Advances in Isorhamnetin Treatment of Malignant Tumors: Mechanisms and Applications
Abstract
1. Introduction
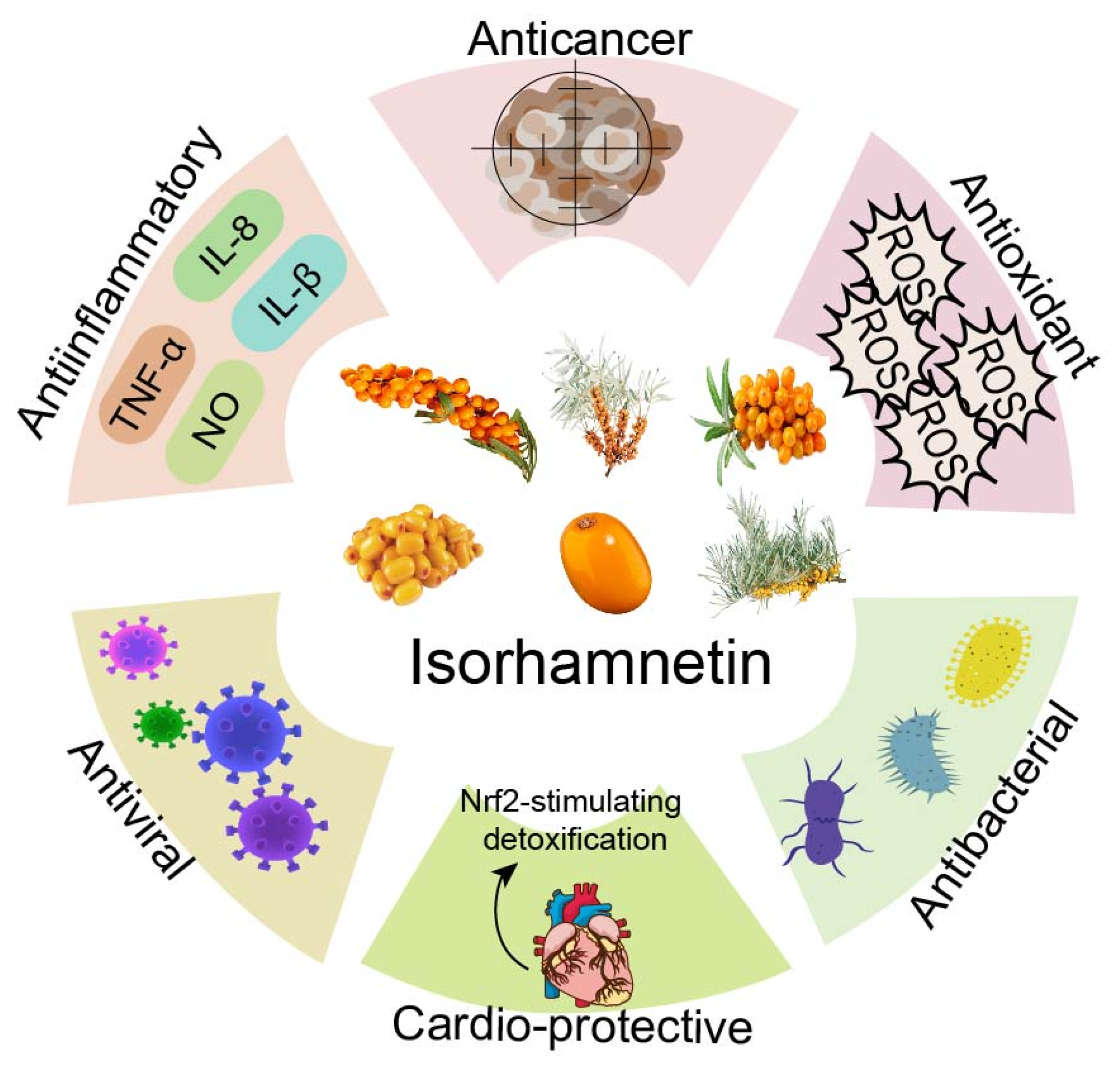
2. Different Effects of ISO
3. Mechanism of Inhibition of Malignant Tumor Cell Proliferation by ISO In Vitro
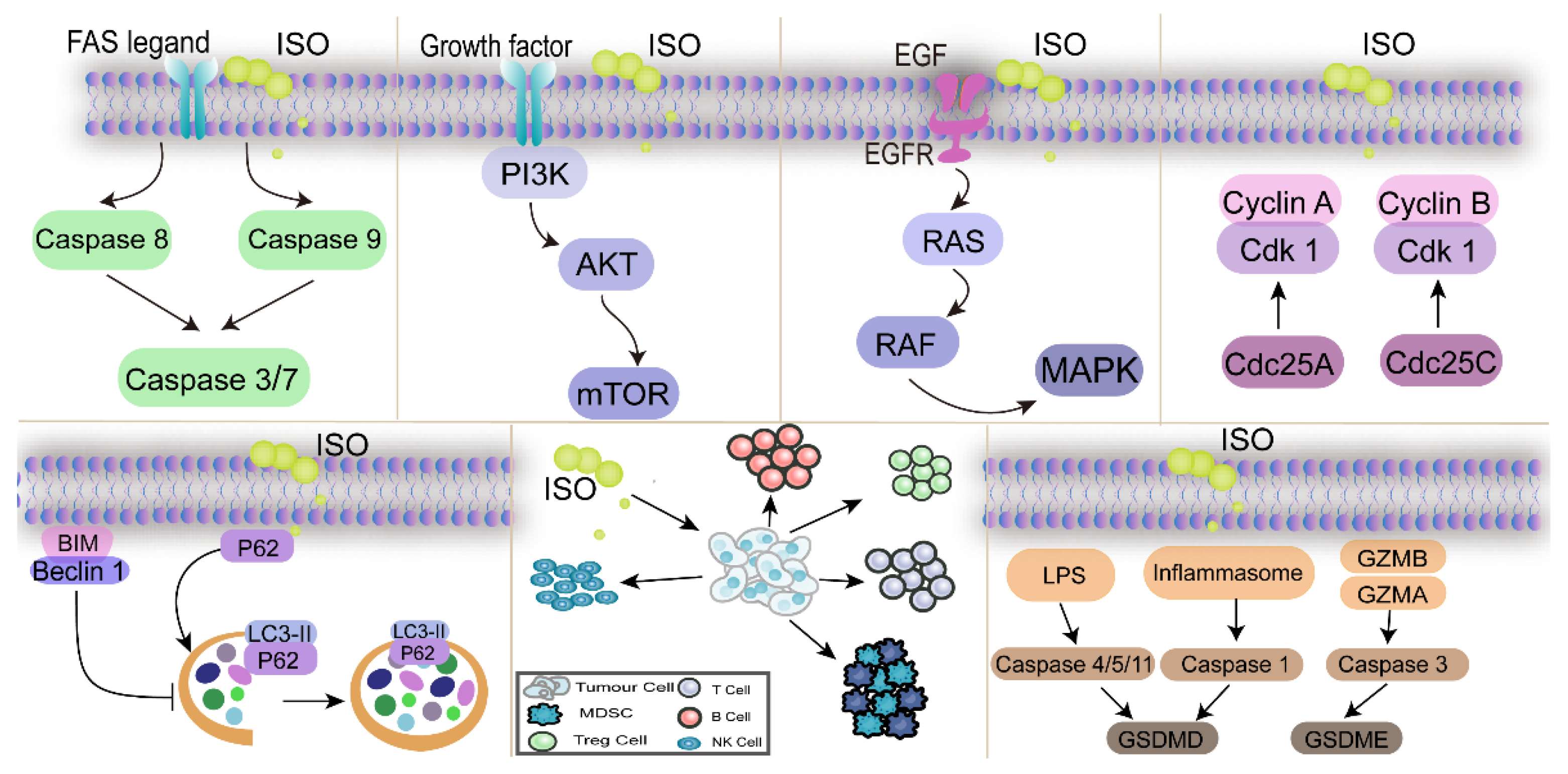
3.1. ISO Regulates the Cell Cycle to Inhibit Cancer Cell Proliferation
3.2. ISO Inhibits Cancer Cell Proliferation by Regulating the PI3K/AKT Pathway
3.3. ISO Inhibits Cancer Cell Proliferation by Regulating the MAPK Pathway
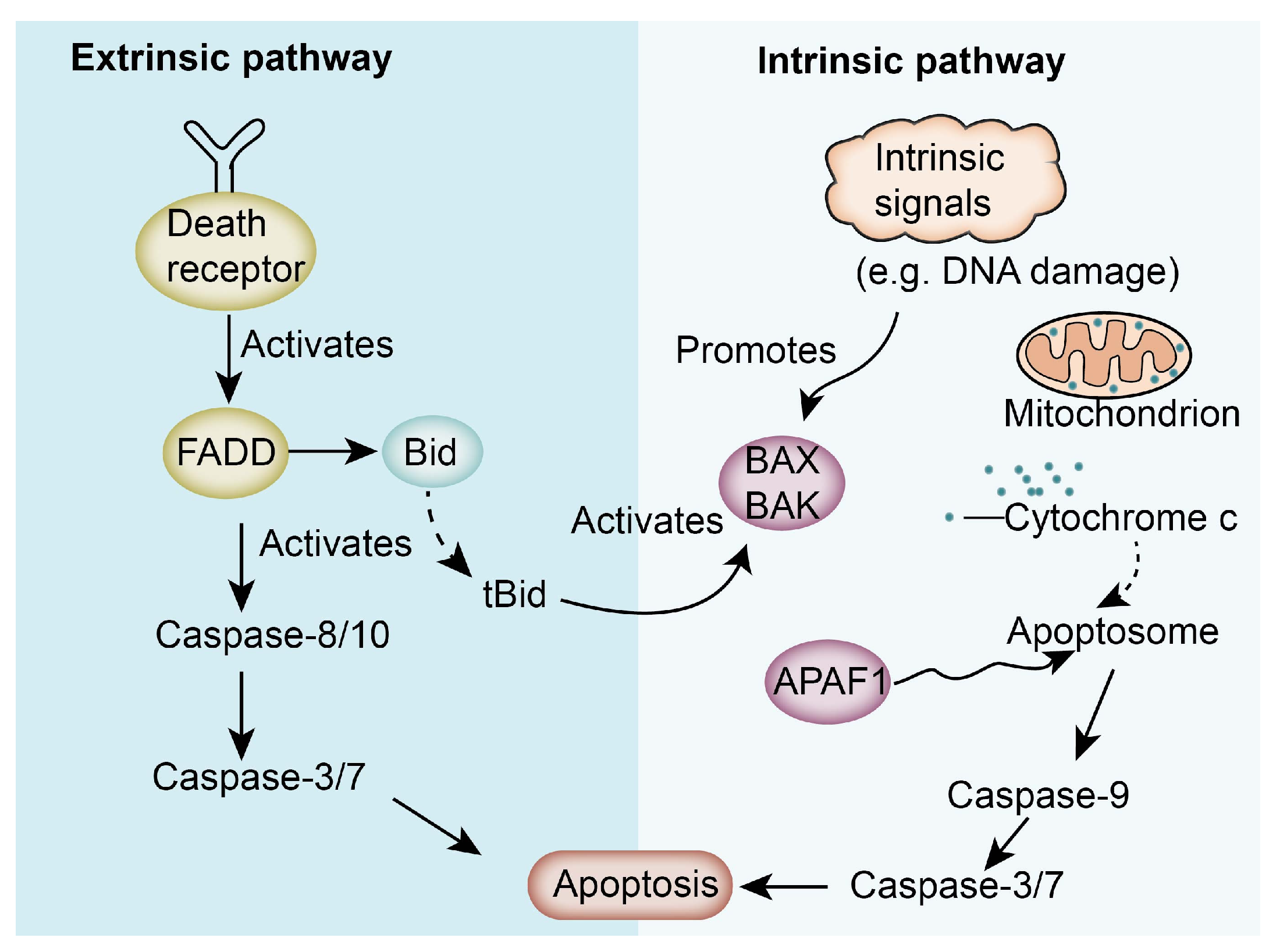
3.4. ISO Inhibits Cancer Cell Proliferation by Regulating Apoptosis-Related Pathways
3.5. ISO Inhibits Cancer Cell Proliferation by Regulating Autophagy-Related Pathways
3.6. ISO Inhibits Cell Proliferation by Modulating the Tumor Microenvironment and Non-Coding RNA-Related Pathway
3.7. ISO Inhibits Cell Proliferation by Modulating the Pyroptosis Pathway
4. The Role of ISO in Targeted Therapies and Its Molecular Mechanism of Action Based on Molecular Docking
5. Advantages of ISO in Clinical Use
5.1. Limitations of Chemotherapy and the Potential of ISO Combination Therapy
5.2. The Use of ISO in Mitigating the Toxic Effects of Chemotherapy
5.3. Enhancement of Chemotherapeutic Drug Sensitivity by ISO
5.4. ISO Has a Protective Effect on Normal Cell
6. Pharmacokinetics and Safety Assessment of ISO
7. ISO Can Be Prepared in Different Forms
8. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin-converting enzyme 2 |
| AKT | protein kinase B |
| Apaf-1 | apoptotic protease activator |
| Cdc25A | cell division cycle 25A |
| Cdc25C | cell division cycle 25C |
| Cdk1 | cyclin dependent kinase 1 |
| CMT | canine mammary tumor |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| ER | estrogen receptor |
| ERK | extracellular signal-regulated kinase |
| FAS | fatty acid synthase |
| GSDMD | gasdermin D |
| GSDME | gasdermin E |
| GZMA | granzyme A |
| GZMB | granzyme B |
| HBC | human breast cancer |
| HER2 | human epidermal growth factor receptor 2 |
| ISO | isorhamnetin |
| JNK | c-Jun amino-terminal kinase |
| LC3-II | microtubule-associated-proteinlight-chain-3 |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinases |
| MDSCs | myeloid-derived suppressor cells |
| MESC | saussurea costus |
| MET | mesenchymal-epithelial transition |
| MMP | matrix metalloproteinase |
| mTOR | mammalian target of rapamycin |
| PD-L1 | programmed death ligand 1 |
| PI3K | phosphoinositide-3 kinase |
| PR | progesterone receptor |
| PTEN | phosphatase and tensin homolog |
| ROS | reactive oxygen species |
| STAT3 | signal transducer and activator of transcription 3 |
| TNBC | triple-negative breast cancer |
| TNF-R | tumor necrosis factor receptor |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 1, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Yu, C. The Mechanism of APOBEC3B in Hepatitis B Virus Infection and HBV Related Hepatocellular Carcinoma Progression, Therapeutic and Prognostic Potential. Infect. Drug Resist. 2024, 17, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Jokhadze, N.; Das, A.; Dizon, D.S. Global cancer statistics: A healthy population relies on population health. CA Cancer J. Clin. 2024, 74, 224–226. [Google Scholar] [CrossRef]
- He, S.; Xia, C.; Li, H.; Cao, M.; Yang, F.; Yan, X.; Zhang, S.; Teng, Y.; Li, Q.; Chen, W. Cancer profiles in China and comparisons with the USA: A comprehensive analysis in the incidence, mortality, survival, staging, and attribution to risk factors. Sci. China Life Sci. 2024, 67, 122–131. [Google Scholar] [CrossRef]
- Akash, S.; Bibi, S.; Biswas, P.; Mukerjee, N.; Khan, D.A.; Hasan, M.N.; Sultana, N.A.; Hosen, M.E.; Jardan, Y.A.B.; Nafidi, H.A.; et al. Revolutionizing anti-cancer drug discovery against breast cancer and lung cancer by modification of natural genistein: An advanced computational and drug design approach. Front. Oncol. 2023, 13, 1228865. [Google Scholar] [CrossRef]
- Georgaki, S.; Skopeliti, M.; Tsiatas, M.; Nicolaou, K.A.; Ioannou, K.; Husband, A.; Bamias, A.; Dimopoulos, M.A.; Constantinou, A.I.; Tsitsilonis, O.E. Phenoxodiol, an anticancer isoflavene, induces immunomodulatory effects in vitro and in vivo. J. Cell. Mol. Med. 2009, 13, 3929–3938. [Google Scholar] [CrossRef]
- Mia, M.A.R.; Dey, D.; Sakib, M.R.; Biswas, M.Y.; Prottay, A.A.S.; Paul, N.; Rimti, F.H.; Abdullah, Y.; Biswas, P.; Iftehimul, M.; et al. The efficacy of natural bioactive compounds against prostate cancer: Molecular targets and synergistic activities. Phytother. Res. 2023, 37, 5724–5754. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Ishola, I.O.; Osele, M.O.; Chijioke, M.C.; Adeyemi, O.O. Isorhamnetin enhanced cortico-hippocampal learning and memory capability in mice with scopolamine-induced amnesia: Role of antioxidant defense, cholinergic and BDNF signaling. Brain Res. 2019, 1712, 188–196. [Google Scholar] [CrossRef]
- Zheng, Q.; Tong, M.; Ou, B.; Liu, C.; Hu, C.; Yang, Y. Isorhamnetin protects against bleomycin-induced pulmonary fibrosis by inhibiting endoplasmic reticulum stress and epithelial-mesenchymal transition. Int. J. Mol. Med. 2019, 43, 117–126. [Google Scholar] [CrossRef]
- Jnawali, H.N.; Jeon, D.; Jeong, M.C.; Lee, E.; Jin, B.; Ryoo, S.; Yoo, J.; Jung, I.D.; Lee, S.J.; Park, Y.M.; et al. Antituberculosis Activity of a Naturally Occurring Flavonoid, Isorhamnetin. J. Nat. Prod. 2016, 79, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Ta, W.; Tang, W.; Hua, R.; Wang, J.; Wang, C.; Lu, W. Potential antiviral activity of isorhamnetin against SARS-CoV-2 spike pseudotyped virus in vitro. Drug Dev. Res. 2021, 82, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, H.; Wang, L.; Song, Z.; Shi, L.; Li, W.; Deng, X.; Wang, J. Isorhamnetin Attenuates Staphylococcus aureus-Induced Lung Cell Injury by Inhibiting Alpha-Hemolysin Expression. J. Microbiol. Biotechnol. 2016, 26, 596–602. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Ghosh, D.; Bhattacharya, S.; Sarkar, S.; Karmakar, P.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of Kombucha against Vibrio cholerae: Targeting cell membrane. Lett. Appl. Microbiol. 2018, 66, 145–152. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Shi, L.; Sun, D.; Wang, L.; Feng, D.; Ding, C. Effect of isorhamnetin on carbonic anhydrase IX expression and tumorigenesis of bladder cancer by activating PPARgamma/PTEN/AKT pathway. Tissue Cell 2023, 82, 102048. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, Y.; Liu, S.; Luo, F.; Tang, D.; Yu, Y.; Xie, Y. Isorhamnetin induces cell cycle arrest and apoptosis by triggering DNA damage and regulating the AMPK/mTOR/p70S6K signaling pathway in doxorubicin-resistant breast cancer. Phytomedicine 2023, 114, 154780. [Google Scholar] [CrossRef]
- Chen, Q.; Song, S.J.; Wang, Z.; Shen, Y.Q.; Xie, L.; Li, J.; Jiang, L.; Zhao, H.; Feng, X.D.; Zhou, Y.; et al. Isorhamnetin induces the paraptotic cell death through ROS and the ERK/MAPK pathway in OSCC cells. Oral Dis. 2021, 27, 240–250. [Google Scholar] [CrossRef]
- Wang, J.L.; Quan, Q.H.; Ji, R.F.; Guo, X.Y.; Zhang, J.M.; Li, X.; Liu, Y.G. Isorhamnetin suppresses PANC-1 pancreatic cancer cell proliferation through S phase arrest. Biomed. Pharmacother. 2018, 108, 925–933. [Google Scholar] [CrossRef]
- Wei, F.; Wang, A.X.; Wang, Q.; Han, W.R.; Rong, R.; Wang, L.J.; Liu, S.J.; Zhang, Y.M.; Dong, C.; Li, Y.L. Plasma endothelial cells-derived extracellular vesicles promote wound healing in diabetes through YAP and the PI3K/Akt/mtOR pathway. Aging-Us 2020, 12, 12002–12018. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Chen, M.; Liu, M.D.; Chen, X.; Zhu, L.J.; Xu, J.Y.; Xue, J.; Wu, H.X.; Du, Y. Semaphorin 5A suppresses ferroptosis through activation of PI3K-AKT-mTOR signaling in rheumatoid arthritis. Cell Death Dis. 2023, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wei, J.; Liu, P.D. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.Y.; Zhang, X.Y.; Hei, Z.Y.; Jin, L.Y.; Han, C.; Ko, A.T.; Yu, X.F.; Wang, J.D. Isorhamnetin Inhibits Human Gallbladder Cancer Cell Proliferation and Metastasis via PI3K/AKT Signaling Pathway Inactivation. Front. Pharmacol. 2021, 12, 792330. [Google Scholar] [CrossRef]
- Cai, F.Z.; Zhang, Y.M.; Li, J.W.; Huang, S.H.; Gao, R.L. Isorhamnetin inhibited the proliferation and metastasis of androgen-independent prostate cancer cells by targeting the mitochondrion-dependent intrinsic apoptotic and PI3K/Akt/mTOR pathway. Biosci. Rep. 2020, 40, BSR20192826. [Google Scholar] [CrossRef]
- Li, C.H.; Li, J.W.; Li, Y.; Li, L.; Luo, Y.L.; Li, J.J.; Zhang, Y.M.; Wang, Y.R.; Liu, X.Z.; Zhou, X.T.; et al. Isorhamnetin Promotes MKN-45 Gastric Cancer Cell Apoptosis by Inhibiting PI3K-Mediated Adaptive Autophagy in a Hypoxic Environment. J. Agr. Food Chem. 2021, 69, 8130–8143. [Google Scholar] [CrossRef]
- Drosten, M.; Barbacid, M. Targeting the MAPK Pathway in KRAS-Driven Tumors. Cancer Cell 2020, 37, 543–550. [Google Scholar] [CrossRef]
- Shi, X.F.; Yu, Q.; Wang, K.B.; Fu, Y.D.; Zhang, S.; Liao, Z.Y.; Li, Y.; Cai, T. Active ingredients Isorhamnetin of Croci Srigma inhibit stomach adenocarcinomas progression by MAPK/mTOR signaling pathway. Sci. Rep. 2023, 13, 12607. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Lin, T.; Liao, X.Z.; Li, Z.Y.; Lin, R.T.; Qi, X.J.; Chen, G.M.; Sun, L.L.; Lin, L.Z. Network pharmacology based research into the effect and mechanism of Yinchenhao Decoction against Cholangiocarcinoma. Chin. Med. 2021, 16, 13. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Guardado-Félix, D.; Rocha-Pizaña, M.R.; Garza-Martínez, J.; Acevedo-Pacheco, L.; Gutiérrez-Uribe, J.A.; Villela-Castrejón, J.; López-Pacheco, F.; Serna-Saldívar, S.O. Opuntia ficus-indica Extract and Isorhamnetin-3-O-Glucosyl-Rhamnoside Diminish Tumor Growth of Colon Cancer Cells Xenografted in Immune-Suppressed Mice through the Activation of Apoptosis Intrinsic Pathway. Plant Foods Hum. Nutr. 2021, 76, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Fan, B.Q.; Pu, N.; Ran, X.; Lian, T.C.; Cai, Y.F.; Xing, W.; Sun, K. Isorhamnetin Suppresses Human Gastric Cancer Cell Proliferation through Mitochondria-Dependent Apoptosis. Molecules 2022, 27, 5191. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Liang, X.; Chai, B.D.; Zhou, Y.W.; Du, H.Y.; Suo, Y.J.; Chen, Z.H.; Li, Q.F.; Huang, X.L. Isorhamnetin Induces Melanoma Cell Apoptosis via the PI3K/Akt and NF-kB Pathways. Biomed Res. Int. 2020, 2020, 1057943. [Google Scholar] [CrossRef]
- El Gizawy, H.A.; El-Haddad, A.E.; Saadeldeen, A.M.; Boshra, S.A. Tentatively Identified (UPLC/T-TOF-MS/MS) Compounds in the Extract of Saussurea costus Roots Exhibit In Vivo Hepatoprotection via Modulation of HNF-1α, Sirtuin-1, C/ebpα, miRNA-34a and miRNA-223. Molecules 2022, 27, 2802. [Google Scholar] [CrossRef]
- Ye, L.; Ma, R.H.; Zhang, X.X.; Thakur, K.R.; Zhang, J.G.; Khan, M.R.; Busquets, R.; Wei, Z.J. Isorhamnetin Induces Apoptosis and Suppresses Metastasis of Human Endometrial Carcinoma Ishikawa Cells via Endoplasmic Reticulum Stress Promotion and Matrix Metalloproteinase-2/9 Inhibition In Vitro and In Vivo. Foods 2022, 11, 3415. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, C.; Liang, Z.X.; Zhi, Y.; Xu, H.J.; Wang, H.J.; Dong, H. Homoharringtonine induces apoptosis of mammary carcinoma cells by inhibiting the AKT/mTOR signaling pathway. Vet Comp. Oncol. 2024, 22, 57–69. [Google Scholar] [CrossRef]
- Mei, C.; Zhang, X.; Zhi, Y.; Liang, Z.X.; Xu, H.J.; Liu, Z.Y.; Liu, Y.; Lyu, Y.; Wang, H.J. Isorhamnetin Regulates Programmed Death Ligand-1 Expression by Suppressing the EGFR-STAT3 Signaling Pathway in Canine Mammary Tumors. Int. J. Mol. Sci. 2024, 25, 670. [Google Scholar] [CrossRef]
- Mei, C.; Liu, Y.; Liu, Z.Y.; Zhi, Y.; Jiang, Z.L.; Lyu, X.; Wang, H.J. Dysregulated Signaling Pathways in Canine Mammary Tumor and Human Triple Negative Breast Cancer: Advances and Potential Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 145. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Bio. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Ruan, Y.; Hu, K.; Chen, H. Autophagy inhibition enhances isorhamnetin-induced mitochondria-dependent apoptosis in non-small cell lung cancer cells. Mol. Med. Rep. 2015, 12, 5796–5806. [Google Scholar] [CrossRef]
- Zhang, H.W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, Y.N.; Deng, Q.; Luo, Q.S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Han, J.X.; Lv, Y.; Zhao, Z.H.; Zheng, S.T.; Sun, Y.; Sun, T. Isorhamnetin and anti-PD-L1 antibody dual-functional mesoporous silica nanoparticles improve tumor immune microenvironment and inhibit YY1-mediated tumor progression. J. Nanobiotechnol. 2023, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.W.; Huang, J.; Tang, L.; Xu, Y.H.; Sun, H.; Tang, J.; Wang, G. Pyroptosis, a target for cancer treatment? Apoptosis 2022, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wendlocha, D.; Kubina, R.; Krzykawski, K.; Mielczarek-Palacz, A. Selected Flavonols Targeting Cell Death Pathways in Cancer Therapy: The Latest Achievements in Research on Apoptosis, Autophagy, Necroptosis, Pyroptosis, Ferroptosis, and Cuproptosis. Nutrients 2024, 16, 1201. [Google Scholar] [CrossRef]
- Li, L.; Jin, X.J.; Li, J.W.; Li, C.H.; Zhou, S.Y.; Li, J.J.; Feng, C.Q.; Liu, D.L.; Liu, Y.Q. Systematic insight into the active constituents and mechanism of Guiqi Baizhu for the treatment of gastric cancer. Cancer Sci. 2021, 112, 1772–1784. [Google Scholar] [CrossRef]
- Wang, L.K.; Xie, Y.C.; Xiao, B.N.; He, X.L.; Ying, G.H.; Zha, H.Y.; Yang, C.; Jin, X.J.; Li, G.L.; Ping, L.; et al. Isorhamnetin alleviates cisplatin-induced acute kidney injury via enhancing fatty acid oxidation. Free Radic. Biol. Med. 2024, 212, 22–33. [Google Scholar] [CrossRef]
- Hu, J.J.; Zhang, Y.H.; Jiang, X.X.; Zhang, H.W.; Gao, Z.Y.; Li, Y.N.; Fu, R.Q.; Li, L.R.; Li, J.; Cui, H.J.; et al. ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine. J. Exp. Clin. Cancer Res. 2019, 38, 225. [Google Scholar] [CrossRef]
- Wu, Q.; Kroon, P.A.; Shao, H.J.; Needs, P.W.; Yang, X.B. Differential Effects of Quercetin and Two of Its Derivatives, Isorhamnetin and Isorhamnetin-3-glucuronide, in Inhibiting the Proliferation of Human Breast-Cancer MCF-7 Cells. J. Agr. Food Chem. 2018, 66, 7181–7189. [Google Scholar] [CrossRef]
- Rassu, G.; Vlckova, H.K.; Giunchedi, P.; Dias, P.; Cossu, M.; Pourova, J.; Harcarova, P.; Lomozova, Z.; Novakova, L.; Gavini, E.; et al. A water-soluble preparation for intravenous administration of isorhamnetin and its pharmacokinetics in rats. Chem. Biol. Interact. 2024, 396, 111064. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Z.; Zhang, Y.; Gu, H.; Li, W. Bioinformatics-based drug repositioning and prediction of the main active ingredients and potential mechanisms of action for the efficacy of Dan-Lou tablet. Sci. Rep. 2024, 14, 23297. [Google Scholar] [CrossRef]
- Wang, C.; Gao, H.; Huang, L.; Wang, Z.; Ding, X. Network Pharmacological Analysis and Experimental Study of the Antipharyngitis Mechanism of the Chaiqin Qingning Capsule. Biomed Res. Int. 2022, 2022, 5616942. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Shen, R.; Yu, X. Efficacy of active compounds of Chanqin granules on airway neurogenic inflammation induced by PM2.5 in vivo. J. Tradit. Chin. Med. 2020, 40, 792–802. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, C.; Liu, Y.; Lyu, X.; Jiang, Z.; Liu, Z.; Zhi, Y.; Xu, X.; Wang, H. Advances in Isorhamnetin Treatment of Malignant Tumors: Mechanisms and Applications. Nutrients 2025, 17, 1853. https://doi.org/10.3390/nu17111853
Mei C, Liu Y, Lyu X, Jiang Z, Liu Z, Zhi Y, Xu X, Wang H. Advances in Isorhamnetin Treatment of Malignant Tumors: Mechanisms and Applications. Nutrients. 2025; 17(11):1853. https://doi.org/10.3390/nu17111853
Chicago/Turabian StyleMei, Chen, Ying Liu, Xueze Lyu, Zhaoling Jiang, Zhenyi Liu, Yan Zhi, Xiaolong Xu, and Hongjun Wang. 2025. "Advances in Isorhamnetin Treatment of Malignant Tumors: Mechanisms and Applications" Nutrients 17, no. 11: 1853. https://doi.org/10.3390/nu17111853
APA StyleMei, C., Liu, Y., Lyu, X., Jiang, Z., Liu, Z., Zhi, Y., Xu, X., & Wang, H. (2025). Advances in Isorhamnetin Treatment of Malignant Tumors: Mechanisms and Applications. Nutrients, 17(11), 1853. https://doi.org/10.3390/nu17111853





