ALBACOVIDIOL Study: Effect of Calcifediol Treatment on Mortality in Patients Hospitalized for COVID-19: A Retrospective Analysis
Abstract
1. Introduction
2. Patients and Methods
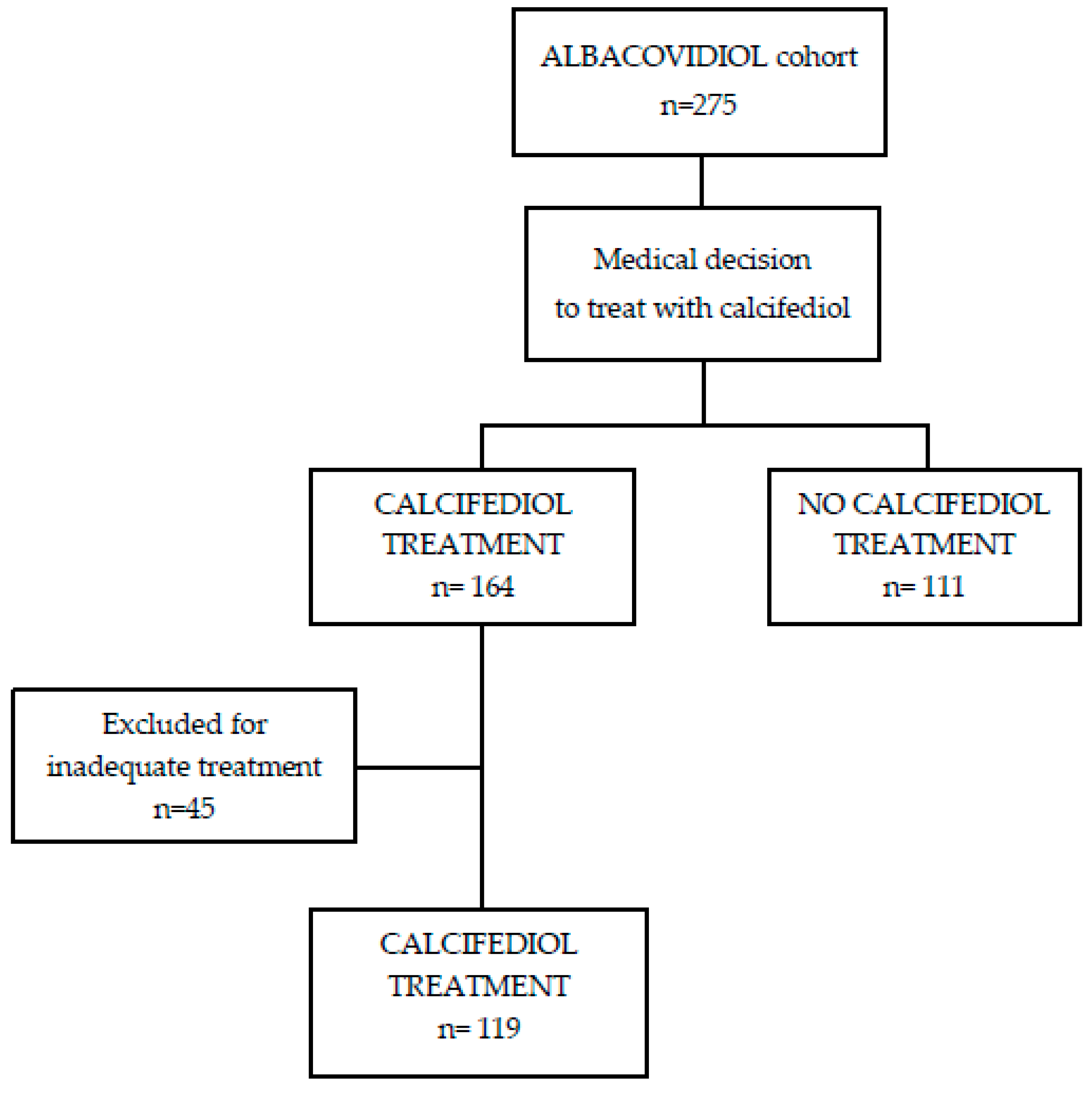
2.1. Overview of the Study
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Pre-Admission Treatment with Vitamin D Metabolites: 25(OH)D Levels and Severity Parameters
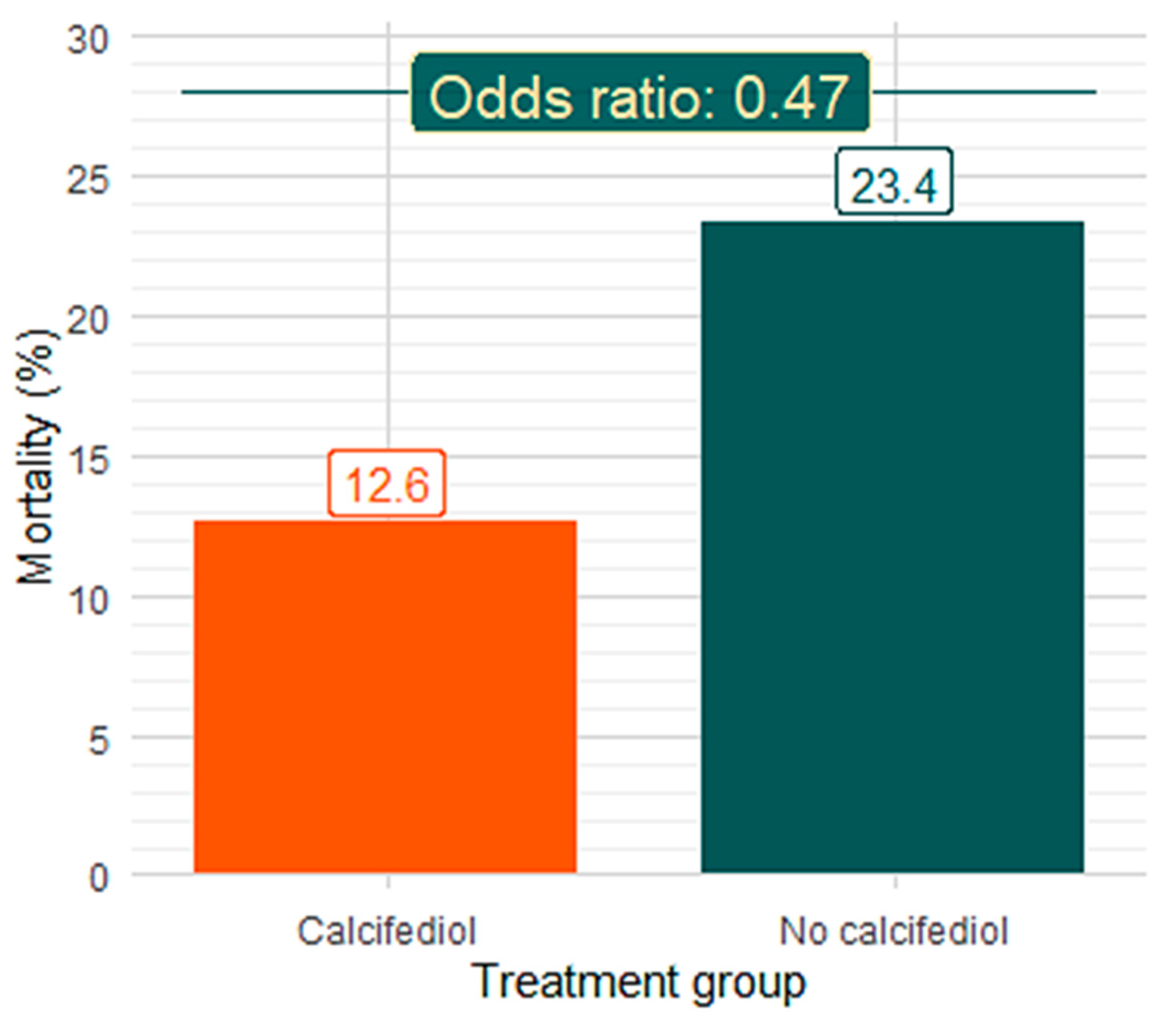
3.3. Effect of In-Hospital Treatment with Calcifediol on Mortality
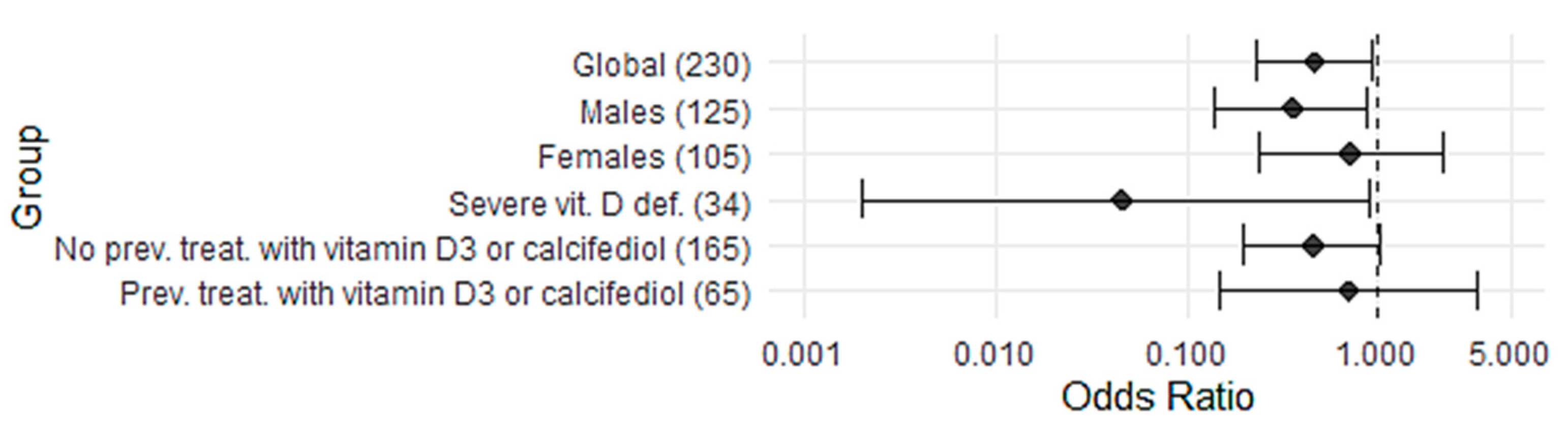
3.4. Effect of Calcifediol Treatment on Mortality According to Severe 25(OH)D Deficiency
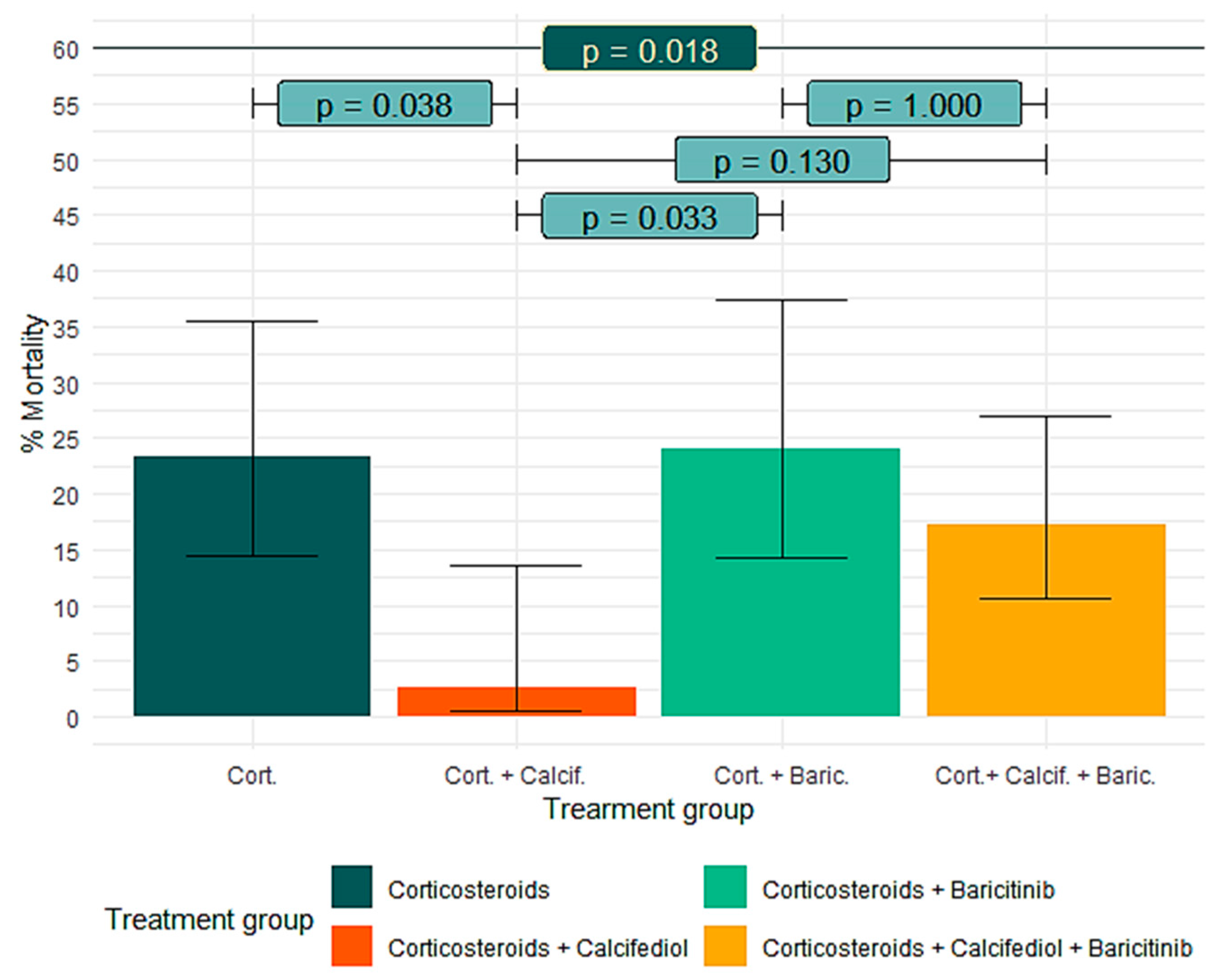
3.5. Effect of Calcifediol on Mortality According to Baricitinib Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Collaborators in Data Collection
References
- Capano, G.; Howlett, M.; Jarvis, D.S.L.; Ramesh, M. Long-Term Policy Impacts of the Coronavirus: Normalization, Adaptation, and Acceleration in the Post-COVID State. Policy Soc. 2022, 41, 1–12. [Google Scholar] [CrossRef]
- Ranabhat, C.L.; Jakovljevic, M.; Kim, C.B.; Simkhada, P. COVID-19 Pandemic: An Opportunity for Universal Health Coverage. Front. Public. Health 2021, 9, 673542. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Yuan, C.; Ma, Z.; Xie, J.; Li, W.; Su, L.; Zhang, G.; Xu, J.; Wu, Y.; Zhang, M.; Liu, W. The Role of Cell Death in SARS-CoV-2 Infection. Signal Transduct. Target. Ther. 2023, 8, 357. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The Immunology and Immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Li, Y.; Huang, J.-A.; Jiang, J.; Su, N. Specific Cytokines in the Inflammatory Cytokine Storm of Patients with COVID-19-Associated Acute Respiratory Distress Syndrome and Extrapulmonary Multiple-Organ Dysfunction. Virol. J. 2021, 18, 117. [Google Scholar] [CrossRef]
- Rysz, S.; Al-Saadi, J.; Sjöström, A.; Farm, M.; Campoccia Jalde, F.; Plattén, M.; Eriksson, H.; Klein, M.; Vargas-Paris, R.; Nyrén, S.; et al. COVID-19 Pathophysiology May Be Driven by an Imbalance in the Renin-Angiotensin-Aldosterone System. Nat. Commun. 2021, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Group, T.R.C. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic Strategies for COVID-19: Progress and Lessons Learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Raharinirina, N.A.; Gubela, N.; Börnigen, D.; Smith, M.R.; Oh, D.Y.; Budt, M.; Mache, C.; Schillings, C.; Fuchs, S.; Dürrwald, R.; et al. SARS-CoV-2 Evolution on a Dynamic Immune Landscape. Nature 2025, 639, 196–204. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune Regulation by Glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Dagens, A.; Sigfrid, L.; Cai, E.; Lipworth, S.; Cheung, V.; Harris, E.; Bannister, P.; Rigby, I.; Horby, P. Scope, Quality, and Inclusivity of Clinical Guidelines Produced Early in the Covid-19 Pandemic: Rapid Review. BMJ 2020, 369, m2371. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf (accessed on 14 April 2025).
- Liu, J.; Zhang, S.; Dong, X.; Li, Z.; Xu, Q.; Feng, H.; Cai, J.; Huang, S.; Guo, J.; Zhang, L.; et al. Corticosteroid Treatment in Severe COVID-19 Patients with Acute Respiratory Distress Syndrome. J. Clin. Investig. 2020, 130, 6417–6428. [Google Scholar] [CrossRef]
- Wang, J.; Yang, W.; Chen, P.; Guo, J.; Liu, R.; Wen, P.; Li, K.; Lu, Y.; Ma, T.; Li, X.; et al. The Proportion and Effect of Corticosteroid Therapy in Patients with COVID-19 Infection: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0249481. [Google Scholar] [CrossRef]
- Sarzani, R.; Spannella, F.; Giulietti, F.; Di Pentima, C.; Giordano, P.; Giacometti, A. Possible Harm from Glucocorticoid Drugs Misuse in the Early Phase of SARS-CoV-2 Infection: A Narrative Review of the Evidence. Intern. Emerg. Med. 2022, 17, 329–338. [Google Scholar] [CrossRef]
- Stebbing, J.; Nievas, G.S.; Falcone, M.; Youhanna, S.; Richardson, P.; Ottaviani, S.; Shen, J.X.; Sommerauer, C.; Tiseo, G.; Ghiadoni, L.; et al. JAK Inhibition Reduces SARS-CoV-2 Liver Infectivity and Modulates Inflammatory Responses to Reduce Morbidity and Mortality. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining Antiviral and Anti-Inflammatory Treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef]
- Damsky, W.; Peterson, D.; Ramseier, J.; Al-Bawardy, B.; Chun, H.; Proctor, D.; Strand, V.; Flavell, R.A.; King, B. The Emerging Role of Janus Kinase Inhibitors in the Treatment of Autoimmune and Inflammatory Diseases. J. Allergy Clin. Immunol. 2021, 147, 814–826. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Entrenas-Castillo, M.; Bouillon, R. Vitamin D Receptor Stimulation to Reduce Acute Respiratory Distress Syndrome (ARDS) in Patients with Coronavirus SARS-CoV-2 Infections: Revised Ms SBMB 2020_166. J. Steroid Biochem. Mol. Biol. 2020, 202, 105719. [Google Scholar] [CrossRef] [PubMed]
- Ismailova, A.; White, J.H. Vitamin D, Infections and Immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Bouillon, R.; Quesada-Gomez, J.M. Vitamin D Endocrine System and COVID-19. JBMR Plus 2021, 5, e10576. [Google Scholar] [CrossRef] [PubMed]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Alcala-diaz, J.F.; Limia-perez, L.; Gomez-huelgas, R.; Martin-escalante, M.D.; Cortes-rodriguez, B.; Zambrana-garcia, J.L.; Entrenas-castillo, M.; Perez-caballero, A.I.; López-carmona, M.D.; Garcia-alegria, J.; et al. Calcifediol Treatment and Hospital Mortality Due to Covid-19: A Cohort Study. Nutrients 2021, 13, 1760. [Google Scholar] [CrossRef]
- Nogues, X.; Ovejero, D.; Pineda-Moncusí, M.; Bouillon, R.; Arenas, D.; Pascual, J.; Ribes, A.; Guerri-Fernandez, R.; Villar-Garcia, J.; Rial, A.; et al. Calcifediol Treatment and COVID-19-Related Outcomes. J. Clin. Endocrinol. Metab. 2021, 106, e4017–e4027. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P. Statistical Algorithms in Review Manager 5. 2010. Available online: https://training.cochrane.org/handbook/current/statistical-methods-revman5 (accessed on 14 April 2025).
- Pagano, M.; Gauvreau, K. Principles of Biostatistics, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2018; pp. 1–585. [Google Scholar] [CrossRef]
- Entrenas-Castillo, M.; Entrenas-Costa, L.M.; Pata, M.P.; Jurado-Gamez, B.; Muñoz-Corroto, C.; Gomez-Rebollo, C.; Mira-Padilla, E.; Bouillon, R.; Quesada-Gómez, J.M. Calcifediol or Corticosteroids in the Treatment of COVID-19: An Observational Study. Nutrients 2024, 16, 1910. [Google Scholar] [CrossRef]
- Entrenas-Castillo, M.; Entrenas-Costa, L.M.; Pata, M.P.; Gámez, B.J.; Muñoz-Corroto, C.; Gómez-Rebollo, C.; Mira-Padilla, E.; Bouillon, R.; Quesada-Gomez, J.M. Latent Class Analysis Reveals, in Patient Profiles, COVID-19-Related Better Prognosis by Calcifediol Treatment than Glucocorticoids. J. Steroid Biochem. Mol. Biol. 2025, 245, 106609. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment with 25-Hydroxyvitamin D3 (Calcifediol) Is Associated with a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients with COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Bli. Endocr. Pract. 2021, 27, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, A.; Leopold, K.; Jackson, S.; Christensen, S.; Nayfeh, T.; Mohammed, K.; Creo, A.; Tebben, P.; Kumar, S. Association of Vitamin D Deficiency with COVID-19 Infection Severity: Systematic Review and Meta-analysis. Clin. Endocrinol. 2021, 96, 281–287. [Google Scholar] [CrossRef]
- Lugg, S.T.; Thickett, D.R. The Role of Vitamin D in COVID-19. In Feldman and Pike’s Vitamin D: Volume Two: Disease and Therapeutics; Academic Press: Cambridge, MA, USA, 2024; pp. 1091–1108. [Google Scholar] [CrossRef]
- González-Molero, I.; Morcillo, S.; Valdés, S.; Pérez-Valero, V.; Botas, P.; Delgado, E.; Hernández, D.; Olveira, G.; Rojo, G.; Gutierrez-Repiso, C.; et al. Vitamin D Deficiency in Spain: A Population-Based Cohort Study. Eur. J. Clin. Nutr. 2011, 65, 321–328. [Google Scholar] [CrossRef]
- Diaz-Curiel, M.; Cabello, A.; Arboiro-Pinel, R.; Mansur, L.; Heili-Frades, S.; Mahillo-Fernandez, I.; Herrero-González, A.; Andrade-Poveda, M. The Relationship between 25(OH) Vitamin D Levels and COVID-19 Onset and Disease Course in Spanish Patients. J. Steroid Biochem. Mol. Biol. 2021, 212, 105928. [Google Scholar] [CrossRef]
- Griffith, G.J.; Morris, T.T.; Tudball, M.J.; Herbert, A.; Mancano, G.; Pike, L.; Sharp, G.C.; Sterne, J.; Palmer, T.M.; Davey Smith, G.; et al. Collider Bias Undermines Our Understanding of COVID-19 Disease Risk and Severity. Nat. Commun. 2020, 11, 5749. [Google Scholar] [CrossRef]
- Smolders, J.; van den Ouweland, J.; Geven, C.; Pickkers, P.; Kox, M. Letter to the Editor: Vitamin D Deficiency in COVID-19: Mixing up Cause and Consequence. Metabolism 2021, 115, 154434. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 Positivity Rates Associated with Circulating 25-Hydroxyvitamin D Levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef]
- Quesada-gomez, J.M.; Lopez-miranda, J.; Entrenas-castillo, M.; Casado-díaz, A.; Nogues, Y.; Solans, X.; Mansur, J.L.; Bouillon, R. Vitamin D Endocrine System and COVID-19. Treatment with Calcifediol. Nutrients 2022, 14, 2716. [Google Scholar] [CrossRef]
- Loucera, C.; Peña-Chilet, M.; Esteban-Medina, M.; Muñoyerro-Muñiz, D.; Villegas, R.; Lopez-Miranda, J.; Rodriguez-Baño, J.; Túnez, I.; Bouillon, R.; Dopazo, J.; et al. Real World Evidence of Calcifediol or Vitamin D Prescription and Mortality Rate of COVID-19 in a Retrospective Cohort of Hospitalized Andalusian Patients. Sci. Rep. 2021, 11, 23380. [Google Scholar] [CrossRef] [PubMed]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Domínguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D Supplementation and COVID-19 Risk: A Population-Based, Cohort Study. J. Endocrinol. Investig. 2021, 45, 167–179. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.S.; Li, B.X.; Song, X.Y.; Zhou, X. Prognostic Value of Interleukin-6, C-Reactive Protein, and Procalcitonin in Patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Gomez, J.M.; Bouillon, R. Is Calcifediol Better than Cholecalciferol for Vitamin D Supplementation? Osteoporos. Int. 2018, 29, 1697–1711. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Stöcklin, E.; Sidelnikov, E.; Willett, W.C.; Edel, J.O.; Stähelin, H.B.; Wolfram, S.; Jetter, A.; Schwager, J.; et al. Oral Supplementation with 25(OH)D3 versus Vitamin D3: Effects on 25(OH)D Levels, Lower Extremity Function, Blood Pressure, and Markers of Innate Immunity. J. Bone Miner. Res. 2012, 27, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Bikle, D. Vitamin D Metabolism Revised: Fall of Dogmas. J. Bone Miner. Res. 2019, 34, 1985–1992. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Matsuyama, T.; Kubli, S.P.; Yoshinaga, S.K.; Pfeffer, K.; Mak, T.W. An Aberrant STAT Pathway Is Central to COVID-19. Cell Death Differ. 2020, 27, 3209–3225. [Google Scholar] [CrossRef]
- Hafezi, S.; Saheb Sharif-Askari, F.; Saheb Sharif-Askari, N.; Ali Hussain Alsayed, H.; Alsafar, H.; Al Anouti, F.; Hamid, Q.; Halwani, R. Vitamin D Enhances Type I IFN Signaling in COVID-19 Patients. Sci. Rep. 2022, 12, 17778. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, B.; Mao, X.; He, J.; Huang, Z.; Zheng, P.; Yu, J.; Han, G.; Liang, X.; Chen, D. Effect of 25-Hydroxyvitamin D3 on Rotavirus Replication and Gene Expressions of RIG-I Signalling Molecule in Porcine Rotavirus-Infected IPEC-J2 Cells. Arch. Anim. Nutr. 2015, 69, 227–235. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef] [PubMed]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine Vitamin D Signaling Switches off Pro-Inflammatory Programs of TH1 Cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef]
- Altmann, D.M.; Whettlock, E.M.; Liu, S.; Arachchillage, D.J.; Boyton, R.J. The Immunology of Long COVID. Nat. Rev. Immunol. 2023, 23, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Efird, J.T.; Anderson, E.J.; Jindal, C.; Suzuki, A. Interaction of Vitamin D and Corticosteroid Use in Hospitalized COVID-19 Patients: A Potential Explanation for Inconsistent Findings in the Literature. Curr. Pharm. Des. 2022, 28, 1695–1702. [Google Scholar] [CrossRef]
- Marcellini, A.; Swieboda, D.; Guedán, A.; Farrow, S.N.; Casolari, P.; Contoli, M.; Johnston, S.L.; Papi, A.; Solari, R. Glucocorticoids Impair Type I IFN Signalling and Enhance Rhinovirus Replication. Eur. J. Pharmacol. 2021, 893, 173839. [Google Scholar] [CrossRef] [PubMed]
- Plaçais, L.; Richier, Q.; Noël, N.; Lacombe, K.; Mariette, X.; Hermine, O. Immune Interventions in COVID-19: A Matter of Time? Mucosal Immunol. 2022, 15, 198–210. [Google Scholar] [CrossRef]
- Kulkarni, N.N.; Gunnarsson, H.I.; Yi, Z.; Gudmundsdottir, S.; Sigurjonsson, O.E.; Agerberth, B.; Gudmundsson, G.H. Glucocorticoid Dexamethasone Down-Regulates Basal and Vitamin D3 Induced Cathelicidin Expression in Human Monocytes and Bronchial Epithelial Cell Line. Immunobiology 2016, 221, 245–252. [Google Scholar] [CrossRef]
- Young, M.J.; Clyne, C.D.; Chapman, K.E. Endocrine Aspects of ACE2 Regulation: RAAS, Steroid Hormones and SARS-CoV-2. J. Endocrinol. 2020, 247, R45–R62. [Google Scholar] [CrossRef]
- Caiazzo, E.; Rezig, A.O.M.; Bruzzese, D.; Ialenti, A.; Cicala, C.; Cleland, J.G.F.; Guzik, T.J.; Maffia, P.; Pellicori, P. Systemic Administration of Glucocorticoids, Cardiovascular Complications and Mortality in Patients Hospitalised with COVID-19, SARS, MERS or Influenza: A Systematic Review and Meta-Analysis of Randomised Trials. Pharmacol. Res. 2022, 176, 106053. [Google Scholar] [CrossRef]
- Pérez-Alba, E.; Nuzzolo-Shihadeh, L.; Aguirre-García, G.M.; Espinosa-Mora, J.; Lecona-Garcia, J.D.; Flores-Pérez, R.O.; Mendoza-Garza, M.; Camacho-Ortiz, A. Baricitinib plus Dexamethasone Compared to Dexamethasone for the Treatment of Severe COVID-19 Pneumonia: A Retrospective Analysis. J. Microbiol. Immunol. Infect. 2021, 54, 787–793. [Google Scholar] [CrossRef]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, J.; Abbas, K.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abbott, A.; et al. Baricitinib in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial and Updated Meta-Analysis. Lancet 2022, 400, 359–368. [Google Scholar] [CrossRef]
- Mizuno, T.; Suzuki, J.; Takahashi, S.; Imai, H.; Itagaki, H.; Yoshida, M.; Endo, S. The Effect of Baricitinib and Corticosteroid Compared to That of Corticosteroid Monotherapy in Severely and Critically Ill Patients with COVID-19: A Japanese Multicenter Inpatient Database Study. J. Infect. Chemother. 2025, 31, 102531. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Calcifediol (N = 119, 51.7%) | Non-Calcifediol (N = 111, 48.3%) | p-Value |
|---|---|---|---|
| Age—years | 72.9 ± 14.2 | 74.0 ± 16.1 | 0.358 |
| Sex, males—n (%) | 63 (52.9) | 62 (55.9) | 0.692 |
| Hypertension—n (%) | 85 (71.4) | 70 (63.1) | 0.206 |
| Diabetes mellitus—n (%) | 34 (28.6) | 31 (29.9) | 1.000 |
| Obesity—n (%) | 30 (25.2) | 22 (19.8) | 0.348 |
| Cardiovascular disease—n (%) δ | 31 (26.1) | 23 (20.7) | 0.355 |
| COPD/Asthma—n (%) | 18 (15.1) | 14 (12.6) | 0.704 |
| Charlson Index—n (%) | |||
| 0 | 48 (43.2%) | 43 (38.7%) | 0.428 |
| 1 | 30 (27.0%) | 20 (18.0%) | |
| 2 | 12 (10.8%) | 21 (18.9%) | |
| 3 | 13 (11.7%) | 8 (7.2%) | |
| 4–8 | 15 (12.6%) | 18 (16.2%) | |
| Corticosteroids—n (%) | 119 (100.0) | 108 (97.2) | 0.111 |
| Baricitinib—n (%) | 81 (61.8) | 50 (38.2) | 0.001 |
| Previous vitamin D or calcifediol treatment—n (%) | 45 (37.8) | 20 (18.0) | 0.001 |
| 25(OH)D (ng/mL)—Median (IQR) ₴ | 17.2 (13.5) | 17.8 (15.6) | 0.761 |
| SpO2 (%) | 89.4 ± 5.5 | 90.2 ± 4.6 | 0.308 |
| SpO2/FiO2 | 319.3 ± 88.6 | 330.9 ± 90.7 | 0.274 |
| Lymphocytes(1000/mL) | 0.93 ± 0.48 | 1.07 ± 1.05 | 0.469 |
| CRP (mg/dL) | 101.1 ± 77.0 | 106.7 ± 75.5 | 0.496 |
| Creatinine (mg/dL) | 1.08 ± 0.44 | 1.36 ± 1.34 | 0.288 |
| LDH (U/L) | 312.4 ± 117.7 | 314.4 ± 126.1 | 0.876 |
| Ferritin (ng/mL)—Median (IQR) | 446 (610) | 603 (1003) | 0.022 |
| IL6 (pg/mL)—Median (IQR) & | 45.8 (75.6) | 53.4 (78.4) | 0.746 |
| D-dimer (ng/mL)—Median (IQR) | 851.5 (734) | 1093 (1725.5) | 0.008 |
| Characteristic | Previous Treatment (N = 65, 28.3%) | No Previous Treatment (N = 165, 71.7%) | p-Value |
|---|---|---|---|
| Age—years | 76.2 ± 13.2 | 72.3 ± 15.7 | 0.118 |
| Sex, males—n (%) | 30 (46.2%) | 95 (57.6%) | 0.142 |
| 25 (OH)D (ng/mL)—Median (IQR) δ | 22.2 (18.9) | 15.2 (13.1) | <0.001 |
| SpO2 (%) | 90.1 ± 4.5 | 89.6 ± 5.3 | 0.584 |
| SpO2/FiO2 | 320.7 ± 94.6 | 326.5 ± 87.8 | 0.608 |
| Lymphocytes (1000/mL) | 0.95 ± 0.43 | 1.01 ± 0.92 | 0.668 |
| CRP (mg/dL) | 102.5 ± 76.8 | 104.1 ± 76.1 | 0.786 |
| Creatinine (mg/dL) | 1.20 ± 0.52 | 1.23 ± 1.13 | 0.223 |
| LDH (U/L) | 297.1 ± 115.5 | 319.8 ± 123.7 | 0.158 |
| Ferritin (ng/mL)—Median (IQR) | 446 (682) | 575 (894) | 0.194 |
| IL6 (pg/mL)—Median (IQR) & | 42.7 (57.1) | 52.6 (82.1) | 0.518 |
| D-dimer (ng/mL)—Median (IQR) | 915.5 (1045.5) 8 | 971 (960.5) | 0.789 |
| Mortality (%) [95% CI] | 8 (12.3%) [6.4–22.5] | 33 (20.0%) [14.6–26.8] | 0.187 |
| Characteristic | Severe vit D Deficiency (N = 34, 14.8%) | No Severe vit D Deficiency (N = 114, 49.6%) | p-Value |
|---|---|---|---|
| Age—years | 78.0 ± 13.7 | 71.6 ± 14.5 | 0.012 |
| Sex, males—n (%) | 17 (50.0%) | 67 (58.8%) | 0.432 |
| Mortality—n (%) | 7 (20.6%) | 19 (16.7%) | 0.612 |
| Hypertension—n (%) | 26 (65.8%) | 75 (65.8%) | 0.297 |
| Diabetes—n (%) | 12 (35.3%) | 30 (26.3%) | 0.386 |
| Obesity—n (%) | 3 (8.8%) | 20 (26.3%) | 0.035 |
| Cardiovascular disease—n (%) | 9 (26.5%) | 28 (24.6%) | 0.824 |
| COPD/Asthma—n (%) | 3 (8.8%) | 15 (13.2%) | 0.765 |
| Corticosteroids—n (%) | 32 (94.1%) | 114 (100%) | 0.052 |
| Baricitinib—n (%) | 16 (47.1%) | 74 (65.5%) | 0.071 |
| Previous vitamin D3 or calcifediol treatment | 4 (11.8%) | 37 (32.5%) | 0.017 |
| SpO2 (%) | 89.5 ± 4.5 | 90.0 ± 4.1 | 0.095 |
| SpO2/FiO2 | 306.5 ± 89.3 | 331.6 ± 91.7 | 0.117 |
| Lymphocytes (1000/mL) | 1.03 ± 0.81 | 1.0 ± 0.96 | 0.834 |
| CRP (mg/dL) | 119.8 ± 93.4 | 103.9 ± 71.1 | 0.545 |
| Creatinine (mg/dL) | 1.23 ± 0.66 | 1.10 ± 0.49 | 0.309 |
| LDH (U/L) | 299.7 ± 135.9 | 326.1 ± 130.8 | 0.149 |
| Ferritin (ng/mL)—Median (IQR) | 450 (1111 | 541 (728) | 0.826 |
| IL6 (pg/mL)—Median (IQR) & | 76.9 (84.4) | 40.7 (68.5) | 0.019 |
| D-dimer (ng/mL)—Median (IQR) | 965.5 (1682.8) | 910.0 (849.0) | 0.082 |
| Calcifediol Mortality (n, %, 95%CI ) | No Calcifediol Mortality (n, %, 95% CI) | OR (95% CI) | p Value | |
|---|---|---|---|---|
| Global—n = 230 | 15/119 (12.6%, 7.8–19.8) | 26/111 (23.4%, 16.5–32.1) | 0.47 (0.23–0.45) | 0.039 |
| Males | 8/63 (12.7%, 6.6–23.1) | 18/62 (29.0%, 19.2–41.3) | 0.36 (0.14–0.89) | 0.029 |
| Women | 7/56 (12.5%, 6.2–23.6) | 8/49 (16.3%, 8.5–29.0) | 0.73 (0.24–2.19) | 0.590 |
| Severe vitamin D deficiency—n = 34 | 0/16 (0%, 0.0–19.4) | 7/18 (38.9%, 20.3–61.4) | 0.05 (0.002–0.90) | 0.008 |
| No previous vitamin D3 or calcifediol treatment—n = 165 | 10/74 (13.5%, 7.5–23.1) | 23/91 (25.3%, 17.5–35.1) | 0.46 (0.20–1.04) | 0.078 |
| Previous vitamin D3 or calcifediol treatment—n = 65 | 5/45 (11.1%, 4.8–23.5) | 3/20 (15.0%, 5.2–36.0) | 0.71 (0.15–3.3) | 0.693 |
| Corticosteroids (N = 60, 61.2%) | Corticosteroids and Calcifediol (N = 38, 38.8%) | Corticosteroids and Baricitinib (N = 50, 38.2%) | Corticosteroids and Calcifediol and Baricitinib (N = 81, 61.8%) | p Value | |
|---|---|---|---|---|---|
| Mortality (%) [95%] CI | 14 (23.3%) [14.4–35.4] | 1 (2.6%) [0.5–13.5] | 12 (24.0%) [14–37.4] | 14 (17.3%) [10.6–26.9] | 0.018 |
| Age—years | 73.8 ± 18.4 | 72.2 ± 15.1 | 74.4 ± 13.3 | 73.1 ± 13.8 | 0.922 |
| Serum 25(OH)D levels—mean | N = 29 17.0 ± 10.6 | N = 28 20.0 ± 15.2 | N = 36 24.2 ± 20.0 | N = 54 20.8 ± 12.3 | 0.285 |
| Previous vitamin D3 or calcifediol treatment (%) | 11 (18.3%) | 19 (50.0%) | 9 (18.0%) | 26 (32.1%) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blázquez-Cabrera, J.A.; Torres-Hernández, J.; Bouillon, R.; Casado-Díaz, A.; Quesada-Gomez, J.M.; Navarro-Casado, L. ALBACOVIDIOL Study: Effect of Calcifediol Treatment on Mortality in Patients Hospitalized for COVID-19: A Retrospective Analysis. Nutrients 2025, 17, 1968. https://doi.org/10.3390/nu17121968
Blázquez-Cabrera JA, Torres-Hernández J, Bouillon R, Casado-Díaz A, Quesada-Gomez JM, Navarro-Casado L. ALBACOVIDIOL Study: Effect of Calcifediol Treatment on Mortality in Patients Hospitalized for COVID-19: A Retrospective Analysis. Nutrients. 2025; 17(12):1968. https://doi.org/10.3390/nu17121968
Chicago/Turabian StyleBlázquez-Cabrera, José Antonio, Javier Torres-Hernández, Roger Bouillon, Antonio Casado-Díaz, José Manuel Quesada-Gomez, and Laura Navarro-Casado. 2025. "ALBACOVIDIOL Study: Effect of Calcifediol Treatment on Mortality in Patients Hospitalized for COVID-19: A Retrospective Analysis" Nutrients 17, no. 12: 1968. https://doi.org/10.3390/nu17121968
APA StyleBlázquez-Cabrera, J. A., Torres-Hernández, J., Bouillon, R., Casado-Díaz, A., Quesada-Gomez, J. M., & Navarro-Casado, L. (2025). ALBACOVIDIOL Study: Effect of Calcifediol Treatment on Mortality in Patients Hospitalized for COVID-19: A Retrospective Analysis. Nutrients, 17(12), 1968. https://doi.org/10.3390/nu17121968






