Caffeine: A Neuroprotectant and Neurotoxin in Traumatic Brain Injury (TBI)
Abstract
1. Introduction to Traumatic Brain Injury (TBI)
2. Pharmacology of Caffeine
| Impact | Mechanism | Pathway | Genes/Proteins |
|---|---|---|---|
| Neuroprotection [29,30] | Reduces cell death and improves cognitive function post-injury [31] | Activates adenosine receptors, leading to downstream neurotransmitter release and neuroinflammatory pathways [30] | Modulates expression of genes involved in inflammation, like TNF-α and IL-1β, and neuronal survival, like BDNF and NGF [32] |
| Anti-inflammatory [31] | Inhibits microglial activation and reduces the release of pro-inflammatory cytokines [31] | Downregulates NF-κB signaling and modulates MAPK pathways [31] | Alters expression of inflammatory mediators (e.g., TNF-α, IL-1β) [30,31] |
| Improvement of Cognitive Function [33] | Enhances synaptic plasticity and neurotransmitter systems (e.g., acetylcholine) [33] | Increases intracellular calcium by inhibiting cAMP phosphodiesterase and directly activating ryanodine receptors [29,32,34] | Upregulates neurotrophic factors (e.g., BDNF, NGF) [29,34] |
3. Numerous Effects of Caffeine in TBI
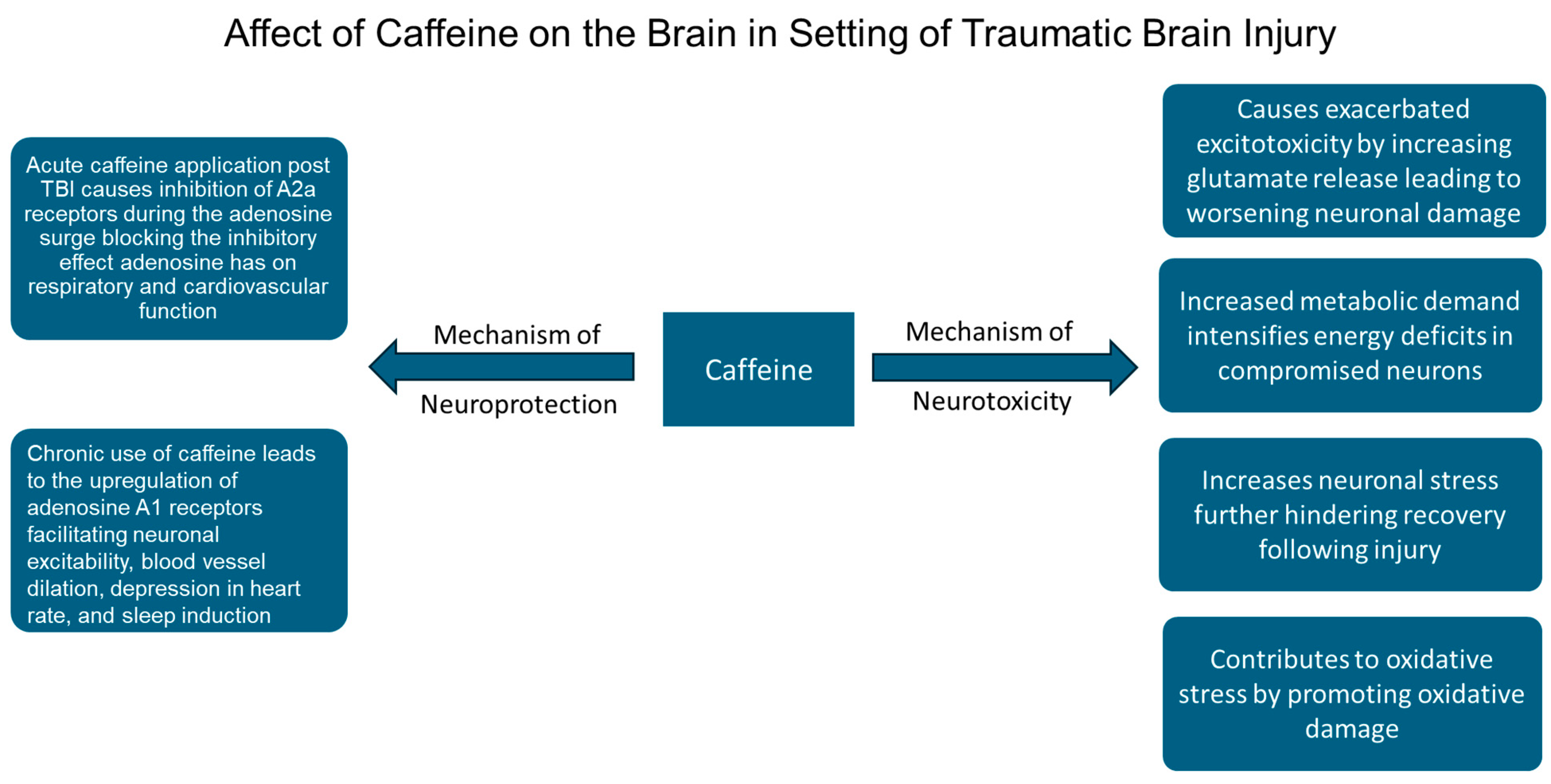
3.1. Caffeine as a Neuroprotectant and Neurotoxin
Caffeine as an Antioxidant and Anti-Inflammatory Pathway Independent of Adenosine
3.2. Effects of Caffeine on a Healthy Brain and an Injured Brain
3.2.1. Healthy Brain
3.2.2. Injured Brain
3.3. Effect of Caffeine on the Immune System in TBI
3.4. Effect of Caffeine on Various Physiological Proteins and Elements in TBI
3.5. How Does Caffeine Interfere with Concussion and TBI Recovery?
3.6. Effect of Preinjury and Post-Injury Exposure to Caffeine
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position Statement: Definition of Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Saatman, K.E.; Duhaime, A.-C.; Bullock, R.; Maas, A.I.R.; Valadka, A.; Manley, G.T. Classification of Traumatic Brain Injury for Targeted Therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Ellenbogen, L.N.; Sekhar, H.B.S. Principles of Neurological Surgery, 4th ed.; Elsevier: New York, NY, USA, 2017; ISBN 9780323461276/9780323431408. [Google Scholar]
- Rincon, S.; Gupta, R.; Ptak, T. Imaging of Head Trauma. Handb. Clin. Neurol. 2016, 135, 447–477. [Google Scholar] [CrossRef] [PubMed]
- Lerner, J.T.; Giza, C.C. Traumatic Brain Injury in Children. In Swaiman’s Pediatric Neurology; Elsevier: New York, NY, USA, 2012; pp. 1087–1125. [Google Scholar] [CrossRef]
- Lafta, G.; Sbahi, H. Factors Associated with the Severity of Traumatic Brain Injury. Med. Pharm. Rep. 2023, 96, 58–64. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention 2023. Available online: https://www.cdc.gov/ (accessed on 3 March 2025).
- Bramlett, H.M.; Dietrich, W.D. Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J. Neurotrauma 2015, 32, 1834–1848. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Jacinto, T.A.; Gonçalves, A.C.; Gaspar, D.; Silva, L.R. Overview of Caffeine Effects on Human Health and Emerging Delivery Strategies. Pharmaceuticals 2023, 16, 1067. [Google Scholar] [CrossRef]
- Justin, E.; John, R.R.; Amanda, S.B. Caffeine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Faudone, G.; Arifi, S.; Merk, D. The Medicinal Chemistry of Caffeine. J. Med. Chem. 2021, 64, 7156–7178. [Google Scholar] [CrossRef] [PubMed]
- Burdan, F. Caffeine in Coffee. In Coffee in Health and Disease Prevention; Elsevier: New York, NY, USA, 2015; pp. 201–207. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sharma, A.; Verma, K.K.; Gaur, P.K.; Kaushik, R.; Abdali, B. A comprehensive review on the pharmacological potentials of Caffeine. J. Appl. Pharm. Sci. Res. 2023, 6, 16–26. [Google Scholar] [CrossRef]
- Grzegorzewski, J.; Bartsch, F.; Köller, A.; König, M. Pharmacokinetics of Caffeine: A Systematic Analysis of Reported Data for Application in Metabolic Phenotyping and Liver Function Testing. Front. Pharmacol. 2022, 12, 752826. [Google Scholar] [CrossRef]
- Schapira, A.H.V. Neurology and Clinical Neuroscience; Edward, B., Ed.; Mosby Elsevier: New York, NY, USA, 2007; p. 1626. ISBN 0323033547/9780323033541. [Google Scholar]
- Belayneh, A.; Molla, F. The Effect of Coffee on Pharmacokinetic Properties of Drugs: A Review. BioMed Res. Int. 2020, 2020, 9703. [Google Scholar] [CrossRef] [PubMed]
- Fiani, B.; Zhu, L.; Musch, B.L.; Briceno, S.; Andel, R.; Sadeq, N.; Ansari, A.Z. The Neurophysiology of Caffeine as a Central Nervous System Stimulant and the Resultant Effects on Cognitive Function. Cureus 2021, 13, e15032. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical, and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Shiva, S.; Manikantan, S.; Ramakrishna, S. Pharmacology of Caffeine and Its Effects on the Human Body. Eur. J. Med. Chem. Rep. 2024, 10, 100138. [Google Scholar] [CrossRef]
- Nowaczewska, M.; Wiciński, M.; Kaźmierczak, W. The Ambiguous Role of Caffeine in Migraine Headache: From Trigger to Treatment. Nutrients 2020, 12, 2259. [Google Scholar] [CrossRef]
- Hong, C.T.; Chan, L.; Bai, C.H. The Effect of Caffeine on the Risk and Progression of Parkinson’s Disease: A Meta-Analysis. Nutrients 2020, 12, 1860, Erratum in Nutrients 2023, 15, 699. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, F.; Liu, X.; Jiang, B.; Li, X.; Wang, Y.; Chen, X.; Su, Y.; Wang, X.; Luo, J.; Chen, L.; et al. Tea, coffee, and caffeine intake and risk of dementia and Alzheimer’s disease: A systematic review and meta-analysis of cohort studies. Food Funct. 2024, 15, 8330–8344. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Hong, C.T.; Bai, C.H. Coffee consumption and the risk of cerebrovascular disease: A meta-analysis of prospective cohort studies. BMC Neurol. 2021, 21, 380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, C.; Tang, H.; Wang, X.; He, J. Coffee Consumption and Stroke Risk: Evidence from a Systematic Review and Meta-Analysis of more than 2.4 Million Men and Women. J. Stroke Cerebrovasc. Dis. 2021, 30, 105452. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.; Calvello, R.; Porro, C.; Messina, G.; Cianciulli, A.; Panaro, M.A. Neurodegenerative Diseases: Can Caffeine Be a Powerful Ally to Weaken Neuroinflammation? Int. J. Mol. Sci. 2022, 23, 12958. [Google Scholar] [CrossRef]
- Kolahdouzan, M.; Hamadeh, M.J. The Neuroprotective Effects of Caffeine in Neurodegenerative Diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Treatment of Traumatic Brain Injury: Nanotherapeutics. J. Clin. Haematol. 2024, 5, 1–3. [Google Scholar] [CrossRef]
- Pugliese, A.M.; Coppi, E.; Spalluto, G.; Corradetti, R.; Pedata, F. A3 Adenosine Receptor Antagonists Delay Irreversible Synaptic Failure Caused by Oxygen and Glucose Deprivation in the Rat CA1 Hippocampus in Vitro. Br. J. Pharmacol. 2006, 147, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Villanueva-García, D.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Domínguez-Oliva, A.; Lezama-García, K.; Miranda-Cortés, A.; Martínez-Burnes, J. Cardiorespiratory and Neuroprotective Effects of Caffeine in Neonate Animal Models. Animals 2023, 13, 1769. [Google Scholar] [CrossRef]
- Vargas-Pozada, E.E.; Ramos-Tovar, E.; Rodriguez-Callejas, J.D.; Cardoso-Lezama, I.; Galindo-Gómez, S.; Talamás-Lara, D.; Vásquez-Garzón, V.R.; Arellanes-Robledo, J.; Tsutsumi, V.; Villa-Treviño, S.; et al. Caffeine Inhibits NLRP3 Inflammasome Activation by Downregulating TLR4/MAPK/NF-ΚB Signaling Pathway in an Experimental NASH Model. Int. J. Mol. Sci. 2022, 23, 9954. [Google Scholar] [CrossRef]
- Tanaka, S.; Koike, T. Caffeine Promotes Survival of Cultured Sympathetic Neurons Deprived of Nerve Growth Factor through a CAMP-Dependent Mechanism. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 1992, 1175, 114–122. [Google Scholar] [CrossRef]
- Lopes, J.P.; Pliássova, A.; Cunha, R.A. The Physiological Effects of Caffeine on Synaptic Transmission and Plasticity in the Mouse Hippocampus Selectively Depend on Adenosine A1 and A2A Receptors. Biochem. Pharmacol. 2019, 166, 313–321. [Google Scholar] [CrossRef]
- Han, K.; Jia, N.; Li, J.; Yang, L.; Min, L.-Q. Chronic Caffeine Treatment Reverses Memory Impairment and the Expression of Brain BNDF and TrkB in the PS1/APP Double Transgenic Mouse Model of Alzheimer’s Disease. Mol. Med. Rep. 2013, 8, 737–740. [Google Scholar] [CrossRef]
- Sachse, K.T.; Jackson, E.K.; Wisniewski, S.R.; Gillespie, D.G.; Puccio, A.M.; Clark, R.S.; Dixon, C.E.; Kochanek, P.M. Increases in Cerebrospinal Fluid Caffeine Concentration Are Associated with Favorable Outcome after Severe Traumatic Brain Injury in Humans. J. Cereb. Blood Flow. Metab. 2008, 28, 395–401. [Google Scholar] [CrossRef]
- Moreira-de-Sá, A.; Lourenço, V.S.; Canas, P.M.; Cunha, R.A. Adenosine A2A Receptors as Biomarkers of Brain Diseases. Front. Neurosci. 2021, 15, 702581. [Google Scholar] [CrossRef]
- Lusardi, T.A.; Lytle, N.K.; Szybala, C.; Boison, D. Caffeine Prevents Acute Mortality after TBI in Rats without Increased Morbidity. Exp. Neurol. 2012, 234, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, T.A.; Lytle, N.K.; Gebril, H.M.; Boison, D. Effects of Preinjury and Postinjury Exposure to Caffeine in a Rat Model of Traumatic Brain Injury. J. Caffeine Adenosine Res. 2020, 10, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.-L.; Yang, N.; Chen, X.; Zhao, Z.-A.; Zhang, X.-Z.; Chen, X.-Y.; Li, P.; Zhao, Y.; Zhou, Y.-G. Chronic Caffeine Exposure Attenuates Blast-Induced Memory Deficit in Mice. Chin. J. Traumatol. 2015, 18, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.-L.; Yang, N.; Chen, X.; Tian, H.-K.; Zhao, Z.-A.; Zhang, X.-Z.; Liu, D.; Li, P.; Zhao, Y.; Peng, Y.; et al. Caffeine Attenuates Brain Injury but Increases Mortality Induced by High-Intensity Blast Wave Exposure. Toxicol. Lett. 2019, 301, 90–97. [Google Scholar] [CrossRef]
- Hall, E.D.; Wang, J.A. Traumatic Brain Injury and Oxidative Stress: Understanding the Role of Reactive Oxygen Species in Brain Injury Pathology. J. Neurochem. 2012, 123, 789–803. [Google Scholar]
- Singh, R.; Kaur, N. N-Acetylcysteine as a Neuroprotective Agent in TBI. Neurotherapeutics 2021, 18, 45–60. [Google Scholar]
- Ahmadipour, M.; Ahmadinejad, M. Effects of Caffeine Administration on GCS and GOSE in Children and Adolescent Patients with Moderate Brain Trauma. Eur. J. Mol. Clin. Med. 2021, 8, 510–520. [Google Scholar]
- Yoon, H.; Ro, Y.S.; Jung, E.; Moon, S.B.; Park, G.J.; Lee, S.G.W.; Shin, S.D. Serum Caffeine Concentration at the Time of Traumatic Brain Injury and Its Long-Term Clinical Outcomes. J. Neurotrauma 2023, 40, 2386–2395. [Google Scholar] [CrossRef]
- Ratliff, W.A.; Saykally, J.N.; Mervis, R.F.; Lin, X.; Cao, C.; Citron, B.A. Behavior, Protein, and Dendritic Changes after Model Traumatic Brain Injury and Treatment with Nanocoffee Particles. BMC Neurosci. 2019, 20, 44. [Google Scholar] [CrossRef]
- Teasdale, G.; Maas, A.; Lecky, F.; Manley, G.; Stocchetti, N.; Murray, G. The Glasgow Coma Scale at 40 Years: Standing the Test of Time. Lancet Neurol. 2014, 13, 844–854. [Google Scholar] [CrossRef]
- Al Moutaery, K.; Al Deeb, S.; Khan, H.A.; Tariq, M. Caffeine Impairs Short-Term Neurological Outcome after Concussive Head Injury in Rats. Neurosurgery 2003, 53, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Yamakawa, G.R.; Salberg, S.; Wang, M.; Kolb, B.; Mychasiuk, R. Caffeine Consumption during Development Alters Spine Density and Recovery from Repetitive Mild Traumatic Brain Injury in Young Adult Rats. Synapse 2020, 74, 22142. [Google Scholar] [CrossRef] [PubMed]
- Eyolfson, E.; Bhatt, D.; Wang, M.; Lohman, A.W.; Mychasiuk, R. Paternal exposure to exercise and/or caffeine and alcohol modify offspring behavioral and pathophysiological recovery from repetitive mild traumatic brain injury in adolescence. Genes Brain Behav. 2021, 20, egbb12736. [Google Scholar] [CrossRef] [PubMed]
- Paiva, I.; Cellai, L.; Meriaux, C.; Poncelet, L.; Nebie, O.; Saliou, J.M.; Lacoste, A.S.; Papegaey, A.; Drobecq, H.; Le Gras, S.; et al. Caffeine intake exerts dual genome-wide effects on hippocampal metabolism and learning-dependent transcription. J. Clin. Investig. 2022, 132, e149371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nehlig, A. Is Caffeine a Cognitive Enhancer? J. Alzheimer’s Dis. 2010, 20, S85–S94. [Google Scholar] [CrossRef]
- Cornelis, M.C.; Weintraub, S.; Morris, M.C. Recent Caffeine Drinking Associates with Cognitive Function in the UK Biobank. Nutrients 2020, 12, 1969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ajjimaporn, A.; Noppongsakit, P.; Ramyarangsi, P.; Siripornpanich, V.; Chaunchaiyakul, R. A low-dose of caffeine suppresses EEG alpha power and improves working memory in healthy University males. Physiol. Behav. 2022, 256, 113955. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Weibel, J.; Landolt, H.P.; Santini, F.; Slawik, H.; Borgwardt, S.; Cajochen, C.; Reichert, C.F. Brain activity during a working memory task after daily caffeine intake and caffeine withdrawal: A randomized double-blind placebo-controlled trial. Sci. Rep. 2023, 13, 1002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kapellou, A.; King, A.; Graham, C.A.M.; Pilic, L.; Mavrommatis, Y. Genetics of caffeine and brain-related outcomes—A systematic review of observational studies and randomized trials. Nutr. Rev. 2023, 81, 1571–1598. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Porkka-Heiskanen, T. Methylxanthines and Sleep. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Djordjevic, N.; Ghotbi, R.; Jankovic, S.; Aklillu, E. Induction of CYP1A2 by Heavy Coffee Consumption Is Associated with the CYP1A2-163C>A Polymorphism. Eur. J. Clin. Pharmacol. 2010, 66, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Rétey, J.V.; Adam, M.; Khatami, R.; Luhmann, U.F.O.; Jung, H.H.; Berger, W.; Landolt, H.P. A Genetic Variation in the Adenosine A2A Receptor Gene (ADORA2A) Contributes to Individual Sensitivity to Caffeine Effects on Sleep. Clin. Pharmacol. Ther. 2007, 81, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Coris, E.E.; Moran, B.; Sneed, K.; Del Rossi, G.; Bindas, B.; Mehta, S.; Narducci, D. Stimulant Therapy Utilization for Neurocognitive Deficits in Mild Traumatic Brain Injury. Sports Health A Multidiscip. Approach. 2022, 14, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Everson, C.A.; Szabo, A.; Plyer, C.; Hammeke, T.A.; Stemper, B.D.; Budde, M.D. Sleep Loss, Caffeine, Sleep Aids, and Sedation Modify Brain Abnormalities of Mild Traumatic Brain Injury. Exp. Neurol. 2024, 372, 114620. [Google Scholar] [CrossRef]
- Temple, J.L. Caffeine Use in Children: What We Know, What We Have Left to Learn, and Why We Should Worry. Neurosci. Biobehav. Rev. 2009, 33, 793–806. [Google Scholar] [CrossRef]
- Landolt, H.-P.; Rétey, J.V.; Tönz, K.; Gottselig, J.M.; Khatami, R.; Buckelmüller, I.; Achermann, P. Caffeine Attenuates Waking and Sleep Electroencephalographic Markers of Sleep Homeostasis in Humans. Neuropsychopharmacology 2004, 29, 1933–1939. [Google Scholar] [CrossRef]
- Yamakawa, G.R.; Lengkeek, C.; Salberg, S.; Spanswick, S.C.; Mychasiuk, R. Behavioral and Pathophysiological Outcomes Associated with Caffeine Consumption and Repetitive Mild Traumatic Brain Injury (RmTBI) in Adolescent Rats. PLoS ONE 2017, 12, e0187218. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Dai, S.; An, J.; Li, P.; Chen, X.; Xiong, R.; Liu, P.; Wang, H.; Zhao, Y.; Zhu, M.; et al. Chronic but Not Acute Treatment with Caffeine Attenuates Traumatic Brain Injury in the Mouse Cortical Impact Model. Neuroscience 2008, 151, 1198–1207. [Google Scholar] [CrossRef]
- Sanjakdar, S.S.; Flerlage, W.J.; Kang, H.S.; Napier, D.A.; Dougherty, J.R.; Mountney, A.; Gilsdorf, J.S.; Shear, D.A. Differential Effects of Caffeine on Motor and Cognitive Outcomes of Penetrating Ballistic-Like Brain Injury. Mil. Med. 2019, 184 (Suppl. S1), 291–300. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Qu, W.-M.; Eguchi, N.; Chen, J.-F.; Schwarzschild, M.A.; Fredholm, B.B.; Urade, Y.; Hayaishi, O. Adenosine A2A, but Not A1, Receptors Mediate the Arousal Effect of Caffeine. Nat. Neurosci. 2005, 8, 858–859. [Google Scholar] [CrossRef]
- Chen, J.-F.; Xu, K.; Petzer, J.P.; Staal, R.; Xu, Y.-H.; Beilstein, M.; Sonsalla, P.K.; Castagnoli, K.; Castagnoli, N.; Schwarzschild, M.A. Neuroprotection by Caffeine and A2A Adenosine Receptor Inactivation in a Model of Parkinson’s Disease. J. Neurosci. 2001, 21, RC143. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, P.M.; Vagni, V.A.; Janesko, K.L.; Washington, C.B.; Crumrine, P.K.; Garman, R.H.; Jenkins, L.W.; Clark, R.S.; Homanics, G.E.; Dixon, C.E.; et al. Adenosine A1 Receptor Knockout Mice Develop Lethal Status Epilepticus after Experimental Traumatic Brain Injury. J. Cereb. Blood Flow. Metab. 2006, 26, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Haselkorn, M.L.; Shellington, D.K.; Jackson, E.K.; Vagni, V.A.; Janesko-Feldman, K.; Dubey, R.K.; Gillespie, D.G.; Cheng, D.; Bell, M.J.; Jenkins, L.W.; et al. Adenosine A1 Receptor Activation as a Brake on the Microglial Response after Experimental Traumatic Brain Injury in Mice. J. Neurotrauma 2010, 27, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Li, P.; Ning, Y.-L.; Jiang, Y.-L.; Yang, N.; Chen, X.; Zhou, Y.-G. Reduction in Blood Glutamate Levels Combined With the Genetic Inactivation of A2AR Significantly Alleviates Traumatic Brain Injury-Induced Acute Lung Injury. Shock 2019, 51, 502–510. [Google Scholar] [CrossRef]
- Venkata Charan Tej, G.N.; Neogi, K.; Verma, S.S.; Chandra Gupta, S.; Nayak, P.K. Caffeine-Enhanced Anti-Tumor Immune Response through Decreased Expression of PD1 on Infiltrated Cytotoxic T Lymphocytes. Eur. J. Pharmacol. 2019, 859, 172538. [Google Scholar] [CrossRef]
- Fletcher, D.K.; Bishop, N.C. Effect of a High and Low Dose of Caffeine on Antigen-Stimulated Activation of Human Natural Killer Cells After Prolonged Cycling. Int. J. Sport. Nutr. Exerc. Metab. 2011, 21, 155–165. [Google Scholar] [CrossRef]
- Abbasi, A.; Abtahi Froushani, S.M.; Delirezh, N.; Mostafaei, A. Caffeine Alters the Effects of Bone Marrow-Derived Mesenchymal Stem Cells on Neutrophils. Adv. Clin. Exp. Med. 2018, 27, 463–468. [Google Scholar] [CrossRef]
- Nehlig, A. Are We Dependent upon Coffee and Caffeine? A Review on Human and Animal Data. Neurosci. Biobehav. Rev. 1999, 23, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Ofluoglu, E.; Pasaoglu, H.; Pasaoglu, A. The Effects of Caffeine on L-Arginine Metabolism in the Brain of Rats. Neurochem. Res. 2009, 34, 395–399. [Google Scholar] [CrossRef]
- Rodak, K.; Bęben, D.; Birska, M.; Siwiela, O.; Kokot, I.; Moreira, H.; Radajewska, A.; Szyjka, A.; Kratz, E.M. Evaluating the Neuroprotective Potential of Caffeinated Coffee in the Context of Aluminum-Induced Neurotoxicity: Insights from a PC12 Cell Culture Model. Antioxidants 2024, 13, 342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shirley, D.G.; Walter, S.J.; Noormohamed, F.H. Natriuretic Effect of Caffeine: Assessment of Segmental Sodium Reabsorption in Humans. Clin. Sci. 2002, 103, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Seal, A.D.; Bardis, C.N.; Gavrieli, A.; Grigorakis, P.; Adams, J.D.; Arnaoutis, G.; Yannakoulia, M.; Kavouras, S.A. Coffee with High but Not Low Caffeine Content Augments Fluid and Electrolyte Excretion at Rest. Front. Nutr. 2017, 4, 40. [Google Scholar] [CrossRef]
- Pin-on, P.; Saringkarinkul, A.; Punjasawadwong, Y.; Kacha, S.; Wilairat, D. Serum Electrolyte Imbalance and Prognostic Factors of Postoperative Death in Adult Traumatic Brain Injury Patients. Medicine 2018, 97, e13081. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.T.; Rzigalinski, B.A.; Ellis, E.F. Calcium Responses to Caffeine and Muscarinic Receptor Agonists Are Altered in Traumatically Injured Neurons. J. Neurotrauma 2002, 19, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Neurological Disorders and Stroke. Traumatic Brain Injury (TBI). 2024. Available online: https://www.ninds.nih.gov/health-information/disorders/traumatic-brain-injury-tbi (accessed on 22 May 2025).
- Bláha, M.; Vajnerová, O.; Bednár, M.; Vajner, L.; Tichý, M. TBI—Effects of Alcohol and Caffeine on Intracranial Pressure and Cerebral Blood Flow. Rozhl. Chir. 2009, 88, 682–686. [Google Scholar]
- Boison, D.; Lusardi, T. Survival and Injury Outcome After TBI: Influence of Pre- and Post-Exposure to Caffeine; Defense Technical Information Center (DTIC): Fort Belvoir, VA, USA, 2012. [Google Scholar]
- Johnson, J.M. Effects of Caffeine on Measures of Clinical Outcome and Recovery Following Mild Traumatic Brain Injury in Adolescents. Master’s Thesis, University of South Carolina, Columbia, SC, USA, 2023. Available online: https://scholarcommons.sc.edu/etd/7497 (accessed on 22 May 2025).
- Lin, Z.; Jiang, D.; Liu, P.; Ge, Y.; Moghekar, A.; Lu, H. Blood-brain barrier permeability in response to caffeine challenge. Magn. Reason. Med. 2022, 88, 2259–2266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahroo, A.; Buck, M.A.; Konstandin, S.; Huber, J.; Hoinkiss, D.C.; Hirsch, J.; Günther, M. New physiological insights using multi-TE ASL MRI measuring blood-brain barrier water exchange after caffeine intake. Magn. Reson. Mater. Phys. Biol. Med. 2025, 38, 207–219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rincon, F.; Ghosh, S.; Dey, S.; Maltenfort, M.; Vibbert, M.; Urtecho, J.; McBride, W.; Moussouttas, M.; Bell, R.; Ratliff, J.K.; et al. Impact of Acute Lung Injury and Acute Respiratory Distress Syndrome After Traumatic Brain Injury in the United States. Neurosurgery 2012, 71, 795–803. [Google Scholar] [CrossRef]
- Everson, C.A.; Szabo, A.; Plyer, C.; Hammeke, T.A.; Stemper, B.D.; Budde, M.D. Subclinical brain manifestations of repeated mild traumatic brain injury are changed by chronic exposure to sleep loss, caffeine, and sleep aids. Exp. Neurol. 2024, 381, 114928. [Google Scholar] [CrossRef] [PubMed]
| Reference Number | Study Model | Findings |
|---|---|---|
| [35] | N/A | Caffeine acts as an adenosine antagonist, specifically at the A1 and A2A receptors. |
| [67] | N/A | Adenosine receptors are of interest in TBI because they are involved in various brain injury pathways. |
| [36] | Rodent | Adenosine may have a protective role in the recovery of TBI |
| [22] | Humans | Caffeine may have a neuroprotective effect on Parkinson’s disease pathways. |
| [31] | Rodent | Caffeine may have a neuroprotective effect on neurodegenerative diseases |
| [36] | Rodent | Chronic caffeine treatment, rather than acute caffeine treatment, showed better recovery from TBI in mouse models. |
| [68] | Mixed | Review article finding combined evidence of potential benefit of treating Parkinson’s Disease and TBI through targeting the adenosine2A receptor. |
| [65] | Rodent | Pretreatment of rats with caffeine before TBI showed an increase in mortality |
| [39] | Rodent | Chronic caffeine use may have some protective benefits in blast-induced TBI; however, chronic and acute caffeine use both increase mortalities. |
| [37] | Rodent | Caffeine injection given immediately after TBI reduced TBI-induced mortality in rat models. |
| [38] | Rodent | An acute dose of caffeine given 10 s after TBI decreased mortality and morbidity in mouse models. |
| [35] | Humans | An increase in CSF concentration of caffeine in patients with TBI was associated with more favorable outcomes. |
| [44] | Human | Patients with TBI who had a serum caffeine concentration between 0.01 to 1.66 μg/mL had better recovery after 6 months of injury compared to the no caffeine group. |
| [69] | Cats | One of the earliest studies that examined the pathophysiology of oxygen radicals in TBI |
| [70] | Humans | Found potential benefits of adenosine on cerebral blood flow and oxidative metabolism in patients with severe head injury |
| [48] | Rodent | Caffeine in female rats has been shown to decrease oxidative stress and specifically decrease lipid peroxidation in the hippocampus. |
| [71] | Rabbits | Caffeine was seen to protect against oxidative stress and Alzheimer’s dementia-like pathology in rabbit models. |
| [45] | Rodent | Positive neuronal changes were seen when treating mice with nano coffee injections after a TBI. |
| Study/Experiment | Details |
|---|---|
| TBI Mortality and Morbidity | High incidence of mortality and morbidity in TBI patients [37] |
| Caffeine Neuroprotective Effects | Caffeine has neuroprotective benefits in degenerative neurological disorders, antagonizes A2A receptors [33] |
| Serum Caffeine in TBI Patients | Caffeine levels were analyzed within 4 h of injury and categorized into low, intermediate, and high levels; a higher likelihood of 6-month recovery in low- and intermediate-caffeine groups [44] |
| Caffeine Antioxidative Properties | Caffeine protects neuronal cells from ROS damage, modulates inflammatory responses, and lowers pro-inflammatory cytokine production [64,72] |
| Impact on Cytotoxic T Lymphocytes | Caffeine reduced PD1 expression on cytotoxic T lymphocytes, enhanced tumor targeting, and decreased tumor size [72] |
| Effect on Natural Killer Cells | Caffeine enhanced NK cell activation post-exercise in cyclists, effective at low and high doses [73] |
| Developmental Exposure in Rats | Developmental caffeine exposure in rats altered spine density, impaired memory and cognitive function, and different TBI recovery patterns [48] |
| Type of Study | Caffeine Treatment | Conclusion |
|---|---|---|
| Pre-Clinical Study | 0.25 g/L | Chronic caffeine treatment alleviated cerebral injury at 24 h post-severe blast-induced TBI (bTBI) [39] |
| Pre-Clinical Study | 5 mg/kg, 15 mg/kg and 50 mg/kg | Chronic caffeine treatment represses the release of glutamate and inhibits cytokine expression after TBI [65] |
| Clinical Study | 0.01–10.00 μg/mL | A statistically significant association was found between a serum caffeine concentration of from 0.01 to 1.66 μg/mL and good functional recovery at 6 months after injury, compared with the no-caffeine group of patients with TBI with intracranial injury [44] |
| Pre-Clinical Study | Oral bolus dose of 25 mg/kg | Regular caffeine consumption before a penetrating brain injury may moderately improve motor recovery but worsen the neurocognitive sequelae associated with a penetrating brain injury [66] |
| Clinical Study | ≥1 μmol/L (194 ng/mL) | Caffeine may be neuroprotective by long-term upregulation of adenosine A1 receptors or acute inhibition of A2a receptors [35] |
| Pre-Clinical Study | 20 mg/kg | Intracranial pressure decreased by 11% from the baseline value, which can improve clinical outcomes post-TBI [83] |
| Pre-Clinical Study | 25 mg/kg | Chronic treatment initiated after TBI suggested improved motor function with a nonspecific adenosine receptor agonist, but a slight decrease in motor function after an A1 receptor antagonist [47] |
| Pre-Clinical Study | 36 mg/kg | The interventions of caffeine, sleep deprivation, sleep aids, and sedation during the acute post-mTBI period each changed the subclinical characteristics of the brain after mTBI and altered the return toward normal function [61]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, B.; Agriantonis, G.; Dawson-Moroz, S.; Brown, R.; Simon, W.; Ebelle, D.; Chapelet, J.; Cardona, A.; Soni, A.; Siddiqui, M.; et al. Caffeine: A Neuroprotectant and Neurotoxin in Traumatic Brain Injury (TBI). Nutrients 2025, 17, 1925. https://doi.org/10.3390/nu17111925
Sharma B, Agriantonis G, Dawson-Moroz S, Brown R, Simon W, Ebelle D, Chapelet J, Cardona A, Soni A, Siddiqui M, et al. Caffeine: A Neuroprotectant and Neurotoxin in Traumatic Brain Injury (TBI). Nutrients. 2025; 17(11):1925. https://doi.org/10.3390/nu17111925
Chicago/Turabian StyleSharma, Bharti, George Agriantonis, Sarah Dawson-Moroz, Rolanda Brown, Whenzdjyny Simon, Danielle Ebelle, Jessica Chapelet, Angie Cardona, Aditi Soni, Maham Siddiqui, and et al. 2025. "Caffeine: A Neuroprotectant and Neurotoxin in Traumatic Brain Injury (TBI)" Nutrients 17, no. 11: 1925. https://doi.org/10.3390/nu17111925
APA StyleSharma, B., Agriantonis, G., Dawson-Moroz, S., Brown, R., Simon, W., Ebelle, D., Chapelet, J., Cardona, A., Soni, A., Siddiqui, M., Patel, B., Cheerasarn, S., Chang, J., Cobb, L., John, F., Hasan, M. M., Garcia, C., Shaefee, Z., Twelker, K., ... Whittington, J. (2025). Caffeine: A Neuroprotectant and Neurotoxin in Traumatic Brain Injury (TBI). Nutrients, 17(11), 1925. https://doi.org/10.3390/nu17111925






