How the Dietary Saturated/Monounsaturated Fatty Acid Ratio Modulates Brain Function in Older Adults
Abstract
1. Introduction
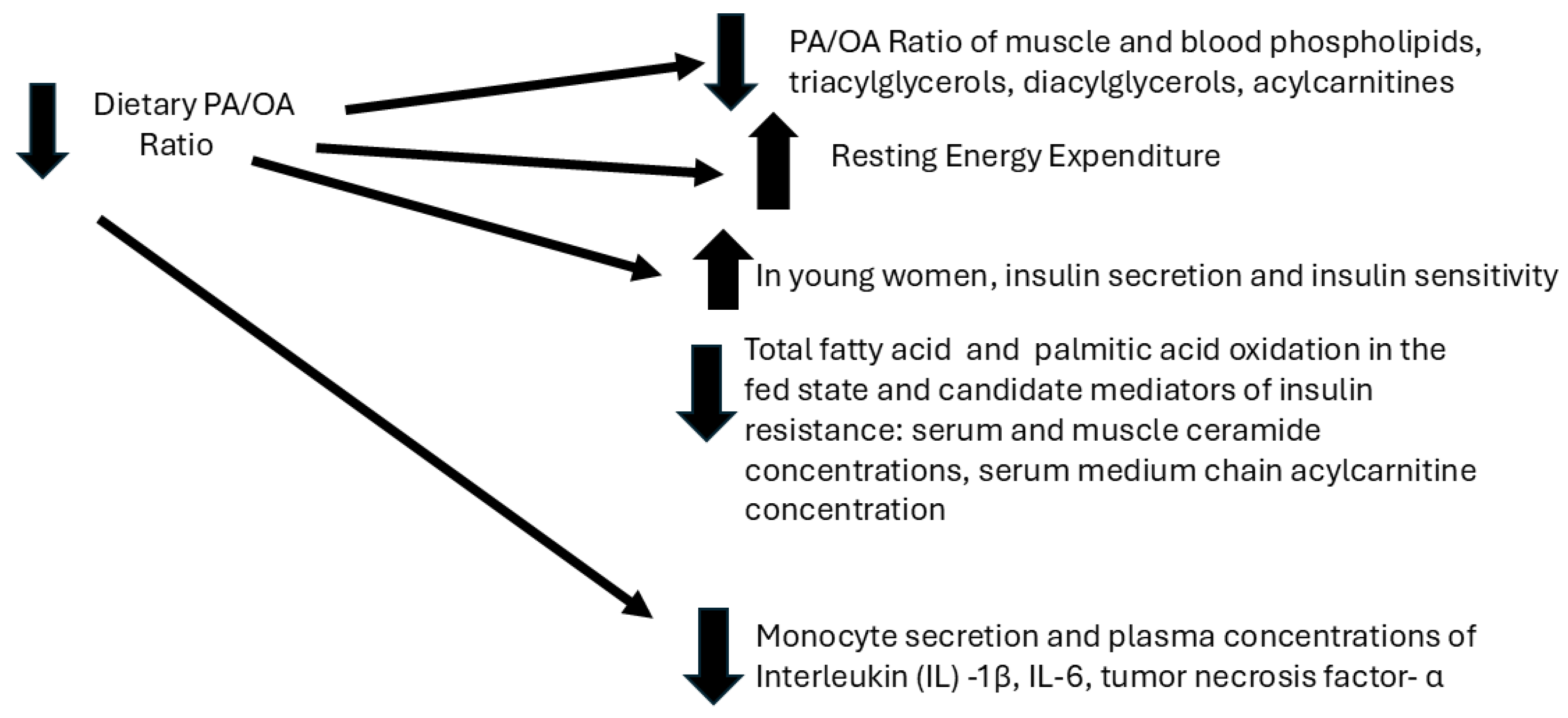
2. Effects of the Dietary PA/OA Ratio on Energy and Lipid Metabolism and Systemic Inflammatory Tone (Figure 1)
3. Effects of the Dietary PA/OA Ratio on Habitual Physical Activity and Mood in Humans
4. Descriptive Human Studies: Effects of the Dietary PA/OA Ratio on Brain Function in Young and Older Adults
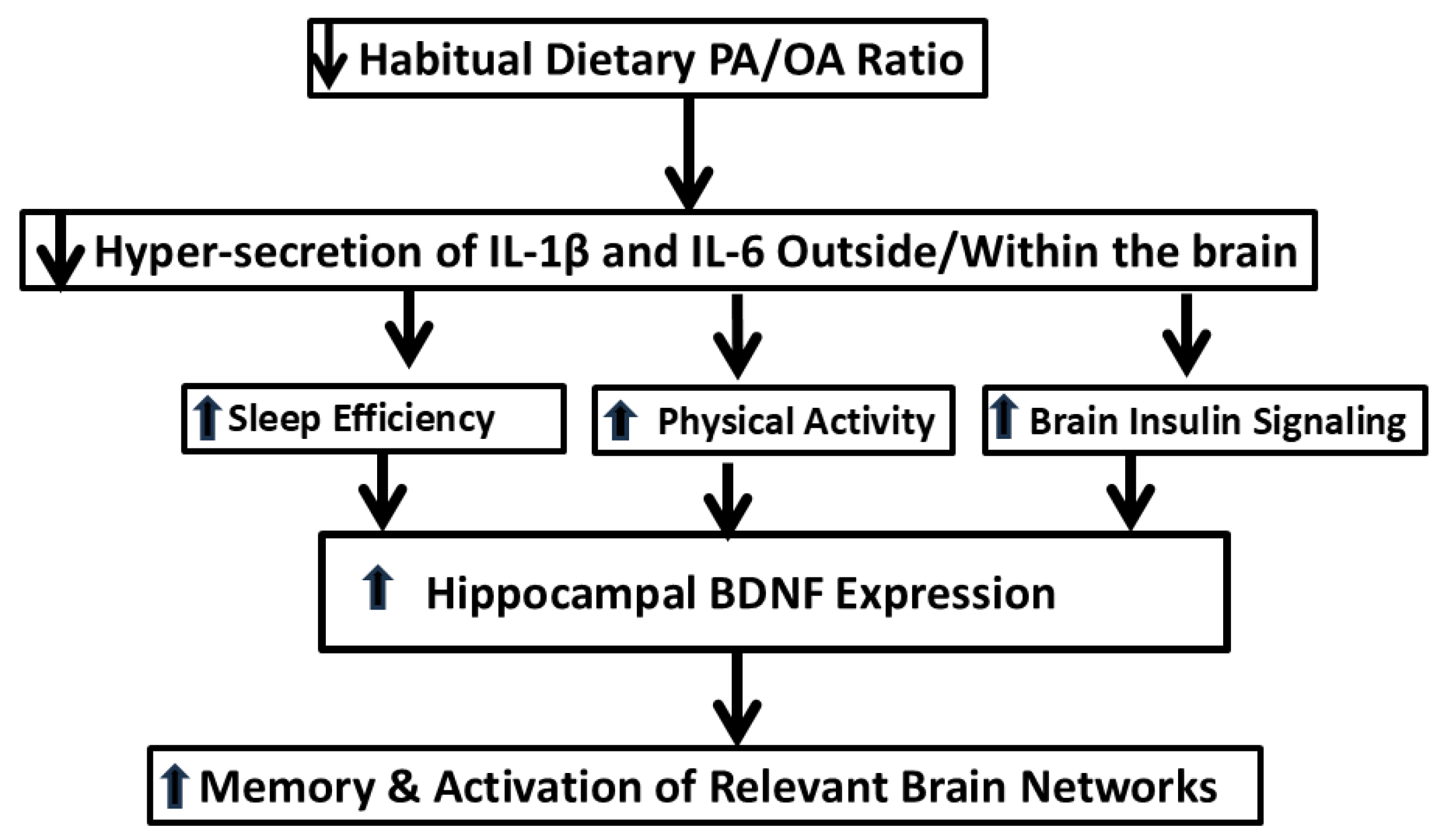
5. Mechanistic Studies Related to How the Dietary PA/OA Ratio May Differentially Affect Brain Function
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BA | Broadman area |
| BDNF | brain-derived neurotrophic factor |
| DLPFC | dorsolateral prefrontal cortex |
| FA | fatty acid |
| fMRI | functional magnetic resonance imaging |
| JNK | c-Jun N-terminal kinase |
| HOA | “high” oleic acid (low PA/OA ratio) diet, typical of the Mediterranean diet |
| HPA | “high” palmitic acid (high PA/OA ratio) diet, typical of the North American diet |
| IL | interleukin |
| MCI | mild cognitive impairment |
| MUFA | monounsaturated fatty acid |
| NLRP3 | nucleotide oligomerization domain (NOD)-like receptor protein |
| OA | oleic acid |
| PA | palmitic acid |
| PBMCs | peripheral blood mononuclear cells |
| PCC | posterior cingulate complex |
| POMS | Profile of Mood States |
| SFA | saturated fatty acid |
| TLR4 | Toll-like receptor-4 |
| TMD | total mood disturbance |
| TNFα | tumor necrosis factor-α |
References
- Grundy, S.; Denke, M. Dietary influences on serum lipids and lipoproteins. J. Lipid Res. 1990, 31, 1149–1172. (In English) [Google Scholar] [CrossRef] [PubMed]
- Berry, E.M. Dietary fatty acids in the management of diabetes mellitus. Am. J. Clin. Nutr. 1997, 66 (Suppl. 4), 991s–997s. (In English) [Google Scholar] [CrossRef] [PubMed]
- Gurr, M.I. Fats in food. In Role of Fats in Food and Nutrition; Gurr, M.I., Ed.; Elsevier Applied Science: London, UK; New York, NY, USA, 1992; pp. 21–53. [Google Scholar]
- Linder, M.C. Nutrition and metabolism of fats. In Nutritional Biochemistry and Metabolism with Clinical Applications; Linder, M.C., Ed.; Elsevier: New York, UK, USA, 1991; pp. 51–85. [Google Scholar]
- Gunstone, F.D.; Harwood, J.L.; Padley, F.B. The Lipid Handbook; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Kien, C.L.; Bunn, J.Y.; Stevens, R.; Bain, J.; Ikayeva, O.; Crain, K.; Koves, T.R.; Muoio, D.M. Dietary intake of palmitate and oleate has broad impact on systemic and tissue lipid profiles in humans. Am. J. Clin. Nutr. 2014, 99, 436–445. (In English) [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Hansson, G.K.; Shah, P.K. Immunomodulation of atherosclerosis: Implications for vaccine development. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 18–28. (In English) [Google Scholar] [CrossRef]
- Basu, A.; Devaraj, S.; Jialal, I. Dietary factors that promote or retard inflammation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 995–1001. (In English) [Google Scholar] [CrossRef]
- Han, S.N.; Leka, L.S.; Lichtenstein, A.H.; Ausman, L.M.; Schaefer, E.J.; Meydani, S.N. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J. Lipid Res. 2002, 43, 445–452. (In English) [Google Scholar] [CrossRef]
- Sudheendran, S.; Chang, C.; Deckelbaum, R. N-3 vs. saturated fatty acids: Effects on the arterial wall. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 205–209. (In English) [Google Scholar] [CrossRef][Green Version]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. 6), 1402s–1406s. (In English) [Google Scholar] [CrossRef]
- Kien, C.L.; Bunn, J.Y.; Poynter, M.E.; Stevens, R.; Bain, J.; Ikayeva, O.; Fukagawa, N.K.; Champagne, C.M.; Crain, K.I.; Koves, T.R.; et al. A lipidomics analysis of the relationship between dietary fatty acid composition and insulin sensitivity in young adults. Diabetes 2013, 62, 1054–1063. (In English) [Google Scholar] [CrossRef]
- Rudel, L.L.; Haines, J.; Sawyer, J.K.; Shah, R.; Wilson, M.S.; Carr, T.P. Hepatic origin of cholesteryl oleate in coronary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J. Clin. Investig. 1997, 100, 74–83. (In English) [Google Scholar] [CrossRef]
- Jones, P.J.; MacKay, D.S.; Senanayake, V.K.; Pu, S.; Jenkins, D.J.; Connelly, P.W.; Lamarche, B.; Couture, P.; Kris-Etherton, P.M.; West, S.G.; et al. High-oleic canola oil consumption enriches LDL particle cholesteryl oleate content and reduces LDL proteoglycan binding in humans. Atherosclerosis 2015, 238, 231–238. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L.; Bunn, J.Y. Gender alters the effects of palmitate and oleate on fat oxidation and energy expenditure. Obesity 2008, 16, 29–33. (In English) [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.A.; Bunn, J.Y.; Nickerson, J.; Crain, K.I.; Ebenstein, D.B.; Tarleton, E.K.; Makarewicz, J.; Poynter, M.E.; Kien, C.L. Dietary saturated fat and monounsaturated fat have reversible effects on brain function and the secretion of pro-inflammatory cytokines in young women. Metab. Clin. Exp. 2016, 65, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L.; Bunn, J.Y.; Fukagawa, N.K.; Anathy, V.; Matthews, D.E.; Crain, K.I.; Ebenstein, D.B.; Tarleton, E.K.; Pratley, R.E.; Poynter, M.E. Lipidomic evidence that lowering the typical dietary palmitate to oleate ratio in humans decreases the leukocyte production of proinflammatory cytokines and muscle expression of redox-sensitive genes. J. Nutr. Biochem. 2015, 26, 1599–1606. (In English) [Google Scholar] [CrossRef]
- Dumas, J.A.; Bunn, J.Y.; LaMantia, M.A.; McIsaac, C.; Miller, A.S.; Nop, O.; Testo, A.; Soares, B.P.; Mank, M.M.; Poynter, M.E.; et al. Alteration of brain function and systemic inflammatory tone in older adults by decreasing the dietary palmitic acid intake. Aging Brain 2023, 3, 100072. (In English) [Google Scholar] [CrossRef]
- Schmidt, D.E.; Allred, J.B.; Kien, C.L. Fractional oxidation of chylomicron-derived oleate is greater than that of palmitate in healthy adults fed frequent small meals. J. Lipid Res. 1999, 40, 2322–2332. (In English) [Google Scholar] [CrossRef]
- Kien, C.L.; Matthews, D.E.; Poynter, M.E.; Bunn, J.Y.; Fukagawa, N.K.; Crain, K.I.; Ebenstein, D.B.; Tarleton, E.K.; Stevens, R.D.; Koves, T.R.; et al. Increased palmitate intake: Higher acylcarnitine concentrations without impaired progression of β-oxidation. J. Lipid Res. 2015, 56, 1795–1807. (In English) [Google Scholar] [CrossRef]
- Kien, C.L.; Bunn, J.Y.; Tompkins, C.L.; Dumas, J.A.; Crain, K.I.; Ebenstein, D.B.; Koves, T.; Muoio, D.M. Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. Am. J. Clin. Nutr. 2013, 97, 689–697. (In English) [Google Scholar] [CrossRef]
- Milner, M.T.; Maddugoda, M.; Götz, J.; Burgener, S.S.; Schroder, K. The NLRP3 inflammasome triggers sterile neuroinflammation and Alzheimer’s disease. Curr. Opin. Immunol. 2021, 68, 116–124. (In English) [Google Scholar] [CrossRef]
- Stienstra, R.; Tack, C.J.; Kanneganti, T.-D.; Joosten, L.A.; Netea, M.G. The inflammasome puts obesity in the danger zone. Cell Metab. 2012, 15, 10–18. (In English) [Google Scholar] [CrossRef]
- McAuley, E.; Mullen, S.P.; Szabo, A.N.; White, S.M.; Wójcicki, T.R.; Mailey, E.L.; Gothe, N.P.; Olson, E.A.; Voss, M.; Erickson, K.; et al. Self-regulatory processes and exercise adherence in older adults: Executive function and self-efficacy effects. Am. J. Prev. Med. 2011, 41, 284–290. (In English) [Google Scholar] [CrossRef] [PubMed]
- Sartorius, T.; Ketterer, C.; Kullmann, S.; Balzer, M.; Rotermund, C.; Binder, S.; Hallschmid, M.; Machann, J.; Schick, F.; Somoza, V.; et al. Monounsaturated fatty acids prevent the aversive effects of obesity on locomotion, brain activity, and sleep behavior. Diabetes 2012, 61, 1669–1679. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hassanain, M.; Zalcman, S.; Bhatt, S.; Siegel, A. Interleukin-1 beta in the hypothalamus potentiates feline defensive rage: Role of serotonin-2 receptors. Neuroscience 2003, 120, 227–233. (In English) [Google Scholar] [CrossRef]
- Zalcman, S.S.; Siegel, A. The neurobiology of aggression and rage: Role of cytokines. Brain Behav. Immun. 2006, 20, 507–514. (In English) [Google Scholar] [CrossRef]
- Suarez, E.C.; Lewis, J.G.; Kuhn, C. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain Behav. Immun. 2002, 16, 675–684. (In English) [Google Scholar] [CrossRef]
- Suarez, E.C.; Lewis, J.G.; Krishnan, R.R.; Young, K.H. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology 2004, 29, 1119–1128. (In English) [Google Scholar] [CrossRef]
- Blazer, D.G.; Yaffe, K.; Liverman, C.T. Committee on the Public Health Dimensions of Cognitive Aging; Board on Health Sciences Policy. In Cognitive Aging: Progress in Understanding and Opportunities for Action; Institute of Medicine, Ed.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Samieri, C.; Grodstein, F.; Rosner, B.A.; Kang, J.H.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Willett, W.C.; Okereke, O.I. Mediterranean diet and cognitive function in older age. Epidemiology 2013, 24, 490–499. (In English) [Google Scholar] [CrossRef]
- Blazer, D.G.; Yaffe, K.; Karlawish, J. Cognitive aging: A report from the Institute of Medicine. JAMA 2015, 313, 2121–2122. (In English) [Google Scholar] [CrossRef]
- Craik, F.I.; Salthouse, T. Handbook of Aging and Cogntion, 2nd ed.; Erlbaum: Mahwah, NJ, USA, 2000. [Google Scholar]
- Baddeley, A.D. Working Memory; Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Verhaeghen, P.; Marcoen, A.; Goossens, L. Facts and fiction about memory aging: A quantitative integration of research findings. J. Gerontol. 1993, 48, P157–P171. [Google Scholar] [CrossRef]
- Labouvie-Vief, G. Dynamic integration: Affect, cognition, and the self in adulthood. Curr. Dir. Psychol. Sci. 2003, 12, 201–206. [Google Scholar] [CrossRef]
- Rypma, B.; D’Esposito, M. Isolating the neural mechanisms of age-related changes in human working memory. Nat. Neurosci. 2000, 3, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Daselaar, S.M.; Dolcos, F.; Prince, S.E.; Budde, M.; Nyberg, L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic memory retrieval. Cereb. Cortex 2004, 14, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.W.; Dennis, N.A.; Daselaar, S.M.; Fleck, M.S.; Cabeza, R. Que PASA? The Posterior Anterior Shift in Aging. Cereb. Cortex 2008, 18, 1201–1209. [Google Scholar] [CrossRef]
- Cabeza, R.; Anderson, N.D.; Locantore, J.K.; McIntosh, A.R. Aging Gracefully: Compensatory Brain Activity in High-Performing Older Adults. NeuroImage 2002, 17, 1394–1402. [Google Scholar] [CrossRef]
- Hsiao, P.Y.; Mitchell, D.C.; Coffman, D.L.; Allman, R.M.; Locher, J.L.; Sawyer, P.; Jensen, G.L.; Hartman, T.J. Dietary patterns and diet quality among diverse older adults: The University of Alabama at Birmingham Study of Aging. J. Nutr. Health Aging 2013, 17, 19–25. (In English) [Google Scholar] [CrossRef]
- Knopman, D.S. Mediterranean diet and late-life cognitive impairment: A taste of benefit. JAMA 2009, 302, 686–687. (In English) [Google Scholar] [CrossRef][Green Version]
- Koyama, A.; Houston, D.K.; Simonsick, E.M.; Lee, J.S.; Ayonayon, H.N.; Shahar, D.R.; Rosano, C.; Satterfield, S.; Yaffe, K. Association between the Mediterranean diet and cognitive decline in a biracial population. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 354–359. (In English) [Google Scholar] [CrossRef]
- Muscat, S.M.; Barrientos, R.M. The Perfect Cytokine Storm: How Peripheral Immune Challenges Impact Brain Plasticity & Memory Function in Aging. Brain Plast. 2021, 7, 47–60. (In English) [Google Scholar] [CrossRef]
- Dinarello, C.A.; Gatti, S.; Bartfai, T. Fever: Links with an ancient receptor. Curr. Biol. 1999, 9, R147–R150. (In English) [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. (In English) [Google Scholar] [CrossRef]
- Resh, M.D. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci. STKE 2006, 2006, re14. (In English) [Google Scholar] [CrossRef] [PubMed]
- Sadaqa, E.; Kurniawan, F.; Mudhakir, D. Mechanistic insights into endosomal escape by sodium oleate-modified liposomes. Beilstein J. Nanotechnol. 2024, 15, 1667–1685. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.-H.; Brickey, W.J.; Ting, J.P.-Y. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. (In English) [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. (In English) [Google Scholar] [CrossRef]
- Cintra, D.E.; Ropelle, E.R.; Moraes, J.C.; Pauli, J.R.; Morari, J.; de Souza, C.T.; Grimaldi, R.; Stahl, M.; Carvalheira, J.B.; Saad, M.J.; et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS ONE 2012, 7, e30571. (In English) [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. (In English) [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. (In English) [Google Scholar] [CrossRef]
- Soto-Guzman, A.; Robledo, T.; Lopez-Perez, M.; Salazar, E.P. Oleic acid induces ERK1/2 activation and AP-1 DNA binding activity through a mechanism involving Src kinase and EGFR transactivation in breast cancer cells. Mol. Cell Endocrinol. 2008, 294, 81–91. (In English) [Google Scholar] [CrossRef]
- Youm, Y.-H.; Grant, R.W.; McCabe, L.R.; Albarado, D.C.; Nguyen, K.Y.; Ravussin, A.; Pistell, P.; Newman, S.; Carter, R.; Laque, A.; et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013, 18, 519–532. (In English) [Google Scholar] [CrossRef]
- Perry, V.H. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010, 120, 277–286. (In English) [Google Scholar] [CrossRef]
- Vitale, G.; Salvioli, S.; Franceschi, C. Oxidative stress and the ageing endocrine system. Nat. Rev. Endocrinol. 2013, 9, 228–240. (In English) [Google Scholar] [CrossRef] [PubMed]
- Poynter, M.E.; Daynes, R.A. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J. Biol. Chem. 1998, 273, 32833–32841. (In English) [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chan, C. The role of inflammasome in Alzheimer’s disease. Ageing Res. Rev. 2014, 15, 6–15. (In English) [Google Scholar] [CrossRef]
- Ojala, J.O.; Sutinen, E.M. The Role of Interleukin-18, Oxidative Stress and Metabolic Syndrome in Alzheimer’s Disease. J. Clin. Med. 2017, 6, 55. (In English) [Google Scholar] [CrossRef]
- Liu, L.; Chan, C. IPAF inflammasome is involved in interleukin-1beta production from astrocytes, induced by palmitate; implications for Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 309–321. (In English) [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. (In English) [Google Scholar] [CrossRef]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9, 857–865. (In English) [Google Scholar] [CrossRef]
- Patterson, S.L. Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1beta, BDNF and synaptic plasticity. Neuropharmacology 2015, 96 Pt A, 11–18. (In English) [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Fagundes, C.P.; Andridge, R.; Peng, J.; Malarkey, W.B.; Habash, D.; Belury, M.A. Depression, daily stressors and inflammatory responses to high-fat meals: When stress overrides healthier food choices. Mol. Psychiatry 2017, 22, 476–482. (In English) [Google Scholar] [CrossRef]
- Cai, Z.; Hussain, M.D.; Yan, L.-J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. (In English) [Google Scholar] [CrossRef]
- Elahi, F.M.; Casaletto, K.B.; La Joie, R.; Walters, S.M.; Harvey, D.; Wolf, A.; Edwards, L.; Rivera-Contreras, W.; Karydas, A.; Cobigo, Y.; et al. Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer’s disease. Alzheimers Dement. 2020, 16, 681–695. (In English) [Google Scholar] [CrossRef] [PubMed]
- Villegas, S.; Roda, A.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666–1674. (In English) [Google Scholar] [CrossRef] [PubMed]
- Van Zeller, M.; Dias, D.M.; Sebastião, A.M.; Valente, C.A. NLRP3 Inflammasome: A Starring Role in Amyloid-β- and Tau-Driven Pathological Events in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 83, 939–961. (In English) [Google Scholar] [CrossRef]
- Ogunmokun, G.; Dewanjee, S.; Chakraborty, P.; Valupadas, C.; Chaudhary, A.; Kolli, V.; Anand, U.; Vallamkondu, J.; Goel, P.; Paluru, H.P.R.; et al. The Potential Role of Cytokines and Growth Factors in the Pathogenesis of Alzheimer’s Disease. Cells 2021, 10, 2790. (In English) [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. (In English) [Google Scholar] [CrossRef]
- Attuquayefio, T.; Stevenson, R.J.; Oaten, M.J.; Francis, H.M. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS ONE 2017, 12, e0172645. (In English) [Google Scholar] [CrossRef]
- Wahl, D.; Cogger, V.C.; Solon-Biet, S.M.; Waern, R.V.; Gokarn, R.; Pulpitel, T.; de Cabo, R.; Mattson, M.P.; Raubenheimer, D.; Simpson, S.J.; et al. Nutritional strategies to optimise cognitive function in the aging brain. Ageing Res. Rev. 2016, 31, 80–92. (In English) [Google Scholar] [CrossRef]
- Chu, C.-Q.; Yu, L.-L.; Qi, G.-Y.; Mi, Y.-S.; Wu, W.-Q.; Lee, Y.-K.; Zhai, Q.-X.; Tian, F.-W.; Chen, W. Can dietary patterns prevent cognitive impairment and reduce Alzheimer’s disease risk: Exploring the underlying mechanisms of effects. Neurosci. Biobehav. Rev. 2022, 135, 104556. (In English) [Google Scholar] [CrossRef]
- Sartorius, T.; Lutz, S.Z.; Hoene, M.; Waak, J.; Peter, A.; Weigert, C.; Rammensee, H.; Kahle, P.J.; Häring, H.; Hennige, A.M. Toll-like receptors 2 and 4 impair insulin-mediated brain activity by interleukin-6 and osteopontin and alter sleep architecture. FASEB J. 2012, 26, 1799–1809. (In English) [Google Scholar] [CrossRef]
- Talbot, K.; Wang, H.-Y.; Kazi, H.; Han, L.-Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012, 122, 1316–1338. (In English) [Google Scholar] [CrossRef]
- Rissman, R.A.; Trojanowski, J.Q.; Shaw, L.M.; Aisen, P.S. Longitudinal plasma amyloid beta as a biomarker of Alzheimer’s disease. J. Neural Transm. 2012, 119, 843–850. (In English) [Google Scholar] [CrossRef] [PubMed]
- Webers, A.; Heneka, M.T.; Gleeson, P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020, 98, 28–41. (In English) [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, R.; Guijarro, A.; Pratley, R.E.; Mason, C.C.; Piomelli, D.; Krakoff, J. Associations of fatty acids in cerebrospinal fluid with peripheral glucose concentrations and energy metabolism. PLoS ONE 2012, 7, e41503. (In English) [Google Scholar] [CrossRef]
- Obici, S.; Feng, Z.; Karkanias, G.; Baskin, D.G.; Rossetti, L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002, 5, 566–572. (In English) [Google Scholar] [CrossRef]
- Molteni, R.; Barnard, R.; Ying, Z.; Roberts, C.; Gómez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112, 803–814. (In English) [Google Scholar] [CrossRef]
- Freeman, L.R.; Granholm, A.-C.E. Vascular changes in rat hippocampus following a high saturated fat and cholesterol diet. J. Cereb. Blood Flow. Metab. 2012, 32, 643–653. (In English) [Google Scholar] [CrossRef]
- Freeman, L.R.; Small, B.J.; Bickford, P.C.; Umphlet, C.; Granholm, A.-C. A high-fat/high-cholesterol diet inhibits growth of fetal hippocampal transplants via increased inflammation. Cell Transplant. 2011, 20, 1499–1514. (In English) [Google Scholar] [CrossRef]
- Granholm, A.-C.; Bimonte-Nelson, H.A.; Moore, A.B.; Nelson, M.E.; Freeman, L.R.; Sambamurti, K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J. Alzheimers Dis. 2008, 14, 133–145. (In English) [Google Scholar] [CrossRef]
- Peters, R.; Breitner, J.; James, S.; Jicha, G.A.; Meyer, P.; Richards, M.; Smith, A.D.; Yassine, H.N.; Abner, E.; Hainsworth, A.H.; et al. Dementia risk reduction: Why haven’t the pharmacological risk reduction trials worked? An in-depth exploration of seven established risk factors. Alzheimers Dement. 2021, 7, e12202. (In English) [Google Scholar] [CrossRef]
- Hanson, A.J.; Bayer, J.L.; Baker, L.D.; Cholerton, B.; VanFossen, B.; Trittschuh, E.; Rissman, R.A.; Donohue, M.C.; Moghadam, S.H.; Plymate, S.R.; et al. Differential Effects of Meal Challenges on Cognition, Metabolism, and Biomarkers for Apolipoprotein E varepsilon4 Carriers and Adults with Mild Cognitive Impairment. J. Alzheimers Dis. 2015, 48, 205–218. (In English) [Google Scholar] [CrossRef]
- Yona, S.; Jung, S. Monocytes: Subsets, origins, fates and functions. Curr. Opin. Hematol. 2010, 17, 53–59. (In English) [Google Scholar] [CrossRef] [PubMed]
- de Kreutzenberg, S.V.; Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Semplicini, A.; Dalla Man, C.; Cobelli, C.; Fadini, G.P.; Avogaro, A. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: Potential biochemical mechanisms. Diabetes 2010, 59, 1006–1015. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. (In English) [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Volle, D.; Jalil, J.; Wu, P.; Small, G.W. Health-Promoting Strategies for the Aging Brain. Am. J. Geriatr. Psychiatry 2019, 27, 213–236. (In English) [Google Scholar] [CrossRef]
- Weuve, J.; Kang, J.H.; Manson, J.E.; Breteler, M.M.; Ware, J.H.; Grodstein, F. Physical activity, including walking, and cognitive function in older women. JAMA 2004, 292, 1454–1461. (In English) [Google Scholar] [CrossRef]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. (In English) [Google Scholar] [CrossRef]
- Smith, J.C.; Nielson, K.A.; Antuono, P.; Lyons, J.-A.; Hanson, R.J.; Butts, A.M.; Hantke, N.C.; Verber, M.D. Semantic memory functional MRI and cognitive function after exercise intervention in mild cognitive impairment. J. Alzheimers Dis. 2013, 37, 197–215. (In English) [Google Scholar] [CrossRef]
- Won, J.; Alfini, A.J.; Weiss, L.R.; Michelson, C.S.; Callow, D.D.; Ranadive, S.M.; Gentili, R.J.; Smith, J.C. Semantic Memory Activation After Acute Exercise in Healthy Older Adults. J. Int. Neuropsychol. Soc. 2019, 25, 557–568. (In English) [Google Scholar] [CrossRef]
- Crowley, K. Sleep and sleep disorders in older adults. Neuropsychol. Rev. 2011, 21, 41–53. (In English) [Google Scholar] [CrossRef]
- Barger, L.K.; Cade, B.E.; Ayas, N.T.; Cronin, J.W.; Rosner, B.; Speizer, F.E.; Czeisler, C.A. Extended work shifts and the risk of motor vehicle crashes among interns. N. Engl. J. Med. 2005, 352, 125–134. (In English) [Google Scholar] [CrossRef]
- Lockley, S.W.; Cronin, J.W.; Evans, E.E.; Cade, B.E.; Lee, C.J.; Landrigan, C.P.; Rothschild, J.M.; Katz, J.T.; Lilly, C.M.; Stone, P.H.; et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. N. Engl. J. Med. 2004, 351, 1829–1837. (In English) [Google Scholar] [CrossRef] [PubMed]
- Tapia-Arancibia, L.; Aliaga, E.; Silhol, M.; Arancibia, S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res. Rev. 2008, 59, 201–220. (In English) [Google Scholar] [CrossRef] [PubMed]
- Karczewska-Kupczewska, M.; Kowalska, I.; Nikołajuk, A.; Adamska, A.; Zielińska, M.; Kamińska, N.; Otziomek, E.; Górska, M.; Strczkowski, M. Circulating brain-derived neurotrophic factor concentration is downregulated by intralipid/heparin infusion or high-fat meal in young healthy male subjects. Diabetes Care 2012, 35, 358–362. (In English) [Google Scholar] [CrossRef] [PubMed]
- Klein, A.B.; Williamson, R.; Santini, M.A.; Clemmensen, C.; Ettrup, A.; Rios, M.; Knudsen, G.M.; Aznar, S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol./Off. Sci. J. Coll. Int. 2011, 14, 347–353. (In English) [Google Scholar] [CrossRef]
- Mueller, K.; Arelin, K.; Möller, H.E.; Sacher, J.; Kratzsch, J.; Luck, T.; Riedel-Heller, S.; Villringer, A.; Schroeter, M.L. Serum BDNF correlates with connectivity in the (pre)motor hub in the aging human brain—A resting-state fMRI pilot study. Neurobiol. Aging 2016, 38, 181–187. (In English) [Google Scholar] [CrossRef]
- Park, H.-R.; Kim, J.-Y.; Park, K.-Y.; Lee, J.-W. Lipotoxicity of palmitic Acid on neural progenitor cells and hippocampal neurogenesis. Toxicol. Res. 2011, 27, 103–110. (In English) [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. (In English) [Google Scholar] [CrossRef]
- Schmitt, K.; Holsboer-Trachsler, E.; Eckert, A. BDNF in sleep, insomnia, and sleep deprivation. Ann. Med. 2016, 48, 42–51. (In English) [Google Scholar] [CrossRef]
- Andrews, S.J.; Das, D.; Cherbuin, N.; Anstey, K.J.; Easteal, S. Association of genetic risk factors with cognitive decline: The PATH through life project. Neurobiol. Aging 2016, 41, 150–158. (In English) [Google Scholar] [CrossRef]
- Canivet, A.; Albinet, C.T.; André, N.; Pylouster, J.; Rodríguez-Ballesteros, M.; Kitzis, A.; Audiffren, M. Effects of BDNF polymorphism and physical activity on episodic memory in the elderly: A cross sectional study. Eur. Rev. Aging Phys. Act. Off. J. Eur. Group Res. Into Elder. Phys. Act. 2015, 12, 15. (In English) [Google Scholar] [CrossRef]
- Sartorius, T.; Heni, M.; Tschritter, O.; Preissl, H.; Hopp, S.; Fritsche, A.; Lievertz, P.-S.; Gertler, A.; Berthou, F.; Taouis, M.; et al. Leptin affects insulin action in astrocytes and impairs insulin-mediated physical activity. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2012, 30, 238–246. (In English) [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H. Lipid overload and overflow: Metabolic trauma and the metabolic syndrome. Trends Endocrinol. Metab. 2003, 14, 398–403. (In English) [Google Scholar] [CrossRef] [PubMed]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. (In English) [Google Scholar] [CrossRef] [PubMed]
| Outcome | Subject Population | Directionality of Change | Methodology | Reference No. |
|---|---|---|---|---|
| Physical Activity | Young Men and Women | ↑ | Wearable Accelerometer | [21] |
| Anger and Total Mood Disturbance | Young Men and Women | ↓ | Profile of Mood States (questionnaire) | [21] |
| Activation of basal ganglia during working memory task | Young Women | ↓ | functional magnetic resonance imaging | [16] |
| Activation of brain working memory network | Men and Women, aged 65–75 yr | ↑ | functional magnetic resonance imaging | [18] |
| Rate of cognitive decline | 6174 women aged ≥ 65 yr | ↓ | Observational study (Women’s Health Study) | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kien, C.L.; Dumas, J.A. How the Dietary Saturated/Monounsaturated Fatty Acid Ratio Modulates Brain Function in Older Adults. Nutrients 2025, 17, 1897. https://doi.org/10.3390/nu17111897
Kien CL, Dumas JA. How the Dietary Saturated/Monounsaturated Fatty Acid Ratio Modulates Brain Function in Older Adults. Nutrients. 2025; 17(11):1897. https://doi.org/10.3390/nu17111897
Chicago/Turabian StyleKien, C. Lawrence, and Julie A. Dumas. 2025. "How the Dietary Saturated/Monounsaturated Fatty Acid Ratio Modulates Brain Function in Older Adults" Nutrients 17, no. 11: 1897. https://doi.org/10.3390/nu17111897
APA StyleKien, C. L., & Dumas, J. A. (2025). How the Dietary Saturated/Monounsaturated Fatty Acid Ratio Modulates Brain Function in Older Adults. Nutrients, 17(11), 1897. https://doi.org/10.3390/nu17111897






