Association Between Malnutrition, Low Muscle Mass, Elevated NT-ProBNP Levels, and Mortality in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
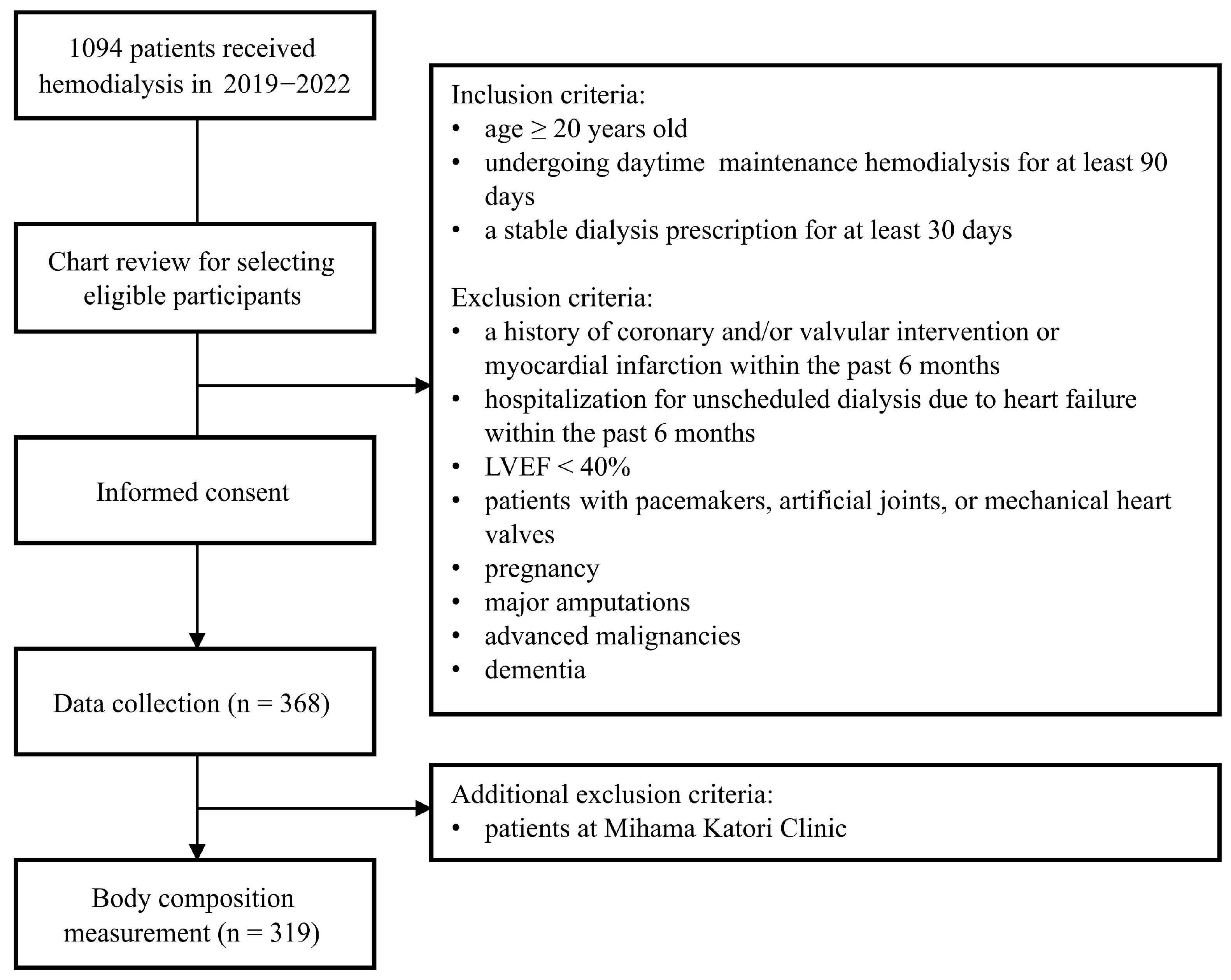
2.1. Study Design and Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
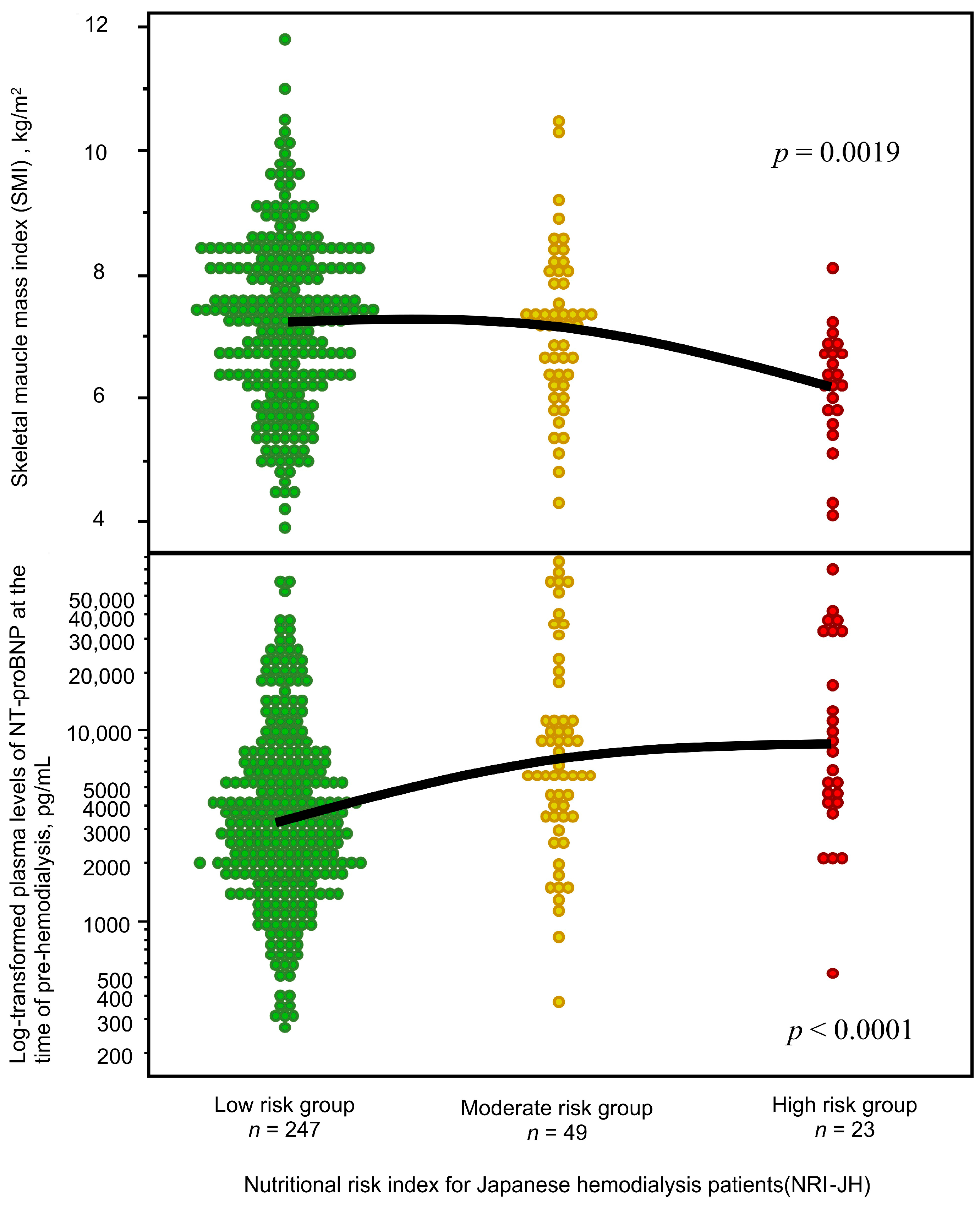
3.2. Associations Between Malnutrition, Low Muscle Mass, and NT-ProBNP Levels
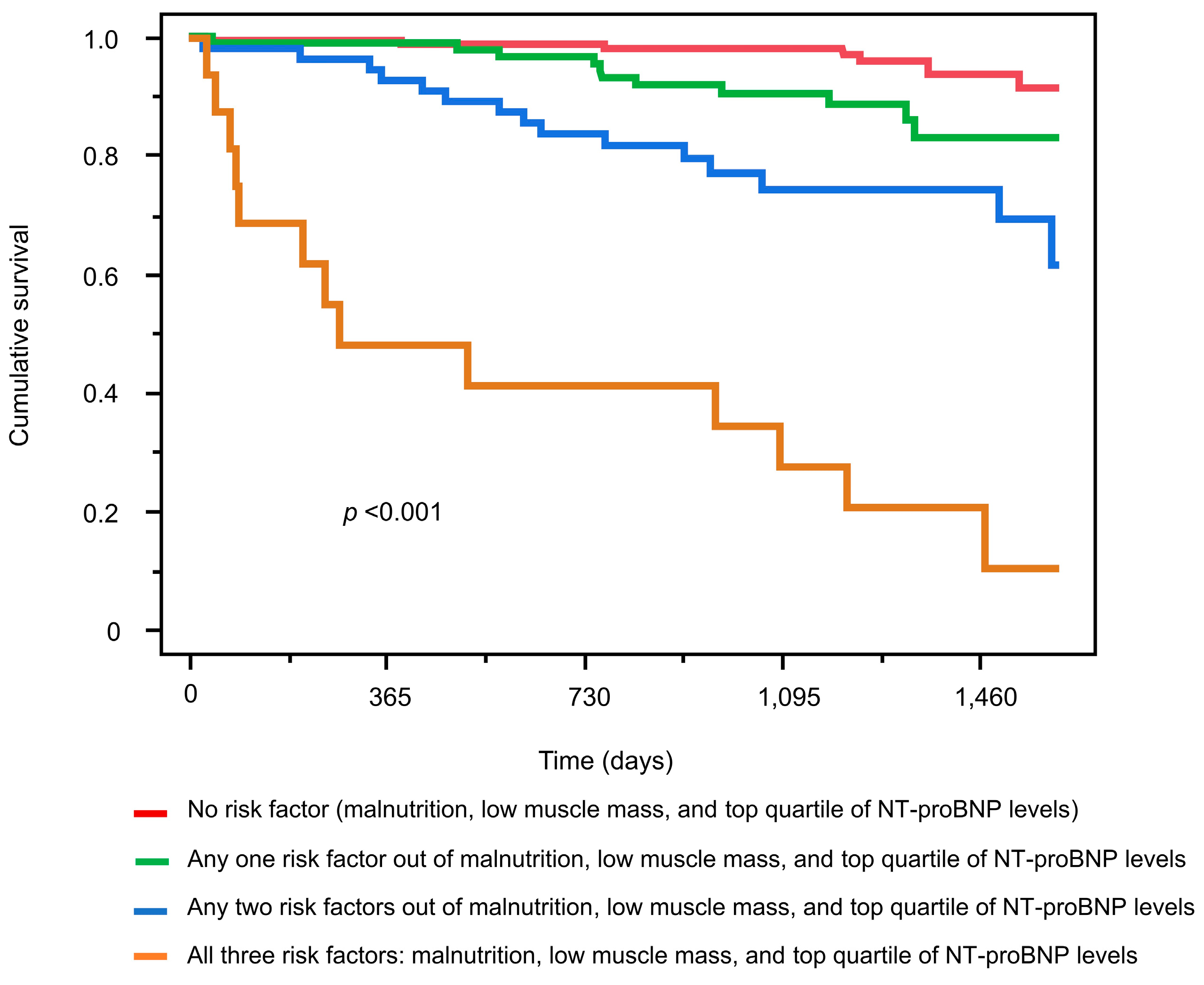
3.3. Mortality Risk Associated with Malnutrition, Low Muscle Mass, and Elevated NT-ProBNP Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inaba, M.; Okuno, S.; Ohno, Y. Importance of Considering Malnutrition and Sarcopenia in Order to Improve the QOL of Elderly Hemodialysis Patients in Japan in the Era of 100-Year Life. Nutrients 2021, 13, 2377. [Google Scholar] [CrossRef] [PubMed]
- de Jorge-Huerta, L.; Marco-Alacid, C.; Grande, C.; Andrés, C.V. A Narrative Review of the Diagnosis and Treatment of Sarcopenia and Malnutrition in Patients with Heart Failure. Nutrients 2024, 16, 2717. [Google Scholar] [CrossRef]
- Dekker, M.J.; Konings, C.; Canaud, B.; van der Sande, F.M.; Stuard, S.; Raimann, J.G.; Öztürk, E.; Usvyat, L.; Kotanko, P.; Kooman, J.P. Interactions Between Malnutrition, Inflammation, and Fluid Overload and Their Associations with Survival in Prevalent Hemodialysis Patients. J. Ren. Nutr. 2018, 28, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Fu, Y.-C.; Wu, M.-J. NT-ProBNP Predicts Total Mortality, Emergency Department Visits, Hospitalization, Intensive-Care Unit Admission, and Cardiovascular Events in Hemodialysis Patients. J. Clin. Med. 2019, 8, 238. [Google Scholar] [CrossRef]
- Guo, Q.; Bárány, P.; Qureshi, A.R.; Snaedal, S.; Heimbürger, O.; Stenvinkel, P.; Lindholm, B.; Axelsson, J. N-Terminal pro-brain natriuretic peptide independently predicts protein energy wasting and is associated with all-cause mortality in prevalent HD patients. Am. J. Nephrol. 2009, 29, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Wathanavasin, W.; Banjongjit, A.; Avihingsanon, Y.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S.; Susantitaphong, P. Prevalence of Sarcopenia and Its Impact on Cardiovascular Events and Mortality among Dialysis Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4077. [Google Scholar] [CrossRef]
- Booth, J.; Pinney, J.; Davenport, A. N-terminal proBNP—Marker of cardiac dysfunction, fluid overload, or malnutrition in hemodialysis patients? Clin. J. Am. Soc. Nephrol. 2010, 5, 1036–1040. [Google Scholar] [CrossRef]
- McKechnie, D.G.; Papacosta, A.O.; Lennon, L.T.; Welsh, P.; Whincup, P.H.; Wannamethee, S.G. Inflammatory Markers and Incident Heart Failure in Older Men: The Role of Nt-Probnp. Biomarkers Med. 2021, 15, 413–425. [Google Scholar] [CrossRef]
- Ikeda, M.; Honda, H.; Takahashi, K.; Shishido, K.; Shibata, T. N-Terminal Pro-B-Type Natriuretic Peptide as a Biomarker for Loss of Muscle Mass in Prevalent Hemodialysis Patients. PLoS ONE 2016, 11, e0166804. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Kanda, E.; Kato, A.; Masakane, I.; Kanno, Y. A new nutritional risk index for predicting mortality in hemodialysis patients: Nationwide cohort study. PLoS ONE 2019, 14, e0214524. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Baos, S.; Prieto-Potin, I.; Román-Blas, J.A.; Sánchez-Pernaute, O.; Largo, R.; Herrero-Beaumont, G. Mediators and Patterns of Muscle Loss in Chronic Systemic Inflammation. Front. Physiol. 2018, 9, 409. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Lindholm, B.; Kaysen, G.A.; Bergström, J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol. Dial. Transplant. 2000, 15, 953–960. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, D.; Locquet, M.; Reginster, J.; Cavalier, E.; Bruyère, O.; Beaudart, C. Mortality in malnourished older adults diagnosed by ESPEN and GLIM criteria in the SarcoPhAge study. J. Cachex- Sarcopenia Muscle 2020, 11, 1200–1211. [Google Scholar] [CrossRef]
- Noda, T.; Maekawa, E.; Maeda, D.; Uchida, S.; Yamashita, M.; Hamazaki, N.; Nozaki, K.; Saito, H.; Saito, K.; Ogasahara, Y.; et al. Prevalence and Prognostic Value of Cachexia Diagnosed by New Definition for Asian People in Older Patients with Heart Failure. J. Cachex- Sarcopenia Muscle 2024, 15, 2660–2668. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Moon, I.; Oh, Y.S.; Yu, B.C.; Park, M.Y.; Kim, J.K.; Choi, S.J. Prediction of Heart Function and Volume Status in End-Stage Kidney Disease Patients through N-Terminal Pro-Brain Natriuretic Peptide. Medicina 2022, 58, 975. [Google Scholar] [CrossRef]
- Yamazaki, K.; Ishii, S.; Hitaka, M.; Masai, M.; Ohashi, Y. Associations between N-Terminal Pro-B-Type Natriuretic Peptide, Body Fluid Imbalance and Quality of Life in Patients Undergoing Hemodialysis: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 7356. [Google Scholar] [CrossRef] [PubMed]
- Bervanlou, R.N.; Hlaváčová, N.; Figueiredo, V.C.; Hosseini, S.R.A.; Rad, M.M. The Impact of Exercise and Protein Intake on Inflammaging: A Meta-Analysis and Systematic Review of Randomized Controlled Trials. Nutr. Rev. 2024, nuae169. [Google Scholar] [CrossRef]
- Theodorakopoulou, M.P.; Dipla, K.; Zafeiridis, A.; Faitatzidou, D.; Koutlas, A.; Doumas, M.; Papagianni, A.; Sarafidis, P. Cerebral oxygenation during exercise deteriorates with advancing chronic kidney disease. Nephrol. Dial. Transplant. 2023, 38, 2379–2388. [Google Scholar] [CrossRef]
- Tabibi, M.A.; Cheema, B.; Salimian, N.; Corrêa, H.d.L.; Ahmadi, S. The effect of intradialytic exercise on dialysis patient survival: A randomized controlled trial. BMC Nephrol. 2023, 24, 100. [Google Scholar] [CrossRef]
- Anding-Rost, K.; von Gersdorff, G.; von Korn, P.; Ihorst, G.; Josef, A.; Kaufmann, M.; Huber, M.; Bär, T.; Zeißler, S.; Höfling, S.; et al. Exercise during Hemodialysis in Patients with Chronic Kidney Failure. NEJM Evid. 2023, 2, EVIDoa2300057. [Google Scholar] [CrossRef] [PubMed]


| Risk Marker | Score Value |
|---|---|
| Body mass index | |
| <20 kg/m2 | 3 |
| Serum albumin | |
| <3.7 g/dL (Age < 65 years); <3.5 g/dL (Age ≥ 65 years) | 4 |
| Serum creatinine | |
| Women <9.7 mg/dL (Age < 65 years); <8.0 mg/dL (Age ≥ 65 years) Men <11.6 mg/dL (Age < 65 years); <9.7 mg/dL (Age ≥ 65 years) | 4 |
| Serum total cholesterol | |
| <130 mg/dL | 1 |
| ≥220 mg/dL | 2 |
| Categorization based on the total score: 0–7 points: low risk, 8–10 points: moderate risk, 11 points or more: high risk | |
| Patient Characteristics | NRI-JH | p | ||
|---|---|---|---|---|
| Low Risk (n = 287) | Moderate Risk (n = 56) | High Risk (n = 25) | ||
| Age, years | 67 (56–74) | 64.5 (59–75) | 76 (56–82) | 0.06 |
| Age < 65, n (%) | 107 (37.3) | 28 (50.0) | 8 (32.0) | |
| Age 65–74, n (%) | 120 (41.8) | 13 (23.2) | 3 (12.0) | <0.001 |
| Age ≥ 75, n (%) | 60 (20.8) | 15 (26.8) | 14 (56.0) | |
| Men, n (%) | 201 (70.0) | 42 (75.0) | 18 (72.0) | 0.75 |
| Diabetes, n (%) | 129 (45.0) | 32 (57.1) | 7 (28.0) | 0.046 |
| Body mass index, kg/m2 | 22.7 (20.2–25.6) | 22.1 (20.8–25.0) | 18.3 (17.0–19.3) | <0.001 |
| Serum albumin, g/dL | 3.6 (3.5–3.8) | 3.4 (3.2–3.5) | 3.3 (3.1–3.4) | <0.001 |
| Serum sodium, mEq/L | 139 (138–141) | 139 (137–141) | 138 (137–139) | 0.047 |
| Serum potassium, mEq/L | 4.9 (4.4–5.4) | 4.7 (4.2–5.0) | 4.7 (4.2–5.2) | 0.028 |
| Serum chloride, mEq/L | 103 (101–105) | 104 (102–106) | 103 (102–106) | 0.48 |
| Serum calcium, mg/dL | 8.7 (8.3–9.0) | 8.6 (8.2–8.9) | 8.3 (8.0–8.7) | 0.011 |
| Serum phosphorus, mg/dL | 5.6 (5.0–6.4) | 5.1 (4.4–5.7) | 5.4 (4.7–6.5) | <0.001 |
| Triglyceride, mg/dL | 105 (75–154) | 91 (67–127) | 64 (51–87) | <0.001 |
| Total cholesterol, mg/dL | 165 (143–193) | 152 (130–185) | 159 (130–191) | 0.040 |
| LDL-C, mg/dL | 87 (71–107) | 77.5 (62–104) | 75 (61–102) | 0.049 |
| HDL-C, mg/dL | 50 (39–60) | 45 (37–57) | 54 (42–70) | 0.11 |
| Uric acid, mg/dL | 7.9 (7.1–8.6) | 6.9 (6.2–8.0) | 6.9 (6.5–8.0) | <0.001 |
| Blood urea nitrogen, mg/dL | 60.8 (52.2–71.4) | 49.6 (43.9–56.8) | 48.9 (45.5–59.0) | <0.001 |
| Serum creatinine, mg/dL | 10.7 (9.4–12.3) | 8.8 (7.7–9.8) | 8.6 (7.4–9.0) | <0.001 |
| Intact PTH, pg/mL | 154 (102–223) | 157 (99–197) | 138 (79–188) | 0.41 |
| β2MG, mg/L | 26.0 (23.4–29.2) | 27.0 (22.6–29.7) | 26.6 (25.4–34) | 0.27 |
| C-reactive protein, mg/dL | 0.1 (0–0.3) | 0.1 (0.1–0.5) | 0.3 (0–0.6) | 0.19 |
| Hemoglobin, g/dL | 11.3 (10.7–11.9) | 11.0 (10.5–11.5) | 10.6 (9.8–11.4) | 0.002 |
| NT-proBNP, pg/mL | 3000 (1610–6610) | 5900 (3478–11,075) | 7650 (4045–32,450) | <0.001 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| Age | 1.06 | (1.04–1.09) | <0.001 | 1.05 | (1.02–1.08) | <0.001 |
| Diabetes | 1.66 | (0.93–2.98) | 0.09 | 1.48 | (0.80–2.77) | 0.21 |
| Men | 2.15 | (1.00–4.62) | 0.049 | 2.15 | (0.95–4.86) | 0.07 |
| CRP ≥ 0.3 mg/dL | 1.33 | (0.71–2.50 | 0.37 | 0.74 | (0.38–1.45) | 0.38 |
| Moderate to high risk by NRI-JH | 4.98 | (2.79–8.91) | <0.001 | 3.52 | (1.92–6.44) | <0.001 |
| Low muscle mass | 3.25 | (1.81–5.82) | <0.001 | 2.63 | (1.42–4.86) | 0.002 |
| Top-quartile NT-proBNP levels | 5.45 | (3.02–9.88) | <0.001 | 3.40 | (1.81–6.38) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, S.; Tanaka, T.; Suzuki, Y.; Yoshida, N.; Hitaka, M.; Ishii, S.; Yamazaki, K.; Masai, M.; Yamada, Y.; Ohashi, Y. Association Between Malnutrition, Low Muscle Mass, Elevated NT-ProBNP Levels, and Mortality in Hemodialysis Patients. Nutrients 2025, 17, 1896. https://doi.org/10.3390/nu17111896
Takahashi S, Tanaka T, Suzuki Y, Yoshida N, Hitaka M, Ishii S, Yamazaki K, Masai M, Yamada Y, Ohashi Y. Association Between Malnutrition, Low Muscle Mass, Elevated NT-ProBNP Levels, and Mortality in Hemodialysis Patients. Nutrients. 2025; 17(11):1896. https://doi.org/10.3390/nu17111896
Chicago/Turabian StyleTakahashi, Sadamu, Tatsuki Tanaka, Yusuke Suzuki, Norihito Yoshida, Mai Hitaka, Shingo Ishii, Keisuke Yamazaki, Motoyuki Masai, Yosuke Yamada, and Yasushi Ohashi. 2025. "Association Between Malnutrition, Low Muscle Mass, Elevated NT-ProBNP Levels, and Mortality in Hemodialysis Patients" Nutrients 17, no. 11: 1896. https://doi.org/10.3390/nu17111896
APA StyleTakahashi, S., Tanaka, T., Suzuki, Y., Yoshida, N., Hitaka, M., Ishii, S., Yamazaki, K., Masai, M., Yamada, Y., & Ohashi, Y. (2025). Association Between Malnutrition, Low Muscle Mass, Elevated NT-ProBNP Levels, and Mortality in Hemodialysis Patients. Nutrients, 17(11), 1896. https://doi.org/10.3390/nu17111896






