The Alleviating Effect of Abalone Viscera Collagen Peptide in DSS-Induced Colitis Mice: Effect on Inflammatory Cytokines, Oxidative Stress, and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. AVCP Preparation
2.2. Molecular Weight Distribution Analysis
2.3. Hydroxyproline Content Analysis
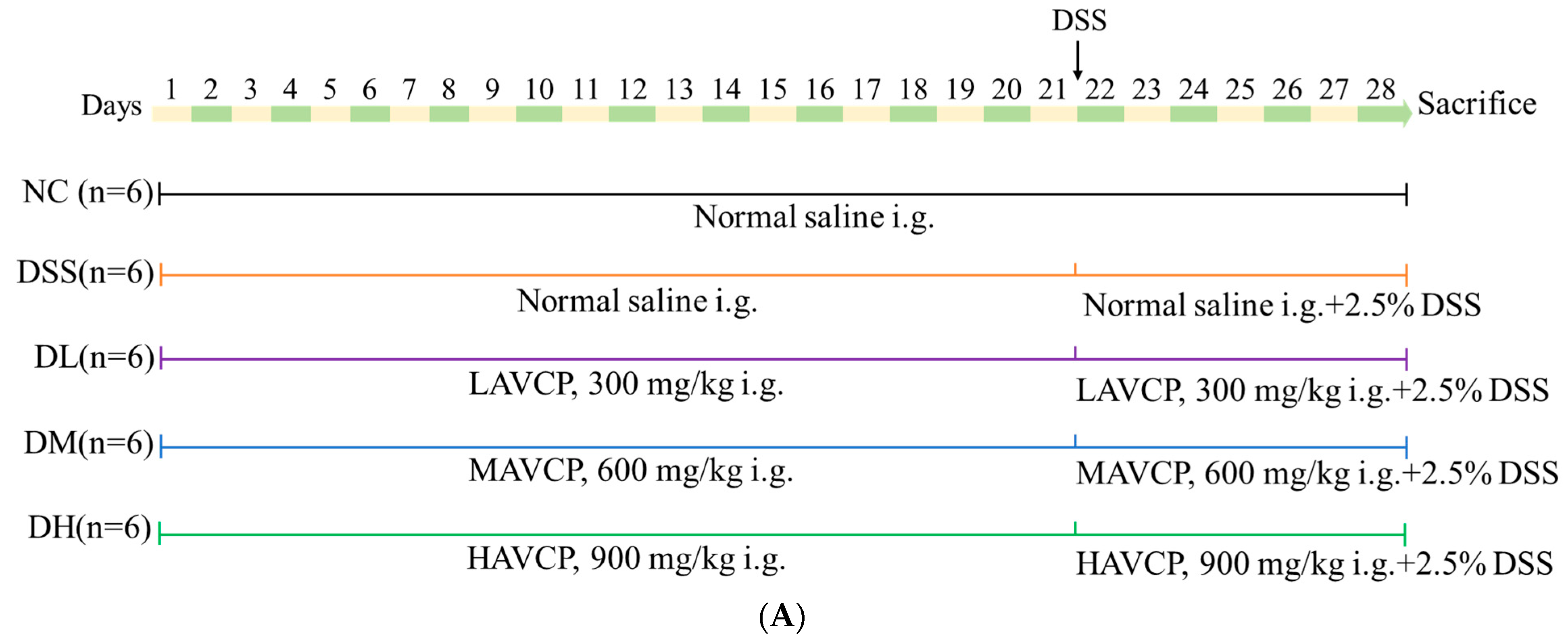
2.4. Animal Experiments
2.5. Immune Organ Index Analysis
2.6. Histopathological Analysis
2.7. Inflammatory Mediators and Oxidative Stress Factors Analysis
2.8. 16S rRNA Sequencing of Gut Microbiota
2.9. Statistical Analysis
3. Results
3.1. Characterization and In Vivo Safety Evaluation of AVCP
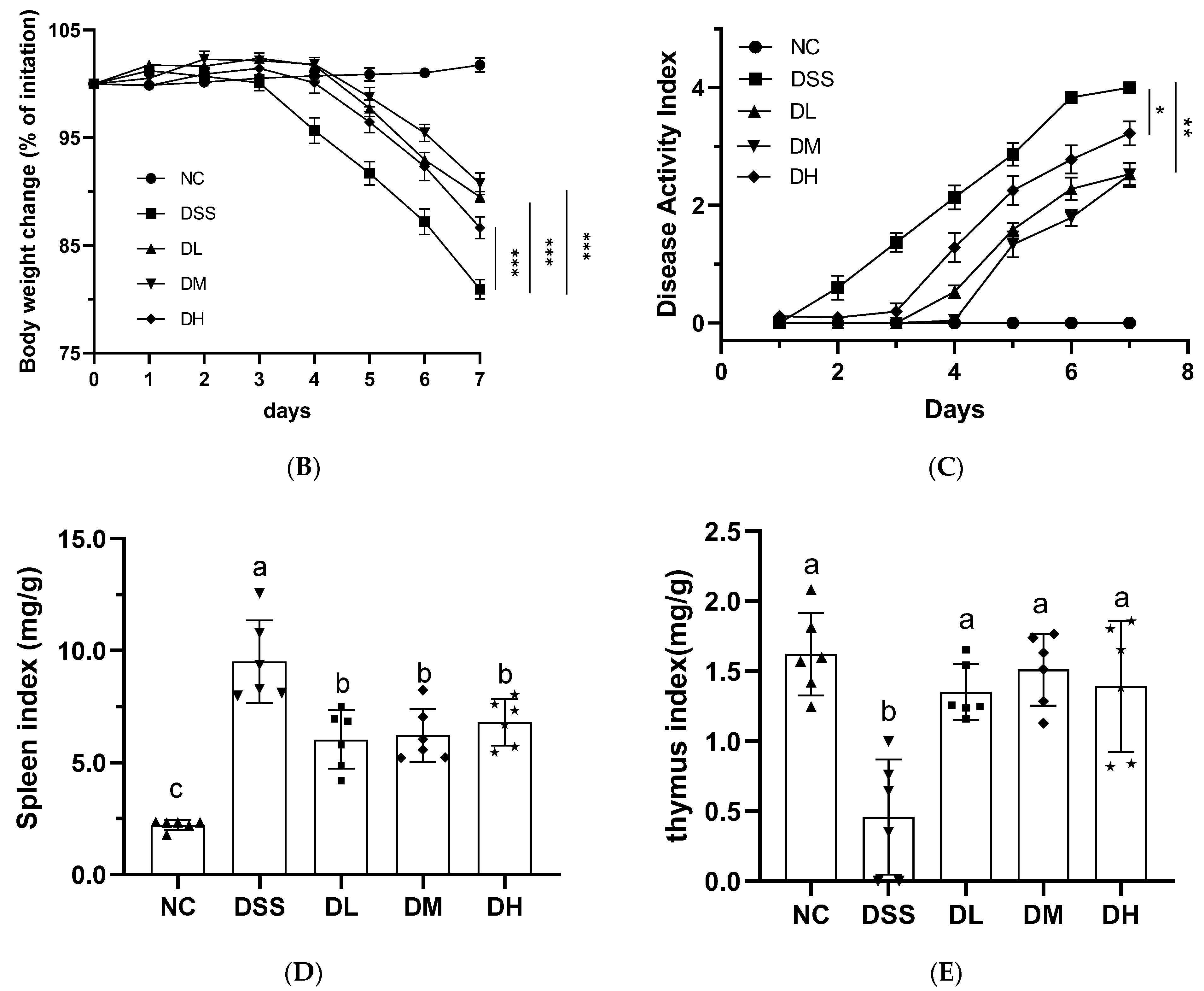
3.2. AVCP Administration Alleviates DSS-Induced Acute Colitis
3.2.1. Influence of AVCP on Body Weight, DAI, and Organ Index
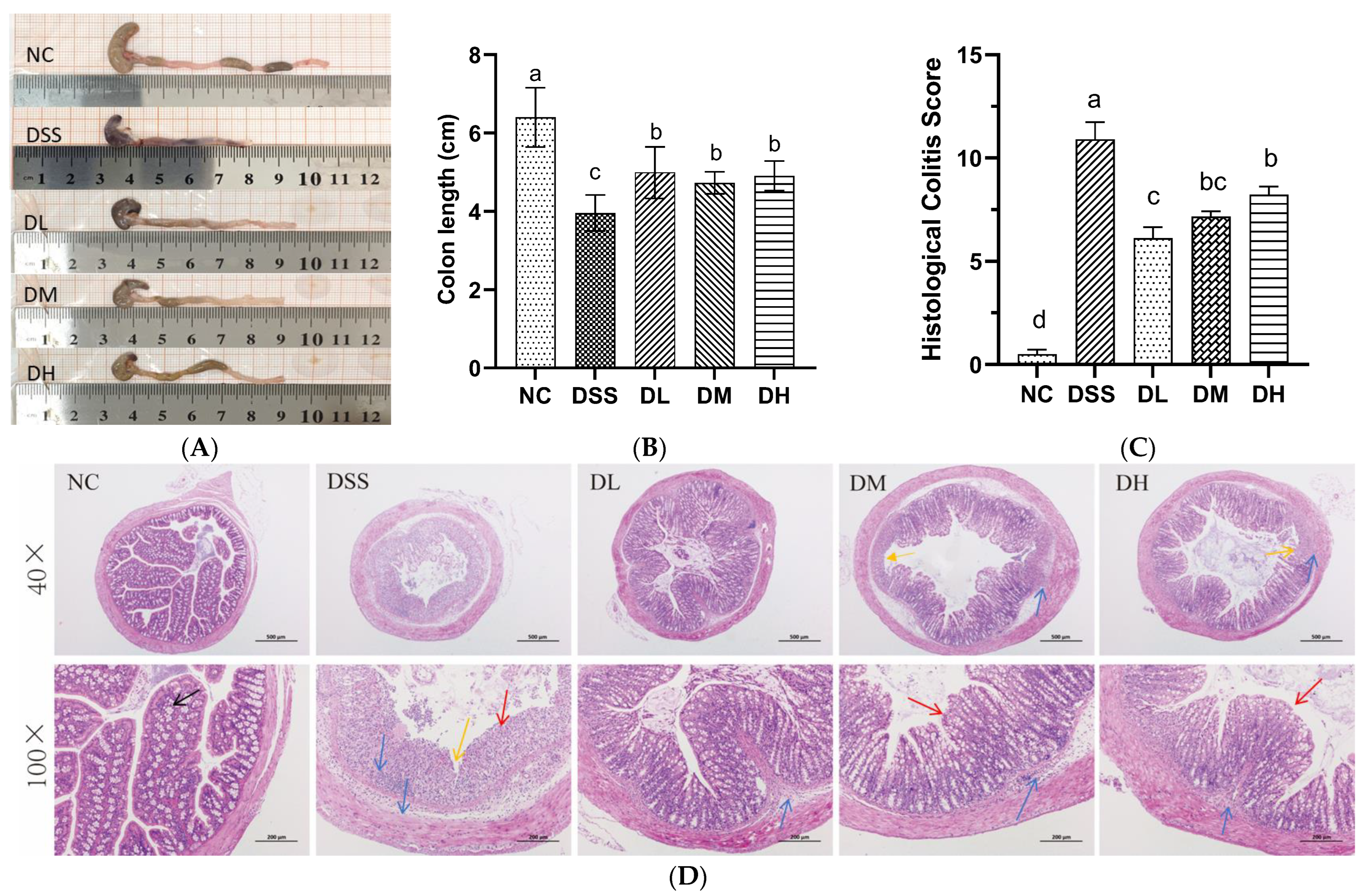
3.2.2. Influence of AVCP on Colon Histopathology
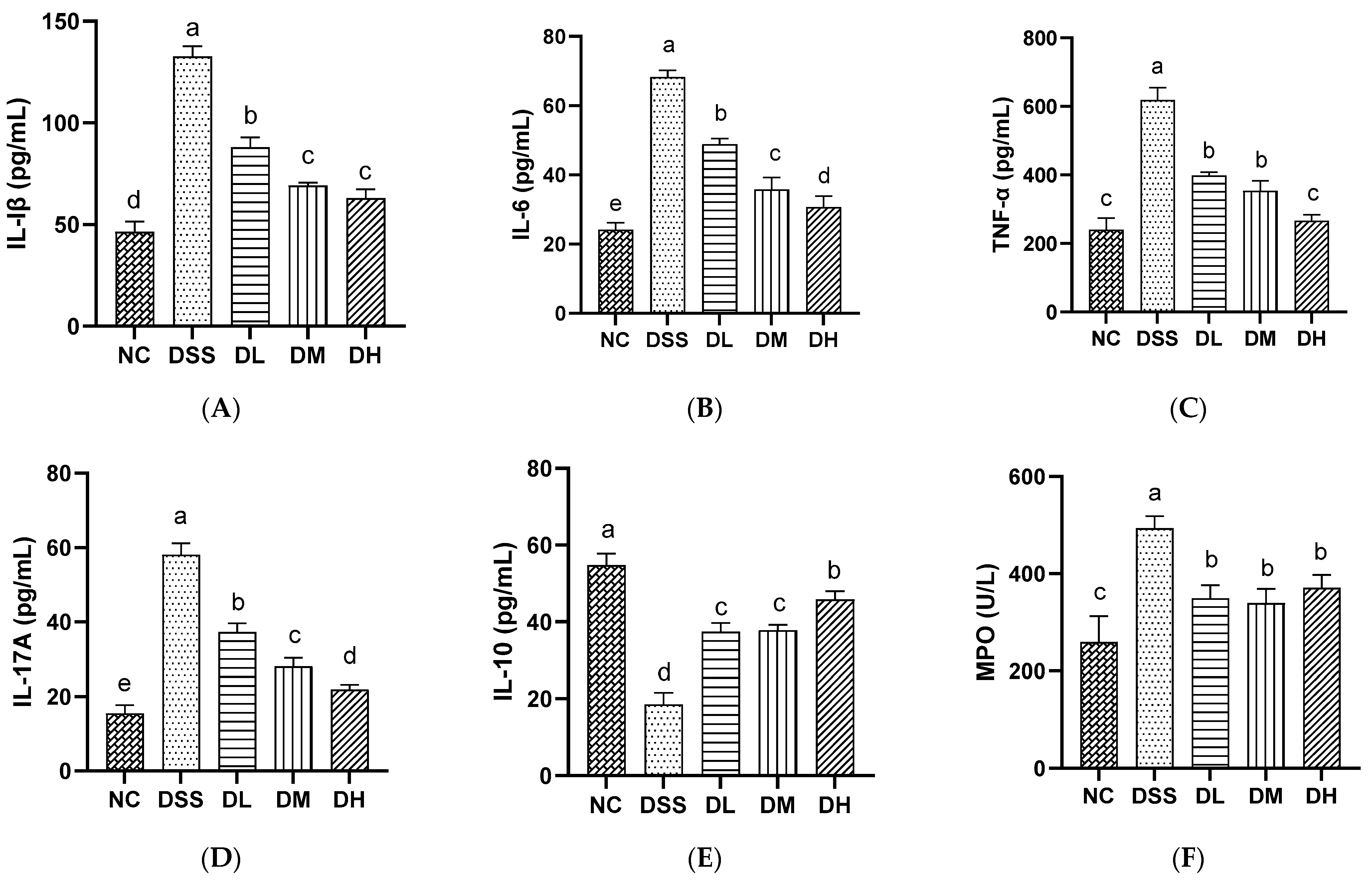
3.2.3. Influence of AVCP on Inflammatory Mediators in Serum
3.2.4. Influence of AVCP on Oxidative Stress Factors in Serum
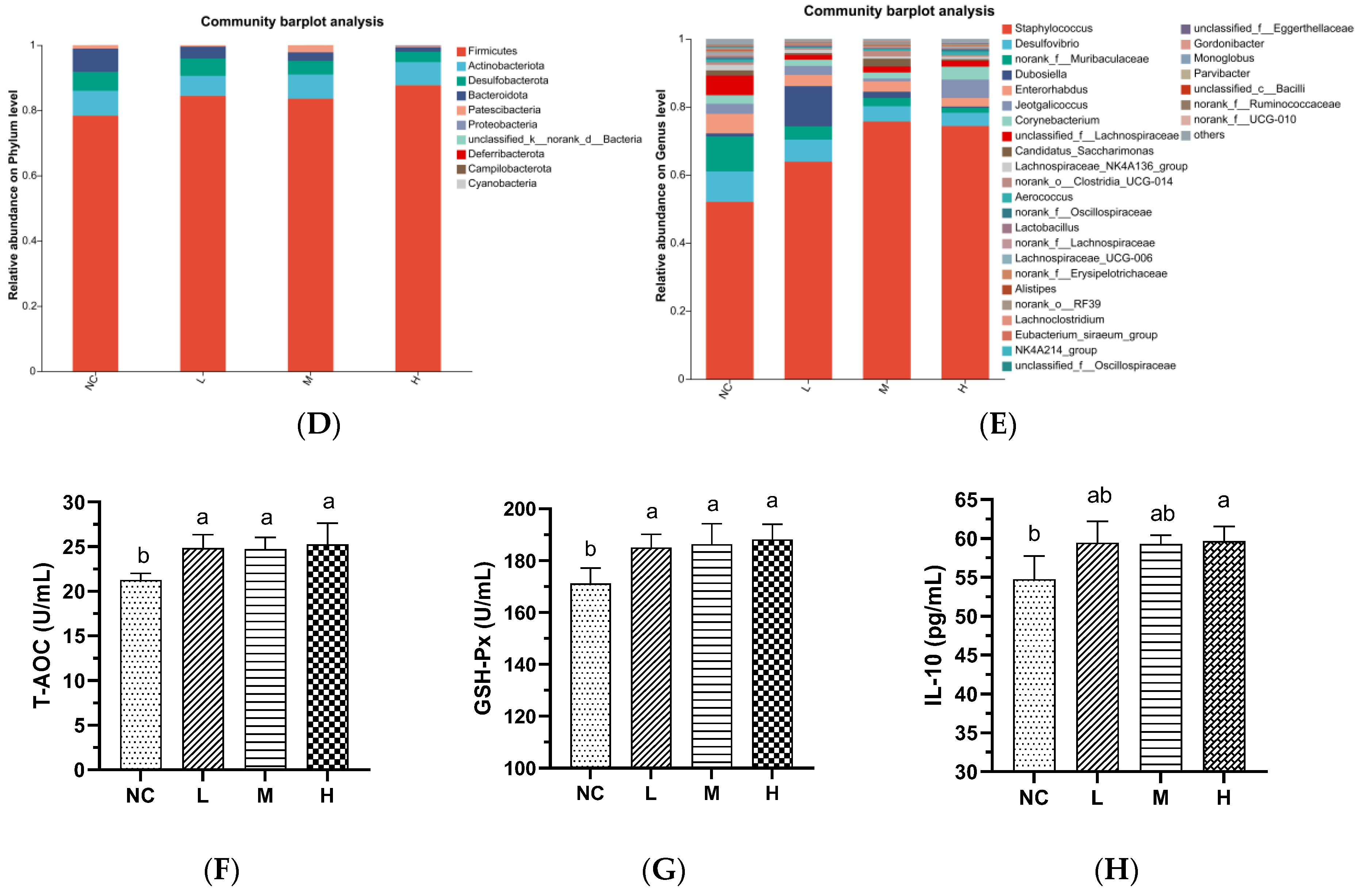
3.3. AVCP Administration Alleviates DSS-Induced Gut Microbiota Dysbiosis
3.3.1. Alpha and Beta-Diversity Analysis of Gut Microbiota
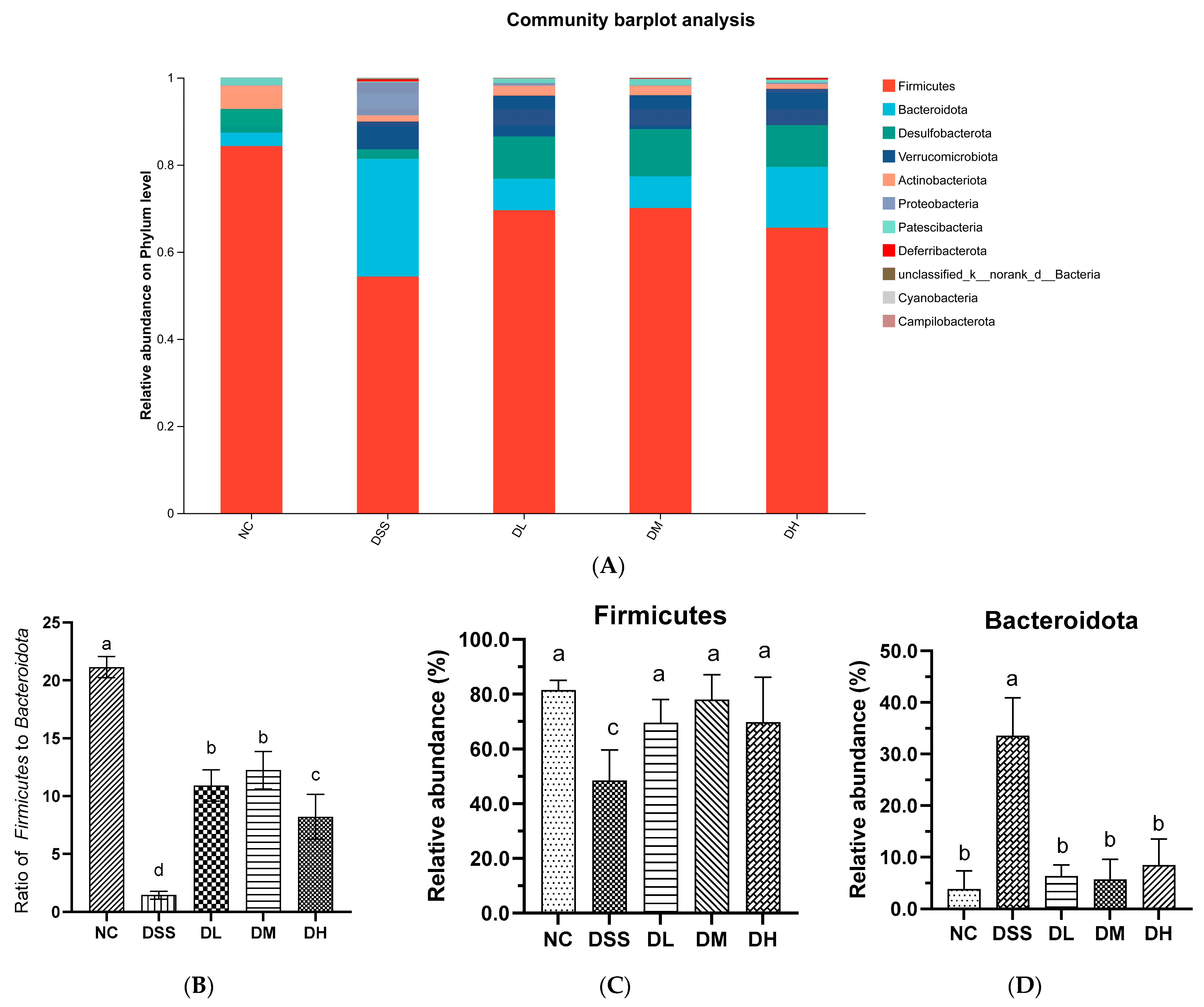
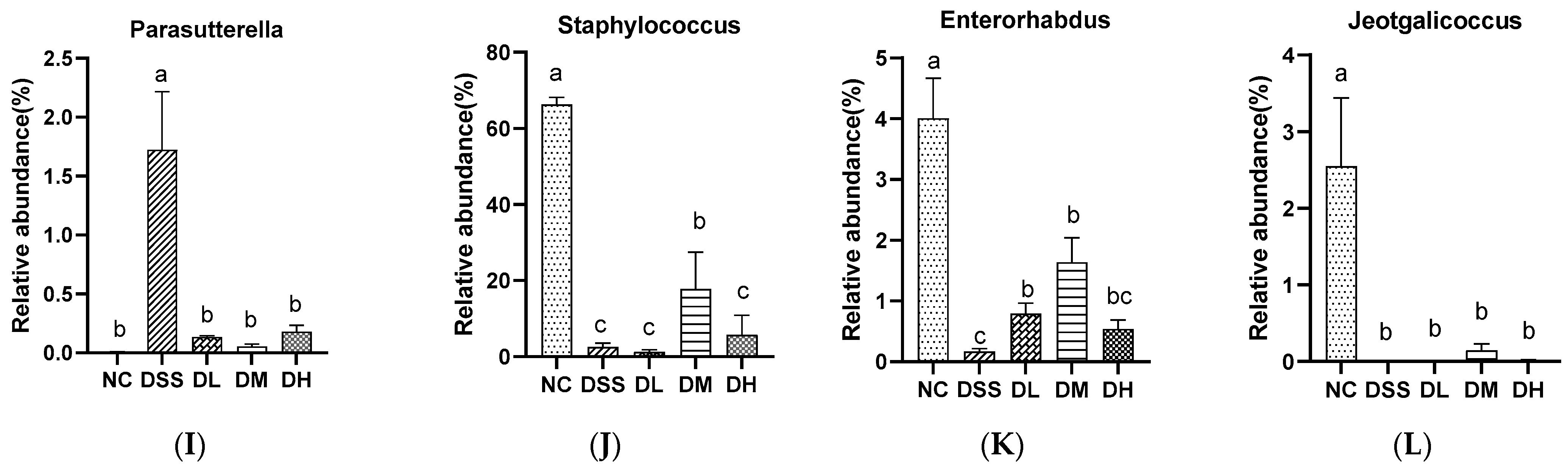
3.3.2. Composition of Gut Microbiota
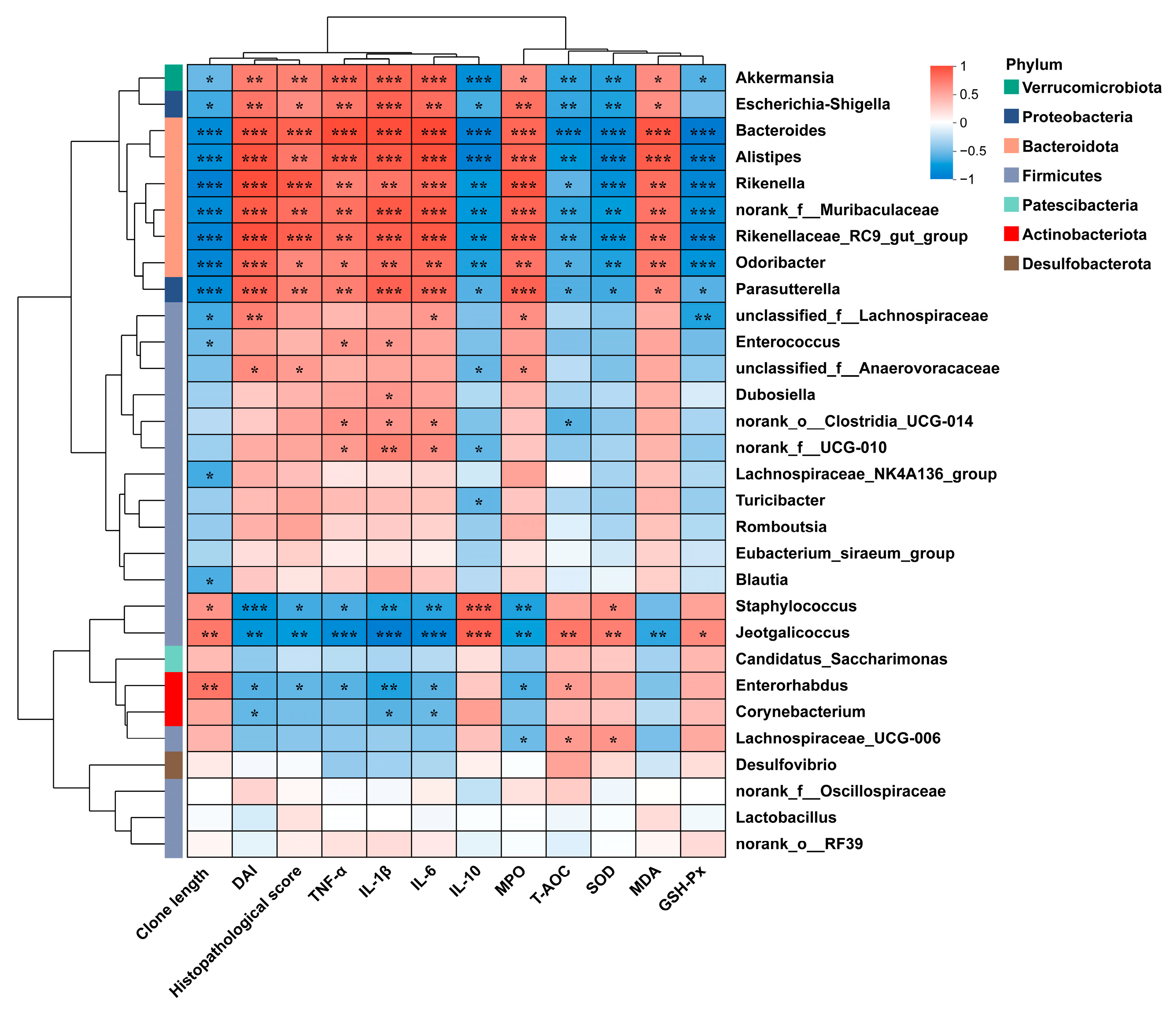
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVCP | abalone viscera collagen peptide |
| UC | ulcerative colitis |
| IBD | inflammatory bowel disease |
| DSS | dextran sulfate sodium |
| DAI | disease activity index |
| MPO | myeloperoxidase |
| TNF-α | tumor necrosis factor-α |
| IL | interleukins |
| MDA | malondialdehyde |
| T-AOC | total antioxidant capacity |
| SOD | superoxide dismutase |
| GSH-Px | glutathione peroxidase |
| PCoA | principal coordinate analysis |
| NMDS | non-metric multidimensional scaling |
References
- Wangchuk, P.; Yeshi, K.; Loukas, A. Ulcerative colitis: Clinical biomarkers, therapeutic targets, and emerging treatments. Trends Pharmacol. Sci. 2024, 45, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Buie, M.J.; Quan, J.; Windsor, J.W.; Coward, S.; Hansen, T.M.; King, J.A.; Kotze, P.G.; Gearry, R.B.; Ng, S.C.; Mak, J.W.Y.; et al. Global Hospitalization Trends for Crohn’s Disease and Ulcerative Colitis in the 21st Century: A Systematic Review with Temporal Analyses. J. Gastroenterol. 2023, 21, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- McCoubrey, L.E.; Seegobin, N.; Sangfuang, N.; Moens, F.; Duyvejonck, H.; Declerck, E.; Dierick, A.; Marzorati, M.; Basit, A.W. The colon targeting efficacies of mesalazine medications and their impacts on the gut microbiome. J. Control. Release 2024, 369, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Itzkowitz, S.H. Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef]
- Guo, P.; Wang, W.; Xiang, Q.; Pan, C.; Qiu, Y.; Li, T.; Wang, D.; Ouyang, J.; Jia, R.; Shi, M.; et al. Engineered probiotic ameliorates ulcerative colitis by restoring gut microbiota and redox homeostasis. Cell Host Microbe 2024, 32, 1502–1518. [Google Scholar] [CrossRef]
- Lo Presti, E.; Di Mitri, R.; Mocciaro, F.; Di Stefano, A.B.; Scibetta, N.; Unti, E.; Cicero, G.; Pecoraro, G.; Conte, E.; Dieli, F.; et al. Characterization of γδ T cells in intestinal mucosa from patients with early-onset or long-standing inflammatory bowel disease and their correlation with clinical status. J. Crohns. Colitis 2019, 13, 873–883. [Google Scholar] [CrossRef]
- Jia, J.; Liu, Q.; Liu, H.; Yang, C.; Zhao, Q.; Xu, Y.; Wu, W. Structure characterization and antioxidant activity of abalone visceral peptides-selenium in vitro. Food Chem. 2024, 433, 137398. [Google Scholar] [CrossRef]
- Chen, J.; Cao, W.; Wei, P.; Li, T.; Weng, W. Speciation transformation of arsenic in abalone viscera hydrolysate fraction: In vitro digestion and in vivo metabolism. Food Res. Int. 2019, 123, 340–345. [Google Scholar] [CrossRef]
- MARA; BFFA; NFTEC; CSF. China Fishery Statistical Yearbook 2023; Production (Chapter 2); China Agriculture Press: Beijing, China, 2024; pp. 28–29. [Google Scholar]
- Je, J.; Park, S.Y.; Hwang, J.; Ahn, C. Amino acid composition and in vitro antioxidant and cytoprotective activity of abalone viscera hydrolysate. J. Funct. Foods 2015, 16, 94–103. [Google Scholar] [CrossRef]
- Liu, J.; Wu, G.; Yang, J.; He, C.; Xiong, H.; Ma, Y. Abalone visceral peptides containing Cys and Tyr exhibit strong in vitro antioxidant activity and cytoprotective effects against oxidative damage. Food Chem. X 2023, 17, 100582. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Pérez, C.; Ponce González, X.P.; Hernández-Savedra, N.Y. Antimicrobial and anticarcinogenic activity of bioactive peptides derived from abalone viscera (Haliotis fulgens and Haliotis corrugata). Sci. Rep. 2023, 13, 15185. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, J.; He, C.; Wei, H.; Wu, G.; Xiong, H.; Ma, Y. Fractionation and purification of antioxidant peptides from abalone viscera by a combination of Sephadex G-15 and Toyopearl HW-40F chromatography. Int. J. Food Sci. Technol. 2022, 57, 1218–1225. [Google Scholar] [CrossRef]
- Heo, J.H.; Kim, E.A.; Kang, N.; Heo, S.Y.; Ahn, G.; Heo, S.J. The antioxidant effects of trypsin-hydrolysate derived from abalone viscera and fishery by-products, and the angiotensin-I converting enzyme (ACE) inhibitory activity of its purified bioactive peptides. Mar. Drugs 2024, 22, 461. [Google Scholar] [CrossRef]
- Iwamoto, N.; Sasaki, A.; Maizawa, T.; Hamada-Sato, N. Abalone viscera fermented with aspergillus oryzae 001 prevents pressure elevation by inhibiting angiotensin converting enzyme. Nutrients 2023, 15, 947. [Google Scholar] [CrossRef]
- Øiseth, S.K.; Delahunty, C.; Cochet, M.; Lundin, L. Why is abalone so chewy? Structural characterization and relationship to textural attributes. J. Shellfish Res. 2013, 32, 73–79. [Google Scholar] [CrossRef]
- Li, J.; Kim, B.; Kang, S. Analysis and comparison of general compositions, amino acids, fatty acids and collagen of abalone harvested in three different regions in Korea. Korean J. Food Preserv. 2013, 20, 441–450. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Liu, D. Collagen peptides and the related synthetic peptides: A review on improving skin health. J. Funct. Foods 2021, 86, 104680. [Google Scholar] [CrossRef]
- Lu, B.; Han, S.; Wang, Z.; Xie, L.; Zhan, J.; Zhang, J.; Zhang, J. Antioxidant and anti-aging potential of collagen peptide conjugated with ionic liquid. J. Mol. Liq. 2024, 394, 123739. [Google Scholar] [CrossRef]
- Al-Atif, H. Collagen supplements for aging and wrinkles: A paradigm shift in the fields of dermatology and cosmetics. Dermatol. Pract. Concept. 2022, 12, e2022018. [Google Scholar] [CrossRef]
- Guo, X.; Li, X.; Dong, Y.; Xie, W.; Jin, T.; Xu, D.; Liu, L. Cod (Gadus) skin collagen peptide powder reduces inflammation, restores mucosal barrier function, and inhibits fibrosis in dextran sodium sulfate-induced colitis in mice. J. Ethnopharmacol. 2023, 316, 116728. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.Y.; Zhou, J.; Liu, G.G.; Zhang, M.Y.; Li, X.Z.; Wang, Y. Anti-inflammatory activity of collagen peptide in vitro and its effect on improving ulcerative colitis. npj Sci. Food 2025, 9, 1–13. [Google Scholar] [CrossRef]
- Tian, M.; Li, D.; Ma, C.; Feng, Y.; Hu, X.; Chen, F. Barley Leaf Insoluble Dietary Fiber Alleviated Dextran Sulfate Sodium-Induced Mice Colitis by Modulating Gut Microbiota. Nutrients 2021, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, H.; Shi, F.; Song, J.; Zhang, Z. The potential therapeutic effects of hydroxypropyl cellulose on acute murine colitis induced by DSS. Carbohydr. Polym. 2022, 289, 119430. [Google Scholar] [CrossRef]
- GB 31645-2018; National Food Safety Standard-Collagen Peptide. China Standards Press: Beijing, China, 2018.
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef]
- Lin, W.; Chen, H.; Chen, X.; Guo, C. The Roles of Neutrophil-Derived Myeloperoxidase (MPO) in Diseases: The New Progress. Antioxidants 2024, 13, 132. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar] [CrossRef]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, K.; Shanthi, C. Purified fish skin collagen hydrolysate attenuates TNF-α induced barrier dysfunction in-vitro and DSS induced colitis in-vivo model. Int. J. Biol. Macromol. 2022, 222, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; Yi, J.; Liu, Y.; Yu, Z.; Chen, S.; Liu, X. Increased mucin-degrading bacteria by high protein diet leads to thinner mucus layer and aggravates experimental colitis. J. Gastroen. Hepatol. 2021, 36, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Hansberry, D.R.; Shah, K.; Agarwal, P.; Agarwal, N. Fecal myeloperoxidase as a biomarker for inflammatory bowel disease. Cureus 2017, 9, e1004. [Google Scholar] [CrossRef]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef]
- Theiss, A.L.; Vijay-Kumar, M.; Obertone, T.S.; Jones, D.P.; Hansen, J.M.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology 2009, 137, 199–208. [Google Scholar] [CrossRef]
- Neubauer, K.; Kempinski, R.; Matusiewicz, M.; Bednarz-Misa, I.; Krzystek-Korpacka, M. Nonenzymatic serum antioxidant capacity in IBD and its association with the severity of bowel inflammation and corticosteroids treatment. Medicina 2019, 55, 88. [Google Scholar] [CrossRef]
- Haro Girón, S.; Monserrat Sanz, J.; Ortega, M.A.; Garcia-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Boaru, D.L.; de Leon-Oliva, D.; Guijarro, L.G.; Atienza-Perez, M.; et al. Prognostic value of malondialdehyde (MDA) in the temporal progression of chronic spinal cord injury. J. Pers. Med. 2023, 13, 626. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Świrkosz, G.; Szczygieł, A.; Logoń, K.; Wrześniewska, M.; Gomułka, K. The Role of the microbiome in the pathogenesis and treatment of ulcerative colitis-a literature review. Biomedicines 2023, 11, 3144. [Google Scholar] [CrossRef] [PubMed]
- Herrera-deGuise, C.; Varela, E.; Sarrabayrouse, G.; Pozuelo Del Río, M.; Alonso, V.R.; Sainz, N.B.; Casellas, F.; Mayorga, L.F.; Manichanh, C.; Vidaur, F.A.; et al. Gut Microbiota composition in long-remission ulcerative colitis is close to a healthy gut microbiota. Inflamm. Bowel Dis. 2023, 29, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Cheng, L.; Kang, J.; Liu, Y.; Zhao, Y.; Xiao, M.; Liu, H.; Zhu, Q.; Guo, Q.; et al. Structural characterization of water-soluble pectin from the fruit of Diospyros lotus L. and its protective effects against DSS-induced colitis in mice. J. Agric. Food Chem. 2025, 73, 1630–1641. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Nie, H.; Yan, J.; Ma, Z.; Li, H.; Tang, S.; Yin, Q.; Qiu, J. Lactobacillus from fermented bamboo shoots prevents inflammation in DSS-induced colitis mice via modulating gut microbiome and serum metabolites. Food Sci. Hum. Wellness 2024, 13, 2833–2846. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Tai, W.C.; Liang, C.M.; Wu, C.K.; Tsai, M.C.; Hu, W.H.; Huang, P.Y.; Chen, C.H.; Kuo, Y.H.; Yao, C.C.; et al. Alternations of the gut microbiota and the Firmicutes/Bacteroidetes ratio after biologic treatment in inflammatory bowel disease. J. Microbiol. Immunol. Infect. 2025, 58, 62–69. [Google Scholar] [CrossRef] [PubMed]
- He, X.X.; Li, Y.H.; Yan, P.G.; Meng, X.C.; Chen, C.Y.; Li, K.M.; Li, J.N. Relationship between clinical features and intestinal microbiota in Chinese patients with ulcerative colitis. World J. Gastroenterol. 2021, 27, 4722–4737. [Google Scholar] [CrossRef]
- Vega-Magaña, N.; Delgado-Rizo, V.; García-Benavides, L.; Del Toro-Arreola, S.; Segura-Ortega, J.; Morales, A.S.M.Z.; Zepeda-Nuño, J.S.; Escarra-Senmarti, M.; Gutiérrez-Franco, J.; Haramati, J.; et al. Bacterial translocation is linked to increased intestinal IFN-γ, IL-4, IL-17, and mucin-2 in cholestatic rats. Ann. Hepatol. 2018, 17, 318–329. [Google Scholar] [CrossRef]
- Gilliland, A.; Chan, J.J.; De Wolfe, T.J.; Yang, H.; Vallance, B.A. Pathobionts in inflammatory bowel disease: Origins, underlying mechanisms, and implications for clinical care. Gastroenterology 2024, 166, 44–58. [Google Scholar] [CrossRef]
- Mills, R.H.; Dulai, P.S.; Vázquez-Baeza, Y.; Sauceda, C.; Daniel, N.; Gerner, R.R.; Batachari, L.E.; Malfavon, M.; Zhu, Q.; Weldon, K. Multi-omics analyses of the ulcerative colitis gut microbiome link Bacteroides vulgatus proteases with disease severity. Nat. Microbioli. 2022, 7, 262–276. [Google Scholar] [CrossRef]
- Kim, M.J.; Jung, D.R.; Lee, J.M.; Kim, I.; Son, H.; Kim, E.S.; Shin, J.H. Microbial dysbiosis index for assessing colitis status in mouse models: A systematic review and meta-analysis. iScience 2023, 27, 108657. [Google Scholar] [CrossRef]
- Alam, M.T.; Amos, G.C.A.; Murphy, A.R.J.; Murch, S.; Wellington, E.M.H.; Arasaradnam, R.P. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. 2020, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.M.; Huang, H.L.; Liu, Y.D.; Zhu, J.Q.; Zhou, Y.L.; Chen, H.T.; Xu, J.; Zhao, H.L.; Guo, X.; Shi, W.; et al. Selection strategy of dextran sulfate sodium-induced acute or chronic colitis mouse models based on gut microbial profile. BMC Microbiol. 2021, 21, 279. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.H.; Ko, G.; Jo, H.; Oh, T.; Ahn, B.; Unno, T. Anti-inflammatory properties and gut microbiota modulation of Porphyra tenera extracts in dextran sodium sulfate-induced colitis in mice. Antioxidants 2020, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.; Liu, J.; Zhang, X.; Wu, X.; Tang, S.; Sun, R.; Qian, C.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef]
- Liu, A.; Lv, H.; Wang, H.; Yang, H.; Li, Y.; Qian, J. Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J. Gerontol. A Biol. Sci. Medi. Sci. 2020, 75, 1284–1292. [Google Scholar] [CrossRef]


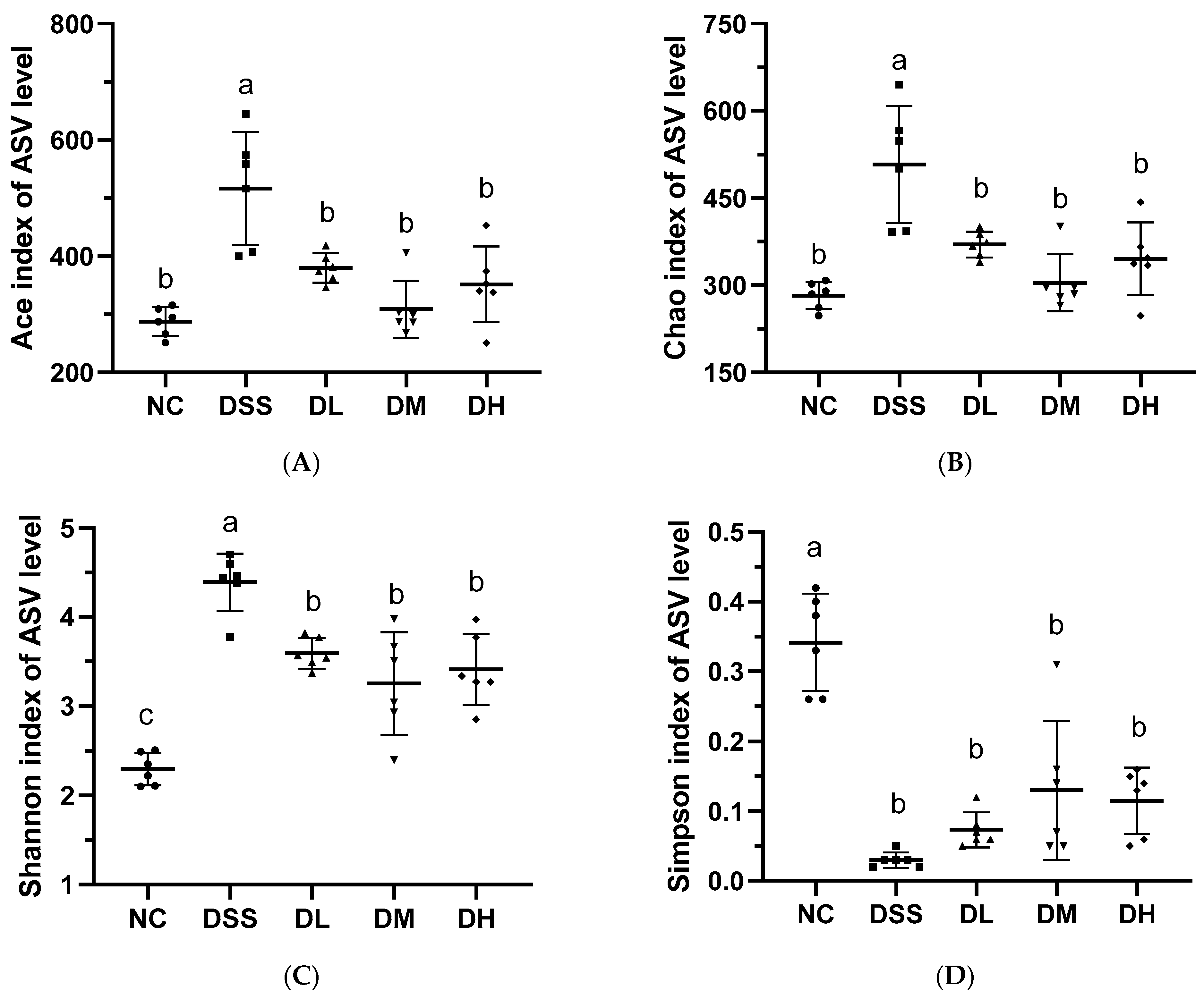



| Molecular Weight Distribution | Hydroxyproline Content | |
|---|---|---|
| <1000 Da | 19.3% | 6.56 ± 0.23% |
| 1000–2000 Da | 21.0% | |
| 2000–3000 Da | 25.6% | |
| 3000–5000 Da | 23.1% | |
| 5000–10,000 Da | 8.1% | |
| >10,000 Da | 2.9% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Liu, L.; Li, C.; Guo, T.; Li, C.; Tian, M.; Fang, T. The Alleviating Effect of Abalone Viscera Collagen Peptide in DSS-Induced Colitis Mice: Effect on Inflammatory Cytokines, Oxidative Stress, and Gut Microbiota. Nutrients 2025, 17, 1926. https://doi.org/10.3390/nu17111926
Liu B, Liu L, Li C, Guo T, Li C, Tian M, Fang T. The Alleviating Effect of Abalone Viscera Collagen Peptide in DSS-Induced Colitis Mice: Effect on Inflammatory Cytokines, Oxidative Stress, and Gut Microbiota. Nutrients. 2025; 17(11):1926. https://doi.org/10.3390/nu17111926
Chicago/Turabian StyleLiu, Binxiong, Lili Liu, Chunjiang Li, Tengming Guo, Changcheng Li, Meiling Tian, and Ting Fang. 2025. "The Alleviating Effect of Abalone Viscera Collagen Peptide in DSS-Induced Colitis Mice: Effect on Inflammatory Cytokines, Oxidative Stress, and Gut Microbiota" Nutrients 17, no. 11: 1926. https://doi.org/10.3390/nu17111926
APA StyleLiu, B., Liu, L., Li, C., Guo, T., Li, C., Tian, M., & Fang, T. (2025). The Alleviating Effect of Abalone Viscera Collagen Peptide in DSS-Induced Colitis Mice: Effect on Inflammatory Cytokines, Oxidative Stress, and Gut Microbiota. Nutrients, 17(11), 1926. https://doi.org/10.3390/nu17111926





