Effects of Intermittent Fasting and Calorie Restriction on Exercise Performance: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Trial Registration
2.2. Search Strategy
2.3. Study Selection and Inclusion Criteria
2.4. Quality Assessment and Sensitivity Analyses
2.5. Statistical Analysis
3. Results
3.1. Included Studies
3.2. Participant Characteristics
3.3. Intervention Characteristics
3.4. Meta-Analysis
3.4.1. The Effects of IF and CR Combined with Exercise Training on Exercise Performance
VO2max
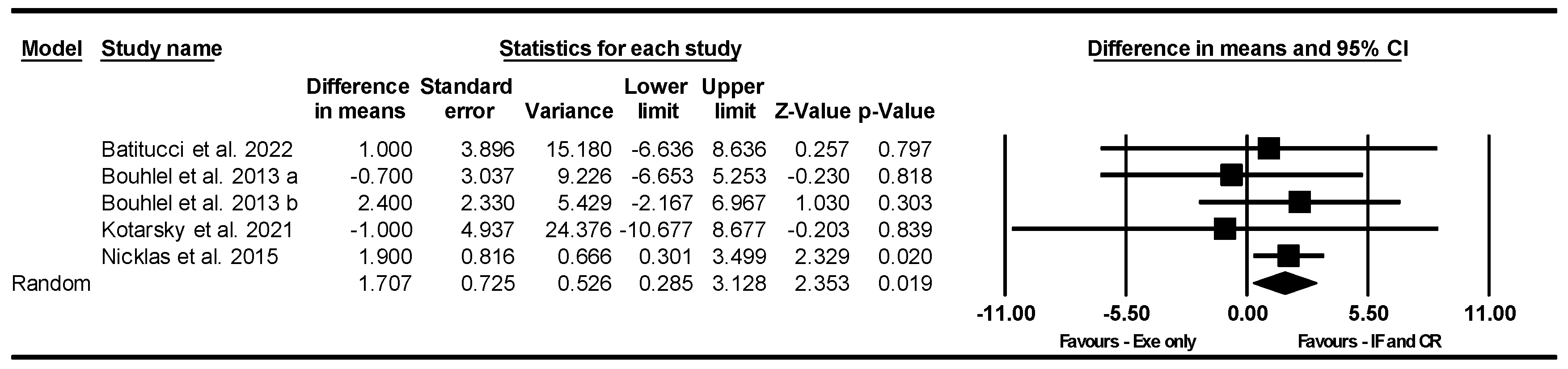
Handgrip Strength
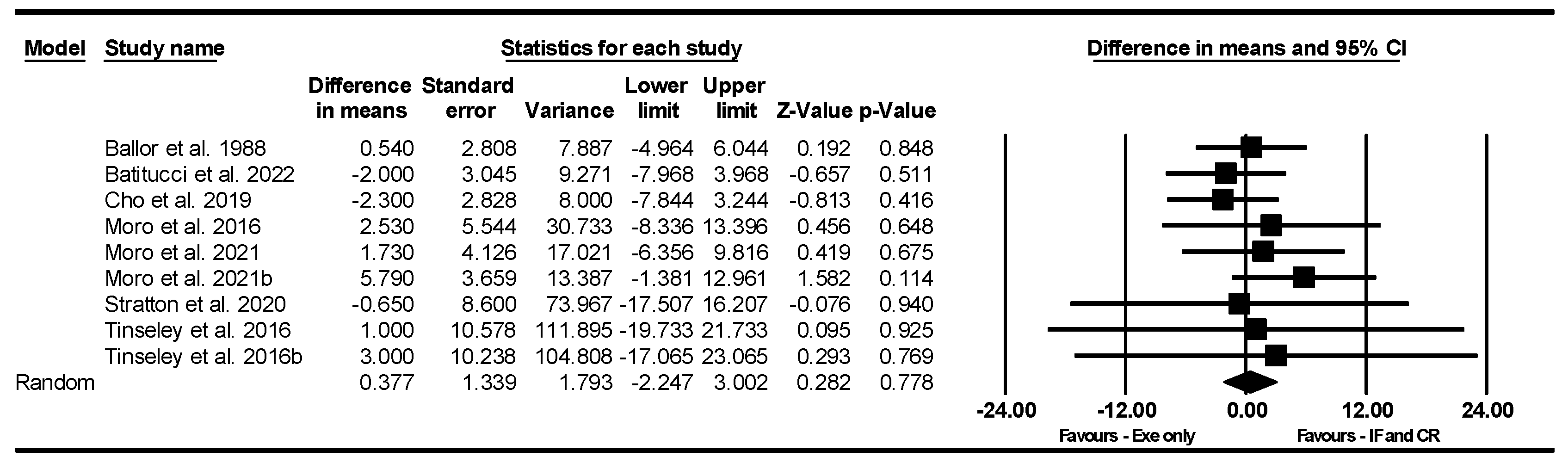
Bench Press Strength
Knee Extensor Strength
Leg Press Strength
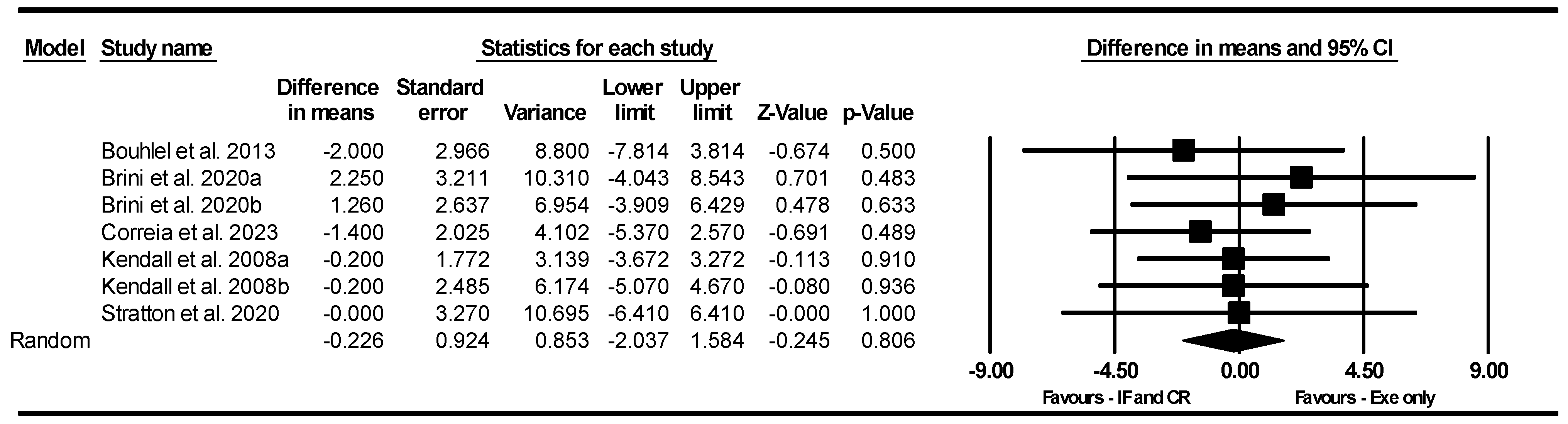
CMJ
The 400 m Walk Test
Gait Speed
3.4.2. The Effects of IF and CR Combined with Exercise Training on Body Weight and Body Composition
Body Weight
BMI
FFM
FM
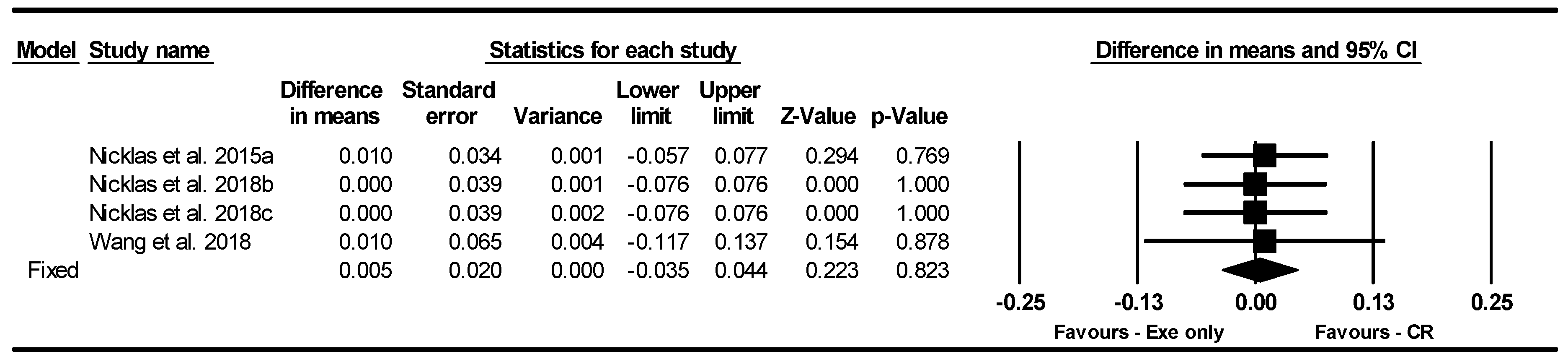
BFP
3.5. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santos, H.O. Intermittent fasting and fat mass: What is the clinical magnitude? Obesities 2022, 2, 1–7. [Google Scholar] [CrossRef]
- Conde-Pipó, J.; Mora-Fernandez, A.; Martinez-Bebia, M.; Gimenez-Blasi, N.; Lopez-Moro, A.; Latorre, J.A.; Almendros-Ruiz, A.; Requena, B.; Mariscal-Arcas, M. Intermittent fasting: Does it affect sports performance? A systematic review. Nutrients 2024, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Cioffi, I.; Evangelista, A.; Ponzo, V.; Goitre, I.; Ciccone, G.; Ghigo, E.; Bo, S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 17–33. [Google Scholar] [CrossRef]
- Park, J.; Seo, Y.-G.; Paek, Y.-J.; Song, H.J.; Park, K.H.; Noh, H.-M. Effect of alternate-day fasting on obesity and cardiometabolic risk: A systematic review and meta-analysis. Metabolism 2020, 111, 154336. [Google Scholar] [CrossRef]
- Elortegui Pascual, P.; Rolands, M.R.; Eldridge, A.L.; Kassis, A.; Mainardi, F.; Lê, K.A.; Karagounis, L.G.; Gut, P.; Varady, K.A. A Meta-Analysis comparing the effectiveness of alternate day fasting, the 5, 2 diet, and Time-Restricted eating for weight loss. Obesity 2023, 31, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Bagheri, R.; Triki, R.; Saeidi, A.; Wong, A.; Hackney, A.C.; Laher, I.; Suzuki, K.; Ben Abderrahman, A. Effects of Ramadan intermittent fasting on gut hormones and body composition in males with obesity. Int. J. Environ. Res. Public Health 2020, 17, 5600. [Google Scholar] [CrossRef]
- Santos, H.O.; Genario, R.; Tinsley, G.M.; Ribeiro, P.; Carteri, R.B.; de Faria Coelho-Ravagnani, C.; Mota, J.F. A scoping review of intermittent fasting, chronobiology, and metabolism. Am. J. Clin. Nutr. 2022, 115, 991–1004. [Google Scholar] [CrossRef]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Bang, E.; Yu, B.P. Impacts of calorie restriction and intermittent fasting on health and diseases: Current trends. Nutrients 2020, 12, 2948. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Tinsley, G.M.; Asbaghi, O.; Paoli, A.; Moro, T. Effects of intermittent fasting combined with resistance training on body composition: A systematic review and meta-analysis. Physiol. Behav. 2021, 237, 113453. [Google Scholar] [CrossRef]
- Levy, E.; Chu, T. Intermittent fasting and its effects on athletic performance: A review. Curr. Sports Med. Rep. 2019, 18, 266–269. [Google Scholar] [CrossRef]
- Correia, J.M.; Santos, I.; Pezarat-Correia, P.; Minderico, C.; Mendonca, G.V. Effects of intermittent fasting on specific exercise performance outcomes: A systematic review including meta-analysis. Nutrients 2020, 12, 1390. [Google Scholar] [CrossRef]
- Grundler, F.; Mesnage, R.; Ruppert, P.M.; Kouretas, D.; Wilhelmi de Toledo, F. Long-Term Fasting-Induced Ketosis in 1610 Subjects: Metabolic Regulation and Safety. Nutrients 2024, 16, 1849. [Google Scholar] [CrossRef]
- Murphy, C.; Koehler, K. Energy deficiency impairs resistance training gains in lean mass but not strength: A meta-analysis and meta-regression. Scand. J. Med. Sci. Sports 2022, 32, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Thompson, L.A.; Niro, P.J.; Margolis, L.M.; McClung, J.P.; Cao, J.J.; Whigham, L.D.; Combs, G.F., Jr.; Young, A.J.; Lieberman, H.R.; et al. Transient decrements in mood during energy deficit are independent of dietary protein-to-carbohydrate ratio. Physiol. Behav. 2015, 139, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, R. The effect of fasting on human metabolism and psychological health. Dis. Markers 2022, 2022, 5653739. [Google Scholar] [CrossRef] [PubMed]
- Abaidia, A.-E.; Daab, W.; Bouzid, M.A. Effects of Ramadan fasting on physical performance: A systematic review with meta-analysis. Sports Med. 2020, 50, 1009–1026. [Google Scholar] [CrossRef]
- BaHammam, A. Assessment of sleep patterns, daytime sleepiness, and chronotype during Ramadan in fasting and nonfasting individuals. Saudi Med. J. 2005, 26, 616–622. [Google Scholar]
- Waterhouse, J.; Alkib, L.; Reilly, T. Effects of Ramadan upon fluid and food intake, fatigue, and physical, mental, and social activities: A comparison between the UK and Libya. Chronobiol. Int. 2008, 25, 697–724. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Sexual dimorphism in glucose and lipid metabolism during fasting, hypoglycemia, and exercise. Front. Endocrinol. 2015, 6, 61. [Google Scholar] [CrossRef]
- Soeters, M.R.; Sauerwein, H.P.; Groener, J.E.; Aerts, J.M.; Ackermans, M.T.; Glatz, J.F.; Fliers, E.; Serlie, M.J. Gender-related differences in the metabolic response to fasting. J. Clin. Endocrinol. Metab. 2007, 92, 3646–3652. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Kolnes, K.J.; Nilsen, E.T.; Brufladt, S.; Meadows, A.M.; Jeppesen, P.B.; Skattebo, Ø.; Johansen, E.I.; Birk, J.B.; Højlund, K.; Hingst, J.; et al. Effects of seven days’ fasting on physical performance and metabolic adaptation during exercise in humans. Nat. Commun. 2025, 16, 122. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2008. [Google Scholar]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Stephenson, M.; Aromataris, E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. JBI Evid. Implement. 2015, 13, 196–207. [Google Scholar] [CrossRef]
- Correia, J.M.; Pezarat-Correia, P.; Minderico, C.; Infante, J.; Mendonca, G.V. Effects of Time-Restricted Eating on Aerobic Capacity, Body Composition, and Markers of Metabolic Health in Healthy Male Recreational Runners: A Randomized Crossover Trial. J. Acad. Nutr. Diet. 2024, 124, 1041–1050. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Brinkley, T.E.; Houston, D.K.; Lyles, M.F.; Hugenschmidt, C.E.; Beavers, K.M.; Leng, X. Effects of Caloric Restriction on Cardiorespiratory Fitness, Fatigue, and Disability Responses to Aerobic Exercise in Older Adults With Obesity: A Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1084–1090. [Google Scholar] [CrossRef]
- Kirkendall, D.T.; Leiper, J.B.; Bartagi, Z.; Dvorak, J.; Zerguini, Y. The influence of Ramadan on physical performance measures in young Muslim footballers. J. Sports Sci. 2008, 26 (Suppl. 3), S15–S27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Leng, X.; Messi, M.L.; Choi, S.J.; Marsh, A.P.; Nicklas, B.; Delbono, O. Relationship of Physical Function to Single Muscle Fiber Contractility in Older Adults: Effects of Resistance Training With and Without Caloric Restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Bouhlel, H.; Shephard, R.J.; Gmada, N.; Aouichaoui, C.; Peres, G.; Tabka, Z.; Bouhlel, E. Effect of Ramadan observance on maximal muscular performance of trained men. Clin. J. Sport Med. 2013, 23, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Longo, G.; Grigoletto, D.; Bianco, A.; Ferraris, C.; Guglielmetti, M.; Veneto, A.; Tagliabue, A.; Marcolin, G.; et al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 65. [Google Scholar] [CrossRef]
- Yassine, H.N.; Marchetti, C.M.; Krishnan, R.K.; Vrobel, T.R.; Gonzalez, F.; Kirwan, J.P. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults—A randomized clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 90–95. [Google Scholar] [CrossRef]
- Christiansen, T.; Paulsen, S.K.; Bruun, J.M.; Ploug, T.; Pedersen, S.B.; Richelsen, B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J. Clin. Endocrinol. Metab. 2010, 95, 911–919. [Google Scholar] [CrossRef]
- Coker, R.H.; Williams, R.H.; Yeo, S.E.; Kortebein, P.M.; Bodenner, D.L.; Kern, P.A.; Evans, W.J. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J. Clin. Endocrinol. Metab. 2009, 94, 4258–4266. [Google Scholar] [CrossRef]
- Cooke, M.B.; Deasy, W.; Ritenis, E.J.; Wilson, R.A.; Stathis, C.G. Effects of Intermittent Energy Restriction Alone and in Combination with Sprint Interval Training on Body Composition and Cardiometabolic Biomarkers in Individuals with Overweight and Obesity. Int. J. Environ. Res. Public Health 2022, 19, 7969. [Google Scholar] [CrossRef]
- Hagan, R.D.; Upton, S.J.; Wong, L.; Whittam, J. The effects of aerobic conditioning and/or caloric restriction in overweight men and women. Med. Sci. Sports Exerc. 1986, 18, 87–94. [Google Scholar] [CrossRef]
- Jefferson, M.E.; Nicklas, B.J.; Chmelo, E.A.; Crotts, C.I.; Shaltout, H.A.; Diz, D.I.; Marsh, A.P.; Brinkley, T.E. Effects of resistance training with and without caloric restriction on arterial stiffness in overweight and obese older adults. Am. J. Hypertens. 2016, 29, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Kotarsky, C.J.; Johnson, N.R.; Mahoney, S.J.; Mitchell, S.L.; Schimek, R.L.; Stastny, S.N.; Hackney, K.J. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol. Rep. 2021, 9, e14868. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; Chmelo, E.; Delbono, O.; Carr, J.J.; Lyles, M.F.; Marsh, A.P. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 991–999. [Google Scholar] [CrossRef]
- Weiss, E.P.; Albert, S.G.; Reeds, D.N.; Kress, K.S.; McDaniel, J.L.; Klein, S.; Villareal, D.T. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: A randomized intervention trial. Am. J. Clin. Nutr. 2016, 104, 576–586. [Google Scholar] [CrossRef]
- Xu, R.; Cao, Y.X.; Chen, Y.T.; Jia, Y.Q. Differential effects of intermittent energy restriction vs. continuous energy restriction combined high-intensity interval training on overweight/obese adults: A randomized controlled trial. Front. Nutr. 2022, 9, 979618. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.J.; Langton, H.M.; Mulligan, M.; Egan, B. Effects of 8 wk of 16, 8 Time-restricted Eating in Male Middle- and Long-Distance Runners. Med. Sci. Sports Exerc. 2021, 53, 633–642. [Google Scholar] [CrossRef]
- Brini, S.; Marzouki, H.; Ouerghi, N.; Ouergui, I.; Castagna, C.; Bouassida, A. Effects of Ramadan observance combined with two training programs on plasma lipids and testosterone/cortisol ratio in male senior basketball players. Med. Dello Sport 2019, 72, 47–58. [Google Scholar] [CrossRef]
- Brini, S.; Ouerghi, N.; Bouassida, A. Small sided games vs repeated sprint training effects on agility in fasting basketball players. Rev. Bras. Med. Esporte 2020, 26, 248–252. [Google Scholar] [CrossRef]
- Brini, S.; Castillo, D.; Raya-González, J.; Castagna, C.; Bouassida, A.; Khalifa, R.; Chortane, S.G.; Clemente, F.M. Basketball-specific small-sided games training during Ramadan intermitting fasting: Do changes in body composition, sleep habits, and perceived exertion affect technical performance? Int. J. Environ. Res. Public Health 2021, 16, 12008. [Google Scholar] [CrossRef]
- Brisswalter, J.; Bouhlel, E.; Falola, J.M.; Abbiss, C.R.; Vallier, J.M.; Hauswirth, C. Effects of Ramadan intermittent fasting on middle-distance running performance in well-trained runners. Clin. J. Sport Med. 2011, 21, 422–427. [Google Scholar] [CrossRef]
- Correia, J.M.; Santos, P.D.G.; Pezarat-Correia, P.; Minderico, C.S.; Infante, J.; Mendonca, G.V. Effect of Time-Restricted Eating and Resistance Training on High-Speed Strength and Body Composition. Nutrients 2023, 15, 285. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.L.; Burke, V.; Morton, A.R.; Beilin, L.J.; Puddey, I.B. Independent and additive effects of energy restriction and exercise on glucose and insulin concentrations in sedentary overweight men. Am. J. Clin. Nutr. 2004, 80, 308–316. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Pacelli, F.Q.; Marcolin, G.; Bianco, A.; Paoli, A. Twelve Months of Time-restricted Eating and Resistance Training Improves Inflammatory Markers and Cardiometabolic Risk Factors. Med. Sci. Sports Exerc. 2021, 53, 2577–2585. [Google Scholar] [CrossRef]
- Stratton, M.T.; Hester, G.M.; Alesi, M.G.; Modjeski, A.S.; King, K.; Olmos, A.A.; Serafini, P.R.; Savage, S.N.; Webb, A.T.; Tinsley, G.M.; et al. Four Weeks of Time-Restricted Feeding Combined with Resistance Training Does Not Differentially Influence Measures of Body Composition, Muscle Performance, Resting Energy Expenditure, and Blood Biomarkers. Nutrients 2020, 12, 1126. [Google Scholar] [CrossRef]
- Tovar, A.P.; Richardson, C.E.; Keim, N.L.; Van Loan, M.D.; Davis, B.A.; Casazza, G.A. Four weeks of 16/8 time restrictive feeding in endurance trained male runners decreases fat mass, without affecting exercise performance. Nutrients 2021, 13, 2941. [Google Scholar] [CrossRef] [PubMed]
- Ballor, D.L.; Katch, V.L.; Becque, M.D.; Marks, C.R. Resistance weight training during caloric restriction enhances lean body weight maintenance. Am. J. Clin. Nutr. 1988, 47, 19–25. [Google Scholar] [CrossRef]
- Batitucci, G.; Faria Junior, E.V.; Nogueira, J.E.; Brandão, C.F.; Abud, G.F.; Ortiz, G.U.; Marchini, J.S.; Freitas, E.C. Impact of Intermittent Fasting Combined With High-Intensity Interval Training on Body Composition, Metabolic Biomarkers, and Physical Fitness in Women With Obesity. Front. Nutr. 2022, 9, 884305. [Google Scholar] [CrossRef] [PubMed]
- Habermann, N.; Makar, K.W.; Abbenhardt, C.; Xiao, L.; Wang, C.Y.; Utsugi, H.K.; Alfano, C.M.; Campbell, K.L.; Duggan, C.; Foster-Schubert, K.E.; et al. No effect of caloric restriction or exercise on radiation repair capacity. Med. Sci. Sports Exerc. 2015, 47, 896–904. [Google Scholar] [CrossRef]
- Haganes, K.L.; Silva, C.P.; Eyjolfsdottir, S.K.; Steen, S.; Grindberg, M.; Lydersen, S.; Hawley, J.A.; Moholdt, T. Time-restricted eating and exercise training improve HbA1c and body composition in women with overweight/obesity: A randomized controlled trial. Cell Metab. 2022, 34, 1457–1471.e4. [Google Scholar] [CrossRef]
- Hammer, R.L.; Barrier, C.A.; Roundy, E.S.; Bradford, J.M.; Fisher, A.G. Calorie-restricted low-fat diet and exercise in obese women. Am. J. Clin. Nutr. 1989, 49, 77–85. [Google Scholar] [CrossRef]
- Nieman, D.C.; Custer, W.F.; Butterworth, D.E.; Utter, A.C.; Henson, D.A. Psychological response to exercise training and/or energy restriction in obese women. J. Psychosom. Res. 2000, 48, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.R.; Moon, J.Y.; Kim, S.; An, K.Y.; Oh, M.; Jeon, J.Y.; Jung, D.-H.; Choi, M.H.; Lee, J.-W. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: A pilot randomized controlled trial. Metabolism 2019, 93, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Brown, J.; Ramirez, S.; Prado, C.M.; Tinsley, G.M.; Gonzalez, M.C. Are Lean Body Mass and Fat-Free Mass the Same or Different Body Components? A Critical Perspective. Adv. Nutr. 2024, 15, 100335. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Gonzalez, M.C.; Shen, W.; Redman, L.; Thomas, D. Weight loss composition is one-fourth fat-free mass: A critical review and critique of this widely cited rule. Obes. Rev. 2014, 15, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.E.; Saltin, B. Variation in total body water with muscle glycogen changes in man. Acta Physiol. Scand. 1970, 80, 11–18. [Google Scholar] [CrossRef]
- de Moraes, W.M.; de Almeida, F.N.; Dos Santos, L.E.; Cavalcante, K.D.; Santos, H.O.; Navalta, J.W.; Prestes, J. Carbohydrate loading practice in bodybuilders: Effects on muscle thickness, photo silhouette scores, mood states and gastrointestinal symptoms. J. Sports Sci. Med. 2019, 18, 772. [Google Scholar]
- Doering, T.M.; Cox, G.R.; Areta, J.L.; Coffey, V.G. Repeated muscle glycogen supercompensation with four days’ recovery between exhaustive exercise. J. Sci. Med. Sport 2019, 22, 907–911. [Google Scholar] [CrossRef]
- Macedo, R.C.; Santos, H.O.; Tinsley, G.M.; Reischak-Oliveira, A. Low-carbohydrate diets: Effects on metabolism and exercise–A comprehensive literature review. Clin. Nutr. ESPEN 2020, 40, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Vlahoyiannis, A.; Andreou, E.; Aphamis, G.; Felekkis, K.; Pieri, M.; Sakkas, G.K.; Giannaki, C.D. Evaluating the evening carbohydrate dilemma: The effect of within-the-day carbohydrate periodization on body composition and physical fitness. Eur. J. Nutr. 2025, 64, 23. [Google Scholar] [CrossRef]
- Methenitis, S.; Feidantsis, K.; Kaprara, A.; Hatzitolios, A.; Skepastianos, P.; Papadopoulou, S.K.; Panayiotou, G. Body Composition, Fasting Blood Glucose and Lipidemic Indices Are Not Primarily Determined by the Nutritional Intake of Middle-Aged Endurance Trained Men—Another “Athletes’ Paradox”? J. Clin. Med. 2022, 11, 6057. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [PubMed]
- Parr, E.B.; Kouw, I.W.; Wheeler, M.J.; Radford, B.E.; Hall, R.C.; Senden, J.M.; Goessens, J.P.; Van Loon, L.J.; Hawley, J.A. Eight-hour time-restricted eating does not lower daily myofibrillar protein synthesis rates: A randomized control trial. Obesity 2023, 31, 116–126. [Google Scholar] [CrossRef]
- Kouw, I.W.; Parr, E.B.; Wheeler, M.J.; Radford, B.E.; Hall, R.C.; Senden, J.M.; Goessens, J.P.; van Loon, L.J.; Hawley, J.A. Short-term intermittent fasting and energy restriction do not impair rates of muscle protein synthesis: A randomised, controlled dietary intervention. Clin. Nutr. 2024, 43, 174–184. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie restriction with or without time-restricted eating in weight loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: A randomized clinical trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: The TREAT randomized clinical trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Polidori, D.; Sanghvi, A.; Seeley, R.J.; Hall, K.D. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity 2016, 24, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.K.; Blond, M.B.; Sandsdal, R.M.; Olsen, L.M.; Juhl, C.R.; Lundgren, J.R.; Janus, C.; Stallknecht, B.M.; Holst, J.J.; Madsbad, S.; et al. Healthy weight loss maintenance with exercise, GLP-1 receptor agonist, or both combined followed by one year without treatment: A post-treatment analysis of a randomised placebo-controlled trial. EClinicalMedicine 2024, 69, 102475. [Google Scholar] [CrossRef]
- Fothergill, E.; Guo, J.; Howard, L.; Kerns, J.C.; Knuth, N.D.; Brychta, R.; Chen, K.Y.; Skarulis, M.C.; Walter, M.; Walter, P.J.; et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 2016, 24, 1612–1619. [Google Scholar] [CrossRef]
- Berg, S.; Stickle, H.; Rose, S.J.; Nemec, E.C. Discontinuing glucagon-like peptide-1 receptor agonists and body habitus: A systematic review and meta-analysis. Obes. Rev. 2025, e13929. [Google Scholar] [CrossRef]
- Vizthum, D.; Katz, S.E.; Pacanowski, C.R. The impact of time restricted eating on appetite and disordered eating in adults: A mixed methods systematic review. Appetite 2023, 183, 106452. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Tinsley, G.M. Is breakfast consumption detrimental, unnecessary, or an opportunity for health promotion? A review of cardiometabolic outcomes and functional food choices. Diabetes/Metab. Res. Rev. 2024, 40, e3684. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Genario, R.; Macedo, R.C.; Pareek, M.; Tinsley, G.M. Association of breakfast skipping with cardiovascular outcomes and cardiometabolic risk factors: An updated review of clinical evidence. Crit. Rev. Food Sci. Nutr. 2021, 62, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Herzog, N.; Janka, S.; Baumann, T.; Kistenmacher, A.; Oltmanns, K.M. Twice as high diet-induced thermogenesis after breakfast vs dinner on high-calorie as well as low-calorie meals. J. Clin. Endocrinol. Metab. 2020, 105, e211–e221. [Google Scholar] [CrossRef]
- LeCheminant, G.M.; LeCheminant, J.D.; Tucker, L.A.; Bailey, B.W. A randomized controlled trial to study the effects of breakfast on energy intake, physical activity, and body fat in women who are nonhabitual breakfast eaters. Appetite 2017, 112, 44–51. [Google Scholar] [CrossRef]
- Wewege, M.A.; Desai, I.; Honey, C.; Coorie, B.; Jones, M.D.; Clifford, B.K.; Leake, H.B.; Hagstrom, A.D. The effect of resistance training in healthy adults on body fat percentage, fat mass and visceral fat: A systematic review and meta-analysis. Sports Med. 2022, 52, 287–300. [Google Scholar] [CrossRef]
- Jayedi, A.; Soltani, S.; Emadi, A.; Zargar, M.-S.; Najafi, A. Aerobic Exercise and Weight Loss in Adults: A Systematic Review and Dose-Response Meta-Analysis. JAMA Netw. Open 2024, 7, e2452185. [Google Scholar] [CrossRef]
- Pontzer, H.; Durazo-Arvizu, R.; Dugas, L.R.; Plange-Rhule, J.; Bovet, P.; Forrester, T.E.; Lambert, E.V.; Cooper, R.S.; Schoeller, D.A.; Luke, A. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr. Biol. 2016, 26, 410–417. [Google Scholar] [CrossRef]
- Hudelmaier, M.; Wirth, W.; Himmer, M.; Ring-Dimitriou, S.; Sänger, A.; Eckstein, F. Effect of exercise intervention on thigh muscle volume and anatomical cross-sectional areas—Quantitative assessment using MRI. Magn. Reson. Med. 2010, 64, 1713–1720. [Google Scholar] [CrossRef]
- Aube, D.; Wadhi, T.; Rauch, J.; Anand, A.; Barakat, C.; Pearson, J.; Bradshaw, J.; Zazzo, S.; Ugrinowitsch, C.; De Souza, E.O. Progressive resistance training volume: Effects on muscle thickness, mass, and strength adaptations in resistance-trained individuals. J. Strength Cond. Res. 2022, 36, 600–607. [Google Scholar] [CrossRef]
- Fujita, T.; Brechue, W.F.; Kurita, K.; Sato, Y.; Abe, T. Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int. J. KAATSU Train. Res. 2008, 4, 1–8. [Google Scholar] [CrossRef]
- Konopka, A.R.; Suer, M.K.; Wolff, C.A.; Harber, M.P. Markers of human skeletal muscle mitochondrial biogenesis and quality control: Effects of age and aerobic exercise training. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 371–378. [Google Scholar] [CrossRef]
- Thomas, A.C.; Brown, A.; Hatt, A.A.; Manta, K.; Costa-Parke, A.; Kamal, M.; Joanisse, S.; McGlory, C.; Phillips, S.M.; Kumbhare, D.; et al. Short-term aerobic conditioning prior to resistance training augments muscle hypertrophy and satellite cell content in healthy young men and women. FASEB J. 2022, 36, e22500. [Google Scholar] [CrossRef]
- Eglseer, D.; Traxler, M.; Embacher, S.; Reiter, L.; Schoufour, J.D.; Weijs, P.J.; Voortman, T.; Boirie, Y.; Cruz-Jentoft, A.; Bauer, S. Nutrition and exercise interventions to improve body composition for persons with overweight or obesity near retirement age: A systematic review and network meta-analysis of randomized controlled trials. Adv. Nutr. 2023, 14, 516–538. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Moon, J.Y.; Kim, J.-H. Association of absolute and relative hand grip strength with all-cause mortality among middle-aged and old-aged people. BMC Geriatr. 2023, 23, 321. [Google Scholar] [CrossRef] [PubMed]
- Mey, R.; Calatayud, J.; Casaña, J.; Torres-Castro, R.; Cuenca-Martínez, F.; Suso-Martí, L.; Andersen, L.L.; López-Bueno, R. Handgrip strength and respiratory disease mortality: Longitudinal analyses from SHARE. Pulmonology 2024, 30, 445–451. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Baharlooie, M.; Karimi, B.; Mokhtari, K.; Rosenkranz, S.K.; Santos, H.O. Effects of intermittent fasting combined with physical exercise on cardiometabolic outcomes: Systematic review and meta-analysis of clinical studies. Nutr. Rev. 2024, 82, 1726–1740. [Google Scholar] [CrossRef]
- Wallis, G.A.; Gonzalez, J.T. Is exercise best served on an empty stomach? Proc. Nutr. Soc. 2019, 78, 110–117. [Google Scholar] [CrossRef]
- Soraya, N.; Parwanto, E. The controversial relationship between body mass index and handgrip strength in the elderly: An overview. Malays. J. Med. Sci. 2023, 30, 73. [Google Scholar] [CrossRef]
- Palacio, A.C.; Díaz-Torrente, X.; Quintiliano-Scarpelli, D. Higher abdominal adiposity is associated with lower muscle strength in Chilean adults. Front. Nutr. 2022, 9, 812928. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Periodized nutrition for athletes. Sports Med. 2017, 47 (Suppl. 1), 51–63. [Google Scholar] [CrossRef]
- Keenan, S.; Cooke, M.B.; Belski, R. The effects of intermittent fasting combined with resistance training on lean body mass: A systematic review of human studies. Nutrients 2020, 12, 2349. [Google Scholar] [CrossRef]
- Vieira, A.F.; Costa, R.R.; Macedo, R.C.O.; Coconcelli, L.; Kruel, L.F.M. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Travers, R.L.; Walhin, J.P.; Gonzalez, J.T.; Koumanov, F.; Betts, J.A.; Thompson, D. Feeding influences adipose tissue responses to exercise in overweight men. Am. J. Physiol.-Endocrinol. Metab. 2017, 313, E84–E93. [Google Scholar] [CrossRef] [PubMed]
- Civitarese, A.E.; Hesselink, M.K.; Russell, A.P.; Ravussin, E.; Schrauwen, P. Glucose ingestion during exercise blunts exercise-induced gene expression of skeletal muscle fat oxidative genes. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, E1023–E1029. [Google Scholar] [CrossRef] [PubMed]
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Ramaekers, M.; Hespel, P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J. Appl. Physiol. 2011, 110, 236–245. [Google Scholar] [CrossRef]
- Santos, H.O.; Macedo, R.C. Impact of intermittent fasting on the lipid profile: Assessment associated with diet and weight loss. Clin. Nutr. ESPEN 2018, 24, 14–21. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Chen, S.-Q. Effects of low/medium-intensity exercise on fat metabolism after a 6-h fast. Int. J. Environ. Res. Public Health 2022, 19, 15502. [Google Scholar] [CrossRef]
- Meng, H.; Zhu, L.; Kord-Varkaneh, H.; Santos, H.O.; Tinsley, G.M.; Fu, P. Effects of intermittent fasting and energy-restricted diets on lipid profile: A systematic review and meta-analysis. Nutrition 2020, 77, 110801. [Google Scholar] [CrossRef]
- Kord, H.V.; Tinsley, G.M.; Santos, H.O.; Zand, H.; Nazary, A.; Fatahi, S.; Mokhtari, Z.; Salehi-Sahlabadi, A.; Tan, S.C.; Rahmani, J.; et al. The influence of fasting and energy-restricted diets on leptin and adiponectin levels in humans: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 1811–1821. [Google Scholar] [CrossRef]
- Kord-Varkaneh, H.; Salehi-Sahlabadi, A.; Tinsley, G.M.; Santos, H.O.; Hekmatdoost, A. Effects of time-restricted feeding (16/8) combined with a low-sugar diet on the management of non-alcoholic fatty liver disease: A randomized controlled trial. Nutrition 2023, 105, 111847. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.A.; Santos, H.O.; Găman, M.A.; Cerqueira, H.S.; Zaher, E.A.; Alromaih, W.R.; Arafat, N.S.; Adi, A.R.; Adly, H.M.; Alyoubi, R.; et al. Effects of intermittent fasting regimens on glycemic, hepatic, anthropometric, and clinical markers in patients with non-alcoholic fatty liver disease: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2024, 59, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Kord Varkaneh, H.; Salehi sahlabadi, A.; Găman, M.A.; Rajabnia, M.; Sedanur Macit-Çelebi, M.; Santos, H.O.; Hekmatdoost, A. Effects of the 5, 2 intermittent fasting diet on non-alcoholic fatty liver disease: A randomized controlled trial. Front. Nutr. 2022, 9, 948655. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O. Intermittent fasting in the management of diabetes: A review of glycemic control and safety. Nutr. Rev. 2024, 82, 1437–1443. [Google Scholar] [CrossRef]










| Source, Year | Study Characteristics | Participant Characteristics | Intermittent Fasting/Calorie Restriction Characteristics | Non-Fasting/Non-Calorie Restriction Control Eating | Exercise Characteristics | Energy Intake (kcal/Day) | Hypocaloric Diet/Eucaloric Diet | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size (Sex) | Groups | Intervention Duration (Weeks) | Outcomes | Health Status | Age (Years) Mean ± SD | BMI (kg·m−2) Mean ± SD | ||||||
| Ballor et al., 1988 [57] | 20 F | Exe + CR Exe alone | 8 | Chest press strength FM FFM BFP BW | Obesity | Exe + CR 32.9 ± 1.5 Exe alone: 32.9 ± 1.5 | Exe + CR: - Exe alone: - | Energy needs were estimated based on body metrics and activity level, then reduced by 1000 kcal/day. The final intake averaged 2200–2500 kcal/day. The diet provided 50% carbohydrate, 27% protein, 23% fat, and included a daily protein supplement. | In the Exe alone group, participants were asked to keep their usual dietary intake unchanged. | Participants performed bench press, leg press, lat pulldown, biceps curl, triceps extension, calf raise, leg extension, and hamstring curl, following the protocol by Sprague and Reynolds. Each exercise was performed three times per week: 2 sets of 10 reps, followed by a third set to voluntary fatigue | A daily caloric deficit of 1000 kcal was applied to baseline needs, estimated at 2200–2500 kcal/day. | Hypocaloric diet |
| Batitucci et al., 2022 [58] | 26 F | TRF + Exe Exe alone | 8 | Leg press strength Chest press strength Handgrip strength VO2max BFP BMI BW | Obesity | TRF + Exe: 32.2 ± 4.4 Exe alone: 33 ± 3.0 | TRF + Exe: 34 ± 3.2 Exe alone: 31.8 ± 2.02 | The 5:2 intermittent fasting protocol was implemented twice weekly on non-consecutive days over 8 weeks. Each fasting day followed a 6:18 schedule, allowing two meals within a 6 h window, followed by 18 h of complete fasting | The Exe alone group was instructed to maintain their habitual diet during the intervention. | HIIT was performed three times per week for 8 weeks, with each 225 min session including a 4 min warm-up, 18 min of intervals at 70–85% HRmax (30–45 s effort, 15–30 s recovery), and a 3 min cool-down. | 1700–1800 kcal/day | Hypocaloric diet |
| Bouhlel et al., 2013 [35] | 20 M | RIF + Exe Exe alone | 4 | Vertical jump Handgrip strength BMI BW | Healthy participants (physical education students) Trained | RIF + Exe: 21.8 ± 1.9 Exe alone: 21.8 ± 1.9 | RIF + Exe: 22.4 ± 1.9 Exe alone: 21.4 ± 2.2 | During Ramadan, participants consumed their last meal between 8:00 and 9:00 p.m. and fasted daily from 5:00 a.m. to approximately 6:30 p.m. | NR | They engaged in 16 h per week of training across various sports, including soccer, handball, basketball, volleyball, gymnastics, and athletics. | NR | Hypocaloric diet (10% reduction in calorie intake) |
| Brady et al., 2021 [47] | 17 M | TRF + Exe Exe alone | 8 | VO2peak FM FFM BW | Healthy participants (Middle and long-distance runners) Trained | TRF + Exe: 35.9 ± 8.6 Exe alone: 39.9 ± 3 | TRF + Exe: NR Exe alone: NR | Participants were directed to eat all meals within an 8 h period, usually from 12:00 to 20:00, with only water allowed outside this window. | Participants were advised to maintain their usual dietary habits throughout the intervention period. | Participants were required to be current competitors in middle- and long-distance events (≥1500 m) and to self-report training a minimum of five days weekly. | The average daily energy intake in the TRF group declined by 5.2 ± 3.6 kcal/kg/day from PRE to MID. | Hypocaloric diet |
| Brini et al., 2019 [48] | 16 M | RIF + SSG SSG RIF + RSA RSA | 4 | SJ CMJ BFP BMI | Healthy participants (Basketball players) Trained | RIF + SSG: 23.4 ± 2.3 SSG: 23.4 ± 2.3 RIF + RSA: 23.4 ± 2.3 RSA: 23.4 ± 2.3 | RIF + SSG: 22.6 ± 1.95 SSG: 22.6 ± 1.95 RIF + RSA: 22.6 ± 1.95 RSA: 22.6 ± 1.95 | During the Ramadan phase, participants fasted from dawn until sunset, abstaining from both food and drink. | Participants maintained stable nutrition and hydration routines, consuming customary Iftar and Sahur meals in proximity to each testing session. | Players from the same basketball team followed SSG and RSA protocols based on their group allocation (GSSG and GRSA), with sessions scheduled at least 24 h apart, twice per week. | NR | Eucaloric diet |
| Brini et al., 2020 [49] | 24 M | RIF + Exe Exe alone | 4 | BFP BMI BW | Healthy participants (Basketball players) Trained | RIF + Exe: 25.32 ± 2.56 Exe alone: 24.85 ± 1.55 | RIF + Exe: 23.43 ± 1.19 Exe alone: 23.41 ± 1.46 | Participants fasted for roughly 16–17 h each day. | Participants documented all meals consumed during the study, including the quantity and type of food and beverages. Additionally, a nutritionist conducted interviews and analyzed their records. | Training sessions began with a 115 min warm-up, followed by technical and tactical drills focusing on fundamental and basketball-specific movements, including defense. Both groups completed approximately 90 min per session, three times per week. | RIF + Exe: 2741.66 ± 172.98 kcal/day Exe alone: 2829.17 ± 73.04 kcal/day | Hypocaloric diet (20% reduction in calorie intake) |
| Brini et al., 2021 [50] | 24 M | RIF + Exe Exe alone | 4 | BMI BW | Professional male basketball players | RIF + Exe: 25.32 ± 2.56 Exe alone: 24.85 ± 1.55 | RIF + Exe: 23.43 ± 1.19 Exe alone: 23.41 ± 1.46 | Ramadan intermittent fasting | Normal diet | Training sessions started with a 15 min warm-up program, followed by technical and tactical drills based on basic basketball movements, specific basketball movements, and basic defensive movements, Both groups completed the same training volume (~90 min per session). 3 day/week | RIF + Exe: 2741.66 ± 172.98 Exe alone: 2829.17 ± 73.04 | Eucaloric diet |
| Brisswalter et al., 2011 [51] | 18 M | RIF + Exe Exe alone | 4 | VO2max BW | Healthy participants (well-trained runners) Trained | RIF + Exe: 23.6 ± 2.9 Exe alone: 23.6 ± 2.9 | RIF + Exe: NR Exe alone: NR | Ramadan intermittent fasting | Normal diet | Training consisted of (1) 30 min of slow running followed by intervals of 30 s running and 30 s rest at maximal aerobic speed (100% MAS), (2) a continuous 30 min run at 100% MAS, and (3) 20 min of slow running at the athlete’s individual competition speed, conducted three times weekly. | NR | Eucaloric diet |
| Cho et al., 2019 [63] | 18 M&F | ADF + Exe Exe alone | 8 | Chest press strength Leg press strength VO2max FM BFP BMI BW | Obesity | ADF + Exe: 34.5 ± 5.7 Exe alone: 38.6 ± 8.2 | ADF + Exe: 28 ± 2.6 Exe alone: 26.9 ± 3.9 | During the 8-week intervention, ADF + Exe participants consumed about 25% of their daily energy needs (~500 kcal) on each fast day (24 h) and ate freely on feed days (24 h). Fast and feed days alternated every other day, with fast days occurring three times weekly. On fast days, participants were instructed to eat a single meal between 12 p.m. and 2 p.m. to standardize fasting periods | Control group participants were instructed to maintain their usual diet. | Resistance training sessions lasted 40 min and involved weight machines, barbells, and dumbbells. Intensity was tailored to each participant’s muscle strength and progressively increased weekly. Aerobic training consisted of 20 min on motorized treadmills, with intensity set according to individual maximal oxygen consumption. Both training types were performed three times per week. | ADF + Exe: 1785.9 ± 311.3 kcal/day Exe alone: 1532.8 ± 410.4 kcal/day | Hypocaloric diet |
| Christiansen et al., 2010 [38] | 40 M&F | CR + Exe Exe alone | 12 | VO2max BMI BW | Obesity | CR + Exe: 37.5 ± 8 Exe alone: 37.2 ± 7 | CR + Exe: 34.2 ± 3 Exe alone: 33.4 ± 4 | Participants in the CR + Exe group were assigned a daily intake of 800 kcal. | NR | Participants completed supervised aerobic training three times weekly, each session lasting 60–75 min, with an estimated energy expenditure of 500–600 kcal per session. | Participants in the DIO and DEX groups 600 and 800 kcal/day for 8 weeks followed by a weight maintenance diet for 4 weeks. The participants in the DEX group were allowed to consume 150–200 kcal more per day compared with the DIO group | Hypocaloric diet |
| Coker et al., 2009 [39] | 17 M&F | CR + Exe Exe alone | 12 | VO2peak BFP BMI BW | Obesity | CR + Exe: 54 ± 6 Exe alone: 55 ± 5.64 | CR + Exe: 32 ± 3 Exe alone: 32 ± 2.82 | Caloric restriction began with a 1000 kcal reduction in the first week, followed by weekly decreases of 500 kcal until reaching a total weekly deficit of 2500 kcal. | NR | Training intensity was prescribed at 50% of participants’ peak oxygen consumption. | NR | Hypocaloric diet |
| Cooke et al., 2022 [40] | 22 M&F | TRF + Exe Exe alone | 8 16 | VO2peak FM FFM BW | Obesity | TRF + Exe: 39 ± 6.8 Exe alone: 32 ± 8.3 | TRF + Exe: 34 ± 2.1 Exe alone: 32 ± 4.4 | The 5:2 IF group restricted energy intake on two non-consecutive days per week for 16 weeks, consuming ad libitum on the other five days. On fasting days, men and women consumed a single meal of 600 kcal and 500 kcal, respectively, along with unlimited water and unsweetened black tea. | NR | SIT involved supervised sessions of 4 × 20 s efforts at 150% VO2peak, each followed by 40 s of active rest at 50 watts on a magnetic braked cycle ergometer. The number of intervals increased from four to six during the first four weeks and stayed at six thereafter. Sessions included 3 min warm-up and cool-down periods, performed three times per week. | NR | Hypocaloric diet |
| Correia et al., 2023 [52] | 18 M | TRF + Exe Exe alone | 4 | CMJ SJ FM FFM BW | Healthy participants Trained | TRF + Exe: 23.7 ± 2.6 Exe alone: 23.7 ± 2.6 | TRF + Exe: NR Exe alone: NR | During TRF, participants ate two to three ad libitum meals within an 8 h window (1–9 p.m.), consuming only water, tea, and black coffee outside this period. | During the Exe-only condition, participants consumed 100% of their energy requirements and maintained their usual dietary habits. | Participants followed a structured training program during each dietary intervention, performing four sets of 8–10 repetitions at 85% of their 1-RM on leg press, bench press, leg extension, leg curl, shoulder press, and lat pulldown, three times per week. | Before TRF + Exe: 2433.3 ± 760.5 kcal/day Before Exe alone: 2427 ± 556.8 kcal/day | Eucaloric diet |
| Correia et al., 2024 [31] | 15 M | TRF + Exe Exe alone | 4 | VO2max FM FFM BW | Healthy participants Trained | TRF + Exe: 23.7 ± 2.6 Exe alone: 23.7 ± 2.6 | TRF + Exe: Exe alone: NR | The TRF intervention used a 16/8 protocol, where participants consumed 2 to 3 meals within an 8 h window (1:00–9:00 p.m.) and were allowed only water, tea, and black coffee outside this period. | During the Exe-only condition, participants were asked to maintain their usual dietary habits without any restrictions on meal timing throughout the intervention. | During the first 2 weeks of the intervention, participants completed 10 km runs per session with 24 h of rest between workouts. In the final 2 weeks, they continued the 10-km runs and added 1 km running intervals after each continuous run, separated by 4 min of active recovery, totaling approximately 39 km per week. Sessions were performed three times per week. | TRF + Exe: 2311 ± 681 kcal/day Exe alone: 2285 ± 659 kcal/day | Eucaloric diet |
| Cox et al., 2004 [53] | 30 M | CR + High intensity Exe High intensity Exe CR + Low intensity Exe Low intensity Exe | 16 | VO2max FM FFM BMI BW | Overweight participants | CR + High intensity Exe: 41.1 ± 5.4 High intensity Exe: 43 ± 4.2 CR + Low intensity Exe: 41.1 ± 5.4 Low intensity Exe: 43 ± 4.2 | CR + High intensity Exe: 33.3 ± 3.6 High intensity Exe: 30.5 ± 3.9 CR + Low intensity Exe: 33.3 ± 3.6 Low intensity Exe: 30.5 ± 3.9 | Participants decreased their daily energy intake by 1000 to 1500 kcal. | Control participants were instructed to keep their regular dietary habits. | The exercise consisted solely of stationary cycling, with participants cycling for 30 min at 60–70% of their maximum workload, as determined by their fitness assessment. Each session included a 5 min warm-up and a 5-min cool-down, performed three times per week. | Participants in the other two groups followed personalized programs targeting a daily energy intake reduction of 1000 to 1500 kcal. | Hypocaloric diet |
| Habermann et al., 2015 [59] | 120 F | CR + Exe Exe alone | 52 | VO2max BW | Postmenopausal women with obesity/overweight | CR + Exe: 58.4 ± 4.7 Exe alone: 59.1 ± 5.1 | CR + Exe: 31.2 ± 4.5 Exe alone: 30.5 ± 3.6 | The diet targeted a daily energy intake of 1200–2000 kcal, adjusted by weight, with less than 30% of calories from fat. The goal was to achieve a 10% weight loss within 6 months, followed by maintenance. | Participants were instructed to maintain their usual dietary habits. | The exercise intervention aimed for 45 min of moderate-to-vigorous activity. Participants attended three supervised sessions per week and exercised twice weekly at home. Training duration increased from 15 min at 60–70% of maximal heart rate to 70–85% by week 7, which was then maintained. Facility sessions included treadmill walking, stationary cycling, and other aerobic machines, performed five days per week. | The diet had a total energy intake goal of 1200–2000 kcal/day based on weight | Hypocaloric diet |
| Hagan et al., 1986 [41] | 24 M 24 F | CR + Exe (male) Exe alone (male) CR + Exe (female) Exe alone (female) | 12 | VO2max FM FFM BW | Overweight | M CR + Exe: 34.4 ± 5.6 Exe alone: 33.9 ± 7.6 F CR + Exe: 34.2 ± 6.5 Exe alone: 37.2 ± 7.4 | NR | Intake was limited to 1200 kcal per day. | Participants continued their usual dietary habits. | Aerobic training was performed for 30 min per session, five days per week. | NR | Hypocaloric diet |
| Haganes et al., 2022 [60] | 52 F | TRF + Exe Exe alone | 7 | VO2peak FM BW | Obesity | TRF + Exe: 37.3 ± 5.7 Exe alone: 34.9 ± 7 | TRF + Exe: 31.4 ± 4 Exe alone: 32.5 ± 4.5 | In the TRF + Exe group, participants restricted their energy intake to a maximum 10 h eating window each day. | NR | Participants engaged in three supervised treadmill workouts per week. | During the baseline week, total daily energy intake averaged 2066 ± 484 kcal in the TRF group, 1996 ± 387 kcal in the Exe alone group, 1943 ± 346 kcal in the TRF + Exe group, and 2056 ± 433 kcal in the Exe alone group. | Eucaloric diet |
| Hammer et al., 1989 [61] | 20 F | CR + Exe Exe alone | 4 16 | VO2max FFM BFP BW | Obesity | CR + Exe: 33 ± 8 Exe alone: 32 ± 7 | CR + Exe: 32.2 ± 7.8 Exe alone: 31.3 ± 6.7 | Participants in the CR + Exe group followed an 800 kcal/day exchange list diet, with specified servings for each food group. The CR diet was set at an 800 kcal daily intake. | No effort was made to restrict calories in the Exe group | Participants started the first week by walking or jogging 1.6 km per session. The distance increased by 0.8 km each week until reaching 4.8 km, which was maintained for the rest of the study. Exercise intensity was set between 70 and 85% of HRmax, performed five days per week. | The estimated caloric deficits were 1203 kcal/day | Hypocaloric diet |
| Jefferson et al., 2016 [42] | 32 M&F | CR + Exe Exe alone | 20 | Knee extensor strength FM FFM BFP BMI BW | Obesity | CR + Exe: 69 ± 3 Exe alone: 68 ± 3 | CR + Exe: 30.7 ± 2.5 Exe alone: 31 ± 2.7 | Participants were guided to decrease their daily energy intake by about 600 kcal below estimated expenditure, aiming for a 5–10% weight loss. | The Exe alone group was asked to maintain their usual diet during the intervention. | Upper and lower body exercises were performed on different machines, with intensity set at 70% of 1RM. Exercise volume and intensity gradually increased over the first month, conducted three times per week. | NR | Hypocaloric diet |
| Kirkendall et al., 2008 [33] | 85 M | RIF + Exe Exe alone | 4 | Vertical jump | Healthy participants (young football players) Trained | RIF + Exe: 18 Exe alone: 18 | RIF + Exe: 22.4 Exe alone: 22.4 | Participants were instructed to adhere to fasting protocols throughout the month of Ramadan. | Participants were instructed not to fast during that period. | NR | NR | Eucaloric diet |
| Kotarsky et al., 2021 [43] | 21 M&F | TRF + Exe Exe alone | 8 | Handgrip strength Knee extensor strength FM FFM BMI BW | Obesity | TRF + Exe: 45 ± 3 Exe alone: 44 ± 2 | TRF + Exe: 29.8 ± 0.8 Exe alone: 29.4 ± 0.8 | TRF + Exe participants consumed all calories daily between 12:00 p.m. and 8:00 p.m., creating a 16 h fasting window. During fasting, TRF participants were advised to drink only water, black coffee, or tea. | Exe alone participants were instructed to keep their usual eating patterns. | Resistance training included three sets of 12 reps with up to 60 s rest between sets. Load was based on a percentage of body weight and adjusted via trial loads, performed three times per week. Aerobic training consisted of 75 min at moderate-to-vigorous intensity (≥55% HRR) on a treadmill or similar equipment. Intensity was increased by 5–10% during weeks 5 and 7 to ensure progression. Sessions were performed immediately after resistance training, three times per week. | A modest but significant energy restriction occurred from pre-intervention to week 7, with approximately 300 kcal/day (14.5%) in the TRF group and 250 kcal/day (11.4%) in the Exe group. | Hypocaloric diet |
| Moro et al., 2016 [23] | 34 M | TRF + Exe Exe alone | 8 | Chest press strength Leg press strength FM FFM | Healthy participants (Resistance trained) Trained | TRF + Exe: 29.94 ± 4.07 Exe alone: 28.47 ± 3.48 | TRF + Exe: NR Exe alone: NR | TRF participants met 100% of their energy needs through three meals at 1 p.m., 4 p.m., and 8 p.m., fasting for the remaining 16 h each day. | The Exe alone group distributed their daily caloric intake across three meals, which were consumed at approximately 8:00 a.m., 1:00 p.m., and 8:00 p.m. | Participants engaged in a split resistance training routine, completing three weekly sessions. Each session comprised three sets of 6–8 reps at 85–90% of 1-RM, performed to failure with 3 min rest intervals between sets and exercises. | TRF: 2826 ± 412.3 kcal/day, carbohydrates 53.2 ± 1.4%, fat 24.7 ± 3.1%, protein 22.1 ± 2.6%, CON: 3007 ± 444.7 kcal/day, carbohydrates 54.7 ± 2.2%, fat 23.9 ± 3.5%, protein 21.4 ± 1.8% | Eucaloric diet |
| Moro et al., 2020 [36] | 16 M | TRF + Exe Exe alone | 4 | VO2peak FFM BW | Healthy participants (Elite cyclists) Trained | TRF + Exe: 19.38 ± 2.39 Exe alone: 19.38 ± 1.60 | TRF + Exe: 21.85 ± 1.65 Exe alone: 22.47 ± 1.83 | Participants in the TRF group were advised to consume their entire daily caloric requirement across four eating occasions, including the three main meals, within an 8 h feeding window. | Participants in the Exe group consumed their daily caloric intake following a conventional meal timing schedule. | This investigation was carried out during the winter pre-competition phase. The athletes followed a structured training regimen comprising approximately 500 ± 50 km per week, distributed across six sessions, all conducted within the designated feeding period (10:00 a.m. to 6:00 p.m.). | All participants adhered to an identical 7-day dietary regimen, with a fixed daily caloric intake of 4800 kcal. | Eucaloric diet |
| Moro et al., 2021 [54] | 20 M | TRF + Exe Exe alone | 8 52 | Chest press strength Leg press strength FM FFM BW | Healthy participants Trained | TRF + Exe: NR Exe alone: NR | TRF + Exe: NR Exe alone: NR | The TRF group was instructed to meet their total daily energy requirements through three meals consumed within an 8 h eating window (approximately at 1 p.m., 4 p.m., and 8 p.m.), followed by a 116 h fasting period. | Participants in the Exe group followed their usual meal pattern, consuming their total daily energy intake across three meals spread over an approximately 12 h period (~8 a.m., 1 p.m., and 8 p.m.). | Throughout the experimental period, training intensity varied between 75% and 90% of 1 RM to cycle between strength and hypertrophy phases. Sessions were scheduled between 4:00 and 6:00 p.m. to align with the eating window for both groups, occurring three times per week. | In the TRF group, energy intake was allocated as 40% at breakfast, 25% at lunch, and 35% at dinner, while participants following a normal diet (ND) consumed 25%, 40%, and 35%, respectively. Additionally, all participants ingested 20 g of whey protein 30 min post-exercise. | Eucaloric diet |
| Nicklas et al., 2015 [44] | 111 M&F | CR + Exe Exe alone | 20 | Handgrip strength Knee extensor strength 400 m walk test Gait speed FM FFM BFP BW | Overweight and obesity | CR + Exe: 69.6 ± 3.9 Exe alone: 69.4 ± 3.6 | CR + Exe: 30.4 ± 2.2 Exe alone: 30.7 ± 2.4 | Each participant received an individualized daily caloric target, calculated by deducting 600 kcal from their estimated energy requirements for weight maintenance. Up to two meal replacements per day (shakes and bars; Slim-Fast Inc.)—each providing approximately 220 kcal with 7–10 g protein, 33–46 g carbohydrates, 1.5–5 g fat, and 2–5 g fiber—were supplied for breakfast and lunch. | Participants in the resistance training only group were instructed to maintain a eucaloric diet throughout the intervention. | The training protocol aimed for participants to perform 3 sets of 10 repetitions per exercise at 70% of their one-repetition maximum (1 RM). Rest intervals between sets were approximately one minute. Resistance was progressively increased when a participant successfully completed 10 repetitions in the third set across two consecutive sessions. Strength assessments were conducted every four weeks to recalibrate training loads, ensuring alignment with the 70% 1 RM target. Training sessions occurred three times per week. | NR | Hypocaloric diet |
| Nicklas et al., 2019 [32] | 155 M&F | Mod-CR + Exe High-CR + Exe Exe alone | 20 | VO2peak 400 m walk test Gait speed FM FFM BFP BW | Obesity | Mod-CR + Exe: 68.8 ± 3.1 High-CR + Exe: 69.6 ± 3.8 Exe alone: 69.1 ± 3.7 | Mod-CR + Exe: 34.7 ± 3.7 High-CR + Exe: 34.4 ± 3.7 Exe alone: 34.6 ± 3.1 | Participants in the calorie restriction (CR) groups received two meals per day (lunch and dinner), collected thrice weekly. Their prescribed caloric intake was calculated by reducing their estimated daily energy requirements for weight maintenance by either 250 kcal (moderate CR) or 600 kcal (high CR). | Participants in the Exe alone group were instructed to continue their usual dietary habits throughout the intervention. | Aerobic training was conducted on treadmills, beginning with a slow-paced walking warm-up. Training duration increased from 15 to 20 min at 50% heart rate reserve (HRR) during the first week to 30 min at 65–70% HRR by week six. Sessions were performed four times per week. | No female participant received less than 1100 kcal/day, and no male participant received less than 1300 kcal/day. | Hypocaloric diet |
| Nieman et al., 2000 [62] | 43 F | CR + Exe Exe alone | 12 weeks | VO2max BFP BW | Obesity | CR + Exe: 45.6 ± 1.1 Exe alone: 43.2 ± 2.3 | CR + Exe: 33.1 ± 0.62 Exe alone: 33.1 ± 0.62 | Participants with obesity followed a 4.19 to 5.44 MJ/day (1200 to 1300 kcal) diet for 12 weeks. The meal plan was structured using dietary exchanges, including two servings of fruit, three of vegetables, two of dairy, six of grains, two of fats, five of lean protein, and 100 kcal of optional foods. | NR | Participants were instructed to walk for 45 min per session at 60% to 80% of their maximum heart rate over a 12-week period, totaling 60 exercise sessions. Exercise duration and intensity were progressively increased during the initial 3 weeks, starting from 25 to 30 min per session at 60% to 65% MHR in week one, reaching 45 min at 70% to 80% MHR from weeks 4 to 12. Sessions were conducted five days per week. | 1884 ± 84 kcal/day | Hypocaloric diet |
| Stratton et al., 2020 [55] | 26 M | TRF + Exe Exe alone | 4 | Vertical Jump Chest press strength Leg press strength FM BFP BW | Healthy participants Trained | TRF + Exe: 22.9 ± 3.6 Exe alone: 22.5 ± 2.2 | TRF + Exe: NR Exe alone: NR | The TRF + Exe group consumed all calories and macronutrients within an 8 h daily window while adhering to a prescribed 25% caloric deficit. | Exe alone group followed their usual daily feeding schedule while maintaining a prescribed 25% caloric deficit. | Resistance training sessions included 1–2 min of rest between sets. Exercise loads were determined based on pre-intervention estimated 1 RM percentages, and all exercises were performed within a specified repetitions-in-reserve range. Training occurred three times per week. | NR | Hypocaloric diet |
| Tinsley et al., 2017 [22] | 18 M | TRF + Exe Exe alone | 4 8 | Chest press strength FM FFM BFP BW | Healthy participants Trained | TRF + Exe: 22.9 ± 4.1 Exe alone: 22.0 ± 2.4 | TRF + Exe: NR Exe alone: NR | On non-training days (four days per week), participants were instructed to consume all their calories within a flexible four-hour window between 4:00 p.m. and midnight, without restrictions on calorie amount or food types. | Participants in the Exe alone group were instructed to maintain their habitual dietary habits throughout the study. | Participants performed alternating upper and lower body workouts, completing four sets of 8–12 repetitions per exercise, with 90 s of rest between sets, three times per week. | TRF + Exe: 1631 ± 563 kcal/day Exe alone: 2318 ± 977 kcal/day | Eucaloric diet |
| Tovar et al., 2021 [56] | 15 M | TRF + Exe Exe alone | 4 | VO2max HR FM FFM BFP BW | Healthy participants Trained | TRF + Exe: 28.7 ± 5.2 Exe alone: 28.7 ± 5.2 | TRF + Exe: NR Exe alone: NR | The 16/8 protocol required participants to consume all meals within a consistent 8 h daily window. During the fasting period, only water and non-caloric beverages such as unsweetened black coffee or plain tea were permitted. | Under the 12/12 protocol, participants were instructed to consume all their calories within a consistent, self-chosen 12 h daily window. | The weekly schedule consisted of one day with high-intensity exercise, one day with moderate-intensity exercise, one day with low-intensity exercise, and one rest day, totaling three exercise sessions per week. | TRF + Exe: 2421 ± 478 kcal/day Exe alone: 2513 ± 367 kcal/day | Eucaloric diet |
| Wang et al., 2019 [34] | 26 M&F | CR + Exe Exe alone | 20 | 400 m walk test Gait Speed Leg press strength Knee extensor strength BMI BW | Obesity | CR + Exe: 65–80 Exe alone: 65–80 | CR + Exe: 29.7 ± 1.8 Exe alone: 29.7 ± 2.2 | Participants assigned to the CR + Exe group followed a dietary weight-loss protocol aimed at achieving moderate weight reduction of 5–10%. | Participants were instructed to maintain their habitual dietary intake. | One-repetition maximum (1 RM) was used to determine training intensity on weight-stack resistance machines. The protocol aimed for participants to perform 3 sets of 10 repetitions at 70% of their 1 RM, with approximately 1 min of rest between sets. Resistance was increased once a participant successfully completed 10 repetitions on the third set in two consecutive sessions. Training occurred three times per week. | NR | Hypocaloric |
| Weiss et al., 2016 [45] | 35 M&F | CR + Exe Exe alone | 12 | VO2max FM FFM BFP BMI BW | Overweight participants | CR + Exe: 57 ± 7 Exe alone: 56 ± 6 | CR + Exe: 28.3 ± 1.8 Exe alone: 27 ± 1.5 | All three interventions aimed to induce a 20% energy deficit, targeting a body weight reduction of 6–8% over 12 to 14 weeks (approximately 0.5% of baseline body weight lost per week). The duration of the intervention was extended for individual participants as necessary to achieve the prescribed weight loss goal. | Participants were instructed to maintain their usual dietary intake. | The objective of the Exe intervention was to increase total energy expenditure (TEE) by approximately 20% without altering dietary habits. Recommended activities included cardiovascular exercises such as brisk walking and cycling, as well as functional physical activities like walking to work or carrying out yard work, performed six days per week. | CR + Exe: 2310 ± 159 Exe alone: 1908 ± 125 | Hypocaloric diet |
| Xu et al., 2022 [46] | 48 M&F | Intermittent-CR + Exe Continuous-CR + Exe Exe alone | 4 | VO2max BFP BMI | Overweight | Intermittent-CR + Exe: 21.3 ± 2.24 Continuous-CR + Exe: 21.3 ± 2.24 Exe alone: 21.3 ± 2.24 | Intermittent-CR + Exe: 26.90 ± 1.46 Continuous-CR + Exe: 26.39 ± 1.63 Exe alone: 26.12 ± 2.01 | Participants in the Intermittent-CR group consumed 30% of their daily recommended energy intake (approximately 500–1000 kcal) on two non-consecutive days per week, while eating ad libitum on the remaining five days. The Continuous-CR group followed a daily hypoenergetic diet, consuming 70% of their estimated energy requirements—approximately 1300–1600 kcal for females and 1600–1900 kcal for males. | Participants in the Exe alone group consumed their full daily recommended energy intake, distributed across three meals. | Participants completed exercise sessions five times per week, consisting of high-intensity intervals at 80% of their VO2max interspersed with active recovery periods at 50% VO2max. Each session included five 3 min cycling bouts, totaling 30 min of exercise, preceded by a 10 min warm-up and followed by a 10-min cool-down. The HIIT protocol was structured to create an approximate energy deficit of 310 kcal per session, with session duration individually adjusted. | The daily recommended energy intakes were 1800–2250 kcal for females and 2250–2700 kcal for males | Hypocaloric diet |
| Yassine et al., 2009 [37] | 24 M&F | CR + Exe Exe alone | 12 | VO2max FM FFM BMI BW | Older adults with obesity | CR + Exe: 65.5 ± 5 Exe alone: 65.5 ± 5 | CR + Exe: 33.7 ± 4.7 Exe alone: 35.3 ± 5.8 | In the CR + Exe group, participants were instructed to decrease their daily energy intake by 500 kcal. | NR | Participants engaged in exercise sessions lasting 50 to 60 min per day, five days per week. The regimen included treadmill walking and/or cycling on an ergometer, with over 75% of the effort dedicated to treadmill activity. During the initial sessions, exercise intensity was set at 60% to 65% of HRmax, increasing to 80% to 85% HRmax (~70% VO2max) after week 4. | Participants in the Exe + CR group consumed fewer calories (1303 ± 210 kcal/day) than those in the Exe group (2141 ± 1338 kcal/day) | Hypocaloric diet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazeminasab, F.; Sharafifard, F.; Bahrami Kerchi, A.; Bagheri, R.; Carteri, R.B.; Kirwan, R.; Santos, H.O.; Dutheil, F. Effects of Intermittent Fasting and Calorie Restriction on Exercise Performance: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 1992. https://doi.org/10.3390/nu17121992
Kazeminasab F, Sharafifard F, Bahrami Kerchi A, Bagheri R, Carteri RB, Kirwan R, Santos HO, Dutheil F. Effects of Intermittent Fasting and Calorie Restriction on Exercise Performance: A Systematic Review and Meta-Analysis. Nutrients. 2025; 17(12):1992. https://doi.org/10.3390/nu17121992
Chicago/Turabian StyleKazeminasab, Fatemeh, Fatemeh Sharafifard, Ali Bahrami Kerchi, Reza Bagheri, Randhall B. Carteri, Richard Kirwan, Heitor O. Santos, and Fred Dutheil. 2025. "Effects of Intermittent Fasting and Calorie Restriction on Exercise Performance: A Systematic Review and Meta-Analysis" Nutrients 17, no. 12: 1992. https://doi.org/10.3390/nu17121992
APA StyleKazeminasab, F., Sharafifard, F., Bahrami Kerchi, A., Bagheri, R., Carteri, R. B., Kirwan, R., Santos, H. O., & Dutheil, F. (2025). Effects of Intermittent Fasting and Calorie Restriction on Exercise Performance: A Systematic Review and Meta-Analysis. Nutrients, 17(12), 1992. https://doi.org/10.3390/nu17121992








