Calcium Supplement Combined with Dietary Supplement Kidtal Can Promote Longitudinal Growth of Long Bone in Calcium-Deficient Adolescent Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Animal Modeling and Drug Delivery
2.4. Blood Sample Collection
2.5. Bone Sample Collection
2.6. Measurement of Serum Bone Metabolism Markers and Mineral Content
2.7. Bone Biomechanical Parameter Measurement
2.8. Micro-Computed Tomography (CT) Analysis
2.9. Histological Analysis
2.10. Elemental Analysis by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.11. Determination of Hydrolyzed Amino Acids in Bamboo Shoot Extracts
2.12. Analysis of Nutritional Components of Bamboo Shoot Extract
2.13. Statistical Analysis
3. Results
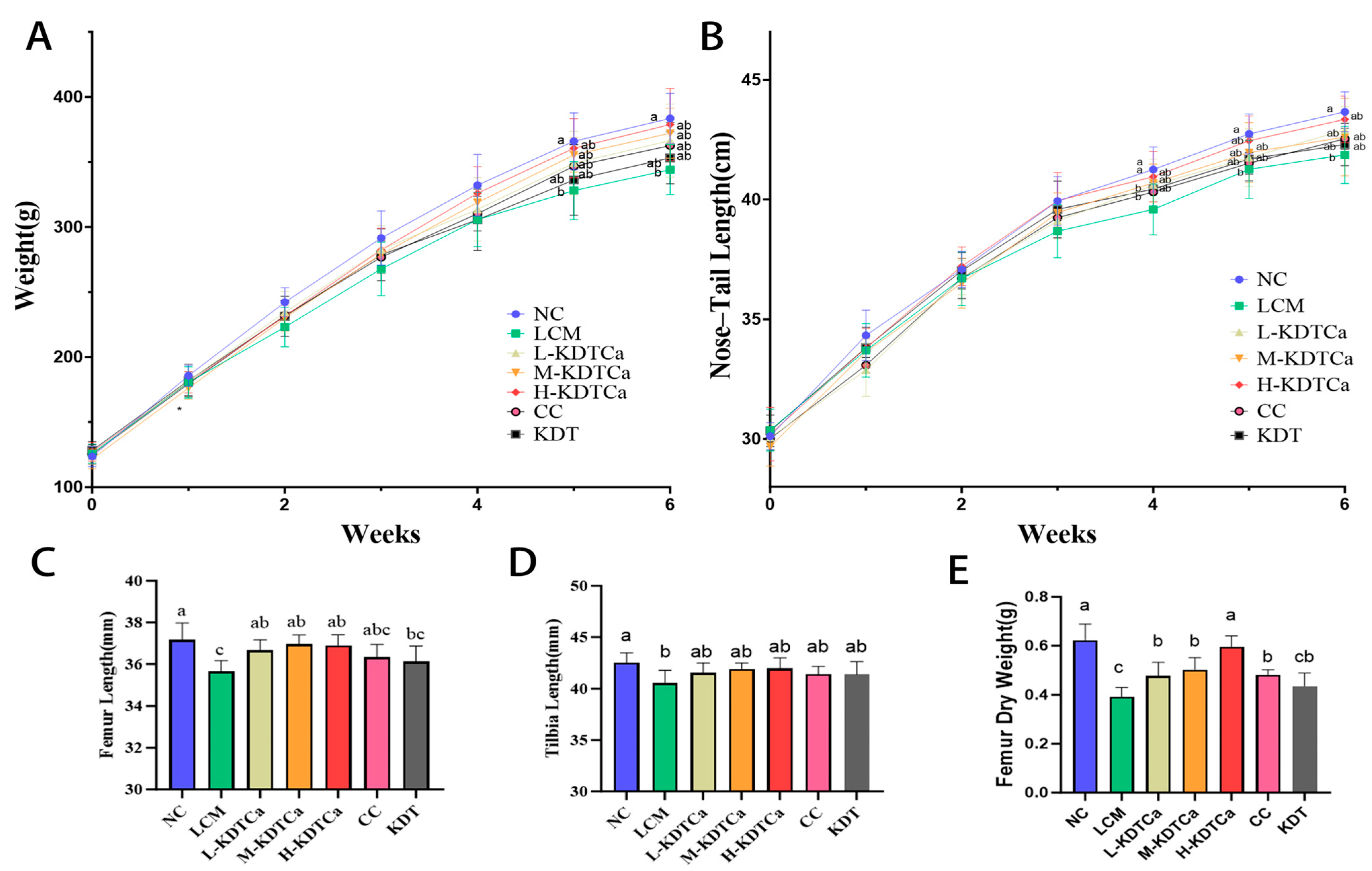
3.1. Effects of KDTCA on Body Weight and Length in Calcium-Deficient Rats
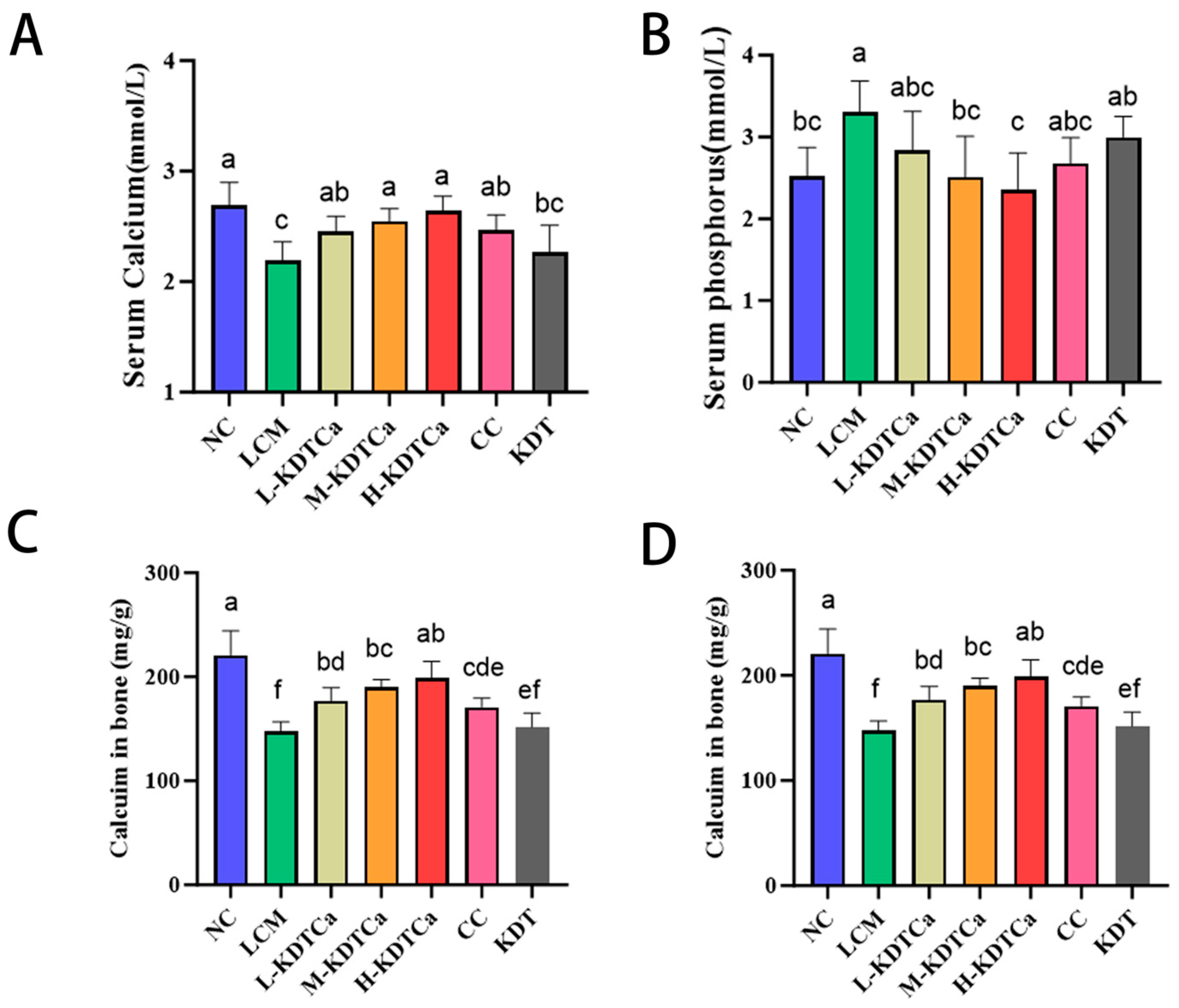
3.2. Biochemical Markers of Bone Metabolism
3.3. Analysis of Serum Biochemical Indices
3.4. Analysis of Bone Biomechanical Indexes
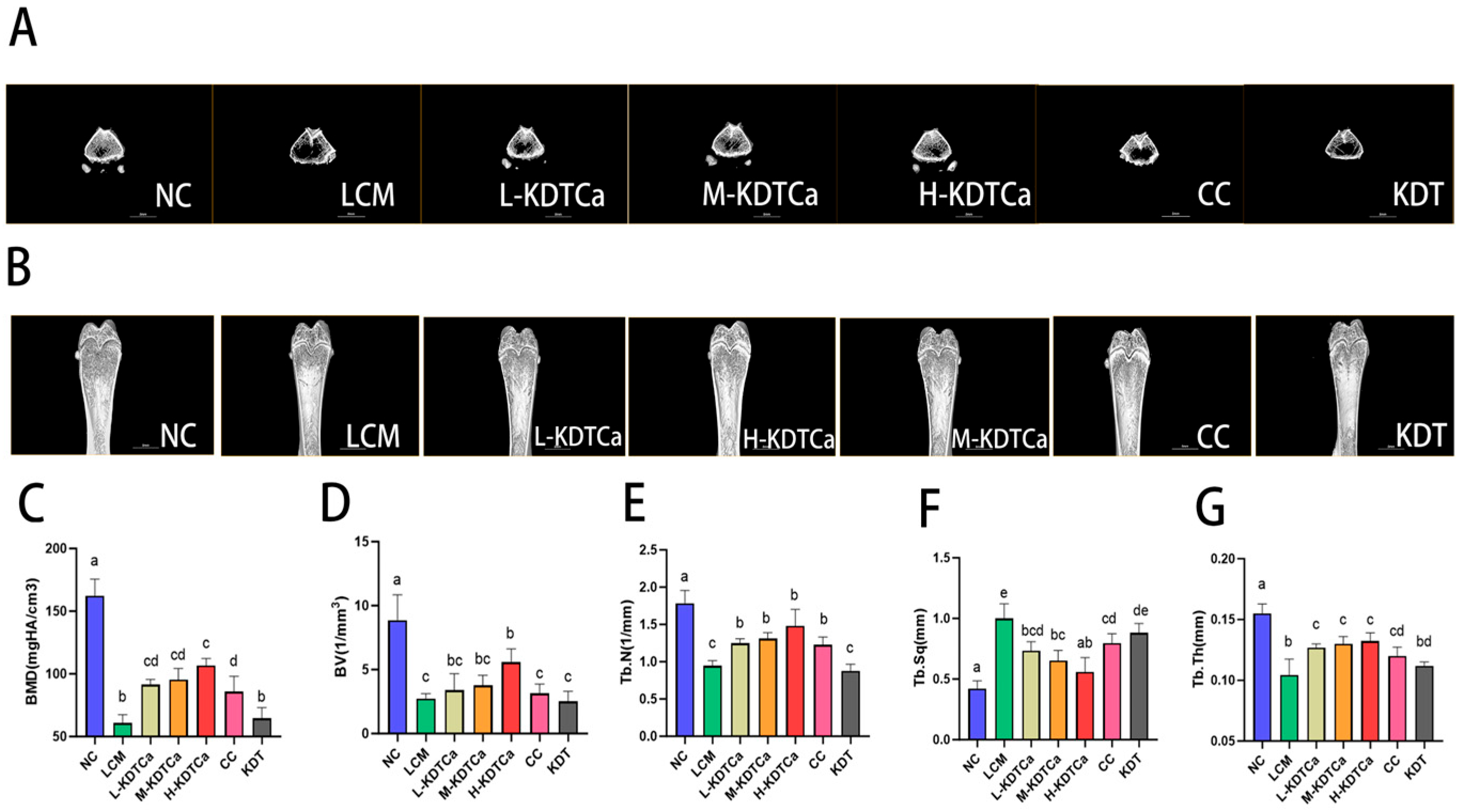
3.5. Bone Microstructural Analysis
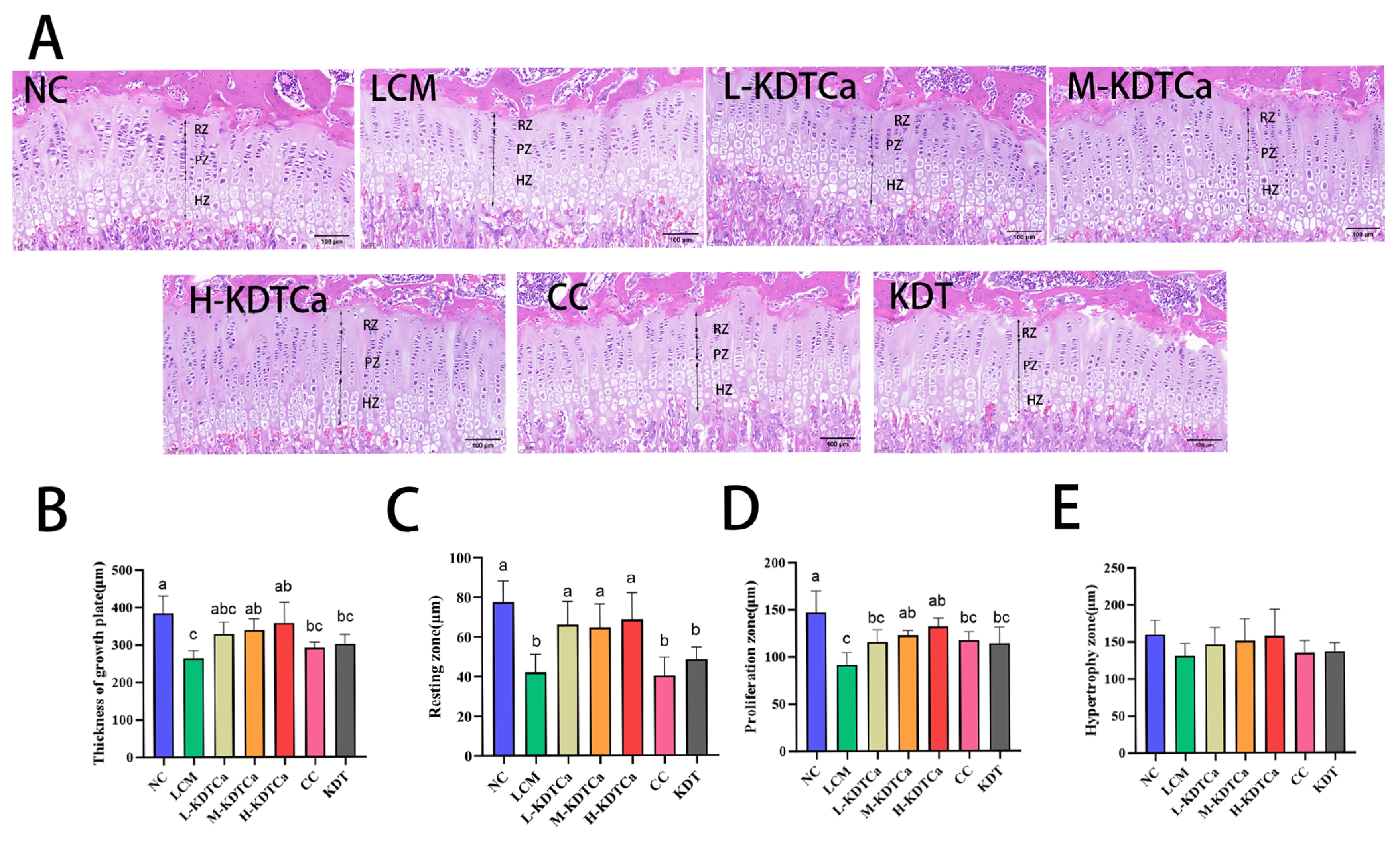
3.6. Effects on the Growth Plate of Tibial
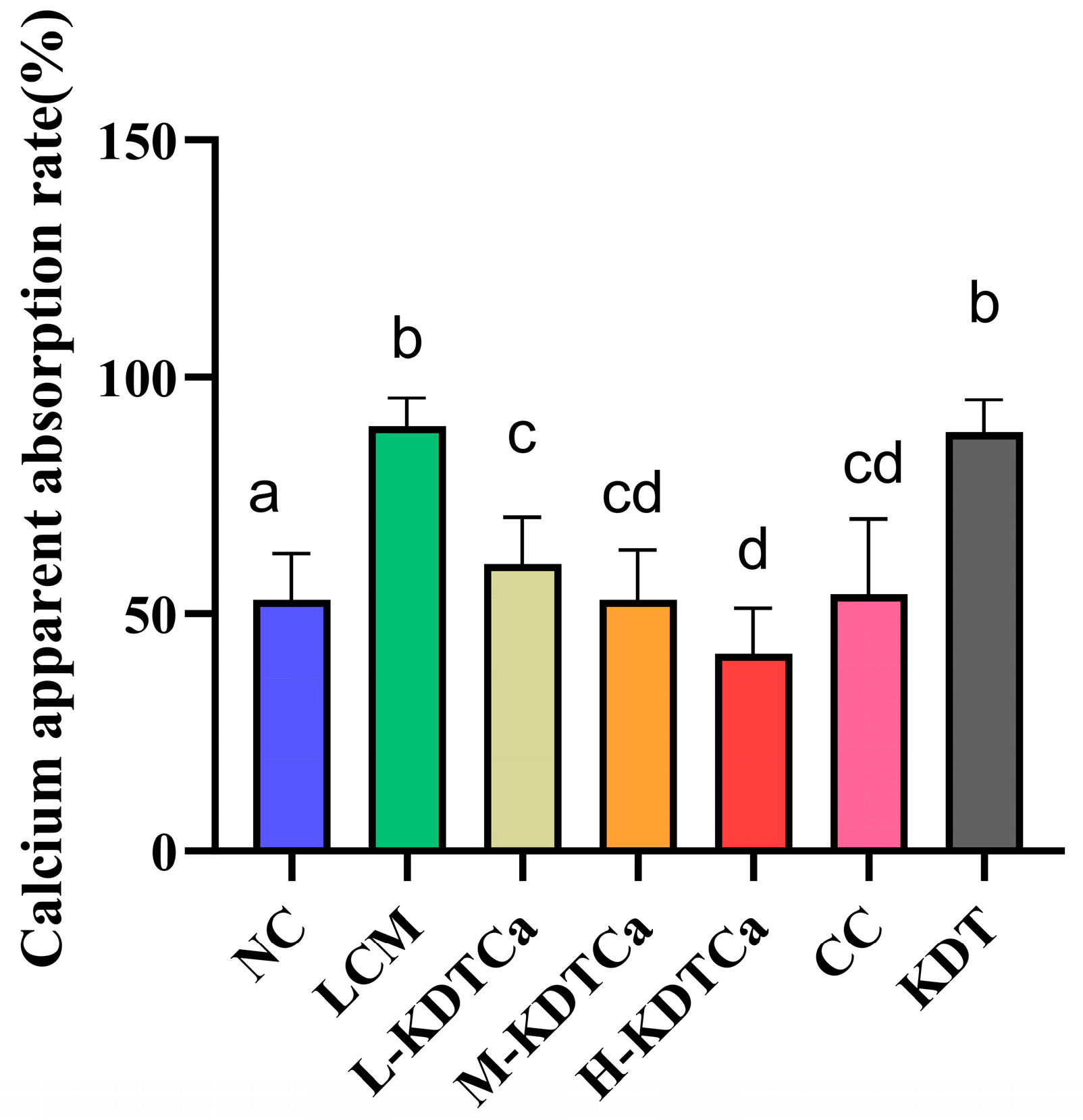
3.7. Effects of Calcium Apparent Absorption Rate
3.8. Nutrient Analysis of Bamboo Shoot Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ala | alanine |
| Arg | arginine |
| Asp | aspartic acid |
| BALP | Bone-Specific Alkaline Phosphatase |
| BMD | bone mineral density |
| KDTCA | bamboo shoot extract + amino acids + calcium |
| KDT | bamboo shoot extract + amino acids |
| BV | bone volume |
| CC | calcium citrate |
| Cys | cysteine |
| GH | growth hormone |
| Glu | glutamic acid |
| Gly | glycine |
| His | histidine |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| IGF-1 | Insulin-like Growth Factor 1 |
| Ile | isoleucine |
| LCM | low-calcium model |
| Leu | leucine |
| Lys | lysine |
| Met | methionine |
| NC | normal calcium |
| Phe | phenylalanine |
| Ser | serine |
| Tb. N | trabecular number |
| Tb. Sq | trabecular separation |
| Tb. Th | trabecular thickness |
| Thr | threonine |
| TRACP | Tartrate-Resistant Acid Phosphatase |
| Tyr | tyrosine |
| Val | valine |
References
- Isaksson, O.G.P.; Lindahl, A.; Nilsson, A.; Isgaard, J. Mechanism of the Stimulatory Effect of Growth Hormone on Longitudinal Bone Growth. Endocr. Rev. 1987, 8, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Johns, R.A.; Stafford, R.S. Americans are not meeting current calcium recommendations. Am. J. Clin. Nutr. 2007, 85, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.L.; Sandberg, D.E.; Rose, S.R.; Leschek, E.W.; Baron, J.; Chipman, J.J.; Cassorla, F.G.; Quigley, C.A.; Crowe, B.J.; Roberts, K.; et al. Psychological Adaptation in Children with Idiopathic Short Stature Treated with Growth Hormone or Placebo. J. Clin. Endocrinol. Metab. 2004, 89, 4873–4878. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, Y.; Bo, Z.; Shunan, W.; Jia, M.; Fen, D.; Min, Y.; Zhixin, Z.; Wenquan, N. Adult Body Height and Cardiometabolic Disease Risk: The China National Health Survey in Shaanxi. Front. Endocrinol. 2020, 11, 587616. [Google Scholar] [CrossRef]
- Yang, Y.X.; Han, J.H.; Shao, X.P.; He, M.; Bian, L.H.; Wang, Z.; Wang, G.D.; Men, J.H. Effect of Micronutrient Supplementation on the Growth of Preschool Children in China. Nutr. Sci. 2002, 15, 196–202. [Google Scholar] [CrossRef]
- Vliet Guy, V.; Styne Dennis, M.; Kaplan Selna, L.; Grumbach Melvin, M. Growth Hormone Treatment for Short Stature. N. Engl. J. Med. 1983, 309, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.J.; Waters, M.J. The growth hormone receptor: Mechanism of activation and clinical implications. Nat. Rev. Endocrinol. 2010, 6, 515–525. [Google Scholar] [CrossRef]
- Cianfarani, S. Safety of Pediatric rhGH Therapy: An Overview and the Need for Long-Term Surveillance. Front. Endocrinol. 2021, 12, 811846. [Google Scholar] [CrossRef]
- Gat-Yablonski, G.; Phillip, M. Nutritionally-Induced Catch-Up Growth. Nutrients 2015, 7, 517–551. [Google Scholar] [CrossRef]
- Carmeliet, G.; Dermauw, V.; Bouillon, R. Vitamin D signaling in calcium and bone homeostasis: A delicate balance. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 621–631. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Y.; Zheng, S.; Chen, Z.; Cai, X.; Wang, S. Food-derived calcium chelating peptides: Biological functional components for better calcium bioavailability. Trends Food Sci. Technol. 2024, 150, 104595. [Google Scholar] [CrossRef]
- Tao, S.; Yu, F.; Song, Y.; Zhou, W.; Lv, J.; Zhao, R.; Wang, C.; Hu, F.; Yuan, H. Water/pH dual responsive in situ calcium supplement collaborates simvastatin for osteoblast promotion mediated osteoporosis therapy via oral medication. J. Control. Release 2021, 329, 121–135. [Google Scholar] [CrossRef]
- Razzaque, M.S. Can adverse effects of excessive vitamin D supplementation occur without developing hypervitaminosis D? J. Steroid Biochem. Mol. Biol. 2018, 180, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Shardell, M.; Sakr Ashour, F.A.; Moaddel, R.; Trehan, I.; Maleta, K.M.; Ordiz, M.I.; Kraemer, K.; Khadeer, M.A.; Ferrucci, L.; et al. Child Stunting is Associated with Low Circulating Essential Amino Acids. eBioMedicine 2016, 6, 246–252. [Google Scholar] [CrossRef]
- Ahmadi, H.; Askari, M.; Suitor, K.; Bellissimo, N.; Azadbakht, L. The association between different types of amino acid intake and physical growth among children. Clin. Nutr. ESPEN 2024, 60, 165–172. [Google Scholar] [CrossRef]
- Yates, H.S.A.; Carter, J.F.; Hungerford, N.L.; Fletcher, M.T. Ion chromatography and ion chromatography/mass spectrometry as a complementary analysis technique for amino acid analysis in food, a review. Food Chem. Adv. 2023, 3, 100415. [Google Scholar] [CrossRef]
- Suen, P.K.; Zheng, L.; Yang, Q.-Q.; Mak, W.S.; Pak, W.Y.; Mo, K.Y.; Chan, M.-L.; Liu, Q.-Q.; Qin, L.; Sun, S.S.-M. Lysine-rich rice partially enhanced the growth and development of skeletal system with better skeletal microarchitecture in young rats. Nutr. Res. 2024, 121, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, H.; Xie, H.; Zhang, J.; Ma, Q.; Wang, S.; Yuan, P.; Xue, H.; Hong, H.; Fan, S.; et al. l-arginine promotes angio-osteogenesis to enhance oxidative stress-inhibited bone formation by ameliorating mitophagy. J. Orthop. Transl. 2024, 46, 53–64. [Google Scholar] [CrossRef]
- Steardo, L.; Iovino, M.; Monteleone, P.; Agrusta, M.; Orio, F. Pharmacological evidence for a dual gabaergic regulation of growth hormone release in humans. Life Sci. 1986, 39, 979–985. [Google Scholar] [CrossRef]
- Arachchilage Hasitha Maduranga Karunarathne, W.; Hyun Choi, Y.; Lee, M.-H.; Kang, C.-H.; Kim, G.-Y. Gamma-aminobutyric acid (GABA)-mediated bone formation and its implications for anti-osteoporosis strategies: Exploring the relation between GABA and GABA receptors. Biochem. Pharmacol. 2023, 218, 115888. [Google Scholar] [CrossRef]
- Wang, H.; Wu, B.; Zhang, J.; Liu, Y.; Zhang, M.; Chen, L.; Zhao, W.; Kan, H.; Cao, C. Bamboo shoots improve the nutritional and sensory quality, and change flavor composition of chicken soup. Food Chem. X 2024, 21, 101140. [Google Scholar] [CrossRef]
- Wang, M.; Yu, A.; Hu, W.; Zhang, Z.; Wang, Z.; Meng, Y.; Yang, B.; Kuang, H. Extraction, purification, structural characteristic, health benefit, and product application of the polysaccharides from bamboo shoot: A review. Int. J. Biol. Macromol. 2024, 271, 132581. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.H.; Cho, N.; Kim, Y.S.; Song, J.; Kim, H. Effects of Eleutherococcus Extract Mixture on Endochondral Bone Formation in Rats. Int. J. Mol. Sci. 2019, 20, 1253. [Google Scholar] [CrossRef]
- Dai, T.Y. Antarctic krill oil promotes longitudinal bone growth in adolescent male mice. Food Biosci. 2019, 28, 170–176. [Google Scholar]
- Xu, Y.; Zhang, D.; Liu, H.; Wang, Z.; Hui, T.; Sun, J. Comprehensive Evaluation of Volatile and Nonvolatile Compounds in Oyster Cuts of Roasted Lamb at Different Processing Stages Using Traditional Nang Roasting. Foods 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Becerra, E.; Jímenez-Mendoza, D.; Mutis-Gonzalez, N.; Pineda-Gomez, P.; Rojas-Molina, I.; Rodríguez-García, M.E. Calcium Deficiency in Diet Decreases the Magnesium Content in Bone and Affects Femur Physicochemical Properties in Growing Rats. Biol. Trace Elem. Res. 2020, 197, 224–232. [Google Scholar] [CrossRef]
- Viguet-Carrin, S.; Hoppler, M.; Membrez Scalfo, F.; Vuichoud, J.; Vigo, M.; Offord, E.A.; Ammann, P. Peak bone strength is influenced by calcium intake in growing rats. Bone 2014, 68, 85–91. [Google Scholar] [CrossRef]
- Zhao, N.; Hu, J.; Hou, T.; Ma, Z.; Wang, C.; He, H. Effects of desalted duck egg white peptides and their products on calcium absorption in rats. J. Funct. Foods 2014, 8, 234–242. [Google Scholar] [CrossRef]
- Peng, Z.; Hou, H.; Zhang, K.; Li, B. Effect of calcium-binding peptide from Pacific cod (Gadus macrocephalus) bone on calcium bioavailability in rats. Food Chem. 2017, 221, 373–378. [Google Scholar] [CrossRef]
- Hua, P.; Xiong, Y.; Yu, Z.; Liu, B.; Zhao, L. Effect of Chlorella Pyrenoidosa Protein Hydrolysate-Calcium Chelate on Calcium Absorption Metabolism and Gut Microbiota Composition in Low-Calcium Diet-Fed Rats. Mar. Drugs 2019, 17, 348. [Google Scholar] [CrossRef] [PubMed]
- Kopic, S.; Geibel, J.P. Gastric Acid, Calcium Absorption, and Their Impact on Bone Health. Physiol. Rev. 2013, 93, 189–268. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, C.; Qin, X.; Zhang, H.; Zhang, Z.; Richel, A. Modulation of gut microbiota by chondroitin sulfate calcium complex during alleviation of osteoporosis in ovariectomized rats. Carbohydr. Polym. 2021, 266, 118099. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Don-Wauchope, A.; Douville, P.; Albert, C.; Vasikaran, S.D. Current use of bone turnover markers in the management of osteoporosis. Clin. Biochem. 2022, 109, 1–10. [Google Scholar] [CrossRef]
- Hu, G.; Sun, X.; Hao, S.; Li, X.; Qian, M.; Dou, L.; Zhang, M.; Hou, P.; Su, L.; Zhao, L.; et al. Effect of sheep bone protein hydrolysate on promoting calcium absorption and enhancing bone quality in low-calcium diet fed rats. Food Chem. 2024, 446, 138763. [Google Scholar] [CrossRef]
- Shi, B.; Lin, C.C.; Lee, C.J.; Ning, D.S.; Lin, C.C.; Zhao, H.W.; Yang, C.S.; Deng, S.X.; Chiu, Y.J.; Wang, C.C. Anti-osteoporotic effects of Yi Mai Jian on bone metabolism of ovariectomized rats. Front. Pharmacol. 2024, 15, 1326415. [Google Scholar] [CrossRef]
- Wang, X. Role of clinical bioinformatics in the development of network-based Biomarkers. J. Clin. Bioinform. 2011, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, X.; Wan, C.; Zhao, Q.; Zhang, L.; Zhou, Q.; Deng, L. Effects of insulin and insulin-like growth factor 1 on osteoblast proliferation and differentiation: Differential signalling via Akt and ERK. Cell Biochem. Funct. 2012, 30, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Nejati, M.; Dehghan, P.; Safari, S.; Jamilian, P.; Zarezadeh, M. The influence of arginine supplementation on IGF-1: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2023, 55, 51–57. [Google Scholar] [CrossRef]
- Stroup, B.M.; Li, X.; Ho, S.; Zhouyao, H.; Chen, Y.; Ani, S.; Dawson, B.; Jin, Z.; Marom, R.; Jiang, M.-M.; et al. Delayed skeletal development and IGF-1 deficiency in a mouse model of lysinuric protein intolerance. Dis. Models Mech. 2023, 16, dmm050118. [Google Scholar] [CrossRef]
- Feng, L.; Lang, Y.; Sun, L.; Shi, W.; Chen, X.; Xia, Y.; Xu, H.; Liu, Y. Ghrelin alleviated TiO2 NPs-induced inhibition of endochondral osteogenesis and promoted longitudinal growth of long bones in juvenile rats via Wnt/β-catenin signaling pathway. Environ. Pollut. 2024, 363, 125185. [Google Scholar] [CrossRef] [PubMed]
- Hallett, S.A.; Ono, W.; Ono, N. Growth Plate Chondrocytes: Skeletal Development, Growth and Beyond. Int. J. Mol. Sci. 2019, 20, 6009. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef]
- Hu, X.; Yang, L.; Du, Y.; Meng, X.; Shi, Y.; Zeng, J. Astragalus polysaccharide promotes osteogenic differentiation of human bone marrow derived mesenchymal stem cells by facilitating ANKFY1 expression through miR-760 inhibition. Bone Jt. Res. 2023, 12, 476–485. [Google Scholar] [CrossRef]
- Lu, S.-H.; Hsia, Y.-J.; Shih, K.-C.; Chou, T.-C. Fucoidan Prevents RANKL-Stimulated Osteoclastogenesis and LPS-Induced Inflammatory Bone Loss via Regulation of Akt/GSK3β/PTEN/NFATc1 Signaling Pathway and Calcineurin Activity. Mar. Drugs 2019, 17, 345. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, X.; Hao, G.; Zhang, M.; Weng, W. Preparation and bioavailability of calcium-chelating peptide complex from tilapia skin hydrolysates. J. Sci. Food Agric. 2017, 97, 4898–4903. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Feng, Y.; Jiang, S.; Zhao, Y.; Zeng, M. Preparation, characterization of L-aspartic acid chelated calcium from oyster shell source and its calcium supplementation effect in rats. J. Funct. Foods 2020, 75, 104249. [Google Scholar] [CrossRef]
- Chen, D.; Mu, X.; Huang, H.; Nie, R.; Liu, Z.; Zeng, M. Isolation of a calcium-binding peptide from tilapia scale protein hydrolysate and its calcium bioavailability in rats. J. Funct. Foods 2014, 6, 575–584. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, H.-M.; Jeong, H.-J. Estrogen-like osteoprotective effects of glycine in in vitro and in vivo models of menopause. Amino Acids 2016, 48, 791–800. [Google Scholar] [CrossRef]
- Han, N.-R.; Kim, H.-Y.; Yang, W.M.; Jeong, H.-J.; Kim, H.-M. Glutamic acid ameliorates estrogen deficiency–induced menopausal-like symptoms in ovariectomized mice. Nutr. Res. 2015, 35, 774–783. [Google Scholar] [CrossRef]
- Hu, G.; Wang, D.; Su, R.; Corazzin, M.; Liu, X.; Sun, X.; Dou, L.; Liu, C.; Yao, D.; Sun, L.; et al. Calcium-binding capacity of peptides obtained from sheep bone and structural characterization and stability of the peptide-calcium chelate. J. Food Meas. Charact. 2022, 16, 4934–4946. [Google Scholar] [CrossRef]



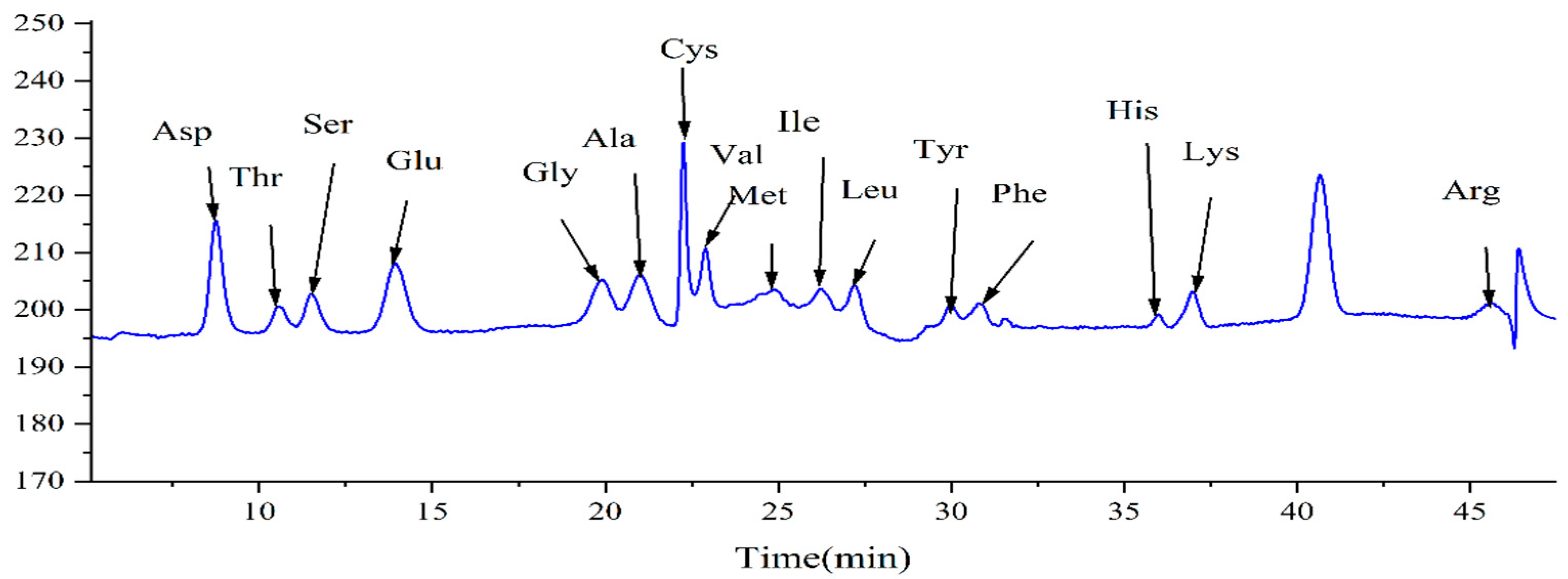
| Name of Amino Acid | Content (mg/g) |
|---|---|
| Asp | 6.283 |
| Thr | 1.624 |
| Ser | 1.990 |
| Glu | 6.218 |
| Gly | 1.502 |
| Ala | 2.958 |
| Cys | 0.661 |
| Val | 2.209 |
| Met | 0.571 |
| Ile | 1.888 |
| Leu | 1.816 |
| Tyr | 1.376 |
| Phe | 1.551 |
| His | 0.478 |
| Lys | 1.847 |
| Arg | 0.996 |
| Total | 33.968 |
| Microelement | Content |
|---|---|
| Total polysaccharides | 27.28% |
| Total proteins | 4.661% |
| Amino acid content | 3.397% |
| Microelement | Content (mg/kg) |
|---|---|
| Na | 13.401 |
| Mg | 2.212 |
| Al | 10.077 |
| K | 2646.339 |
| Mn | 2.117 |
| Fe | 14.374 |
| Cu | 9.421 |
| Zn | 1.977 |
| Ca | 31.828 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Zhang, M.; Zhou, Z.; Guan, H.; Shan, K.; Mi, S.; Ye, X.; Liu, Z.; Yin, J.; Han, N. Calcium Supplement Combined with Dietary Supplement Kidtal Can Promote Longitudinal Growth of Long Bone in Calcium-Deficient Adolescent Rats. Nutrients 2025, 17, 1966. https://doi.org/10.3390/nu17121966
Xie H, Zhang M, Zhou Z, Guan H, Shan K, Mi S, Ye X, Liu Z, Yin J, Han N. Calcium Supplement Combined with Dietary Supplement Kidtal Can Promote Longitudinal Growth of Long Bone in Calcium-Deficient Adolescent Rats. Nutrients. 2025; 17(12):1966. https://doi.org/10.3390/nu17121966
Chicago/Turabian StyleXie, Haosheng, Mingxuan Zhang, Zhengyuan Zhou, Hongyang Guan, Kunmei Shan, Shiwei Mi, Xinfa Ye, Zhihui Liu, Jun Yin, and Na Han. 2025. "Calcium Supplement Combined with Dietary Supplement Kidtal Can Promote Longitudinal Growth of Long Bone in Calcium-Deficient Adolescent Rats" Nutrients 17, no. 12: 1966. https://doi.org/10.3390/nu17121966
APA StyleXie, H., Zhang, M., Zhou, Z., Guan, H., Shan, K., Mi, S., Ye, X., Liu, Z., Yin, J., & Han, N. (2025). Calcium Supplement Combined with Dietary Supplement Kidtal Can Promote Longitudinal Growth of Long Bone in Calcium-Deficient Adolescent Rats. Nutrients, 17(12), 1966. https://doi.org/10.3390/nu17121966





