained support the hypothesis that targeted nutritional interventions based on the adoption of the MedDiet may be an effective strategy for primary prevention of CVD.
Adherence to such a dietary regimen not only improves the lipid and metabolic profile, but also appears to modulate early parameters of subclinical vascular and cardiac damage.
Limitations
However, this study has some limitations, such as the observational nature and the small sample size (n = 55). Therefore, despite the emerging evidence, these limitations do not allow for a definitive causal relationship between MedDiet adherence and cardiovascular risk reduction to be established. Thus, prospective studies on larger cohorts will be needed to confirm the predictive value of the MedDiet in preventing atherosclerosis and reducing long-term cardiovascular risk.
In addition, about one-third of the included subjects (20 of 55) had hypertension under pharmacological treatment. Although all were at the target pressor according to guidelines, the presence of hypertension, even if controlled, may have influenced some vascular and cardiac markers (e.g., cIMT, PWV, LVM), introducing a potential bias in subclinical cardiovascular risk assessment.
It should also be considered that anti-hypertensive therapy might have protective effects on vascular and cardiac remodeling, partially masking any differences between the groups.
Furthermore, the discovery of initial signs of organ damage, despite the anti-hypertensive treatment, suggests the usefulness of a broader and earlier risk assessment based on integrated parameters and a multidisciplinary approach. It also reinforces the idea that early and integrated indicators are critical to intercept residual risk, even in already treated patients.
Since moderate changes in lifestyle, including diet, may influence cardiovascular risk over time, we selected participants who reported stable dietary patterns, not only over the previous year, but also at the time of evaluation. Nevertheless, the potential influence of unreported or subtle changes in lifestyle cannot be entirely ruled out, which remains a limitation of the study.
Moreover, physical activity, although not assessed in our study, may act synergistically with a healthy diet to enhance cardiovascular benefits. Conversely, in individuals with poor dietary habits, regular physical activity might partially compensate for some of the associated metabolic risks. The absence of physical activity data therefore limits our ability to fully interpret the interaction between lifestyle factors and cardiovascular outcomes.
Longitudinal studies will be needed to confirm these findings and assess the evolution of cardiovascular damage over time.
5. Conclusions
This study confirms the protective role of adherence to the MedDiet in the prevention of CVD through multiple mechanisms. Higher MedDiet adherence, reflected by a higher MEDAS score, was significantly associated with lower cIMT, suggesting a beneficial effect in preventing subclinical atherosclerosis. Regular consumption of key MedDiet foods, such as extra virgin olive oil, legumes, and fish, was linked to better body composition and reductions in cardiovascular risk factors, partiularly Lp(a).
Additionally, higher VAT accumulation was associated with increased AS and BP, reinforcing the importance of fat distribution and lipid metabolism in CVD progression. The analysis of cardiac function revealed early signs of LV remodeling in individuals with increased cIMT.
These findings highlight the importance of early monitoring of cardiovascular and metabolic parameters and support the implementation of personalized nutritional strategies based on the MedDiet. Further large-scale studies are needed to confirm these associations and explore the potential of nutrigenomic approaches in optimizing CVD prevention. Overall, promoting the MedDiet, combined with comprehensive cardiovascular risk assessment, may represent an effective strategy to reduce the burden of CVD.
Author Contributions
Conceptualization, L.D.R., P.G. and G.F.; methodology, L.D.R., and P.G.; validation, P.G. and L.D.R.; formal analysis, B.P.; investigation, G.T. and G.L.P.; resources, L.D.R.; data curation, P.G., B.P. and G.F.; writing—original draft preparation, B.P. and G.F.; writing—review and editing, P.G. and G.T.; visualization, G.F., P.G. and B.P.; supervision, L.D.R.; project administration, L.D.R.; funding acquisition, L.D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health, Project T5-AN-08, n259, 3 February 2023.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Calabria Region Central Area Section (protocol no. 97, 20 April 2023).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
This published article includes all of the data generated or analyzed during this study.
Acknowledgments
The authors thank all of the patients who participated as volunteers in the study. The authors also thank Ivan Moreno Nestor Torres for data analysis.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AI@75 | Augmentation index normalized to a heart rate of 75 beats per minute |
| AIP | Atherogenic Index of Plasma |
| APOE | Apolipoprotein E |
| AS | Arterial Stiffness |
| BAI | Body Adiposity Index |
| BCM | Body Cell Mass |
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| BSA | Body Surface Area |
| c-Dia BP | Central Diastolic Blood Pressure |
| C-HDL | High-Density Lipoprotein Cholesterol |
| cIMT | Carotid Intima-Media Thickness |
| C-LDL | Low-Density Lipoprotein Cholesterol |
| c-Sys BP | Central Systolic Blood Pressure |
| C-TOT | Total Cholesterol |
| CVD | Cardiovascular Diseases |
| DXA | Dual-Energy X-Ray Absorptiometry |
| EVO | Extra Virgin Olive Oil |
| FFM | Fat-Free Mass |
| FFQ | Food Frequency Questionnaire |
| FM | Fat Mass |
| FT3 | Free triiodiotironine |
| GOT | Glutamic Oxalo-Acetic Transaminase |
| GPT | Glutamate Pyruvate Transaminase |
| HDL | High-Density Lipoprotein |
| HR | Heart Rate |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| IMAT | Intramuscular Adipose Tissue |
| IVRT | Isovolumic Relaxation Time |
| IVSd | Inter-Ventricular Septum Thickness |
| LA | Left Atrial |
| LDL | Low-Density Lipoprotein |
| LM | Lean Mass |
| Lp(a) | Lipoprotein (a) |
| LV | Left Ventricular |
| LVH | Left Ventricle Hypertrophy |
| LVM | Left Ventricle Mass |
| LVM/BSA | Left Ventricle Mass/Body surface area |
| LVM/h2.7 | Left Ventricle Mass/height2.7 |
| MEDAS | Mediterranean Diet Adherence Screener |
| MedDiet | Mediterranean Diet |
| PhA | Phase Angle |
| PWTd | Posterior Wall Thickness |
| PWV | Pulse Wave Velocity |
| SAT | Subcutaneous Adipose Tissue |
| SD | Standard Deviation |
| Sys | Systolic Blood Pressure |
| TBB | Total Body Bone Mass |
| TBW | Total Body Water |
| TDI | Tissue Doppler Imaging |
| TSA | Supra-Aortic Trunks |
| VAT | Visceral Adipose Tissue |
| WHR | Waist-To-Hip Ratio |
Appendix A
Appendix A.1
The MEDAS questionnaire is reported in Table A1.

Table A1.
MEDAS.
Table A1.
MEDAS.
| Questions | Yes = 1/No = 0 |
|---|---|
| 1. Is olive oil the main culinary fat used? | |
| 2. Are four tablespoons of olive oil used each day? | |
| 3. Are two servings (of 200 g each) of vegetables eaten each day? | |
| 4. Are three servings of fruit (of 80 g each) eaten each day? | |
| 5. Is <1 serving (100–150 g) of red meat/hamburgers/other meat products eaten each day? | |
| 6. Was <1 serving (12 g) of butter, margarine, or cream eaten each day? | |
| 7. Is <1 serving (330 mL) of sweet or sugar-sweetened carbonated beverages consumed each day? | |
| 8. Are three glasses (of 125 mL) of wine consumed each week? | |
| 9. Are three servings (of 150 g) of legumes consumed each week? | |
| 10. Are three servings of fish (100–150 g) or seafood (200 g) eaten each week? | |
| 11. Is <3 servings of commercial sweets/pastries eaten each week? | |
| 12. Is one serving (of 30 g) of nuts consumed each week? | |
| 13. Is chicken, turkey, or rabbit routinely eaten instead of veal, pork, hamburger, or sausage? | |
| 14. Are pasta, vegetable, or rice dishes flavored with garlic, tomato, leek, or onion eaten ≥twice a week? |
Appendix A.2
In Table A2 all of the BIA, DXA, blood test, and cardiovascular parameters of the sample are reported.

Table A2.
Sample Parameters.
Table A2.
Sample Parameters.
| n Sample = 55 | |
|---|---|
| FM% (Siri) | 28.00 ± 9.00 |
| BAI (%) | 28.60 ± 5.50 |
| Mean handgrip max strenght (Kg) | 35.00 ± 11.00 |
| PhA (°) | 5.89 ± 0.88 |
| Z (ohm) | 532.00 ± 92.00 |
| FM (kg) | 23.00 ± 9.00 |
| FM (%) | 29.00 ± 9.00 |
| FFM (kg) | 55.00 ± 13.00 |
| FFM (%) | 70.00 ± 10.00 |
| BCM (kg) | 30.00 ± 8.00 |
| BCM (%) | 39.00 ± 7.00 |
| BCMI (kg/m2) | 10.50 ± 2.18 |
| Total Fat (g) | 22.17 ± 9.77 |
| Total Lean (g) | 51.56 ± 11.41 |
| FM % | 28.00 ± 11.00 |
| IMAT | 0.91 ± 0.45 |
| ASMMI | 8.03 ± 1.24 |
| VAT/SAT | 0.34 ± 0.17 |
| HGB (g/dL) | 13.79 ± 1.18 |
| HTC (%) | 41.55 ± 2.69 |
| Glycemia (mg/dL) | 93.00 ± 13.00 |
| Insulin (uUI/mL) | 8.42 ± 2.12 |
| C-TOT (mg/dL) | 213.00 ± 43.00 |
| C-HDL (mg/dL) | 61.00 ± 22.00 |
| C-LDL (mg/dL) | 131.00 ± 33.00 |
| TG (mg/dL) | 115.00 ± 52.00 |
| GOT (U/lt) | 28.00 ± 19.00 |
| GPT (U/lt) | 26.00 ± 14.00 |
| Azotemia (mg/dL) | 27.00 ± 10.00 |
| Creatinine (mg/dL) | 0.83 ± 0.12 |
| Uric Acid (mg/dL) | 4.97 ± 1.23 |
| Lipoprotein(a) | 43.00 ± 38.00 |
| TSH microUI/mL | 2.17 ± 1.00 |
| ft3/ft4 | 2.71 ± 1.26 |
| PTL | 308.17 ± 363.55 |
| Lymphocytes | 2.08 ± 464.00 |
| Neutrophilis | 3.32 ± 905.00 |
| Homa-Index | 1.63 ± 4.62 |
| TyG | 8.47 ± 0.50 |
| tg/hdl | 2.40 ± 1.97 |
| C-TOT/C-HDL | 4.03 ± 1.96 |
| C-LDL/C-HDL | 2.47 ± 1.58 |
| AIP | 0.26 ± 0.32 |
| PLR | 148.00 ± 150.00 |
| NLR | 1.66 ± 0.51 |
| Systolic BP (mmHg) | 130.00 ± 16.00 |
| Diastolic BP(mmHg) | 84.00 ± 11.00 |
| PAM | 105.00 ± 12.00 |
| Pulse Pressure (mmHg) | 47.00 ± 11.00 |
| Heart Rate (1/min) | 68.00 ± 10.00 |
| cSis (mmHg) | 120.00 ± 21.00 |
| cDia (mmHg) | 85.00 ± 11.00 |
| Peripheral Resistance (Dyn × s/cm5) | 1.81 ± 236.00 |
| AI@75 | 26.00 ± 11.00 |
| PWV (m/s) | 7.87 ± 1.02 |
| cIMT | 0.83 ± 0.16 |
Abbreviations: ASMMI, Appendicular Skeletal Muscle Mass Index; BAI, Body Adiposity Index; BCM, Body Cell Mass; BCMI, Body Cell Mass Index; cIMT, common carotid artery intima-media thickness; FFM, Fat-Free Mass; FM, Fat Mass; FT3/FT4, Triiodothyronine free/thyroxine free, HGB, Hemoglobin; HTC (%): Hematocrit; Glycemia (mg/dL): Blood Glucose; Insulin (uUI/mL): Insulin; C-TOT (mg/dL): Total Cholesterol; C-HDL (mg/dL): High-Density Lipoprotein Cholesterol; C-LDL (mg/dL): Low-Density Lipoprotein Cholesterol; TG (mg/dL): Triglycerides; GOT (U/L): Glutamate Oxaloacetate Transaminase (AST); GPT (U/L): Glutamate Pyruvate Transaminase (ALT); Azotemia (mg/dL): Blood Urea Nitrogen; Creatinine (mg/dL): Serum Creatinine; Uric Acid (mg/dL): Uric Acid; TSH micro UI/mL: Thyroid Stimulating Hormone; fT3/fT4: Free Triiodothyronine / Free Thyroxine; PTL: Platelet Count; Lymphocytes: Lymphocyte Count; Neutrophils: Neutrophil Count; HOMA-Index: Homeostatic Model Assessment of Insulin Resistance; TyG: Triglyceride-Glucose Index; tg/hdl: Triglycerides to HDL Cholesterol Ratio; Ctot/hdl: Total Cholesterol to HDL Cholesterol Ratio; LDL/HDL: LDL Cholesterol to HDL Cholesterol Ratio; AIP: Atherogenic Index of Plasma; PLR: Platelet-to-Lymphocyte Ratio; NLR: Neutrophil-to-Lymphocyte Ratio; Systolic BP (mmHg): Systolic Blood Pressure; Diastolic BP (mmHg): Diastolic Blood Pressure; PAM: Mean Arterial Pressure; Pulse Pressure (mmHg): Pulse Pressure; Heart Rate (1/min): Heart Rate; cSis (mmHg): Central Systolic Blood Pressure; cDia (mmHg): Central Diastolic Blood Pressure; Peripheral Resistance (Dyn·s/cm⁵): Systemic Vascular Resistance; AI@75: Augmentation Index PWV (m/s): Pulse Wave Velocity; IMAT, Intramuscular Adipose Tissue; PhA, Phase Angle; VAT/SAT, Visceral Adipose Tissue-Subcutaneous Adipose Tissue; Z, Impedance.
References
- Tattersall, M.C.; Hansen, S.L.; McClelland, R.L.; Korcarz, C.E.; Hansen, K.M.; Post, W.S.; Shapiro, M.D.; Stein, J.H. Importance of Age and Sex in Carotid Artery Plaque Detection and Cardiovascular Disease Risk. JAMA Cardiol. 2025, 10, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, V.; Suero-Abreu, G.A.; Neilan, T.G. Immune Checkpoint Inhibitors and Myocardial Infarction. J. Thromb. Thrombolysis 2025, 83, 102334. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.; Koenig, W. Inflammation in Atherosclerotic Cardiovascular Disease: From Diagnosis to Treatment. Eur. J. Clin. Investig. 2025, e70020. [Google Scholar] [CrossRef] [PubMed]
- Muiesan, M.L.; Virdis, A.; Tocci, G.; Borghi, C.; Cicero, A.F.G.; Ferri, C.; Pirro, M.; Corsini, A.; Volpe, M. 2024 Consensus Document of the Italian Society of Arterial Hypertension (SIIA) and the Italian Society of Cardiovascular Prevention (SIPREC): Update on LDL Cholesterol Lowering in Patients with Arterial Hypertension. High Blood Press. Cardiovasc. Prev. 2025, 32, 151–163. [Google Scholar] [CrossRef]
- Pala, B.; Tocci, G.; Nardoianni, G.; Barbato, E.; Amedei, A. Gut Microbiome and Carotid Artery Intima-Media Thickness: A Narrative Review of the Current Scenario. Diagnostics 2024, 14, 2463. [Google Scholar] [CrossRef]
- Tocci, G.; Presta, V. Increased Arterial Stiffness and Haemorrhagic Transformation in Ischaemic Stroke after Thrombolysis: A New Marker of Risk for Cerebrovascular Events and Complications. Int. J. Cardiol. 2017, 243, 471–472. [Google Scholar] [CrossRef]
- Wang, M.; McGraw, K.R.; Monticone, R.E.; Pintus, G. Unraveling Elastic Fiber-Derived Signaling in Arterial Aging and Related Arterial Diseases. Biomolecules 2025, 15, 153. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial Fat Links Obesity to Cardiovascular Diseases. Prog. Cardiovasc. Dis. 2023, 78, 27–33. [Google Scholar] [CrossRef]
- Ortega-Loubon, C.; Fernández-Molina, M.; Singh, G.; Correa, R. Obesity and Its Cardiovascular Effects. Diabetes Metab. Res. 2019, 35, e3135. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Antoniades, C. The Interplay between Adipose Tissue and the Cardiovascular System: Is Fat Always Bad? Cardiovasc. Res. 2017, 113, 999–1008. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Antoniades, C. The Role of Adipose Tissue in Cardiovascular Health and Disease. Nat. Rev. Cardiol. 2019, 16, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Frank, G.; Cianci, R.; Caldarelli, M.; Leggeri, G.; Raffaelli, G.; Pizzocaro, E.; Cirillo, M.; De Lorenzo, A. Exploring the Exposome Spectrum: Unveiling Endogenous and Exogenous Factors in Non-Communicable Chronic Diseases. Diseases 2024, 12, 176. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Frank, G.; De Lorenzo, A. Nutrition for Prevention and Control of Chronic Degenerative Diseases and COVID-19. Nutrients 2023, 15, 2253. [Google Scholar] [CrossRef]
- Marchetti, M.; Gualtieri, P.; De Lorenzo, A.; Trombetta, D.; Smeriglio, A.; Ingegneri, M.; Cianci, R.; Frank, G.; Schifano, G.; Bigioni, G.; et al. Dietary ω-3 Intake for the Treatment of Morning Headache: A Randomized Controlled Trial. Front. Neurol. 2022, 13, 987958. [Google Scholar] [CrossRef]
- Nestel, P.J.; Mori, T.A. Dietary Patterns, Dietary Nutrients and Cardiovascular Disease. Rev. Cardiovasc. Med. 2022, 23, 17. [Google Scholar] [CrossRef]
- Fukumoto, Y. Nutrition and Cardiovascular Diseases. Nutrients 2021, 14, 94. [Google Scholar] [CrossRef]
- Jimenez-Torres, J.; Alcalá-Diaz, J.F.; Torres-Peña, J.D.; Gutierrez-Mariscal, F.M.; Leon-Acuña, A.; Gómez-Luna, P.; Fernández-Gandara, C.; Quintana-Navarro, G.M.; Fernandez-Garcia, J.C.; Perez-Martinez, P.; et al. Mediterranean Diet Reduces Atherosclerosis Progression in Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. Stroke 2021, 52, 3440–3449. [Google Scholar] [CrossRef]
- Gualtieri, P.; Marchetti, M.; Frank, G.; Cianci, R.; Bigioni, G.; Colica, C.; Soldati, L.; Moia, A.; De Lorenzo, A.; Di Renzo, L. Exploring the Sustainable Benefits of Adherence to the Mediterranean Diet during the COVID-19 Pandemic in Italy. Nutrients 2022, 15, 110. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Frank, G.; De Santis, G.L.; Cianci, R.; Bigioni, G.; De Lorenzo, A. Sex Differences in the Efficacy of Mediterranean Diet Treatment: A Nutrigenomics Pilot Study. Genes 2023, 14, 1980. [Google Scholar] [CrossRef]
- Buscemi, S.; Rosafio, G.; Vasto, S.; Massenti, F.M.; Grosso, G.; Galvano, F.; Rini, N.; Barile, A.M.; Maniaci, V.; Cosentino, L.; et al. Validation of a Food Frequency Questionnaire for Use in Italian Adults Living in Sicily. Int. J. Food Sci. Nutr. 2015, 66, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Frank, G.; Cianci, R.; Raffaelli, G.; Peluso, D.; Bigioni, G.; De Lorenzo, A. Sex-Specific Adherence to the Mediterranean Diet in Obese Individuals. Nutrients 2024, 16, 3076. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Itani, L.; El Ghoch, M.; Gualtieri, P.; Frank, G.; Raffaelli, G.; Pellegrini, M.; Di Renzo, L. Difference in Body Composition Patterns between Age Groups in Italian Individuals with Overweight and Obesity: When BMI Becomes a Misleading Tool in Nutritional Settings. Nutrients 2024, 16, 2415. [Google Scholar] [CrossRef]
- Haun, D.R.; Pitanga, F.J.G.; Lessa, I. Razão Cintura/Estatura Comparado a Outros Indicadores Antropométricos de Obesidade Como Preditor de Risco Coronariano Elevado. Rev. Assoc. Med. Bras. 2009, 55, 705–711. [Google Scholar] [CrossRef]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A Better Index of Body Adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef]
- Di Renzo, L.; Marchetti, M.; Cioccoloni, G.; Gratteri, S.; Capria, G.; Romano, L.; Soldati, L.; Mele, M.C.; Merra, G.; Cintoni, M.; et al. Role of Phase Angle in the Evaluation of Effect of an Immuno-Enhanced Formula in Post-Surgical Cancer Patients: A Randomized Clinical Trial. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1322–1334. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Sasso, G.F.; Andreoli, A.; Sorge, R.; Candeloro, N.; Cairella, M. Improved Prediction Formula for Total Body Water Assessment in Obese Women. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 535–538. [Google Scholar]
- De Lorenzo, A.; Itani, L.; El Ghoch, M.; Frank, G.; De Santis, G.L.; Gualtieri, P.; Di Renzo, L. The Association between Sarcopenic Obesity and DXA-Derived Visceral Adipose Tissue (VAT) in Adults. Nutrients 2024, 16, 1645. [Google Scholar] [CrossRef]
- Bauer, J.; Thornton, J.; Heymsfield, S.; Kelly, K.; Ramirez, A.; Gidwani, S.; Gallagher, D. Dual-Energy X-Ray Absorptiometry Prediction of Adipose Tissue Depots in Children and Adolescents. Pediatr. Res. 2012, 72, 420–425. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- Hametner, B.; Wassertheurer, S.; Kropf, J.; Mayer, C.; Eber, B.; Weber, T. Oscillometric Estimation of Aortic Pulse Wave Velocity: Comparison with Intra-Aortic Catheter Measurements. Blood Press Monit 2013, 18, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.W.; Singh, B.M. Augmentation Index as a Measure of Peripheral Vascular Disease State. Curr. Opin. Cardiol. 2002, 17, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.M.G.; Mota-Gomes, M.A.; Brandão, A.A.; Silveira, F.S.; Silveira, M.S.; Okawa, R.T.P.; Feitosa, A.D.M.; Sposito, A.C.; Nadruz, W. Reference Values of Office Central Blood Pressure, Pulse Wave Velocity, and Augmentation Index Recorded by Means of the Mobil-O-Graph PWA Monitor. Hypertens. Res. 2020, 43, 1239–1248. [Google Scholar] [CrossRef]
- Lancellotti, P.; Galderisi, M.; Edvardsen, T.; Donal, E.; Goliasch, G.; Cardim, N.; Magne, J.; Laginha, S.; Hagendorff, A.; Haland, T.F.; et al. Echo-Doppler Estimation of Left Ventricular Filling Pressure: Results of the Multicentre EACVI Euro-Filling Study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 961–968. [Google Scholar] [CrossRef]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart. J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Perrone-Filardi, P.; Coca, A.; Galderisi, M.; Paolillo, S.; Alpendurada, F.; de Simone, G.; Donal, E.; Kahan, T.; Mancia, G.; Redon, J.; et al. Non-Invasive Cardiovascular Imaging for Evaluating Subclinical Target Organ Damage in Hypertensive Patients: A Consensus Paper from the European Association of Cardiovascular Imaging (EACVI), the European Society of Cardiology Council on Hypertension, and the European Society of Hypertension (ESH). Eur. Heart J. Cardiovasc. Imaging 2017, 18, 945–960. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Wu, T.-T.; Gao, Y.; Zheng, Y.-Y.; Ma, Y.-T.; Xie, X. Atherogenic Index of Plasma (AIP): A Novel Predictive Indicator for the Coronary Artery Disease in Postmenopausal Women. Lipids Health Dis. 2018, 17, 197. [Google Scholar] [CrossRef]
- Greco, A.; Finocchiaro, S.; Spagnolo, M.; Faro, D.C.; Mauro, M.S.; Raffo, C.; Sangiorgio, G.; Imbesi, A.; Laudani, C.; Mazzone, P.M.; et al. Lipoprotein(a) as a Pharmacological Target: Premises, Promises, and Prospects. Circulation 2025, 151, 400–415. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Molani-Gol, R.; Rafraf, M. Effects of the Mediterranean Diet on the Secondary Prevention of Cardiovascular Diseases: A Systematic Review of Randomised Controlled Trials. Int. J. Food Sci. Nutr. 2025, 76, 226–238. [Google Scholar] [CrossRef]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-Style Diet for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean Diet and Cardiovascular Health: Teachings of the PREDIMED Study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Dietary Patterns, Mediterranean Diet, and Cardiovascular Disease. Curr. Opin. Lipidol. 2014, 25, 20–26. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Ser. A 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Almevall, A.D.; Wennberg, P.; Liv, P.; Nyman, E.; Lindvall, K.; Norberg, M.; Chorell, E.; Wennberg, M. Midlife Mediterranean Diet is Associated with Subclinical Carotid Atherosclerosis in Late Midlife. Eur J Prev Cardiol. 2025, zwaf155. [Google Scholar] [CrossRef]
- Gómez Sánchez, M.; Gómez Sánchez, L.; Patino-Alonso, M.C.; Alonso-Domínguez, R.; Sánchez-Aguadero, N.; Lugones-Sánchez, C.; Rodríguez Sánchez, E.; García Ortiz, L.; Gómez-Marcos, M.A. Adherence to the Mediterranean Diet in Spanish Population and Its Relationship with Early Vascular Aging according to Sex and Age: EVA Study. Nutrients 2020, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Deng, Y.; Ma, Y.; Man, S.; Yang, X.; Yu, C.; Lv, J.; Liu, H.; Wang, B.; Li, L. Adherence to a Healthy Diet and Risk of Multiple Carotid Atherosclerosis Subtypes: Insights from the China MJ Health Check-Up Cohort. Nutrients 2024, 16, 2338. [Google Scholar] [CrossRef] [PubMed]
- Rychter, A.M.; Naskręt, D.; Zawada, A.; Ratajczak, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. What Can We Change in Diet and Behaviour in Order to Decrease Carotid Intima-Media Thickness in Patients with Obesity? J. Pers. Med. 2021, 11, 505. [Google Scholar] [CrossRef]
- Ivey, K.L.; Lewis, J.R.; Hodgson, J.M.; Zhu, K.; Dhaliwal, S.S.; Thompson, P.L.; Prince, R.L. Association between Yogurt, Milk, and Cheese Consumption and Common Carotid Artery Intima-Media Thickness and Cardiovascular Disease Risk Factors in Elderly Women. Am. J. Clin. Nutr. 2011, 94, 234–239. [Google Scholar] [CrossRef]
- Haring, B.; Wang, W.; Fretts, A.; Shimbo, D.; Lee, E.T.; Howard, B.V.; Roman, M.J.; Devereux, R.B. Red Meat Consumption and Cardiovascular Target Organ Damage (from the Strong Heart Study). J. Hypertens. 2017, 35, 1794–1800. [Google Scholar] [CrossRef]
- Wang, D.; Karvonen-Gutierrez, C.A.; Jackson, E.A.; Elliott, M.R.; Appelhans, B.M.; Barinas-Mitchell, E.; Bielak, L.F.; Huang, M.-H.; Baylin, A. Western Dietary Pattern Derived by Multiple Statistical Methods Is Prospectively Associated with Subclinical Carotid Atherosclerosis in Midlife Women. J. Nutr. 2020, 150, 579–591. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Bradbury, K.E.; Sweeting, M.; Wood, A.; Johansson, I.; Kühn, T.; Steur, M.; Weiderpass, E.; Wennberg, M.; et al. Consumption of Meat, Fish, Dairy Products, and Eggs and Risk of Ischemic Heart Disease: A Prospective Study of 7198 Incident Cases Among 409 885 Participants in the Pan-European EPIC Cohort. Circulation 2019, 139, 2835–2845. [Google Scholar] [CrossRef]
- Tzelefa, V.; Tsirimiagkou, C.; Argyris, A.; Moschonis, G.; Perogiannakis, G.; Yannakoulia, M.; Sfikakis, P.; Protogerou, A.D.; Karatzi, K. Associations of Dietary Patterns with Blood Pressure and Markers of Subclinical Arterial Damage in Adults with Risk Factors for CVD. Public Health Nutr. 2021, 24, 6075–6084. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; De Sancti, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-Inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2017, 18, 36–50. [Google Scholar] [CrossRef]
- Wongwarawipat, T.; Papageorgiou, N.; Bertsias, D.; Siasos, G.; Tousoulis, D. Olive Oil-Related Anti-Inflammatory Effects on Atherosclerosis: Potential Clinical Implications. Endocr. Metab. Immune Disord. Drug Targets 2017, 18, 51–62. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr. Metab. Immune Disord. Drug Targets 2017, 18, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Comperatore, M.; Barona, J.; Calle, M.C.; Andersen, C.; McIntosh, M.; Najm, W.; Lerman, R.H.; Fernandez, M.L. A Mediterranean-Style, Low-Glycemic-Load Diet Decreases Atherogenic Lipoproteins and Reduces Lipoprotein (a) and Oxidized Low-Density Lipoprotein in Women with Metabolic Syndrome. Metabolism 2012, 61, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Lampsas, S.; Xenou, M.; Oikonomou, E.; Pantelidis, P.; Lysandrou, A.; Sarantos, S.; Goliopoulou, A.; Kalogeras, K.; Tsigkou, V.; Kalpis, A.; et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules 2023, 28, 969. [Google Scholar] [CrossRef] [PubMed]
- Yari, Z.; Tabibi, H.; Najafi, I.; Hedayati, M.; Movahedian, M. Effects of soy isoflavones on serum lipids and lipoprotein (a) in peritoneal dialysis patients. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1382–1388. [Google Scholar] [CrossRef]
- Nikoohemmat, M.; Ahmadi, A.R.; Valizadeh, A.; Moteshakereh, S.M.; Yari-Boroujeni, R.; Seifi, Z.; Valizadeh, M.; Abiri, B. Association between Body Composition Indices and Vascular Health: A Systematic Review and Meta-Analysis. Eat Weight Disord. 2025, 30, 3. [Google Scholar] [CrossRef]
- Kang, P.S.; Neeland, I.J. Body Fat Distribution, Diabetes Mellitus, and Cardiovascular Disease: An Update. Curr. Cardiol. Rep. 2023, 25, 1555–1564. [Google Scholar] [CrossRef]
- Kubra Ata Ozturk, H.; Zeybek, V.; Kurtulus Dereli, A.; Acar, K.; Dogu Kılıc, I.; Tekin, O.; Akca, A. The Relationships between Anthropometric Measurements, Organ Weights and Intracranial, Carotid and Coronary Atherosclerosis. BMC Cardiovasc. Disord. 2025, 25, 155. [Google Scholar] [CrossRef]
- Lear, S.A.; Sarna, L.K.; Siow, T.J.; Mancini, G.B.J.; Siow, Y.L.; Karmin, O. Oxidative Stress Is Associated with Visceral Adipose Tissue and Subclinical Atherosclerosis in a Healthy Multi-Ethnic Population. Appl. Physiol. Nutr. Metab. 2012, 37, 1164–1170. [Google Scholar] [CrossRef]
- Chartrand, D.J.; Murphy-Després, A.; Alméras, N.; Lemieux, I.; Larose, E.; Després, J.-P. Overweight, Obesity, and CVD Risk: A Focus on Visceral/Ectopic Fat. Curr. Atheroscler. Rep. 2022, 24, 185–195. [Google Scholar] [CrossRef]
- Hyun, Y.J.; Koh, S.J.; Chae, J.S.; Kim, J.Y.; Kim, O.Y.; Lim, H.H.; Jang, Y.; Park, S.; Ordovas, J.M.; Lee, J.H. Atherogenecity of LDL and Unfavorable Adipokine Profile in Metabolically Obese, Normal-weight Woman. Obesity 2008, 16, 784–789. [Google Scholar] [CrossRef]
- Alanazi, M.A.; Alshehri, K.; Alerwy, F.H.; Alrasheed, T.; Lahza, H.F.M.; Aref Albezrah, N.K.; Alghabban, Y.I.; Mohammed Abdulghani, M.A. Abdominal Volume Index Is Associated with Higher Oxidized LDL, High Blood Pressure and Lower HDL among Obese Adults. BMC Endocr. Disord. 2025, 25, 56. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-T.; Rui, Y.-F.; Fan, L.; Yan, Z.-N. Echocardiographic Association of Epicardial Adipose Tissue with Ascending Aorta Elasticity in Patients with Type 2 Diabetes Mellitus. Angiology 2023, 74, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Covassin, N.; Sert-Kuniyoshi, F.H.; Singh, P.; Romero-Corral, A.; Davison, D.E.; Lopez-Jimenez, F.; Jensen, M.D.; Somers, V.K. Experimental Weight Gain Increases Ambulatory Blood Pressure in Healthy Subjects: Implications of Visceral Fat Accumulation. Mayo Clin. Proc. 2018, 93, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, X.; Lo, K.; Huang, Y.; Feng, Y. The Effect of Total Cholesterol/High-Density Lipoprotein Cholesterol Ratio on Mortality Risk in the General Population. Front. Endocrinol. 2022, 13, 1012383. [Google Scholar] [CrossRef]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P.; et al. Lipoprotein Ratios: Physiological Significance and Clinical Usefulness in Cardiovascular Prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar]
- Zeitouni, M.; Sabouret, P.; Kerneis, M.; Silvain, J.; Collet, J.-P.; Bruckert, E.; Montalescot, G. 2019 ESC/EAS Guidelines for Management of Dyslipidaemia: Strengths and Limitations. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 324–333. [Google Scholar] [CrossRef]
- González-González, J.G.; Violante-Cumpa, J.R.; Zambrano-Lucio, M.; Burciaga-Jimenez, E.; Castillo-Morales, P.L.; Garcia-Campa, M.; Solis, R.C.; González-Colmenero, A.D.; Rodríguez-Gutiérrez, R. HOMA-IR as a Predictor of Health Outcomes in Patients with Metabolic Risk Factors: A Systematic Review and Meta-Analysis. High Blood Press. Cardiovasc. Prev. 2022, 29, 547–564. [Google Scholar] [CrossRef]
- Scott, D.A.; Ponir, C.; Shapiro, M.D.; Chevli, P.A. Associations between Insulin Resistance Indices and Subclinical Atherosclerosis: A Contemporary Review. Am. J. Prev. Cardiol. 2024, 18, 100676. [Google Scholar] [CrossRef]
- Armstrong, A.C.; Gidding, S.; Gjesdal, O.; Wu, C.; Bluemke, D.A.; Lima, J.A.C. LV Mass Assessed by Echocardiography and CMR, Cardiovascular Outcomes, and Medical Practice. JACC Cardiovasc. Imaging 2012, 5, 837–848. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).