Beyond the Average: Trends in Extreme Sodium Intake in the U.S. Population, 2003–2018
Abstract
1. Introduction
2. Data and Methods
2.1. Dietary Sodium Assessment
2.2. Sample Selection and Exclusions
2.3. Self-Reported Health Conditions Records
2.4. Statistical Analysis
2.5. Modeling Sodium Intake with Individual Level Data
2.6. Modeling Sodium Intake with Population Level Data
2.7. Sensitivity Analysis
2.8. Programming Software Used
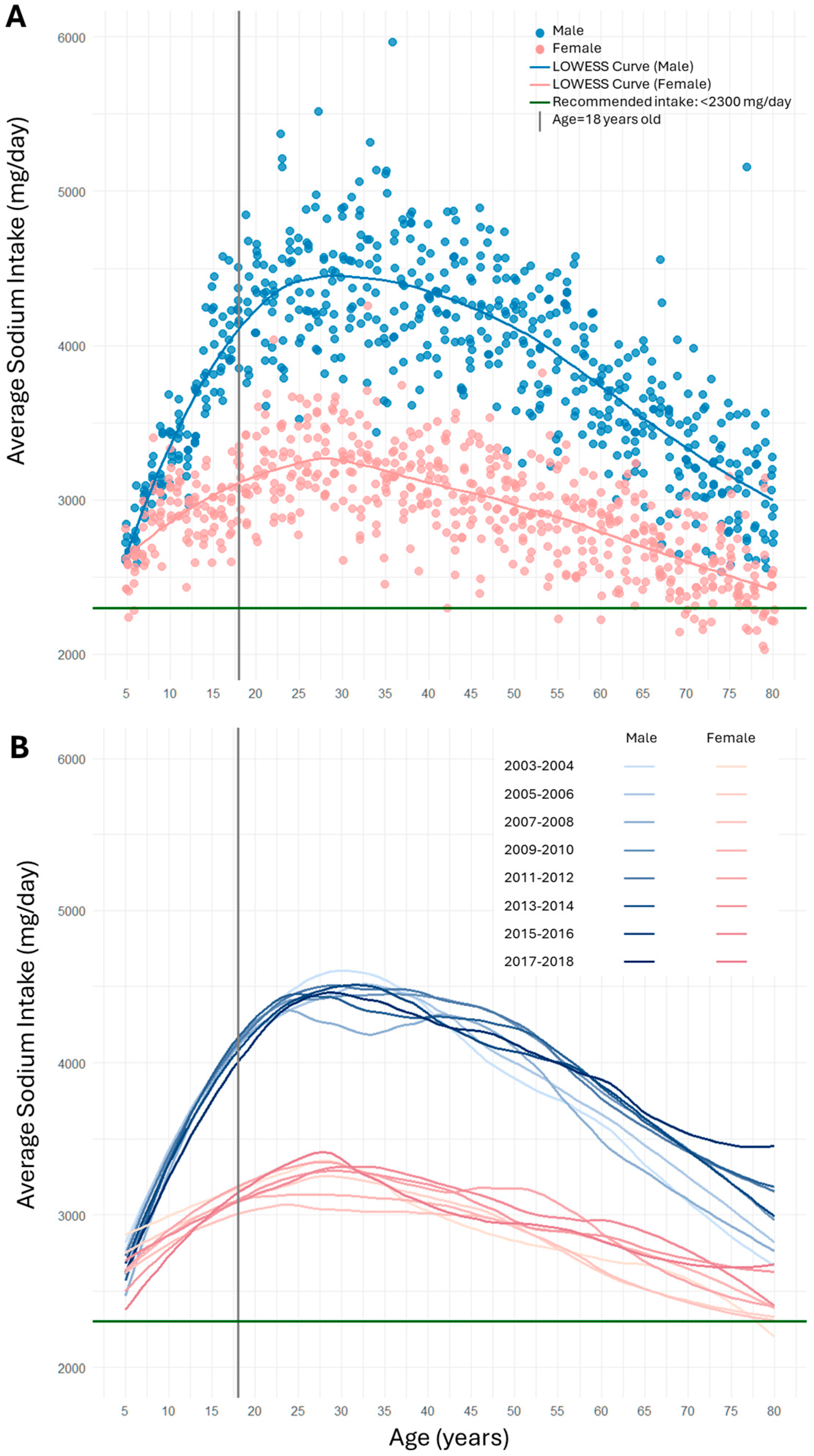
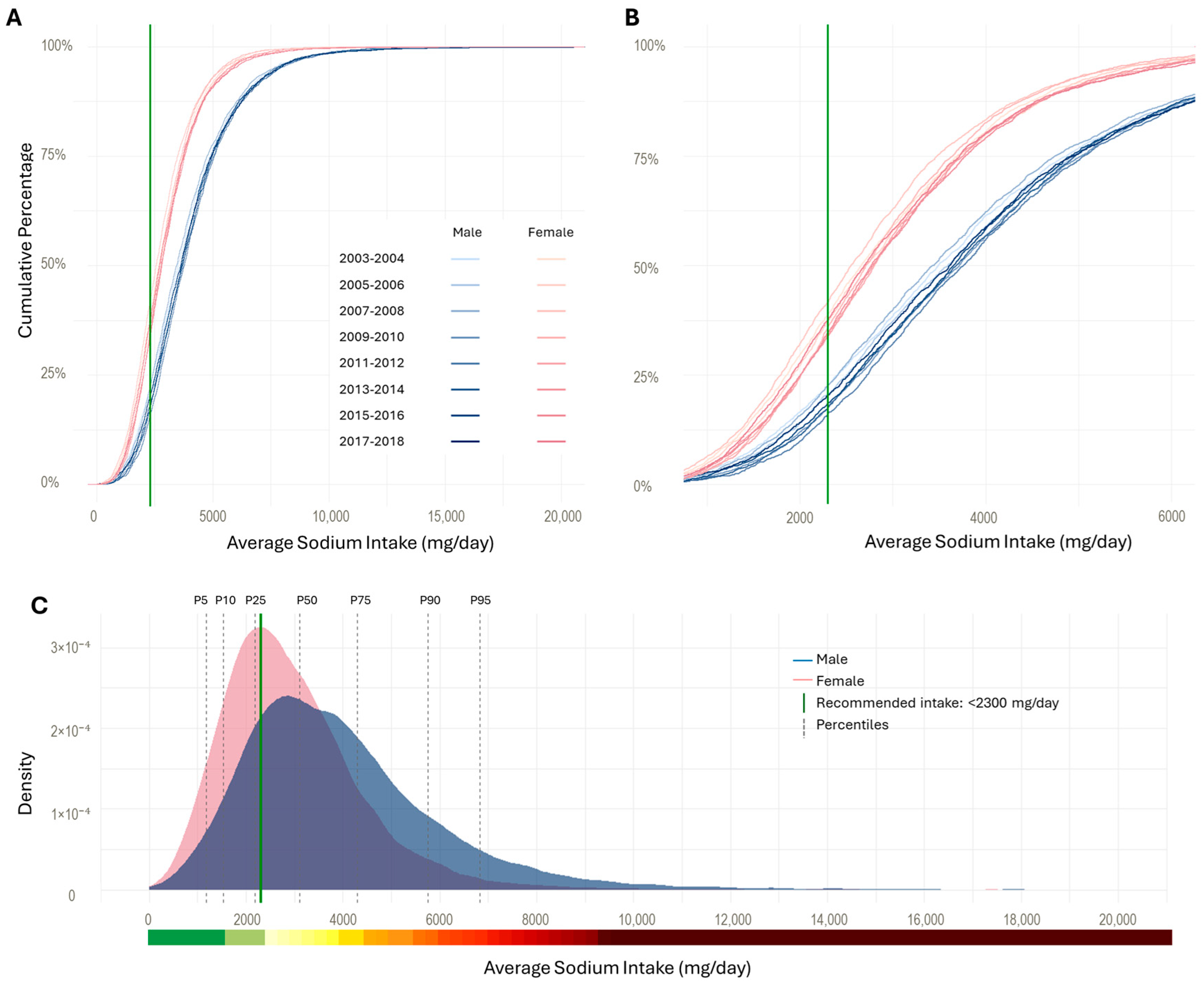
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K.; Teo, K.K.; Rangarajan, S.; Islam, S.; Gupta, R.; Avezum, A.; Bahonar, A.; Chifamba, J.; Dagenais, G.; Diaz, R.; et al. Prevalence, Awareness, Treatment, and Control of Hypertension in Rural and Urban Communities in High-, Middle-, and Low-Income Countries. JAMA 2013, 310, 959–968. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar]
- Stamler, J. The INTERSALT Study: Background, methods, findings, and implications. Am. J. Clin. Nutr. 1997, 65 (Suppl. S2), 626s–642s. [Google Scholar] [CrossRef]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sodium Reduction; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 20 May 2025).
- World Health Organization. The SHAKE Technical Package for Salt Reduction; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Thout, S.R.; Santos, J.A.; McKenzie, B.; Trieu, K.; Johnson, C.; McLean, R.; Arcand, J.; Campbell, N.R.C.; Webster, J. The Science of Salt: Updating the evidence on global estimates of salt intake. J. Clin. Hypertens. 2019, 21, 710–721. [Google Scholar] [CrossRef]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Marklund, M.; Henry, M.E.; Appel, L.J.; Croft, K.D.; Neal, B.; Wu, J.H.Y. A Systematic Review of the Sources of Dietary Salt Around the World. Adv. Nutr. 2020, 11, 677–686. [Google Scholar] [CrossRef]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Sodium in Your Diet; US Food and Drug Administration: Silver Spring, MD, USA, 2021.
- Dietary Guidelines for Americans, 2020–2025. U.S. Department of Agriculture and U.S. Department of Health and Human Services. 2020. Available online: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials (accessed on 20 May 2025).
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 2014, 129, 981–989. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Vaudin, A.; Wambogo, E.; Moshfegh, A.J.; Sahyoun, N.R. Sodium and Potassium Intake, the Sodium to Potassium Ratio, and Associated Characteristics in Older Adults, NHANES 2011–2016. J. Acad. Nutr. Diet. 2022, 122, 64–77. [Google Scholar] [CrossRef]
- Zhang, Z.; Cogswell, M.E.; Gillespie, C.; Fang, J.; Loustalot, F.; Dai, S.; Carriquiry, A.L.; Kuklina, E.V.; Hong, Y.; Merritt, R.; et al. Association between usual sodium and potassium intake and blood pressure and hypertension among U.S. adults: NHANES 2005-2010. PLoS ONE 2013, 8, e75289. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. About NHANES. Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2024. Available online: https://www.cdc.gov/nchs/nhanes/about/ (accessed on 15 November 2024).
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Data; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2025.
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes1. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). NHANES Interviewers Procedures Manual; Centers for Disease Control and Prevention (CDC): Atlanta, GR, USA, 2018.
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National health and nutrition examination survey: Plan and operations, 1999–2010. In Vital and Health Statisctics; U.S. Department of Health and Human Services: Hyattsville, MD, USA, 2013. [Google Scholar]
- Tran, K.M.; Johnson, R.K.; Soultanakis, R.P.; Matthews, D.E. In-person vs. Telephone-administered Multiple-pass 24-hour Recalls in Women: Validation with Doubly Labeled Water. J. Am. Diet. Assoc. 2000, 100, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Brassard, D.; Laramée, C.; Robitaille, J.; Lemieux, S.; Lamarche, B. Differences in Population-Based Dietary Intake Estimates Obtained From an Interviewer-Administered and a Self-Administered Web-Based 24-h Recall. Front. Nutr. 2020, 7, 2020. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA. Available online: https://wwwn.cdc.gov/nchs/nhanes/default.aspx (accessed on 20 May 2025).
- Jacoby, W.G. Loess:: A nonparametric, graphical tool for depicting relationships between variables. Elect. Stud. 2000, 19, 577–613. [Google Scholar] [CrossRef]
- National Center for Health Statistics. Analytic Note Regarding 2007–2010 Survey Design Changes and Combining Data Across Other Survey Cycles; Centers for Disease Control and Prevention (CDC): Atlanta, GR, USA, 2011.
- Centers for Disease Control and Prevention (CDC). Weighting; Centers for Disease Control and Prevention (CDC): Atlanta, GR, USA. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/weighting.aspx (accessed on 15 November 2024).
- Calvani, R.; Picca, A.; Coelho-Júnior, H.J.; Tosato, M.; Marzetti, E.; Landi, F. Diet for the prevention and management of sarcopenia. Metabolism 2023, 146, 155637. [Google Scholar] [CrossRef]
- Newberry, S.J.; Chung, M.; Anderson, C.A.; Chen, C.; Fu, Z.; Tang, A.; Zhao, N.; Booth, M.; Marks, J.; Hollands, S.; et al. Sodium and Potassium Intake: Effects on Chronic Disease Outcomes and Risks [Internet]; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018.
- O’Donnell, M.J.; Yusuf, S.; Mente, A.; Gao, P.; Mann, J.F.; Teo, K.; McQueen, M.; Sleight, P.; Sharma, A.M.; Dans, A.; et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 2011, 306, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Pfister, R.; Michels, G.; Sharp, S.J.; Luben, R.; Wareham, N.J.; Khaw, K.T. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur. J. Heart Fail. 2014, 16, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Olde Engberink, R.H.G.; van den Hoek, T.C.; van Noordenne, N.D.; van den Born, B.H.; Peters-Sengers, H.; Vogt, L. Use of a Single Baseline Versus Multiyear 24-Hour Urine Collection for Estimation of Long-Term Sodium Intake and Associated Cardiovascular and Renal Risk. Circulation 2017, 136, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-S.; Cai, Q.; Yang, J.J.; Lipworth, L.; Cai, H.; Yu, D.; Steinwandel, M.D.; Gupta, D.K.; Blot, W.J.; Zheng, W.; et al. Sodium Intake and Cause-Specific Mortality Among Predominantly Low-Income Black and White US Residents. JAMA Netw. Open 2024, 7, e243802. [Google Scholar] [CrossRef]
- Clarke, L.S.; Overwyk, K.; Bates, M.; Park, S.; Gillespie, C.; Cogswell, M.E. Temporal Trends in Dietary Sodium Intake Among Adults Aged >/=19 Years—United States, 2003–2016. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1478–1482. [Google Scholar] [CrossRef]
- Aubakirova, M.; Sultanov, M.; Izimov, A.; Sakko, Y.; Bex, T.; Mussagazin, A.; Alibekova, R. Factors Influencing Salt-Reducing Behavior in Young Adults: A Pilot Cross-Sectional Study from Kazakhstan. Cent. Asian J. Glob. Health 2020, 9, e415. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Gu, D.; Huang, J.-F.; Cao, J.; Chen, J.-C.; Li, J.; Lu, F.; Mu, J.; Ma, J.; Hu, D.; et al. Physical activity reduces salt sensitivity of blood pressure: The Genetic Epidemiology Network of Salt Sensitivity Study. Am. J. Epidemiol. 2012, 176 (Suppl. S7), S106–S113. [Google Scholar] [CrossRef]
- Veniamakis, E.; Kaplanis, G.; Voulgaris, P.; Nikolaidis, P.T. Effects of Sodium Intake on Health and Performance in Endurance and Ultra-Endurance Sports. Int. J. Environ. Res. Public Health 2022, 19, 3651. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Food and Nutrition Board. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Institute of Medicine (US) Food and Nutrition Board. Dietary Reference Intakes. A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- NHANES 1999–2000; Addendum to the NHANES III Analytic Guidelines. U.S. Department of Health and Human Services Centers for Disease Control and Prevention: Atlanta, GR, USA, 2002.
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Johnson, C.L. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002, 288, 1723–1727. [Google Scholar] [CrossRef]
- Volkert, D.; Kreuel, K.; Heseker, H.; Stehle, P. Energy and nutrient intake of young-old, old-old and very-old elderly in Germany. Eur. J. Clin. Nutr. 2004, 58, 1190–1200. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Sodium Intake Reduction, 1st ed.; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- de Mestral, C.; Mayén, A.L.; Petrovic, D.; Marques-Vidal, P.; Bochud, M.; Stringhini, S. Socioeconomic Determinants of Sodium Intake in Adult Populations of High-Income Countries: A Systematic Review and Meta-Analysis. Am. J. Public Health 2017, 107, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Contreras Navarro, A.; Gallagher, K.; Griffin, S.; Leydon, C.L.; Perry, I.J.; Harrington, J.M. Systematic Review on the Impact of Salt-Reduction Initiatives by Socioeconomic Position to Address Health Inequalities in Adult Populations. Nutr. Rev. 2025, 83, e1090–e1100. [Google Scholar] [CrossRef]
- McLaren, L.; Heidinger, S.; Dutton, D.J.; Tarasuk, V.; Campbell, N.R. A repeated cross-sectional study of socio-economic inequities in dietary sodium consumption among Canadian adults: Implications for national sodium reduction strategies. Int. J. Equity Health 2014, 13, 44. [Google Scholar] [CrossRef][Green Version]
- French, S.A.; Tangney, C.C.; Crane, M.M.; Wang, Y.; Appelhans, B.M. Nutrition quality of food purchases varies by household income: The SHoPPER study. BMC Public Health 2019, 19, 231. [Google Scholar] [CrossRef]
- Leung, C.W.; Fulay, A.P.; Parnarouskis, L.; Martinez-Steele, E.; Gearhardt, A.N.; Wolfson, J.A. Food insecurity and ultra-processed food consumption: The modifying role of participation in the Supplemental Nutrition Assistance Program (SNAP). Am. J. Clin. Nutr. 2022, 116, 197–205. [Google Scholar] [CrossRef]
- Sawyer, A.D.M.; van Lenthe, F.; Kamphuis, C.B.M.; Terragni, L.; Roos, G.; Poelman, M.P.; Nicolaou, M.; Waterlander, W.; Djojosoeparto, S.K.; Scheidmeir, M.; et al. Dynamics of the complex food environment underlying dietary intake in low-income groups: A systems map of associations extracted from a systematic umbrella literature review. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.A.; Tekle, D.; Rosewarne, E.; Flexner, N.; Cobb, L.; Al-Jawaldeh, A.; Kim, W.J.; Breda, J.; Whiting, S.; Campbell, N.; et al. A Systematic Review of Salt Reduction Initiatives Around the World: A Midterm Evaluation of Progress Towards the 2025 Global Non-Communicable Diseases Salt Reduction Target. Adv. Nutr. 2021, 12, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, B.; Naumova, E.N. How well global dietary intake estimates agree: A case of sodium consumption. J. Public Health Policy 2024, 45, 205–211. [Google Scholar] [CrossRef]
- Roberto, C.A.; Lawman, H.G.; LeVasseur, M.T.; Mitra, N.; Peterhans, A.; Herring, B.; Bleich, S.N. Association of a Beverage Tax on Sugar-Sweetened and Artificially Sweetened Beverages With Changes in Beverage Prices and Sales at Chain Retailers in a Large Urban Setting. JAMA 2019, 321, 1799–1810. [Google Scholar] [CrossRef]
- Kaplan, S.; White, J.S.; Madsen, K.A.; Basu, S.; Villas-Boas, S.B.; Schillinger, D. Evaluation of Changes in Prices and Purchases Following Implementation of Sugar-Sweetened Beverage Taxes Across the US. JAMA Health Forum 2024, 5, e234737. [Google Scholar] [CrossRef]
- Falbe, J.; Thompson, H.R.; Becker, C.M.; Rojas, N.; McCulloch, C.E.; Madsen, K.A. Impact of the Berkeley Excise Tax on Sugar-Sweetened Beverage Consumption. Am. J. Public Health 2016, 106, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Leonberg, K.E.; Maski, M.R.; Scott, T.M.; Chen, Y.; Zhou, B.; Naumova, E.N. Trends in chronic kidney disease and calories from ultra-processed foods: NHANES at the highly granular level. Discov. Public Health 2025, 22, 169. [Google Scholar] [CrossRef]
- Gibson, R.S.; Charrondiere, U.R.; Bell, W. Measurement Errors in Dietary Assessment Using Self-Reported 24-Hour Recalls in Low-Income Countries and Strategies for Their Prevention. Adv. Nutr. 2017, 8, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Va, P.; Dodd, K.W.; Zhao, L.; Thompson-Paul, A.M.; Mercado, C.I.; Terry, A.L.; Jackson, S.L.; Wang, C.-Y.; Loria, C.M.; Moshfegh, A.J.; et al. Evaluation of measurement error in 24-hour dietary recall for assessing sodium and potassium intake among US adults—National Health and Nutrition Examination Survey (NHANES), 2014. Am. J. Clin. Nutr. 2019, 109, 1672–1682. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, Z.; May, M.; Tsoi, K.K.-F.; Ingle, S.; Lee, E.K.-P.; Wong, S.Y.-S.; Kim, J.H. The role of body mass index in the association between dietary sodium intake and blood pressure: A mediation analysis with NHANES. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3335–3344. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Z.; Kuklina, E.V.; Fang, J.; Ayala, C.; Hong, Y.; Loustalot, F.F.; Dai, S.; Gunn, J.P.; Tian, N.; et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics 2012, 130, 611–619. [Google Scholar] [CrossRef]
- Ming, L.; Wang, D.; Zhu, Y. Association of sodium intake with diabetes in adults without hypertension: Evidence from the National Health and Nutrition Examination Survey 2009–2018. Front. Public Health 2023, 11, 1118364. [Google Scholar] [CrossRef]
- Jaques, D.A.; Wuerzner, G.; Ponte, B. Sodium Intake as a Cardiovascular Risk Factor: A Narrative Review. Nutrients 2021, 13, 3177. [Google Scholar] [CrossRef]
- He, J.; Ogden, L.G.; Vupputuri, S.; Bazzano, L.A.; Loria, C.; Whelton, P.K. Dietary Sodium Intake and Subsequent Risk of Cardiovascular Disease in Overweight Adults. JAMA 1999, 282, 2027–2034. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Baissa, D.K.; Rainey, C. When BLUE is not best: Non-normal errors and the linear model. Political Sci. Res. Methods 2020, 8, 136–148. [Google Scholar] [CrossRef]
- Carriquiry, A.; Moshfegh, A.J.; Steinfeldt, L.C.; Cogswell, M.E.; Loustalot, F.; Zhang, Z.; Yang, Q.; Tian, N. Trends in the prevalence of excess dietary sodium intake—United States, 2003–2010. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 1021–1025. [Google Scholar]
- Brouillard, A.M.; Kraja, A.T.; Rich, M.W. Trends in Dietary Sodium Intake in the United States and the Impact of USDA Guidelines: NHANES 1999–2016. Am. J. Med. 2019, 132, 1199–1206.e5. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). NHANES August 2021–August 2023. Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?Cycle=2021-2023 (accessed on 10 April 2025).
- Centers for Disease Control and Prevention (CDC). Brief Overview of Sample Design, Nonresponse Bias Assessment, and Analytic Guidelines for NHANES August 2021–August 2023. 2024. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overviewbrief.aspx?Cycle=2021-2023 (accessed on 10 April 2025).
- Chen, Y.; Zhou, B.; Naumova, E.N. Exploring global dietary data gaps in relationship to nutrition inequity: A case of sodium intake. In The Landscape of Global Health Inequity; Zhou, B., Chen, Y., Naumova, E.N., Eds.; Springer: Cham, Switzerland, 2024; pp. 105–128. [Google Scholar]
- Zhou, B.; Chen, Y.; Naumova, E.N. Revealing health disparities with visual analytics in the digital era: From individual attributes to global patterns in nutrition surveillance. In The Landscape of Global Health Inequity; Zhou, B., Chen, Y., Naumova, E.N., Eds.; Springer: Cham, Switzerland, 2024; pp. 129–149. [Google Scholar]

| Condition | Sign | Interpretation: Local Behavior and Global Pattern |
|---|---|---|
| The quadratic term dominates over time: b2x2 > b1x for large x, or equivalently x > b1/b2 | b1 > 0 (increasing linear trend) b2 > 0 (positive curvature) | IA—increase with acceleration (fast incline): the slope increases as x increases, resulting in a steepening upward trend over the observed range. |
| The linear term dominates for low values of x: b1x > ∣b2∣x2 or x ≤ b1/∣b2∣ | b1 > 0 (increasing linear trend) b2 < 0 (negative curvature) | ID—increase with deceleration (slow incline): the slope increases but at a decreasing rate as x increases; The Rise-then-fall patterns: this may appear as a rise-then-fall (∩-shaped) curve if a turning point exists within the observed x-range, or as a gradually flattening upward trend, otherwise. |
| The linear term dominates for low values of x: ∣b1∣x > b2x2, or x ≤ ∣b1∣/b2 | b1 < 0 (decreasing linear trend) b2 > 0 (positive curvature) | DD—decrease with deceleration (slow decline): the slope decreases as x increases, but the rate of decline slows down; The fall-then-rise patterns: this may appear as a fall-then-rise (∪ shape) if a turning point exists within the observed range, or as a flattening downward trend. |
| The quadratic term reinforces the decline: ∣b2∣x2 > ∣b1∣x, or x > ∣b1∣/∣b2∣ | b1 < 0 (decreasing linear trend) b2 < 0 (negative curvature) | DA—decrease with acceleration (fast decline): the slope decreases as x increases, resulting in a downward trend over the observed range. |
| Statistics a | Percentiles and Extremes b | Shape c | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | N | Mean | SD | Range | Min | P05 | P10 | P25 | P50 | P75 | P90 | P95 | Max | Skew | Kurt |
| Children (5–17) | 18,194 | 3157 * | 1601 | 20,325 | 0 | 1190 | 1496 | 2085 | 2861 | 3887 | 5143 | 6135 | 20,325 | 1.62 | 5.50 |
| Adults (18 and older) | 42,469 | 3442 | 1849 | 25,949 | 0 | 1172 | 1521 | 2177 | 3104 | 4300 | 5757 | 6833 | 25,949 | 1.64 | 5.87 |
| Boys | 9198 | 3438 * | 1749 | 20,325 | 0 | 1318 | 1633 | 2257 | 3100 | 4219 | 5644 | 6734 | 20,325 | 1.60 | 5.22 |
| Male adults | 20,637 | 3962 | 2046 | 25,949 | 0 | 1397 | 1804 | 2549 | 3617 | 4940 | 6537 | 7753 | 25,949 | 1.47 | 4.70 |
| Girls | 8996 | 2870 * | 1375 | 15,976 | 0 | 1110 | 1390 | 1940 | 2645 | 3527 | 4580 | 5397 | 15,976 | 1.42 | 4.33 |
| Female adults | 21,832 | 2950 | 1482 | 21,004 | 0 | 1052 | 1360 | 1936 | 2713 | 3688 | 4758 | 5618 | 21,004 | 1.62 | 7.35 |
| Hypertension (Without) d | 28,095 | 3550 | 1899 | 25,949 | 0 | 1207 | 1574 | 2255 | 3206 | 4417 | 5929 | 7048 | 25,949 | 1.64 | 5.92 |
| Hypertension (With) | 14,374 | 3230 * | 1728 | 20,999 | 5 | 1123 | 1440 | 2045 | 2899 | 4047 | 5377 | 6433 | 21,004 | 1.60 | 5.48 |
| Heart disease (Without) | 40,792 | 3457 | 1860 | 25,949 | 0 | 1176 | 1524 | 2187 | 3116 | 4318 | 5785 | 6867 | 25,949 | 1.64 | 5.88 |
| Heart disease (With) | 1677 | 3060 * | 1504 | 11,474 | 5 | 1096 | 1412 | 2004 | 2814 | 3846 | 4990 | 5948 | 11,479 | 1.10 | 1.98 |
| Heart attack (Without) | 40,716 | 3459 | 1854 | 25,949 | 0 | 1182 | 1532 | 2192 | 3117 | 4319 | 5782 | 6866 | 25,949 | 1.64 | 5.87 |
| Heart attack (With) | 1753 | 3039 * | 1675 | 15,720 | 5 | 984 | 1280 | 1893 | 2723 | 3819 | 5118 | 6090 | 15,725 | 1.65 | 5.79 |
| Stroke (Without) | 40,913 | 3463 | 1856 | 25,949 | 0 | 1185 | 1533 | 2193 | 3124 | 4321 | 5788 | 6864 | 25,949 | 1.64 | 5.90 |
| Stroke (With) | 1556 | 2873 * | 1551 | 12,786 | 75 | 926 | 1249 | 1811 | 2582 | 3547 | 4905 | 5737 | 12,861 | 1.38 | 3.12 |
| Having no condition | 27,113 | 3568 | 1906 | 25,949 | 0 | 1215 | 1585 | 2265 | 3223 | 4438 | 5953 | 7083 | 25,949 | 1.64 | 5.90 |
| Having ≥ 1 condition | 15,356 | 3219 * | 1722 | 20,999 | 5 | 1116 | 1436 | 2042 | 2890 | 4027 | 5356 | 6404 | 21,004 | 1.61 | 5.54 |
| Group | Temporal Trend Across Cycle ‡ | Age-Related Effect | ||
|---|---|---|---|---|
| Pattern | Turning Point (in Cycle) | Pattern | Turning Point (in Years) | |
| Children (5–17) | No | ID Increase | † | |
| Adults (18 and older) | ID Slow incline | 6.80 | ID-∩ | 23.14 |
| Boys | No | IA Fast increase | † | |
| Male adults | ID Slow incline | 5.42 | ID-∩ | 26.60 |
| Girls | DD Fast decline | † | ID Slow increase | 12.65 |
| Female adults | No | ID Decline | † | |
| Hypertension (Without) | ID Slow incline | 6.36 | ID-∩ Rise-then-fall | 23.53 |
| Hypertension (With) | ID Slow incline | 7.05 | ID Decline | † |
| Heart disease (Without) | ID Slow incline | 6.62 | ID-∩ Rise-then-fall | 24.05 |
| Heart disease (With) | No | No | ||
| Heart attack (Without) | ID Slow incline | 6.36 | ID-∩ Rise-then-fall | 23.59 |
| Heart attack (With) | No | No | ||
| Stroke (Without) | ID Slow incline | 6.78 | ID-∩ Rise-then-fall | 23.66 |
| Stroke (With) | No | No | ||
| Having no condition | ID Slow incline | 5.99 | ID-∩ Rise-then-fall | 24.27 |
| Having ≥1 condition | ID Slow incline | 7.81 | ID Slow decrease | † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, J.; Leonberg, K.E.; Chui, K.K.H.; Ausman, L.M.; Naumova, E.N. Beyond the Average: Trends in Extreme Sodium Intake in the U.S. Population, 2003–2018. Nutrients 2025, 17, 1975. https://doi.org/10.3390/nu17121975
Chen Y, Wang J, Leonberg KE, Chui KKH, Ausman LM, Naumova EN. Beyond the Average: Trends in Extreme Sodium Intake in the U.S. Population, 2003–2018. Nutrients. 2025; 17(12):1975. https://doi.org/10.3390/nu17121975
Chicago/Turabian StyleChen, Yutong, Jingyan Wang, Kristin E. Leonberg, Kenneth Kwan Ho Chui, Lynne M. Ausman, and Elena N. Naumova. 2025. "Beyond the Average: Trends in Extreme Sodium Intake in the U.S. Population, 2003–2018" Nutrients 17, no. 12: 1975. https://doi.org/10.3390/nu17121975
APA StyleChen, Y., Wang, J., Leonberg, K. E., Chui, K. K. H., Ausman, L. M., & Naumova, E. N. (2025). Beyond the Average: Trends in Extreme Sodium Intake in the U.S. Population, 2003–2018. Nutrients, 17(12), 1975. https://doi.org/10.3390/nu17121975






