Association Between Low Omega-6 Polyunsaturated Fatty Acid Levels and the Development of Delirium in the Coronary Intensive Care Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood Sampling
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
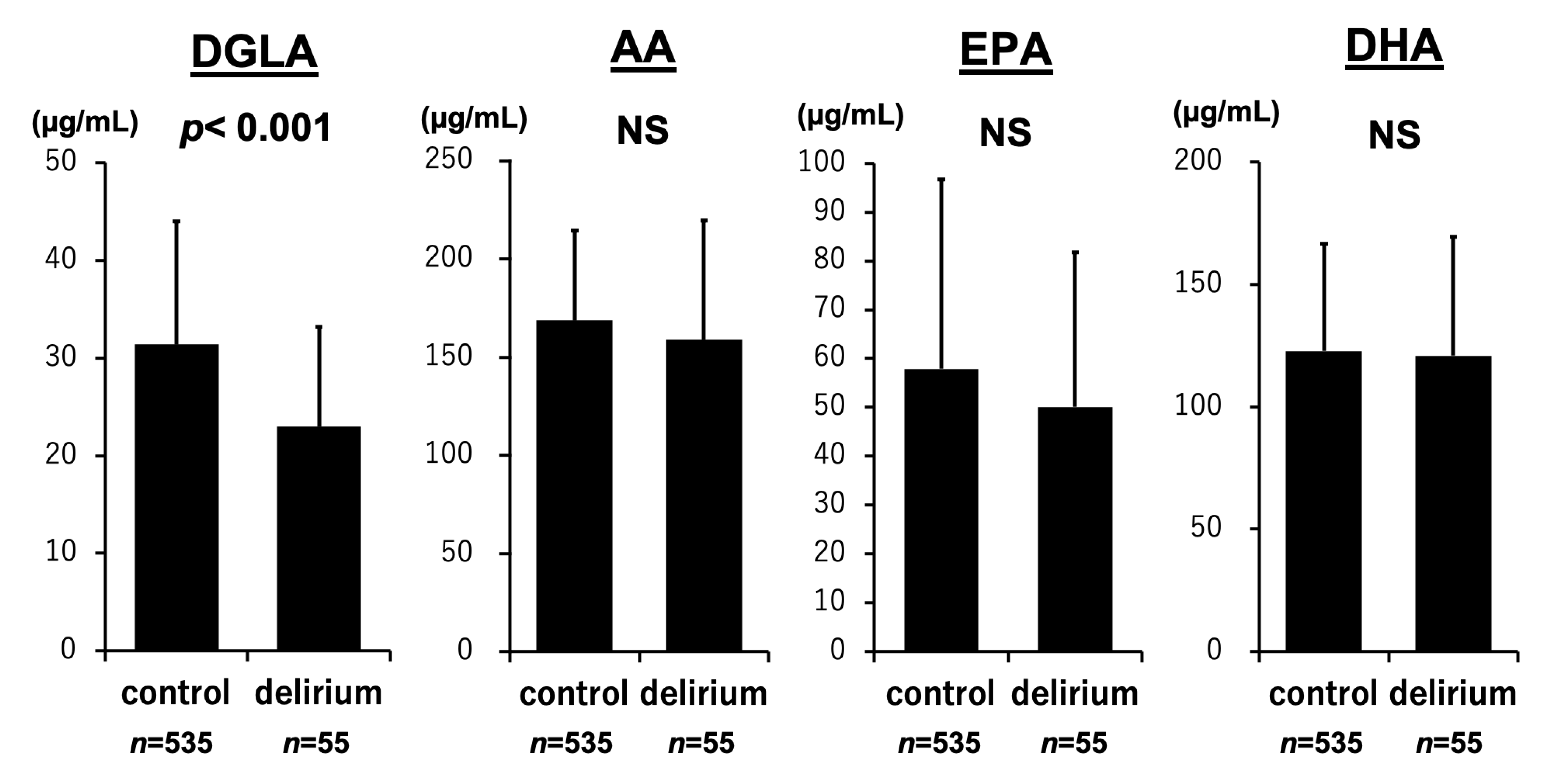
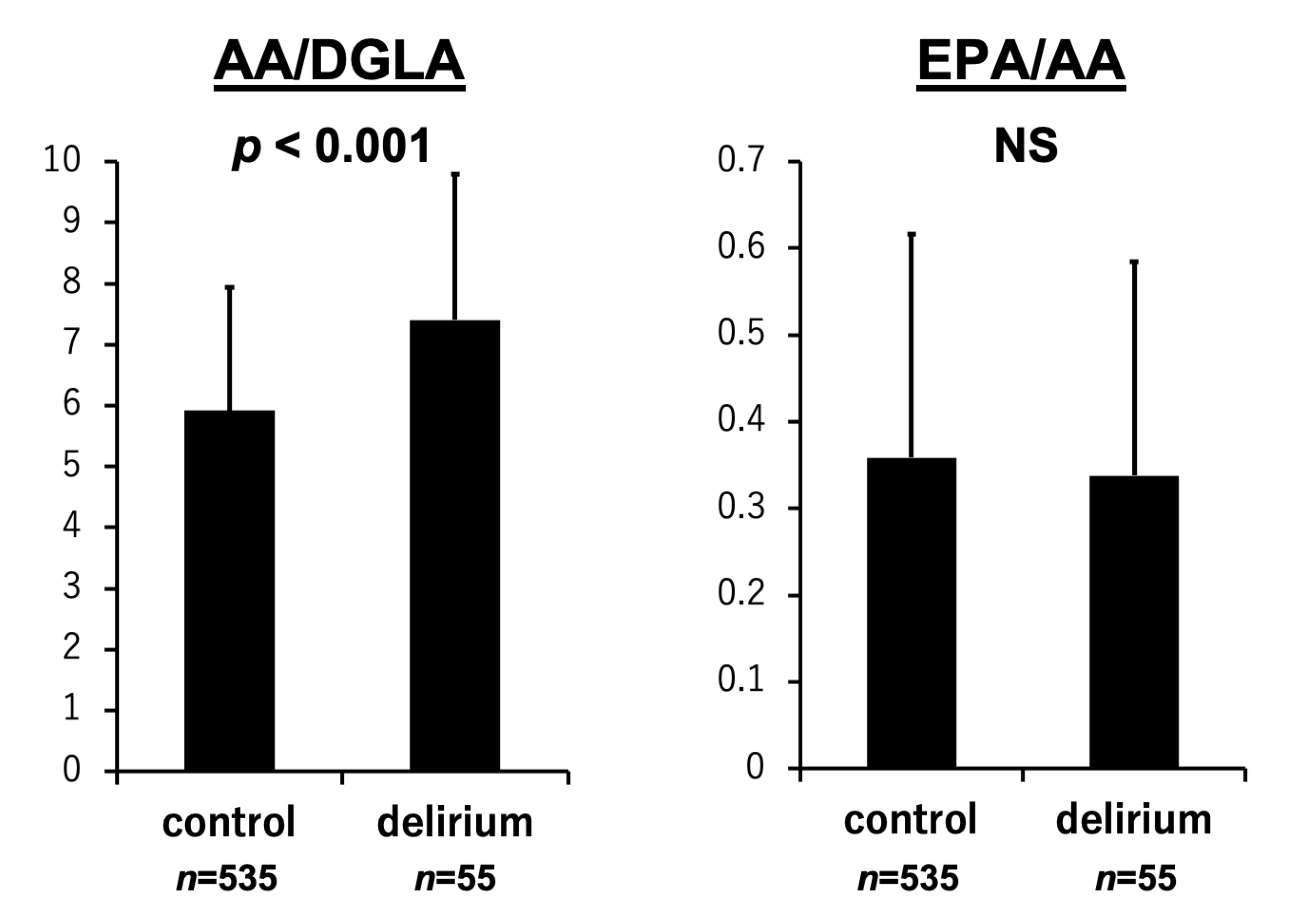
3.2. PUFA Levels and the Development of Delirium
3.3. PUFA Levels, the ICDSC Score, and the Duration of Delirium
3.4. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcantonio, E.R. Delirium in Hospitalized Older Adults. N. Engl. J. Med. 2017, 377, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Bellelli, G.; Morandi, A.; Di Santo, S.G.; Mazzone, A.; Cherubini, A.; Mossello, E.; Bo, M.; Bianchetti, A.; Rozzini, R.; Zanetti, E.; et al. “Delirium Day”: A nationwide point prevalence study of delirium in older hospitalized patients using an easy standardized diagnostic tool. BMC Med. 2016, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Westendorp, R.G.J.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Ringaitiene, D.; Gineityte, D.; Vicka, V.; Zvirblis, T.; Sipylaite, J.; Irnius, A.; Ivaskevicius, J.; Kacergius, T. Impact of malnutrition on postoperative delirium development after on pump coronary artery bypass grafting. J. Cardiothorac. Surg. 2015, 10, 74. [Google Scholar] [CrossRef]
- Mazzola, P.; Ward, L.; Zazzetta, S.; Broggini, V.; Anzuini, A.; Valcarcel, B.; Brathwaite, J.S.; Pasinetti, G.M.; Bellelli, G.; Annoni, G. Association Between Preoperative Malnutrition and Postoperative Delirium After Hip Fracture Surgery in Older Adults. J. Am. Geriatr. Soc. 2017, 65, 1222–1228. [Google Scholar] [CrossRef]
- Sugita, Y.; Miyazaki, T.; Shimada, K.; Shimizu, M.; Kunimoto, M.; Ouchi, S.; Aikawa, T.; Kadoguchi, T.; Kawaguchi, Y.; Shiozawa, T.; et al. Correlation of Nutritional Indices on Admission to the Coronary Intensive Care Unit with the Development of Delirium. Nutrients 2018, 10, 1712. [Google Scholar] [CrossRef]
- Falsini, G.; Grotti, S.; Porto, I.; Toccafondi, G.; Fraticelli, A.; Angioli, P.; Ducci, K.; Liistro, F.; Pieroni, M.; Taddei, T.; et al. Long-term prognostic value of delirium in elderly patients with acute cardiac diseases admitted to two cardiac intensive care units: A prospective study (DELIRIUM CORDIS). Eur. Heart J. Acute Cardiovasc. Care 2017, 7, 661–670. [Google Scholar] [CrossRef]
- Hornstra, G. Essential fatty acids in mothers and their neonates. Am. J. Clin. Nutr. 2000, 71, 1262s–1269s. [Google Scholar] [CrossRef]
- Aaes-Jorgensen, E. Essential fatty acids. Physiol. Rev. 1961, 41, 1–51. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Salem, N., Jr.; Ward, G.R. Are omega 3 fatty acids essential nutrients for mammals? World Rev. Nutr. Diet. 1993, 72, 128–147. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef] [PubMed]
- Mampuya, W.M. Cardiac rehabilitation past, present and future: An overview. Cardiovasc. Diagn. Ther. 2012, 2, 38–49. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar]
- Marchioli, R.; Barzi, F.; Bomba, E.; Chieffo, C.; Di Gregorio, D.; Di Mascio, R.; Franzosi, M.G.; Geraci, E.; Levantesi, G.; Maggioni, A.P.; et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002, 105, 1897–1903. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef]
- Petrone, A.B.; Weir, N.; Hanson, N.Q.; Glynn, R.; Tsai, M.Y.; Gaziano, J.M.; Djoussé, L. Omega-6 fatty acids and risk of heart failure in the Physicians’ Health Study. Am. J. Clin. Nutr. 2013, 97, 66–71. [Google Scholar] [CrossRef]
- Ouchi, S.; Miyazaki, T.; Shimada, K.; Sugita, Y.; Shimizu, M.; Murata, A.; Kato, T.; Aikawa, T.; Suda, S.; Shiozawa, T.; et al. Decreased circulating dihomo-gamma-linolenic acid levels are associated with total mortality in patients with acute cardiovascular disease and acute decompensated heart failure. Lipids Health Dis. 2017, 16, 150. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Watanabe, M.; Kawasaki, C.; Kuroda, I.; Abe, H.; Date, M.; Ueda, Y.; Yasumura, Y.; Koretsune, Y. A novel scoring system to predict delirium and its relationship with the clinical course in patients with acute decompensated heart failure. J. Cardiol. 2017, 71, 564–569. [Google Scholar] [CrossRef]
- McKee, P.A.; Castelli, W.P.; McNamara, P.M.; Kannel, W.B. The natural history of congestive heart failure: The Framingham study. N. Engl. J. Med. 1971, 285, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Mickley, H.; Crea, F.; Van de Werf, F.; et al. Fourth universal definition of myocardial infarction (2018). Kardiol. Pol. 2018, 76, 1383–1415. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, S.; Miyazaki, T.; Shimada, K.; Sugita, Y.; Shimizu, M.; Murata, A.; Kato, T.; Aikawa, T.; Suda, S.; Shiozawa, T.; et al. Low Docosahexaenoic Acid, Dihomo-Gamma-Linolenic Acid, and Arachidonic Acid Levels Associated with Long-Term Mortality in Patients with Acute Decompensated Heart Failure in Different Nutritional Statuses. Nutrients 2017, 9, 956. [Google Scholar] [CrossRef]
- Sanford, A.M.; Flaherty, J.H. Do nutrients play a role in delirium? Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 45–50. [Google Scholar] [CrossRef]
- Kuszewski, J.C.; Wong, R.H.X.; Howe, P.R.C. Effects of Long-Chain Omega-3 Polyunsaturated Fatty Acids on Endothelial Vasodilator Function and Cognition-Are They Interrelated? Nutrients 2017, 9, 487. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. The omega-6/omega-3 ratio and dementia or cognitive decline: A systematic review on human studies and biological evidence. J. Nutr. Gerontol. Geriatr. 2013, 32, 1–23. [Google Scholar] [CrossRef]
- Zamroziewicz, M.K.; Paul, E.J.; Zwilling, C.E.; Barbey, A.K. Predictors of Memory in Healthy Aging: Polyunsaturated Fatty Acid Balance and Fornix White Matter Integrity. Aging Dis. 2017, 8, 372–383. [Google Scholar] [CrossRef]
- Burkhart, C.S.; Dell-Kuster, S.; Siegemund, M.; Pargger, H.; Marsch, S.; Strebel, S.P.; Steiner, L.A. Effect of n-3 fatty acids on markers of brain injury and incidence of sepsis-associated delirium in septic patients. Acta Anaesthesiol. Scand. 2014, 58, 689–700. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef]
- Arai, K.; Koba, S.; Yokota, Y.; Tsunoda, F.; Tsujita, H.; Kondo, S.; Tsukamoto, S.; Shoji, M.; Shinke, T. Relationships of Fatty Acids, Delta-5 Desaturase Activity, and Lipid Profiles in Men with Acute Coronary Syndrome. J. Atheroscler. Thromb. 2020, 27, 1216–1229. [Google Scholar] [CrossRef]
- Tsurutani, Y.; Inoue, K.; Sugisawa, C.; Saito, J.; Omura, M.; Nishikawa, T. Increased Serum Dihomo-γ-linolenic Acid Levels Are Associated with Obesity, Body Fat Accumulation, and Insulin Resistance in Japanese Patients with Type 2 Diabetes. Intern. Med. 2018, 57, 2929–2935. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Shah, H.; Xu, Y.; Qian, S. Delta-5-desaturase: A novel therapeutic target for cancer management. Transl. Oncol. 2021, 14, 101207. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, L.; Qi, H.; Hu, C.; Jia, X.; Lin, H.; Wang, S.; Lin, L.; Zhang, Y.; Zheng, R.; et al. Brain tissue- and cell type-specific eQTL Mendelian randomization reveals efficacy of FADS1 and FADS2 on cognitive function. Transl. Psychiatry 2024, 14, 77. [Google Scholar] [CrossRef]
- Balk, E.M.; Lichtenstein, A.H. Omega-3 Fatty Acids and Cardiovascular Disease: Summary of the 2016 Agency of Healthcare Research and Quality Evidence Review. Nutrients 2017, 9, 865. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.P.; Tremblay, A.J.; Lépine, M.C.; Lemelin, V.; Lamarche, B.; Couture, P. Substitution of dietary ω-6 polyunsaturated fatty acids for saturated fatty acids decreases LDL apolipoprotein B-100 production rate in men with dyslipidemia associated with insulin resistance: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 26–34. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Fleming, J.A. Emerging nutrition science on fatty acids and cardiovascular disease: Nutritionists’ perspectives. Adv. Nutr. 2015, 6, 326s–337s. [Google Scholar] [CrossRef]
- Hegsted, D.M.; Ausman, L.M.; Johnson, J.A.; Dallal, G.E. Dietary fat and serum lipids: An evaluation of the experimental data. Am. J. Clin. Nutr. 1993, 57, 875–883. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Miller, N.H.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk. Circulation 2014, 129, S76–S99. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Kiage, J.N.; Merrill, P.D.; Robinson, C.J.; Cao, Y.; Malik, T.A.; Hundley, B.C.; Lao, P.; Judd, S.E.; Cushman, M.; Howard, V.J.; et al. Intake of trans fat and all-cause mortality in the Reasons for Geographical and Racial Differences in Stroke (REGARDS) cohort. Am. J. Clin. Nutr. 2013, 97, 1121–1128. [Google Scholar] [CrossRef]
| Delirium Group (n = 55) | Non-Delirium Group (n = 535) | p | |
|---|---|---|---|
| Age, years | 80.3 ± 10.9 | 69.0 ± 13.8 | <0.001 |
| Male, n (%) | 28 (51) | 372 (70) | 0.01 |
| Body mass index, kg/m2 | 22.4 ±3.9 | 23.7 ± 4.5 | 0.02 |
| Left ventricular ejection fraction, % | 55 ± 14 | 55 ± 16 | 0.70 |
| Comorbidities on admission | |||
| Dementia, n (%) | 16 (29) | 11 (2) | <0.001 |
| Atrial fibrillation, n (%) | 15 (28) | 83 (16) | 0.03 |
| Malignancy, n (%) | 11 (20) | 59 (11) | 0.05 |
| Hypertension, n (%) | 35 (64) | 279 (52) | 0.10 |
| Diabetes mellitus, n (%) | 23 (42) | 154 (29) | 0.12 |
| Cerebral infarction, n (%) | 6 (11) | 42 (7.9) | 0.43 |
| Reason of hospital admission | |||
| Acute decompensated heart failure, n (%) | 35 (64) | 204 (38) | <0.001 |
| Acute coronary syndrome, n (%) | 7 (13) | 185 (35) | 0.001 |
| Aortic disease, n (%) | 4 (7.3) | 16 (3.0) | 0.10 |
| VT/VF, n (%) | 1 (1.8) | 15 (2.8) | 1.00 |
| PTE/DVT, n (%) | 2 (3.6) | 20 (3.7) | 1.00 |
| Others, n (%) | 6 (11) | 95 (18) | 0.54 |
| Laboratory data | |||
| Albumin, g/dL | 3.13 ± 0.57 | 3.47 ± 0.56 | <0.001 |
| CRP, mg/dL | 4.3 ± 6.0 | 1.9 ± 3.5 | <0.001 |
| NT-pro BNP, pg/mL | 14718 ± 22640 | 6202 ± 15499 | <0.001 |
| Total cholesterol, mg/dL | 147.8 ± 50.5 | 163.0 ± 40.4 | 0.002 |
| Creatinine, mg/dL | 1.92 ± 2.22 | 1.44 ± 1.81 | 0.003 |
| LDL-C, mg/dL | 87.6 ± 34.2 | 100.7 ± 33.3 | 0.003 |
| HDL-C, mg/dL | 39.1 ± 12.5 | 43.3 ± 13.8 | 0.02 |
| Triglycerides, mg/dL | 86.0 ± 57.0 | 93.3 ± 52.7 | 0.11 |
| HbA1c, % | 6.43 ± 2.06 | 6.16 ± 0.99 | 0.51 |
| Medication | |||
| Antipsychotics, n (%) | 2 (3.7) | 2 (0.4) | 0.04 |
| Anticoagulants, n (%) | 14 (26) | 89 (17) | 0.10 |
| Nonbenzodiazepines, n (%) | 2 (3.7) | 6 (1.1) | 0.16 |
| Antiplatelets, n (%) | 16 (29) | 194 (36) | 0.29 |
| β-blockers, n (%) | 20 (36) | 169 (32) | 0.50 |
| Calcium channel blockers, n (%) | 15 (27) | 168 (31) | 0.53 |
| Insulin, n (%) | 4 (7) | 30 (6) | 0.55 |
| Statin, n (%) | 16 (29) | 176 (33) | 0.57 |
| Oral hypoglycemic agents, n (%) | 11 (20) | 93 (18) | 0.59 |
| ACE-I/ARBs, n (%) | 20 (36) | 178 (33) | 0.64 |
| Anti-depressants, n (%) | 0 (0) | 3 (0.6) | 1.0 |
| Anxiolytic drugs, n (%) | 0 (0) | 8 (1.5) | 1.0 |
| Benzodiazepines, n (%) | 1 (1.9) | 19 (3.6) | 1.0 |
| Maximum ISDSC Score | Duration of Delirium | |||
|---|---|---|---|---|
| r | p | r | p | |
| DGLA (mg/dL) | −0.231 | <0.001 | −0.134 | 0.001 |
| AA (mg/dL) | −0.131 | 0.002 | −0.120 | 0.004 |
| EPA (mg/dL) | −0.064 | 0.120 | −0.080 | 0.052 |
| DHA (mg/dL) | −0.048 | 0.236 | −0.079 | 0.055 |
| EPA/AA | −0.001 | 0.976 | −0.031 | 0.456 |
| AA/DGLA | 0.168 | <0.001 | 0.069 | 0.093 |
| Univariate | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| DGLA, 1 increase | 0.92 | 0.90–0.96 | <0.001 | 0.93 | 0.90–0.97 | <0.001 | 0.94 | 0.90–0.97 | 0.001 | 0.95 | 0.91–0.99 | 0.009 |
| DGLA as a categorical variable | ||||||||||||
| 1st quartile | 1.00 | reference | 1.00 | reference | 1.00 | reference | 1.00 | reference | ||||
| 2nd quartile | 0.30 | 0.14–0.62 | 0.001 | 0.33 | 0.15–0.70 | 0.004 | 0.30 | 0.13–0.69 | 0.005 | 0.35 | 0.15–0.83 | 0.02 |
| 3rd quartile | 0.22 | 0.10–0.49 | <0.001 | 0.23 | 0.10–0.55 | <0.001 | 0.20 | 0.08–0.52 | 0.001 | 0.26 | 0.09–0.70 | 0.008 |
| 4th quartile | 0.13 | 0.05–0.35 | <0.001 | 0.20 | 0.07–0.57 | 0.003 | 0.21 | 0.07–0.62 | 0.005 | 0.22 | 0.06–0.79 | 0.02 |
| AA/DGLA, 1 increase | 1.33 | 1.18–1.50 | <0.001 | 1.29 | 1.14–1.47 | <0.001 | 1.30 | 1.13–1.49 | <0.001 | 1.28 | 1.11–1.48 | <0.001 |
| AA/DGLA as a categorical variable | ||||||||||||
| 1st quartile | 1.00 | reference | 1.00 | reference | 1.00 | reference | 1.00 | reference | ||||
| 2nd quartile | 2.28 | 0.77–6.73 | 0.14 | 1.62 | 0.53–4.95 | 0.40 | 1.78 | 0.56–5.70 | 0.33 | 2.20 | 0.62–7.80 | 0.22 |
| 3rd quartile | 2.52 | 0.87–7.36 | 0.09 | 1.51 | 0.50–4.59 | 0.46 | 1.27 | 0.39–4.08 | 0.69 | 1.32 | 0.36–4.78 | 0.67 |
| 4th quartile | 6.34 | 2.37–17.0 | <0.001 | 3.90 | 1.40–10.9 | 0.009 | 3.64 | 1.24–10.7 | 0.02 | 3.74 | 1.12–12.5 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugita-Yamaguchi, Y.; Miyazaki, T.; Shimada, K.; Shimizu, M.; Ouchi, S.; Aikawa, T.; Shiozawa, T.; Takasu, K.; Hiki, M.; Takahashi, S.; et al. Association Between Low Omega-6 Polyunsaturated Fatty Acid Levels and the Development of Delirium in the Coronary Intensive Care Unit. Nutrients 2025, 17, 1979. https://doi.org/10.3390/nu17121979
Sugita-Yamaguchi Y, Miyazaki T, Shimada K, Shimizu M, Ouchi S, Aikawa T, Shiozawa T, Takasu K, Hiki M, Takahashi S, et al. Association Between Low Omega-6 Polyunsaturated Fatty Acid Levels and the Development of Delirium in the Coronary Intensive Care Unit. Nutrients. 2025; 17(12):1979. https://doi.org/10.3390/nu17121979
Chicago/Turabian StyleSugita-Yamaguchi, Yurina, Tetsuro Miyazaki, Kazunori Shimada, Megumi Shimizu, Shohei Ouchi, Tatsuro Aikawa, Tomoyuki Shiozawa, Kiyoshi Takasu, Masaru Hiki, Shuhei Takahashi, and et al. 2025. "Association Between Low Omega-6 Polyunsaturated Fatty Acid Levels and the Development of Delirium in the Coronary Intensive Care Unit" Nutrients 17, no. 12: 1979. https://doi.org/10.3390/nu17121979
APA StyleSugita-Yamaguchi, Y., Miyazaki, T., Shimada, K., Shimizu, M., Ouchi, S., Aikawa, T., Shiozawa, T., Takasu, K., Hiki, M., Takahashi, S., Sumiyoshi, K., & Minamino, T. (2025). Association Between Low Omega-6 Polyunsaturated Fatty Acid Levels and the Development of Delirium in the Coronary Intensive Care Unit. Nutrients, 17(12), 1979. https://doi.org/10.3390/nu17121979





