Potential Applications and Risks of Supranutritional Selenium Supplementation in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Critical Review
Abstract
1. Introduction
2. Methods
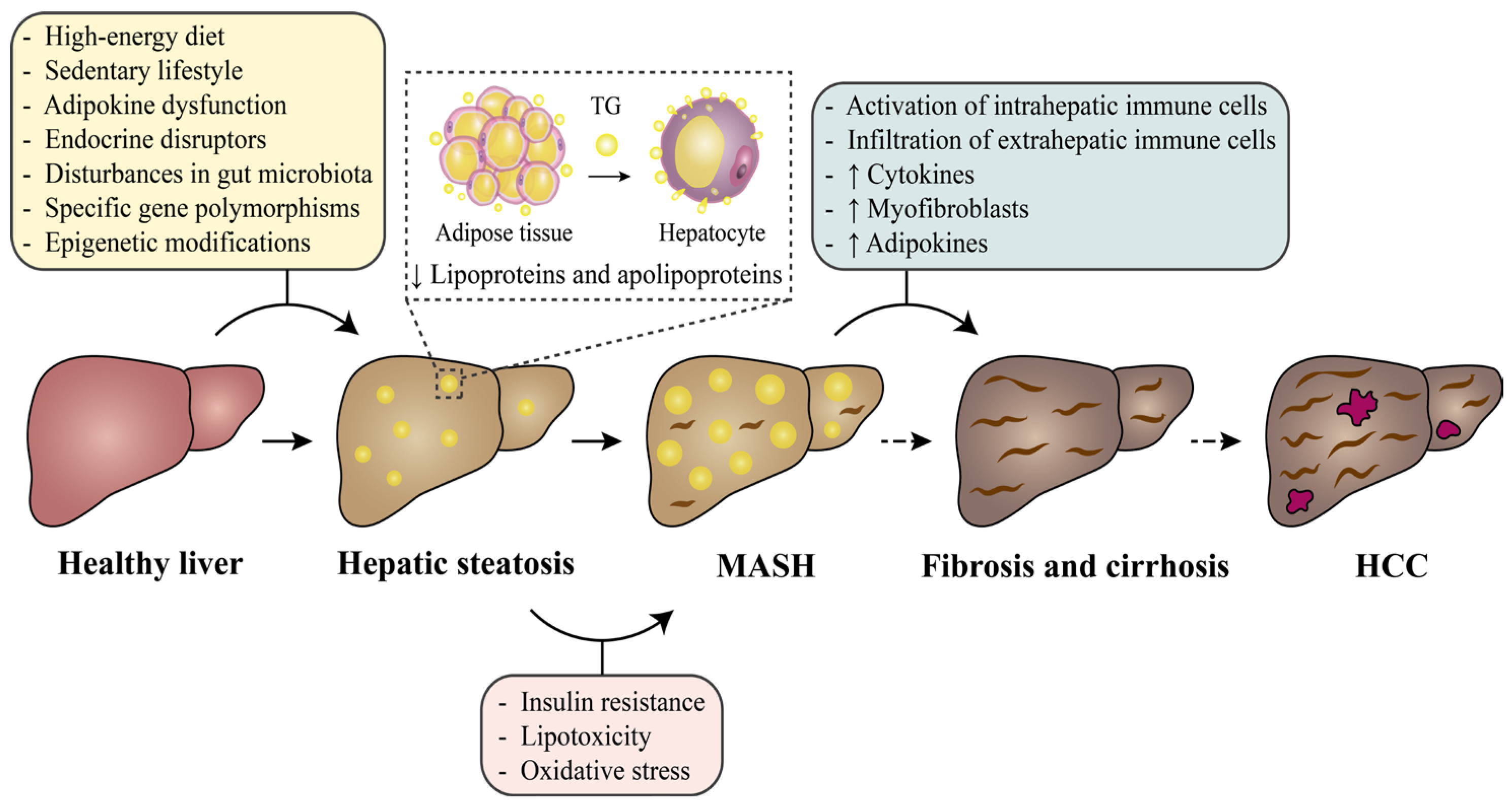
3. Occurrence and Development of MASLD
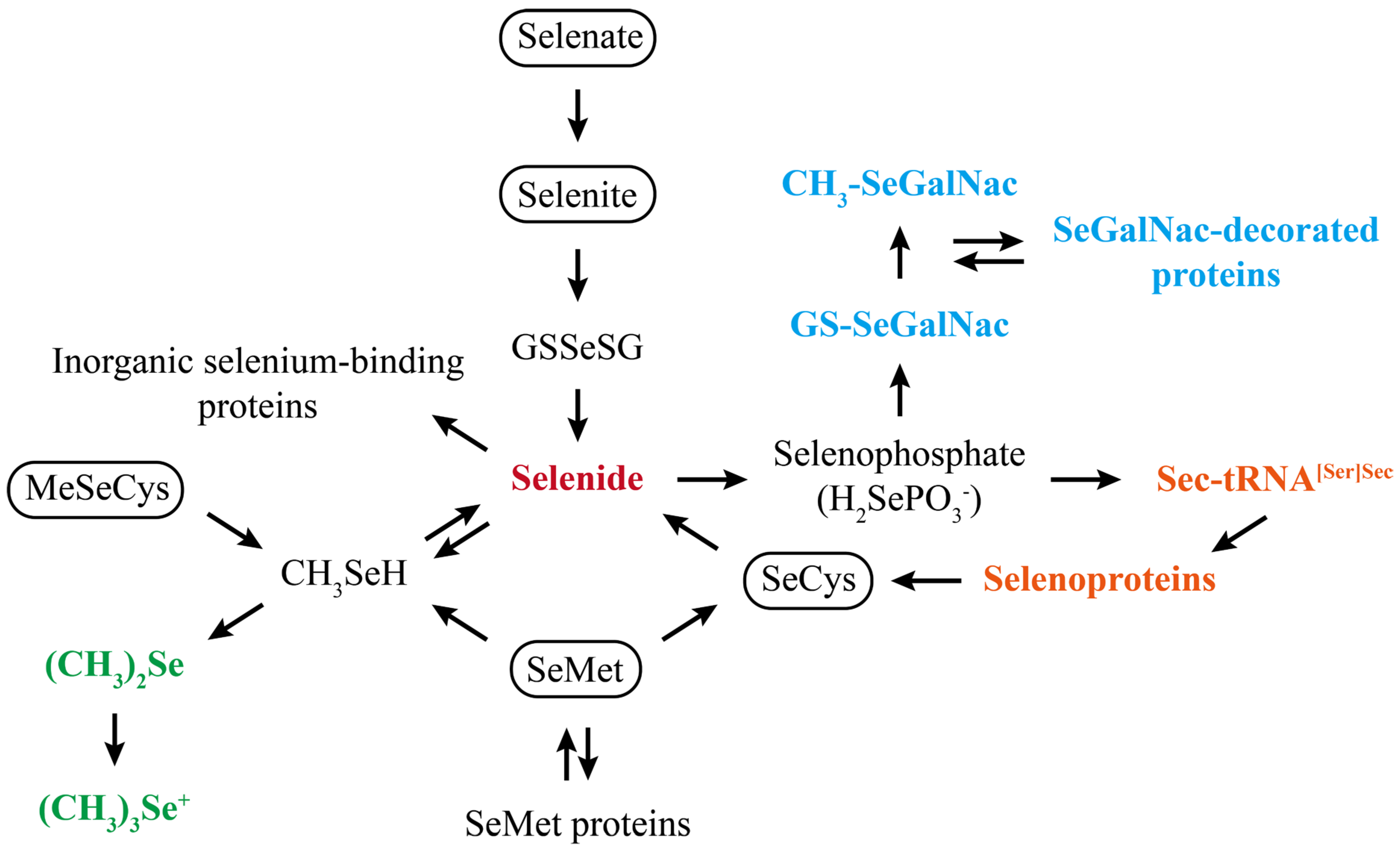
4. Mechanisms of Selenium Action in MASLD
5. Effects of Dose and Form of Selenium Supplementation on MASLD-Associated Hepatic Disorders
6. Results of Epidemiological Studies on Selenium and MASLD
6.1. Associations Between In Vivo Selenium Levels and MASLD
6.2. Associations Between Dietary Selenium Intake and MASLD
7. Crucial Points Regarding Supranutritional Selenium Supplementation in MASLD
7.1. Accurate Assessment of Selenium Nutritional Status in MASLD Patients
7.2. Development of Novel Functional Selenium Forms Like SeNPs for MASLD
8. Conclusions and Future Directions
- (1)
- Based on the effects of different doses and forms of selenium on MASLD-associated hepatic disorders, supranutritional selenium supplementation must establish a tolerable dosage range according to the severity of MASLD and select superior forms of selenium, such as organic selenium and SeNPs.
- (2)
- Numerous epidemiological studies have observed that hepatic fibrosis, cirrhosis, and HCC (the middle or late stages of MASLD) are more prone to selenium functional deficiencies, indicating that patients in these stages are ideal candidates for supranutritional selenium supplementation. Meanwhile, MASLD patients who live in high-selenium areas or who have a history of diabetes, hyperglycemia, hyperinsulinemia, etc., should exercise caution regarding supranutritional selenium supplementation.
- (3)
- Determining selenium nutritional status is a prerequisite for the utilization of supranutritional selenium supplementation in MASLD. Furthermore, novel forms of selenium with enhanced functionality may facilitate broader adoption of supranutritional selenium supplementation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A SeNDs | Amorphous selenium nanodots |
| AI | Adequate intake |
| Akt | Protein kinase B |
| ALT | Alanine aminotransferase |
| AMPK | Adenosine monophosphate-activated protein kinase |
| ATP | Adenosine triphosphate |
| BW | Body weight |
| CD36 | Fatty acid translocase |
| CH3-SeGalNac | 1β-methylseleno-N-acetyl-D-galactosamine |
| ECM | Extracellular matrix |
| EFSA | European Food Safety Authority |
| ER | Endoplasmic reticulum |
| Fxr | Farnesoid X receptor |
| GPX | Glutathione peroxidase |
| GSSeSG | Selenodiglutathione |
| GS-SeGalNac | 1β-glutathionylseleno-N-acetyl-D-galactosamine |
| GST | Glutathione-S-transferase |
| HCC | Hepatocellular carcinoma |
| HFD | High-fat diet |
| IL | Interleukin |
| IR | Insulin resistance |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MeSeCys | Methylselenocysteine |
| MMPs | Metalloproteinases |
| mTOR | Mammalian target of rapamycin |
| NAFLD | Non-alcoholic fatty liver disease |
| NF-κB | Nuclear factor kappa B |
| NHANES | National Health and Nutrition Examination Survey |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| PI3K | Phosphatidylinositol 3-kinase |
| RNI | Recommended nutrient intake |
| ROS | Reactive oxygen species |
| SAM | S-adenosylmethionine |
| SD | Sprague-Dawley |
| SeCys | Selenocysteine |
| Se-enriched | Selenium-enriched |
| Se-GTP | Selenium-containing tea polysaccharide |
| SELENOK | Selenoprotein K |
| SELENOM | Selenoprotein M |
| SELENOP | Selenoprotein P |
| SELENOS | Selenoprotein S |
| SELENOW | Selenoprotein W |
| SeMet | Selenomethionine |
| SeMs | Se-enriched microorganisms |
| SeNPs | Selenium nanoparticles |
| TCA | Tricarboxylic acid |
| TGs | Triglycerides |
| TLR4 | Toll-like receptor 4 |
| TNF | Tumor necrosis factor |
| TXNRD | Thioredoxin reductase |
| UL | Tolerable upper intake level |
| VEGF | Vascular endothelial growth factor |
References
- Younossi, Z.; Dunshea, F.; Arrese, M.; Sharma, B.C.; Mostafa, I.; Bugianesi, E.; Wong, V.W.-S.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Paik, J.M.; Stepanova, M.; Ong, J.; Alqahtani, S.; Henry, L. Clinical Profiles and Mortality Rates Are Similar for Metabolic Dysfunction-Associated Steatotic Liver Disease and Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2024, 80, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology 2018, 155, 282–302.e8. [Google Scholar] [CrossRef]
- Saunders, K.H.; Umashanker, D.; Igel, L.I.; Kumar, R.B.; Aronne, L.J. Obesity Pharmacotherapy. Med. Clin. North Am. 2018, 102, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Fernandes, J.; Hu, X.; Smith, M.R.; Go, Y.-M.; Jones, D.P. Selenium at the redox interface of the genome, metabolome and exposome. Free Radic. Biol. Med. 2018, 127, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Li, S.; Zhang, K.; Li, J.; Han, Y.; Zhan, T.; Zhao, Q.; Guo, X.; Zhang, J. Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver. Redox Biol. 2020, 36, 101519. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Goulas, A.; Duntas, L. Selenium and selenoprotein P in nonalcoholic fatty liver disease. Horm.-Int. J. Endocrinol. Metab. 2020, 19, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, L.; Song, A.; Zhang, C. Selenium status and its antioxidant role in metabolic diseases. Oxidative Med. Cell. Longev. 2022, 2022, 7009863. [Google Scholar] [CrossRef]
- Wang, M.; Li, B.; Li, S.; Song, Z.; Kong, F.; Zhang, X. Selenium in wheat from farming to food. J. Agric. Food Chem. 2021, 69, 15458–15467. [Google Scholar] [CrossRef]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and Phytoremediation of Selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.-Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- McClung, J.P.; Roneker, C.A.; Mu, W.; Lisk, D.J.; Langlais, P.; Liu, F.; Lei, X.G. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 8852–8857. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Lei, X.G. Selenium. Adv. Nutr. 2016, 7, 415–417. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Liu, Y.; Wang, Q.; Huang, Z. Dietary Reference Intakes of Selenium for Chinese Residents. J. Nutr. 2025; in press. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific Opinion on the Tolerable Upper Intake Level for Selenium. EFSA J. 2023, 21, e07704. [Google Scholar] [CrossRef]
- Sasaki, S. Dietary Reference Intakes (DRIs) in Japan. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 2), 420–444. [Google Scholar] [PubMed]
- Choi, K.; Lee, O. 2020 korean dietary reference intakes of selenium and a review of selenium database of foods by evaluating of selenium contents of the recommended menus. J. Nutr. Health 2022, 55, 430. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and Nonalcoholic Fatty Liver Disease: From Pathophysiology to Therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Mahady, S.E.; George, J. Exercise and diet in the management of nonalcoholic fatty liver disease. Metabolism 2016, 65, 1172–1182. [Google Scholar] [CrossRef]
- Gao, Y.; Hua, R.; Peng, K.; Yin, Y.; Zeng, C.; Guo, Y.; Wang, Y.; Li, L.; Li, X.; Qiu, Y.; et al. High-starchy carbohydrate diet aggravates NAFLD by increasing fatty acids influx mediated by NOX2. Food Sci. Hum. Wellness 2023, 12, 1081–1101. [Google Scholar] [CrossRef]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Adipokines in nonalcoholic fatty liver disease. Metab.-Clin. Exp. 2016, 65, 1062–1079. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Deretzi, G.; Zavos, C.; Mantzoros, C.S. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: A concept implicating nonalcoholic fatty liver disease. Curr. Mol. Med. 2012, 12, 68–82. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F.W. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Burt, A.D.; Lackner, C.; Tiniakos, D.G. Diagnosis and assessment of NAFLD: Definitions and histopathological classification. Semin. Liver Dis. 2015, 35, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease: Disease mongering or call to action? Diabetologia 2016, 59, 1145–1147. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Mantzoros, C.S. Leptin in health and disease: Facts and expectations at its twentieth anniversary. Metabolism 2015, 64, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lu, Z.; He, M.; Shi, B.; Lei, X.; Shan, A. The Effects of Endoplasmic-Reticulum-Resident Selenoproteins in a Nonalcoholic Fatty Liver Disease Pig Model Induced by a High-Fat Diet. Nutrients 2020, 12, 692. [Google Scholar] [CrossRef]
- Nati, M.; Haddad, D.; Birkenfeld, A.L.; Koch, C.A.; Chavakis, T.; Chatzigeorgiou, A. The role of immune cells in metabolism-related liver inflammation and development of non-alcoholic steatohepatitis (NASH). Rev. Endocr. Metab. Disord. 2016, 17, 29–39. [Google Scholar] [CrossRef]
- Kisseleva, T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology 2017, 65, 1039–1043. [Google Scholar] [CrossRef]
- Lei, X.G.; Combs, G.F.; Sunde, R.A.; Caton, J.S.; Arthington, J.D.; Vatamaniuk, M.Z. Dietary selenium across species. Annu. Rev. Nutr. 2022, 42, 337–375. [Google Scholar] [CrossRef]
- Cai, J.; Huang, J.; Yang, J.; Chen, X.; Zhang, H.; Zhu, Y.; Liu, Q.; Zhang, Z. The protective effect of selenoprotein M on non-alcoholic fatty liver disease: The role of the AMPKα1–MFN2 pathway and Parkin mitophagy. Cell. Mol. Life Sci. 2022, 79, 354. [Google Scholar] [CrossRef]
- Huang, X.; Yang, X.; Zhang, M.; Li, T.; Zhu, K.; Dong, Y.; Lei, X.; Yu, Z.; Lv, C.; Huang, J. SELENOI Functions as a Key Modulator of Ferroptosis Pathway in Colitis and Colorectal Cancer. Adv. Sci. 2024, 11, e2404073. [Google Scholar] [CrossRef]
- Miyata, M.; Matsushita, K.; Shindo, R.; Shimokawa, Y.; Sugiura, Y.; Yamashita, M. Selenoneine Ameliorates Hepatocellular Injury and Hepatic Steatosis in a Mouse Model of NAFLD. Nutrients 2020, 12, 1898. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, S.; Zhang, N.; Mu, Y.; Ren, H.; Wang, Y.; Li, K. Long-term supranutritional supplementation with selenate decreases hyperglycemia and promotes fatty liver degeneration by inducing hyperinsulinemia in diabetic db/db mice. PLoS ONE 2014, 9, e101315. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Du, Q.; Yao, H.; Chen, X.; Zhang, Z.; Xu, S. Roles of Oxidative Stress and Endoplasmic Reticulum Stress in Selenium Deficiency-Induced Apoptosis in Chicken Liver. Biometals 2015, 28, 255–265. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.; Zhao, Q.; Zhan, T.; Li, Y.; Sun, W.; Li, S.; Sun, D.; Si, X.; Yu, X.; et al. Targeted Metabolomics Analysis Reveals That Dietary Supranutritional Selenium Regulates Sugar and Acylcarnitine Metabolism Homeostasis in Pig Liver. J. Nutr. 2020, 150, 704–711. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.-B.; Zhang, X.-W.; Liu, Y.; Shi, W.-Y.; Hidayat, K.; Xu, J.-Y.; Yuan, L.; Qin, L.-Q. Organic selenium ameliorates non-alcoholic fatty liver disease through 5-hydroxytryptamine/bile acid enterohepatic circulation in mice. J. Funct. Foods 2023, 106, 105596. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Wu, P.; Chu, Y.; Gui, S.; Zheng, Y.; Chen, X. Dietary selenium alleviated mouse liver oxidative stress and NAFLD induced by obesity by regulating the KEAP1/NRF2 pathway. Antioxidants 2022, 11, 349. [Google Scholar] [CrossRef]
- Ren, D.; Hu, Y.; Luo, Y.; Yang, X. Selenium-containing polysaccharides from Ziyang green tea ameliorate high-fructose diet induced insulin resistance and hepatic oxidative stress in mice. Food Funct. 2015, 6, 3342–3350. [Google Scholar] [CrossRef]
- Stahel, P.; Kim, J.J.; Cieslar, S.R.L.; Warrington, J.M.; Xiao, C.; Cant, J.P. Supranutritional selenium intake from enriched milk casein impairs hepatic insulin sensitivity via attenuated IRS/PI3K/AKT signaling and decreased PGC-1α expression in male Sprague–Dawley rats. J. Nutr. Biochem. 2017, 41, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Barcus, M.; Kim, J.; Lum, K.L.; Mills, C.; Lei, X.G. High Dietary Selenium Intake Alters Lipid Metabolism and Protein Synthesis in Liver and Muscle of Pigs. J. Nutr. 2016, 146, 1625–1633. [Google Scholar] [CrossRef]
- Shah, T.; Malhi, M.; Kachiwal, A.B.; Bhutto, B.; Shah, Q.A.; Lei, Y.; Soomro, S.A.; Soomro, J.; Kalhoro, N.H.; Gui, H. Ameliorative Effects of Supranutritional Selenium on TLR-4-NF-κB-TNF-α-Mediated Hepatic Oxidative Injury and Inflammation in Goats Fed High Concentrate Diet. Food Sci. Nutr. 2022, 10, 3842–3854. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; He, Y.; Liu, L.; Tao, W.; Wang, G.; Sun, W.; Pei, X.; Xiao, Z.; Wang, H.; Wang, M. Effects of supranutritional selenium nanoparticles on immune and antioxidant capacity in Sprague-Dawley rats. Biol. Trace Elem. Res. 2021, 199, 4666–4674. [Google Scholar] [CrossRef]
- Qiao, L.; Guo, Z.; Liu, H.; Liu, J.; Lin, X.; Deng, H.; Liu, X.; Zhao, Y.; Xiao, X.; Lei, J.; et al. Protective effect of mitophagy regulated by mTOR signaling pathway in liver fibrosis associated with selenium. Nutrients 2022, 14, 2410. [Google Scholar] [CrossRef]
- Zhu, M.; Niu, Q.; Zhang, J.; Yu, Y.; Wang, H.; Zhu, T.; Wang, G.; Yang, L.; Yin, Y.; Li, P. Amorphous selenium nanodots alleviate non-alcoholic fatty liver disease via activating VEGF receptor 1 to further inhibit phosphorylation of JNK/p38 MAPK pathways. Eur. J. Pharmacol. 2022, 932, 175235. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Liu, Y.J.; Qiu, T.T.; Loon k, S.; Zhou, D. Microplastic-induced NAFLD: Hepatoprotective effects of nanosized selenium. Ecotoxicol. Environ. Saf. 2024, 272, 115850. [Google Scholar] [CrossRef]
- Evenson, J.K.; Sunde, R.A. Metabolism of tracer 75Se selenium from inorganic and organic selenocompounds into selenoproteins in rats, and the missing 75Se metabolites. Front. Nutr. 2021, 8, 699652. [Google Scholar] [CrossRef] [PubMed]
- Bierla, K.; Szpunar, J.; Lobinski, R.; Sunde, R.A. Effect of Graded Levels of Selenium Supplementation as Selenite on Expression of Selenosugars, Selenocysteine, and Other Selenometabolites in Rat Liver. Metallomics 2023, 15, mfad066. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Xu, L.; Lu, Y.; Wang, N.; Feng, Y. The role and mechanisms of selenium supplementation on fatty liver-associated disorder. Antioxidants 2022, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Curran, J.E.; Jowett, J.B.M.; Elliott, K.S.; Gao, Y.; Gluschenko, K.; Wang, J.; Azim, D.M.A.; Cai, G.; Mahaney, M.C.; Comuzzie, A.G.; et al. Genetic variation in selenoprotein S influences inflammatory response. Nat. Genet. 2005, 37, 1234–1241. [Google Scholar] [CrossRef]
- Gao, Y.; Hannan, N.R.F.; Wanyonyi, S.; Konstantopolous, N.; Pagnon, J.; Feng, H.C.; Jowett, J.B.M.; Kim, K.-H.; Walder, K.; Collier, G.R. Activation of the selenoprotein SEPS1 gene expression by pro-inflammatory cytokines in HepG2 cells. Cytokine 2006, 33, 246–251. [Google Scholar] [CrossRef]
- Gong, T.; Hashimoto, A.C.; Sasuclark, A.R.; Khadka, V.S.; Gurary, A.; Pitts, M.W. Selenoprotein M promotes hypothalamic leptin signaling and thioredoxin antioxidant activity. Antioxid. Redox Signal. 2021, 35, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Mita, Y.; Nakayama, K.; Inari, S.; Nishito, Y.; Yoshioka, Y.; Sakai, N.; Sotani, K.; Nagamura, T.; Kuzuhara, Y.; Inagaki, K.; et al. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat. Commun. 2017, 8, 1658. [Google Scholar] [CrossRef]
- Misu, H.; Takamura, T.; Takayama, H.; Hayashi, H.; Matsuzawa-Nagata, N.; Kurita, S.; Ishikura, K.; Ando, H.; Takeshita, Y.; Ota, T.; et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010, 12, 483–495. [Google Scholar] [CrossRef]
- You, M.; Wu, F.; Gao, M.; Chen, M.; Zeng, S.; Zhang, Y.; Zhao, W.; Li, D.; Wei, L.; Ruan, X.Z.; et al. Selenoprotein K contributes to CD36 subcellular trafficking in hepatocytes by accelerating nascent COPII vesicle formation and aggravates hepatic steatosis. Redox Biol. 2022, 57, 102500. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, W.; Miao, Z.; Cao, Q.; Xu, S. Role of Selenoprotein W in Participating in the Progression of Non-Alcoholic Fatty Liver Disease. Redox Biol. 2024, 71, 103114. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, N.; Anan, Y.; Hashimoto, Y.; Tokoro, N.; Mizuno, R.; Hayashi, S.; Yamamoto, S.; Shimada, K.; Kamata, S.; Ishii, I. Dietary selenium deficiency or selenomethionine excess drastically alters organ selenium contents without altering the expression of most selenoproteins in mice. J. Nutr. Biochem. 2019, 69, 120–129. [Google Scholar] [CrossRef]
- Ganther, H.E. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: Complexities with thioredoxin reductase. Carcinogenesis 1999, 20, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-X.; Cao, C.-Y.; Sun, Y.-C.; Wang, L.-L.; Li, N.; Xu, S.-W.; Li, J.-L. Effects on Liver Hydrogen Peroxide Metabolism Induced by Dietary Selenium Deficiency or Excess in Chickens. Biol. Trace Elem. Res. 2014, 159, 174–182. [Google Scholar] [CrossRef]
- Castel, T.; Léon, K.; Gandubert, C.; Gueguen, B.; Amérand, A.; Guernec, A.; Théron, M.; Pichavant-Rafini, K. Comparison of sodium selenite and selenium-enriched spirulina supplementation effects after selenium deficiency on growth, tissue selenium concentrations, antioxidant activities, and selenoprotein expression in rats. Biol. Trace Elem. Res. 2024, 202, 685–700. [Google Scholar] [CrossRef]
- Han, J.; Liang, H.; Yi, J.; Tan, W.; He, S.; Wang, S.; Li, F.; Wu, X.; Ma, J.; Shi, X.; et al. Long-term selenium-deficient diet induces liver damage by altering hepatocyte ultrastructure and MMP1/3 and TIMP1/3 expression in growing rats. Biol. Trace Elem. Res. 2017, 175, 396–404. [Google Scholar] [CrossRef]
- Qiao, L.; Lin, X.; Zhao, Y.; Wang, Q.; Liu, H.; You, M.; Yuan, Q.; Yang, Z.; Bian, W.; Liu, J.; et al. Short-term dietary selenium deficiency induced liver fibrosis by inhibiting the Akt/mTOR signaling pathway in rats. Biol. Trace Elem. Res. 2023, 201, 3825–3833. [Google Scholar] [CrossRef] [PubMed]
- Raines, A.M.; Sunde, R.A. Selenium toxicity but not deficient or super-nutritional selenium status vastly alters the transcriptome in rodents. BMC Genom. 2011, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, X.; Zhang, L.; Bao, Y. Biological effects of a nano red elemental selenium. BioFactors 2001, 15, 27–38. [Google Scholar] [CrossRef]
- Jia, X.; Li, N.; Chen, J. A subchronic toxicity study of elemental Nano-Se in Sprague-Dawley rats. Life Sci. 2005, 76, 1989–2003. [Google Scholar] [CrossRef]
- Kondaparthi, P.; Deore, M.; Naqvi, S.; Flora, S.J.S. Dose-dependent hepatic toxicity and oxidative stress on exposure to nano and bulk selenium in mice. Environ. Sci. Pollut. Res. 2021, 28, 53034–53044. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yamashita, M. Identification of a novel selenium-containing compound, selenoneine, as the predominant chemical form of organic selenium in the blood of bluefin tuna. J. Biol. Chem. 2010, 285, 18134–18138. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of Short-Term Toxicity between Nano-Se and Selenite in Mice. Life Sci. 2005, 76, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef]
- Shakibaie, M.; Shahverdi, A.R.; Faramarzi, M.A.; Hassanzadeh, G.R.; Rahimi, H.R.; Sabzevari, O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm. Biol. 2013, 51, 58–63. [Google Scholar] [CrossRef]
- Benko, I.; Nagy, G.; Tanczos, B.; Ungvari, E.; Sztrik, A.; Eszenyi, P.; Prokisch, J.; Banfalvi, G. Subacute Toxicity of Nano-Selenium Compared to Other Selenium Species in Mice. Environ. Toxicol. Chem. 2012, 31, 2812–2820. [Google Scholar] [CrossRef]
- He, Y.; Chen, S.; Liu, Z.; Cheng, C.; Li, H.; Wang, M. Toxicity of selenium nanoparticles in male Sprague–Dawley rats at supranutritional and nonlethal levels. Life Sci. 2014, 115, 44–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, B.; Zhou, C.; Zhang, P.; Liu, C.; Xiang, Y.; Xia, W.; Sun, G.; You, Y.; Li, D.; et al. Biomimetic Selenium-Nanocomposites Alleviate MASH by Modulating Lipid and Iron Homeostasis. Adv. Healthc. Mater. 2025, 14, e2500467. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Lu, G.Z.; Szpyt, J.; Mariotti, M.; Garrity, R.; Paulo, J.A.; Schweppe, D.K.; Laznik-Bogoslavski, D.; Kazak, L.; Murphy, M.P.; et al. Facultative protein selenation regulates redox sensitivity, adipose tissue thermogenesis, and obesity. Proc. Natl. Acad. Sci. USA 2020, 117, 10789–10796. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Kawada, N.; Japan Study Group of NAFLD (JSG-NAFLD). The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Zhan, S.; Han, F.; Wang, Q.; Liu, Y.; Huang, Z. Possible Metabolic Remodeling Based on de Novo Biosynthesis of L-Serine in Se-Subtoxic or -Deficient Mammals. J. Nutr. 2025, 155, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Diskin, C.J.; Tomasso, C.L.; Alper, J.C.; Glaser, M.L.; Fliegel, S.E. Long-term selenium exposure. Arch. Intern. Med. 1979, 139, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Lazard, M.; Dauplais, M.; Blanquet, S.; Plateau, P. Recent Advances in the Mechanism of Selenoamino Acids Toxicity in Eukaryotic Cells. Biomol. Concepts 2017, 8, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Bierla, K.; Szpunar, J.; Lobinski, R.; Sunde, R.A. Selenomethionine Supplementation and Expression of Selenosugars, Selenocysteine, and Other Selenometabolites in Rat Liver. Metallomics 2023, 15, mfad067. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Zeng, S.; Gao, M.; Guo, S.; Wang, M.; Hong, Y.; Zhao, G. L-selenomethionine affects liver development and glucolipid metabolism by inhibiting autophagy in zebrafish embryos. Ecotoxicol. Environ. Saf. 2023, 252, 114589. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Z.; Wang, L.; Yu, H.; Zhang, X.; Rong, K. Dietary selenium requirement for on-growing grass carp, Ctenopharyngodon idellus. Aquaculture 2023, 573, 739572. [Google Scholar] [CrossRef]
- Sunde, R.A. Gene set enrichment analysis of selenium-deficient and high-selenium rat liver transcript expression and comparison with turkey liver expression. J. Nutr. 2021, 151, 772–784. [Google Scholar] [CrossRef]
- Day, K.; Seale, L.A.; Graham, R.M.; Cardoso, B.R. Selenotranscriptome network in non-alcoholic fatty liver disease. Front. Nutr. 2021, 8, 744825. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Yang, J.; Wang, L.; Sun, G.; Guo, Y.; Xiang, Y.; Zou, Y.; Song, X.; Li, M.; et al. High-Selenium Exposure Is Associated with Modulation of Serum Lipid Metabolism. Ecotoxicol. Environ. Saf. 2025, 289, 117677. [Google Scholar] [CrossRef]
- Nangliya, V.; Sharma, A.; Yadav, D.; Sunder, S.; Nijhawan, S.; Mishra, S. Study of trace elements in liver cirrhosis patients and their role in prognosis of disease. Biol. Trace Elem. Res. 2015, 165, 35–40. [Google Scholar] [CrossRef]
- Shih, C.-W.; Chen, Y.-J.; Chen, W.-L. Inverse Association between Serum Selenium Level and Severity of Liver Fibrosis: A Cross-Sectional Study. Nutrients 2022, 14, 3625. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.J.; Duarte-Salles, T.; Hybsier, S.; Trichopoulou, A.; Stepien, M.; Aleksandrova, K.; Overvad, K.; Tjønneland, A.; Olsen, A.; Affret, A.; et al. Prediagnostic selenium status and hepatobiliary cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Am. J. Clin. Nutr. 2016, 104, 406–414. [Google Scholar] [CrossRef]
- Rohr-Udilova, N.; Sieghart, W.; Eferl, R.; Stoiber, D.; Bjorkhem-Bergman, L.; Eriksson, L.C.; Stolze, K.; Hayden, H.; Keppler, B.; Sagmeister, S.; et al. Antagonistic effects of selenium and lipid peroxides on growth control in early hepatocellular carcinoma. Hepatology 2012, 55, 1112–1121. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E.; Motley, A.K.; Byrne, D.W.; Norsworthy, B.K. Selenium deficiency occurs in some patients with moderate-to-severe cirrhosis and can be corrected by administration of selenate but not selenomethionine: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1126–1133. [Google Scholar] [CrossRef]
- Wang, X.; Seo, Y.A.; Park, S.K. Serum selenium and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2011–2016. Environ. Res. 2021, 197, 111190. [Google Scholar] [CrossRef]
- Urbano, T.; Filippini, T.; Lasagni, D.; De Luca, T.; Grill, P.; Sucato, S.; Polledri, E.; Djeukeu Noumbi, G.; Malavolti, M.; Santachiara, A.; et al. Association of urinary and dietary selenium and of serum selenium species with serum alanine aminotransferase in a healthy Italian population. Antioxidants 2021, 10, 1516. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yan, C.; Liu, G.; Niu, Y.; Zhang, W.; Lu, S.; Li, X.; Zhang, H.; Ning, G.; Fan, J.; et al. Plasma selenium levels and nonalcoholic fatty liver disease in Chinese adults: A cross-sectional analysis. Sci. Rep. 2016, 6, 37288. [Google Scholar] [CrossRef]
- Liu, J.; Tan, L.; Liu, Z.; Shi, R. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with blood selenium level based on the NHANES 2017–2018. Ann. Med. 2022, 54, 2259–2268. [Google Scholar] [CrossRef]
- Aktary, M.L.; Eller, L.K.; Nicolucci, A.C.; Reimer, R.A. Cross-Sectional Analysis of the Health Profile and Dietary Intake of a Sample of Canadian Adults Diagnosed with Non-Alcoholic Fatty Liver Disease. Food Nutr. Res. 2020, 64, 4548. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, C.; Yang, Z.; Li, X.; Lei, G.; Xie, D.; Wang, Y.; Wei, J.; Yang, T. Association between dietary selenium intake and the prevalence of nonalcoholic fatty liver disease: A cross-sectional study. J. Am. Coll. Nutr. 2020, 39, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zarghani, S.S.; Kashkooli, N.R.; Bagheri, Z.; Tabatabaei, M.; Fattahi, M.R.; Safarpour, A.R. Dietary selenium intake in relation to non-alcoholic fatty liver disease assessed by fatty liver index and hepatic steatosis index; a cross-sectional study on the baseline data of prospective PERSIAN Kavar cohort study. BMC Endocr. Disord. 2023, 23, 51. [Google Scholar] [CrossRef]
- Lin, Y.; He, F.; Lian, S.; Xie, B.; Liu, T.; He, J.; Liu, C. Selenium Status in Patients with Chronic Liver Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 952. [Google Scholar] [CrossRef]
- Burk, R.F.; Norsworthy, B.K.; Hill, K.E.; Motley, A.K.; Byrne, D.W. Effects of Chemical Form of Selenium on Plasma Biomarkers in a High-Dose Human Supplementation Trial. Cancer Epidemiol. Biomark. Prev. 2006, 15, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of Long-Term Selenium Supplementation on Mortality: Results from a Multiple-Dose, Randomised Controlled Trial. Free Radic. Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Roberts, B.R.; Malpas, C.B.; Vivash, L.; Genc, S.; Saling, M.M.; Desmond, P.; Steward, C.; Hicks, R.J.; Callahan, J.; et al. Supranutritional Sodium Selenate Supplementation Delivers Selenium to the Central Nervous System: Results from a Randomized Controlled Pilot Trial in Alzheimer’s Disease. Neurotherapeutics 2019, 16, 192–202. [Google Scholar] [CrossRef]
- Demircan, K.; Bengtsson, Y.; Sun, Q.; Brange, A.; Vallon-Christersson, J.; Rijntjes, E.; Malmberg, M.; Saal, L.H.; Rydén, L.; Borg, Å.; et al. Serum selenium, selenoprotein P and glutathione peroxidase 3 as predictors of mortality and recurrence following breast cancer diagnosis: A multicentre cohort study. Redox Biol. 2021, 47, 102145. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Selenoprotein P: An extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu. Rev. Nutr. 2005, 25, 215–235. [Google Scholar] [CrossRef]
- Schomburg, L. Selenoprotein P—Selenium transport protein, enzyme and biomarker of selenium status. Free Radic. Biol. Med. 2022, 191, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Brodin, O.; Hackler, J.; Misra, S.; Wendt, S.; Sun, Q.; Laaf, E.; Stoppe, C.; Björnstedt, M.; Schomburg, L. Selenoprotein P as biomarker of selenium status in clinical trials with therapeutic dosages of selenite. Nutrients 2020, 12, 1067. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Hill, K.E.; Li, P.; Xu, J.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: A placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am. J. Clin. Nutr. 2010, 92, 525–531. [Google Scholar] [CrossRef]
- Sun, Q.; Mehl, S.; Renko, K.; Seemann, P.; Görlich, C.L.; Hackler, J.; Minich, W.B.; Kahaly, G.J.; Schomburg, L. Natural autoimmunity to selenoprotein P impairs selenium transport in Hashimoto’s thyroiditis. Int. J. Mol. Sci. 2021, 22, 13088. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogra, Y.; Ishiwata, K.; Takayama, H.; Aimi, N.; Suzuki, K.T. Selenosugars Are Key and Urinary Metabolites for Selenium Excretion within the Required to Low-Toxic Range. Proc. Natl. Acad. Sci. USA 2002, 99, 15932–15936. [Google Scholar] [CrossRef]
- Kuehnelt, D.; Kienzl, N.; Traar, P.; Le, N.H.; Francesconi, K.A.; Ochi, T. Selenium Metabolites in Human Urine after Ingestion of Selenite, L-Selenomethionine, or DL-Selenomethionine: A Quantitative Case Study by HPLC/ICPMS. Anal. Bioanal. Chem. 2005, 383, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Katarzyna, B.; Taylor, R.M.; Szpunar, J.; Lobinski, R.; Sunde, R.A. Identification and Determination of Selenocysteine, Selenosugar, and Other Selenometabolites in Turkey Liver. Metallomics 2020, 12, 758–766. [Google Scholar] [CrossRef]
- Zambonino, M.C.; Quizhpe, E.M.; Mouheb, L.; Rahman, A.; Agathos, S.N.; Dahoumane, S.A. Biogenic selenium nanoparticles in biomedical sciences: Properties, current trends, novel opportunities and emerging challenges in theranostic nanomedicine. Nanomaterials 2023, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, J.; Zhang, S.; Zhang, J.; Xu, Q.; Xu, Z.; Guo, Y. Amorphous Structure and Crystal Stability Determine the Bioavailability of Selenium Nanoparticles. J. Hazard. Mater. 2024, 465, 133287. [Google Scholar] [CrossRef]
- Yang, X.; Fu, Y.; Zhang, J.; Liu, J.; Liu, X.; Peng, Y.; Kyin, S.L.; Zhang, M.; Zhou, D. Preparation, characterization, and antioxidant and antiapoptotic activities of biosynthesized nano-selenium by yak-derived Bacillus cereus and chitosan-encapsulated chemically synthesized nano-selenium. Int. J. Biol. Macromol. 2023, 242, 124708. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Guo, Y.; Ma, L.; Cheng, Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr. Polym. 2018, 195, 576–585. [Google Scholar] [CrossRef]
- Yan, S.; Qiao, L.; Dou, X.; Song, X.; Chen, Y.; Zhang, B.; Xu, C. Biogenic selenium nanoparticles by Lactobacillus casei ATCC 393 alleviate the intestinal permeability, mitochondrial dysfunction and mitophagy induced by oxidative stress. Food Funct. 2021, 12, 7068–7080. [Google Scholar] [CrossRef]
- Song, X.; Qiao, L.; Dou, X.; Chang, J.; Zhang, Y.; Xu, C. Selenium nanoparticles alleviate deoxynivalenol-induced intestinal epithelial barrier dysfunction by regulating endoplasmic reticulum stress in IPEC-J2 cells. Toxicology 2023, 494, 153593. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qiao, L.; Chang, J.; Dou, X.; Zhang, X.; Pi, S.; Xu, C. Dietary supplementation with selenium nanoparticles-enriched Lactobacillus casei ATCC 393 alleviates intestinal barrier dysfunction of mice exposed to deoxynivalenol by regulating endoplasmic reticulum stress and gut microbiota. Ecotoxicol. Environ. Saf. 2022, 248, 114276. [Google Scholar] [CrossRef]
- Lei, X.; Peng, Y.; Li, Y.; Chen, Q.; Shen, Z.; Yin, W.; Lemiasheuski, V.; Xu, S.; He, J. Effects of selenium nanoparticles produced by Lactobacillus acidophilus HN23 on lipid deposition in WRL68 cells. Bioorganic Chem. 2024, 145, 107165. [Google Scholar] [CrossRef]
- Li, T.; Zhu, K.; Wang, L.; Dong, Y.; Huang, J. Stabilization by chaperone GroEL in biogenic selenium nanoparticles produced from Bifidobacterium animalis H15 for the treatment of DSS-induced colitis. ACS Appl. Mater. Interfaces 2024, 16, 13439–13452. [Google Scholar] [CrossRef] [PubMed]
- EL-Sayed, A.I.M.; El-Sheekh, M.M.; Abo-Neima, S.E. Mycosynthesis of selenium nanoparticles using Penicillium tardochrysogenum as a therapeutic agent and their combination with infrared irradiation against Ehrlich carcinoma. Sci. Rep. 2024, 14, 2547. [Google Scholar] [CrossRef]
- Hassan, M.G.; Hawwa, M.T.; Baraka, D.M.; El-Shora, H.M.; Hamed, A.A. Biogenic selenium nanoparticles and selenium/chitosan-Nanoconjugate biosynthesized by Streptomyces parvulus MAR4 with antimicrobial and anticancer potential. BMC Microbiol. 2024, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, Y.; Zhang, S.; Xu, Q.; Guo, Y. Nitrate reductase involves in selenite reduction in Rahnella aquatilis HX2 and the characterization and anticancer activity of the biogenic selenium nanoparticles. J. Trace Elem. Med. Biol. 2024, 83, 127387. [Google Scholar] [CrossRef] [PubMed]
- Spyridopoulou, K.; Tryfonopoulou, E.; Aindelis, G.; Ypsilantis, P.; Sarafidis, C.; Kalogirou, O.; Chlichlia, K. Biogenic selenium nanoparticles produced by Lactobacillus casei ATCC 393 inhibit colon cancer cell growth in vitro and in vivo. Nanoscale Adv. 2021, 3, 2516–2528. [Google Scholar] [CrossRef]
- Jin, H.; Riaz Rajoka, M.S.; Xu, X.; Liao, N.; Pang, B.; Yan, L.; Liu, G.; Sun, H.; Jiang, C.; Shao, D.; et al. Potentials of orally supplemented selenium-enriched Lacticaseibacillus rhamnosus to mitigate the lead induced liver and intestinal tract injury. Environ. Pollut. 2022, 302, 119062. [Google Scholar] [CrossRef]
- Liu, R.; Sun, W.; Sun, T.; Zhang, W.; Nan, Y.; Zhang, Z.; Xiang, K.; Yang, H.; Wang, F.; Ge, J. Nano selenium-enriched probiotic Lactobacillus enhances alum adjuvanticity and promotes antigen-specific systemic and mucosal immunity. Front. Immunol. 2023, 14, 1116223. [Google Scholar] [CrossRef]
- Sun, N.; Jia, Q.; Tu, J.; Liu, H. Apoptosis of hepatocellular carcinoma HepG2 cells induced by seleno-ovalbumin (Se-OVA) via mitochondrial pathway. Int. J. Biol. Macromol. 2021, 192, 82–89. [Google Scholar] [CrossRef]
- Zheng, G.; Liu, H.; Zhu, Z.; Zheng, J.; Liu, A. Selenium modification of β-lactoglobulin (β-Lg) and its biological activity. Food Chem. 2016, 204, 246–251. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Wang, W.; Wu, D.; Shi, J.; Liu, A. Apoptosis and autophagy induction of Seleno-β-lactoglobulin (Se-β-Lg) on hepatocellular carcinoma cells lines. J. Funct. Foods 2018, 49, 412–423. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, Y.; Yu, J.; Ji, H.; Liu, A. Characterization of Se-enriched Pleurotus ostreatus polysaccharides and their antioxidant effects in vitro. Int. J. Biol. Macromol. 2018, 111, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, W.; Zhu, Y.; Chen, Y.; Zhang, W.; Yu, P.; Mao, G.; Zhao, T.; Feng, W.; Yang, L.; et al. Structural elucidation and antioxidant activity a novel Se-polysaccharide from Se-enriched Grifola frondosa. Carbohydr. Polym. 2017, 161, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, W.; Zhai, T.; You, J.; Chen, Y. Silibinin ameliorates hepatic lipid accumulation and oxidative stress in mice with non-alcoholic steatohepatitis by regulating CFLAR-JNK pathway. Acta Pharm. Sin. B 2019, 9, 745–757. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, N.; Aldhahrani, A.; Soliman, M.M.; Zhang, L.; Zhou, F. Puerarin ameliorates nonalcoholic fatty liver in rats by regulating hepatic lipid accumulation, oxidative stress, and inflammation. Front. Immunol. 2022, 13, 956688. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, Z.-Y.; Zhou, P.-H.; Zhou, X.-L.; Zhang, D.-L.; He, N.; Zhang, J.; Liu, Y.-H.; Gu, Q. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. 2018, 17, 165. [Google Scholar] [CrossRef]
- Mousavi, S.N.; Faghihi, A.; Motaghinejad, M.; Shiasi, M.; Imanparast, F.; Amiri, H.L.; Shidfar, F. Zinc and selenium co-supplementation reduces some lipid peroxidation and angiogenesis markers in a rat model of NAFLD-fed high fat diet. Biol. Trace Elem. Res. 2018, 181, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Nagashimada, M.; Ota, T. Role of vitamin E in nonalcoholic fatty liver disease. IUBMB Life 2019, 71, 516–522. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 2012, 10, 2579. [Google Scholar] [CrossRef]

| Country or Body | RNI (μg/Day) | UL (μg/Day) | Ref. |
|---|---|---|---|
| WHO | 26 (males), 34 (females) | 400 | [16] |
| China | 60 | 400 | [20] |
| USA | 55 | 400 | [16] |
| EFSA | 70 (AI) | 255 | [21,22] |
| Japan | 30–35 (males), 25 (females) | 450 (males), 350 (females) | [23] |
| Korea | 60 | 400 | [24] |
| Form | Dose | Experimental Animal | Experimental Period | Route of Administration | Main Effects | Ref. |
|---|---|---|---|---|---|---|
| Inorganic selenium | ||||||
| Sodium selenate | 0.8 mg sodium selenate/kg BW | Diabetic db/db mice | 9 weeks | Gavage | Increased insulin production and secretion but reduced antioxidant defense capacity, which exacerbated fatty liver degeneration. | [45] |
| Sodium selenite | 0.3, 1.0 and 3.0 mg Se/kg diet | Pigs | 16 weeks | Oral feed | The supplementation of selenium at 1.0 mg/kg may be the optimum concentration against liver damage induced by HFD. | [38] |
| Sodium selenite | 0.033 and 0.2 mg Se/kg diet | Chickens | 15, 25, 35, 45, 55 or 65 days | Oral feed | Selenium deficiency induced oxidative stress, ER stress, and apoptosis in chicken livers. | [46] |
| Organic selenium | ||||||
| SeMet | 0.25 and 2.5 mg Se/kg diet | Pigs | 60 days | Oral feed | High SeMet intake resulted in hyperglycemia, hyperinsulinemia, and fatty acid accumulation in the liver. | [47] |
| SeMet | 0.3 mg Se/kg diet | Pigs | 16 weeks | Oral feed | Improved the redox imbalance, which led to hepatic metabolic reprogramming and inflammation in selenium deficiency. | [9] |
| Selenocystine, SeMet, and MeSeCys | 0.25 and 0.5 mg Se/kg BW | C57BL/6 mice | 16 weeks | Gavage | Improved MASLD in mice through 5-hydroxytryptophan/bile acid enterohepatic circulation. | [48] |
| Se-enriched spirulina | 0.45 mg Se/kg diet | C57BL/6 mice | 12 weeks | Oral feed | Improved the hepatic injury and IR in HFD mice. | [49] |
| Se-GTP | 200, 400 and 800 mg Se-GTP/kg BW | Kunming mice | 8 weeks | Gavage | Ameliorated the high fructose-induced IR and hepatic oxidative injury, which was more effective at a high dose. | [50] |
| Se-enriched milk casein | 0.25, 0.5 and 2.0 mg Se/kg diet | SD rats | 7 weeks | Oral feed | Supranutritional selenium intake up to 8 times the requirement had similar negative effects on hepatic insulin sensitivity as consuming an HFD. | [51] |
| Se-enriched yeast | 0.3 and 3.0 mg Se/kg diet | Pigs | 11 weeks | Oral feed | High dietary selenium intake altered lipid metabolism and protein synthesis in liver and muscle of pigs. | [52] |
| Se-enriched yeast | 0.15 and 0.65 mg Se/kg diet | Goats | 10 weeks | Oral feed | Supranutritional selenium alleviated hepatic oxidative and inflammatory lesions induced by a high-concentrate diet. | [53] |
| Other forms | ||||||
| Selenoneine | 0.3 mg Se/kg diet | Fxr-null mice | 4 months | Oral feed | Attenuated hepatic steatosis and hepatocellular injury in an MASLD mouse model. | [44] |
| SeNPs | 0.2, 0.4 and 0.8 mg Se/kg BW | SD rats | 2 weeks | Gavage | Improved hepatic antioxidant capacity at supranutritional levels. | [54] |
| Chondroitin sulfate SeNPs | 0.1 and 0.2 mg Se/kg diet | SD rats | 12 weeks | Oral feed | Prevented liver fibrosis, maintained normal energy metabolic activity, and decreased mitophagy. | [55] |
| A SeNDs | 0.3 mg Se/kg BW | SD rats | 8 weeks | Gavage | Reduced hepatocyte steatosis, oxidative stress, and inflammatory reactions and improved hepatic structure and liver function in MASLD rats. | [56] |
| SeNPs | 1 mg Se/kg BW | Kunming mice | 4 weeks | Gavage | Attenuated liver lipid accumulation and degeneration caused by polystyrene microplastics. | [57] |
| Size of SeNPs (nm) | Source of SeNPs | Form of Selenium in the Control Group | Dose Gradient | Experimental Animals | Main Conclusions | Ref. |
|---|---|---|---|---|---|---|
| 20–60 | Chemical synthesis | Sodium selenite | 2, 4, and 6 mg Se/kg BW | Kunming mice | A high dose of sodium selenite caused more pronounced oxidative stress, greater liver injury, and prominent retardation of growth than SeNPs. | [83] |
| 20–60 | Chemical synthesis | SeMet | 5 and 10 mg Se/kg BW | Kunming mice | SeNPs functioned as antioxidants with a reduced risk of toxicity and a comparable ability to increase selenoenzymes to SeMet. | [84] |
| 20–60 | Chemical synthesis | MeSeCys | 5 and 10 mg Se/kg BW | Kunming mice | SeNPs could serve as potential chemopreventive agents with reduced risk of toxicity compared to MeSeCys. | [85] |
| 20–60 | Chemical synthesis | Sodium selenite and high-selenium protein | 2, 3, 4, and 5 mg Se/kg diet | SD rats | SeNPs were less toxic than selenite and high-selenium protein in the 13-week rat study. | [80] |
| 70–90 | Chemical synthesis | Sodium selenite | 1 and 4 mg Se/kg BW | Swiss albino mice | SeNPs at low doses exhibited antioxidant effects in the liver compared to the high dose of SeNPs and the high and low doses of sodium selenite. | [81] |
| 80–220 | Biosynthesis | Selenium dioxide | 2.5, 5, 10, and 20 mg Se/kg BW | NMRI mice | The biogenic SeNPs were much less (26-fold) toxic than selenium dioxide, and a dose of 20 mg Se/kg BW was accompanied by signs of toxicity. | [86] |
| 100–500 | Biosynthesis | Sodium selenate, sodium hydroselenite, selenoaminoacids, and Se-enriched yogurt powder | 0.5, 5, and 50 mg Se/kg diet | BDF1 mice | The toxicity of selenium species decreased in the following order: selenate > selenite > SeNPs > selenoaminoacids > Se-enriched yogurt powder. | [87] |
| Microorganisms | Precursor of SeNPs | Size of SeNPs (nm) | Functions of SeNPs and SeMs | Ref. |
|---|---|---|---|---|
| Bacillus cereus YC-3 | Sodium selenite | 116.87 ± 35.45 | Antioxidant and anti-apoptotic activities (SeNPs); attenuate liver lipid accumulation and degeneration (SeNPs). | [57,128] |
| Lactobacillus coryniformis ES23 | Sodium selenite | 127.4 ± 41.2 | Alleviate MASH (SeNPs). | [89] |
| Lactobacillus casei ATCC 393 | Sodium selenite | 50–80 | Anticancer and antioxidant activities (SeNPs); alleviate H2O2-induced intestinal epithelial barrier dysfunction (SeNPs); alleviate intestinal barrier dysfunction induced by deoxynivalenol (SeNPs and SeMs). | [129,130,131,132] |
| Lactobacillus acidophilus HN23 | Sodium selenite | 60–300 | Reduce hepatocyte lipid deposition and oxidative damage (SeNPs). | [133] |
| Bifidobacterium animalis H15 | Sodium selenite | 40–200 | Alleviate dextran sulfate sodium-induced colitis (SeNPs). | [134] |
| Penicillium tardochrysogenum OR059437 | Sodium selenate | 82.31 ± 22.10 | Antioxidant, antimicrobial, and anticancer activities (SeNPs). | [135] |
| Streptomyces parvulus MAR4 | Sodium selenate | 48.8–129.0 | Antimicrobial and anticancer activities (SeNPs). | [136] |
| Rahnella aquatilis HX2 | Sodium selenite | 193–513 | Anticancer activity (SeNPs). | [137] |
| Lactobacillus casei ATCC 393 | Sodium biselenite | 170–550 | Inhibit colon cancer cell growth in vitro and in vivo (SeNPs and SeMs). | [138] |
| Lacticaseibacillus rhamnosus SHA113 | Sodium selenite | 42.4 ± 10.5 | Protect the liver and intestinal tract from injury by lead (SeMs). | [139] |
| Levilactobacillus brevis 23017 | Sodium selenite | 50–80 | Improve the immune effect of the alum adjuvant vaccine (SeMs). | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Chen, K.; Xu, Z.; Wang, L.; Zhu, Y.; Yu, Z.; Li, T.; Huang, J. Potential Applications and Risks of Supranutritional Selenium Supplementation in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Critical Review. Nutrients 2025, 17, 2484. https://doi.org/10.3390/nu17152484
Liu C, Chen K, Xu Z, Wang L, Zhu Y, Yu Z, Li T, Huang J. Potential Applications and Risks of Supranutritional Selenium Supplementation in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Critical Review. Nutrients. 2025; 17(15):2484. https://doi.org/10.3390/nu17152484
Chicago/Turabian StyleLiu, Chuanming, Ke Chen, Zijian Xu, Lianshun Wang, Yinhua Zhu, Zhengquan Yu, Tong Li, and Jiaqiang Huang. 2025. "Potential Applications and Risks of Supranutritional Selenium Supplementation in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Critical Review" Nutrients 17, no. 15: 2484. https://doi.org/10.3390/nu17152484
APA StyleLiu, C., Chen, K., Xu, Z., Wang, L., Zhu, Y., Yu, Z., Li, T., & Huang, J. (2025). Potential Applications and Risks of Supranutritional Selenium Supplementation in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Critical Review. Nutrients, 17(15), 2484. https://doi.org/10.3390/nu17152484








