The Effect of Probiotics on Health in Pregnancy and Infants: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Study Approval and Registration
2.2. Participant Selection
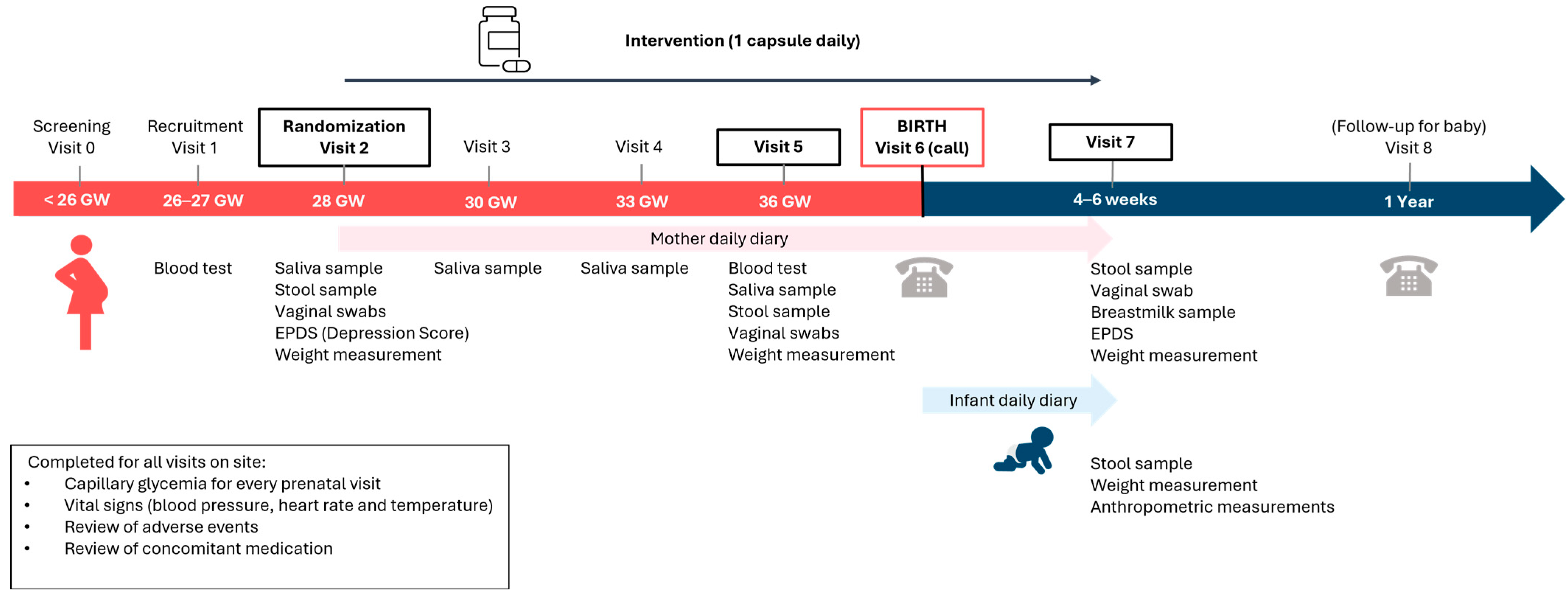
2.3. Study Design and Intervention
2.4. Outcomes
2.5. Data Collection
2.6. Blood Inflammatory Markers
2.7. sIgA Analysis
2.8. Microbiota Composition
2.9. Sample Size Calculation
2.10. Statistical Analysis
2.11. Exploratory Analysis of Bacterial Networks
3. Results
3.1. Participant Characteristics
3.2. Primary Outcome: Infections in Mothers
3.3. Infections in Infants
3.4. Inflammatory Markers and Immunoglobulin Levels
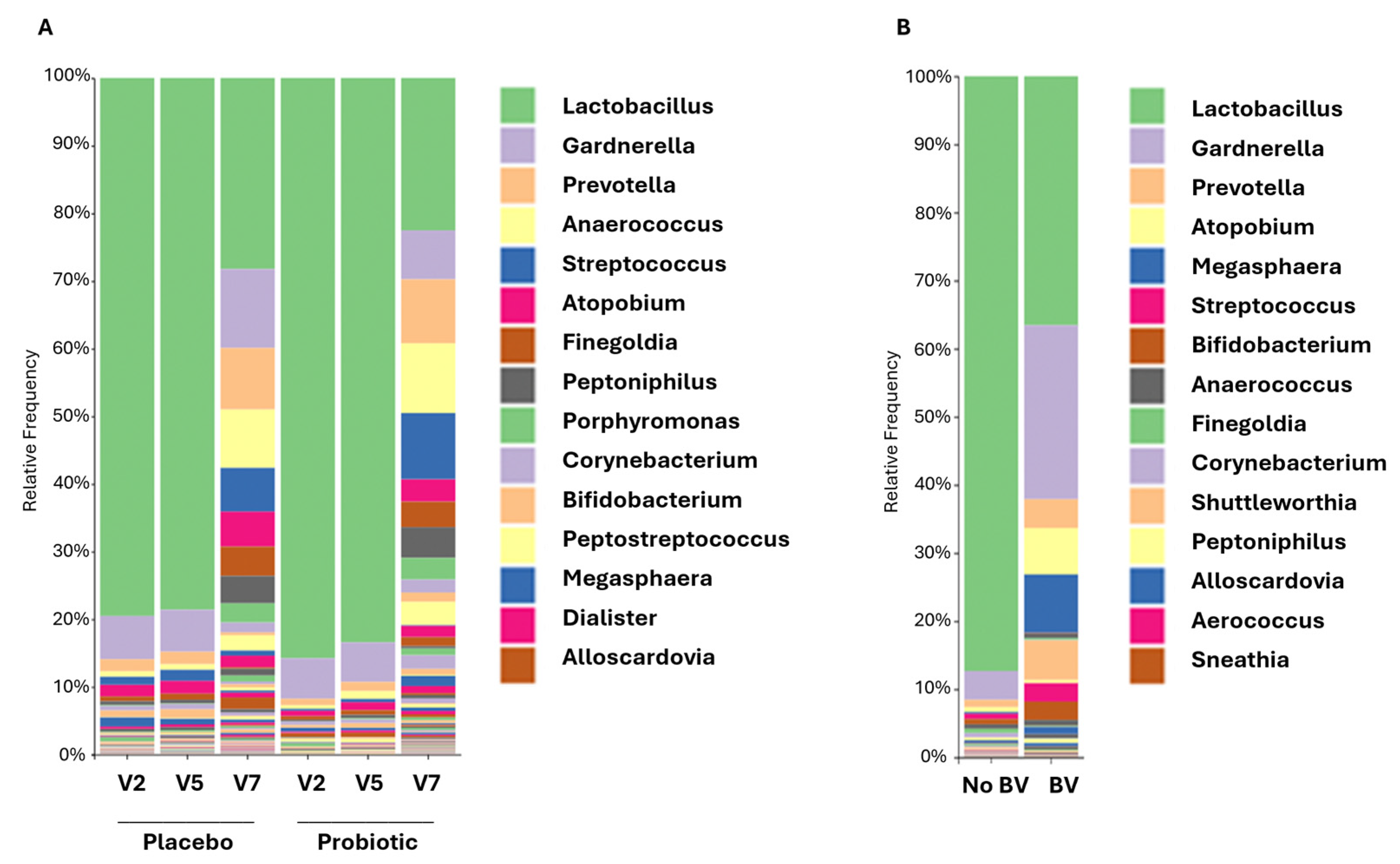
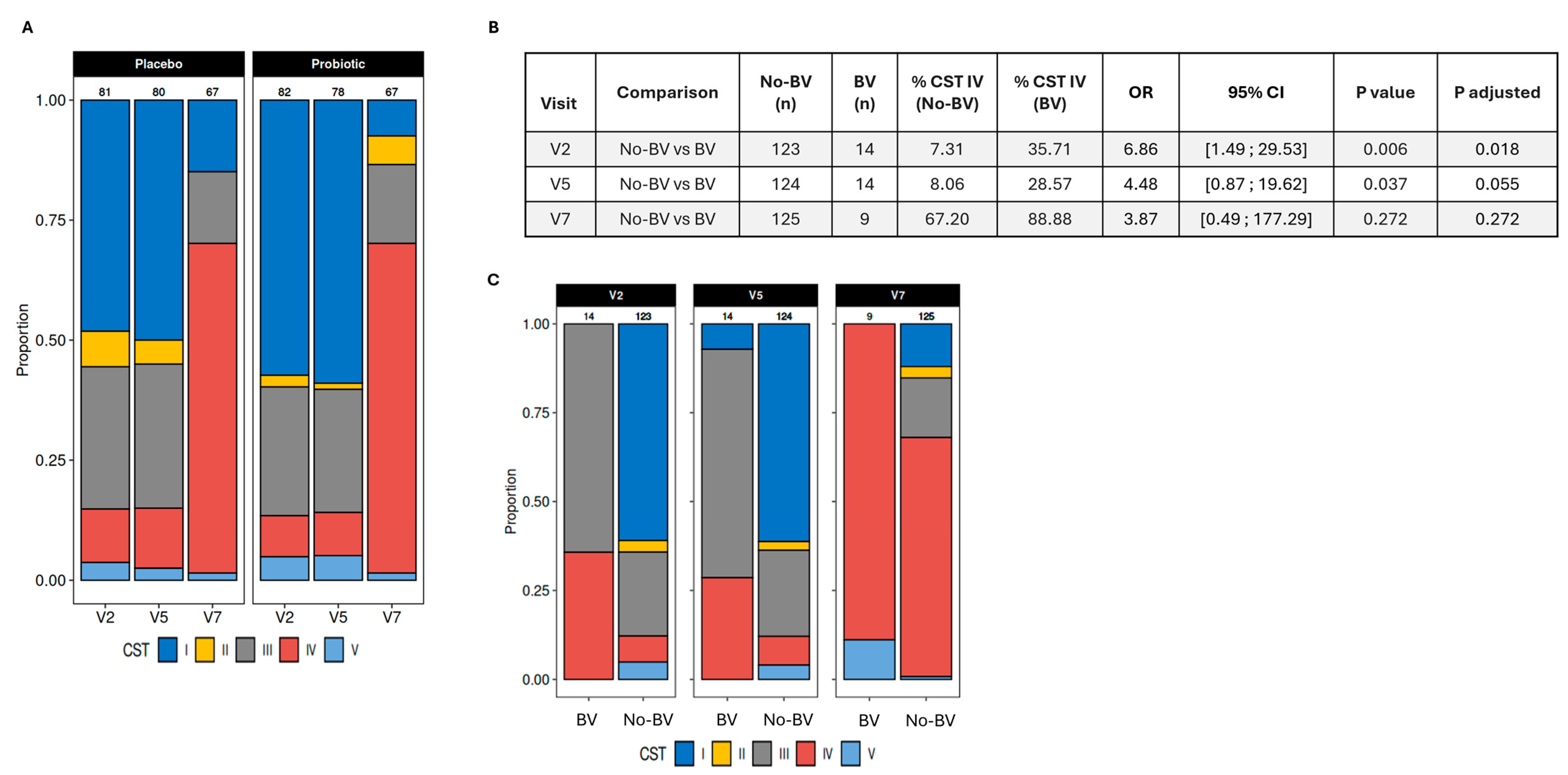
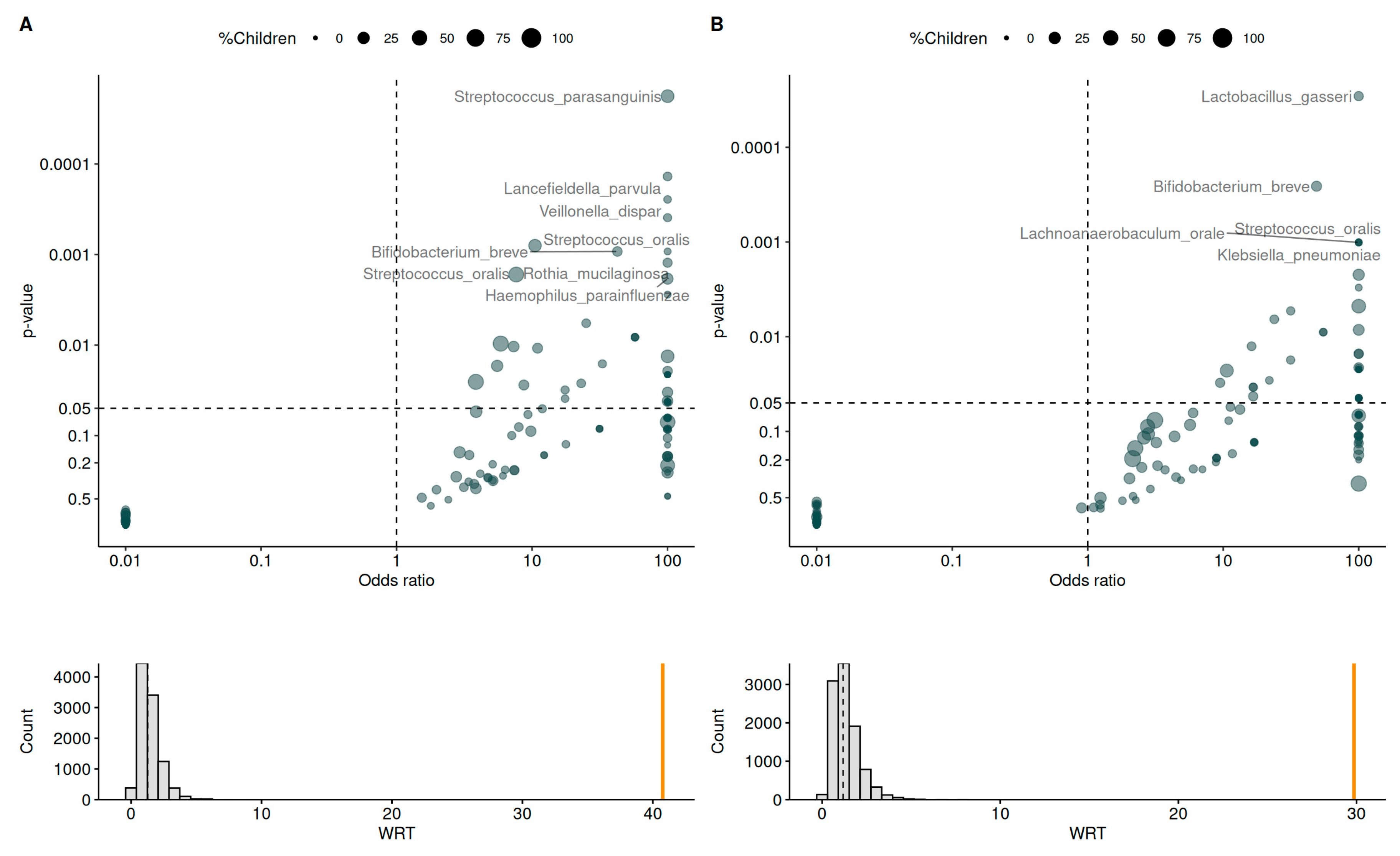
3.5. Vaginal Microbiome and Its Association with Infections
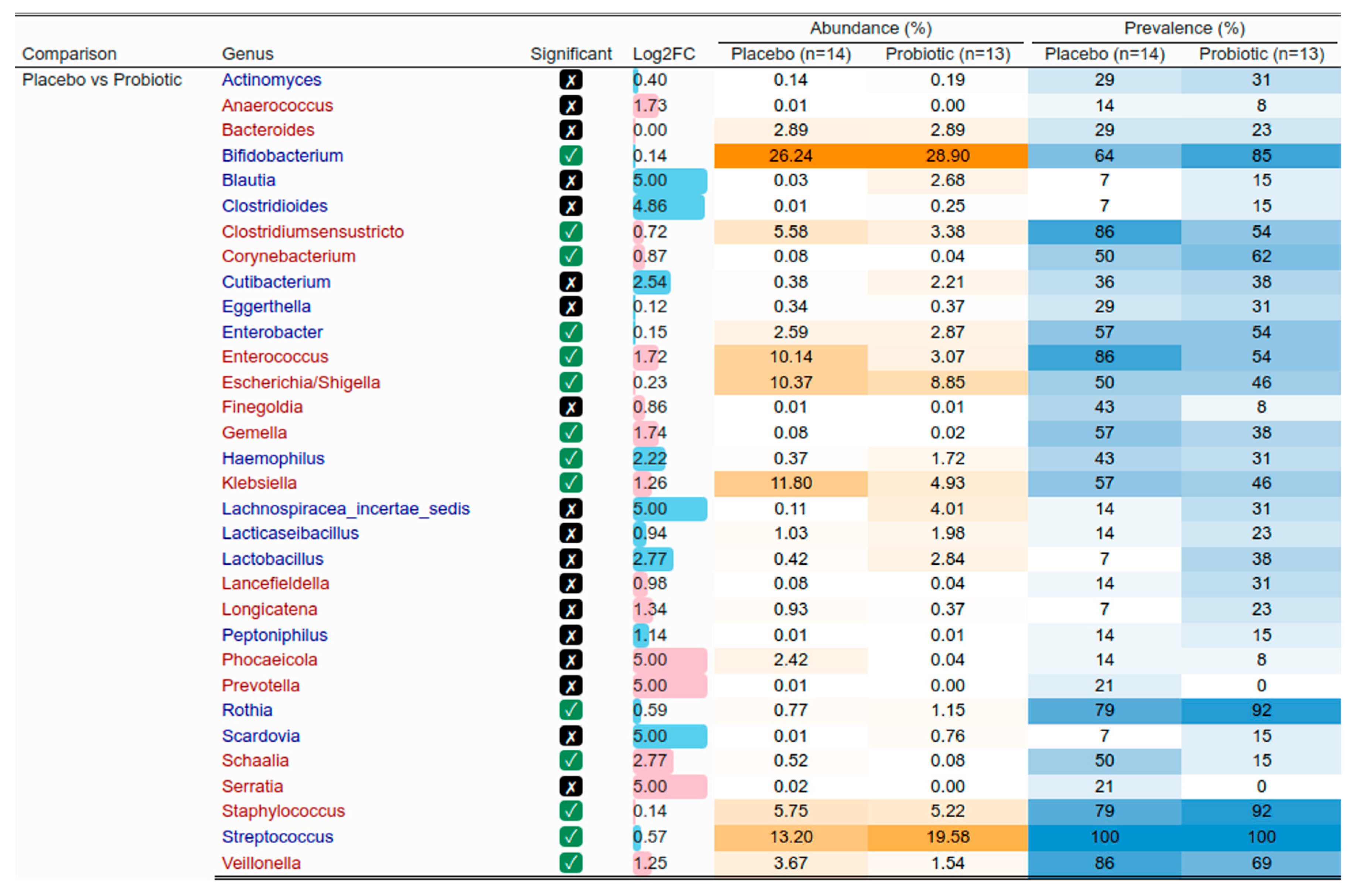
3.6. Mother and Infant Gut Microbiota
3.7. Vertical Transmission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannella, L.; Grelloni, C.; Quintili, D.; Fiorelli, A.; Montironi, R.; Alia, S.; Delli Carpini, G.; Di Giuseppe, J.; Vignini, A.; Ciavattini, A. Microbiome Changes in Pregnancy Disorders. Antioxidants 2023, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.; Brushett, S.; Prins, J.; Zhernakova, A. The maternal gut microbiome during pregnancy and its role in maternal and infant health. Curr. Opin. Microbiol. 2023, 74, 102309. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.Z.; Al-Rumaihi, S.; Al-Absi, R.S.; Farah, H.; Elamin, M.; Nader, R.; Bouabidi, S.; Suleiman, S.E.; Nasr, S.; Al-Asmakh, M. Physiological changes and interactions between microbiome and the host during pregnancy. Front. Cell. Infect. Microbiol. 2022, 12, 824925. [Google Scholar] [CrossRef] [PubMed]
- Gomez Arango, L.F.; Barrett, H.L.; Callaway, L.K.; Nitert, M.D. Probiotics and pregnancy. Curr. Diabetes Rep. 2015, 15, 567. [Google Scholar] [CrossRef]
- Mesa, M.D.; Loureiro, B.; Iglesia, I.; Fernandez Gonzalez, S.; Llurba Olivé, E.; Garcia Algar, O.; Solana, M.J.; Cabero Perez, M.J.; Sainz, T.; Martinez, L. The evolving microbiome from pregnancy to early infancy: A comprehensive review. Nutrients 2020, 12, 133. [Google Scholar] [CrossRef]
- Fuhler, G. The immune system and microbiome in pregnancy. Best Pract. Res. Clin. Gastroenterol. 2020, 44, 101671. [Google Scholar] [CrossRef]
- Sappenfield, E.; Jamieson, D.J.; Kourtis, A.P. Pregnancy and susceptibility to infectious diseases. Infect. Dis. Obstet. Gynecol. 2013, 2013, 752852. [Google Scholar] [CrossRef]
- Aguin, T.J.; Sobel, J.D. Vulvovaginal candidiasis in pregnancy. Curr. Infect. Dis. Rep. 2015, 17, 30. [Google Scholar] [CrossRef]
- Guaschino, S.; De Seta, F.; Piccoli, M.; Maso, G.; Alberico, S. Aetiology of preterm labour: Bacterial vaginosis. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 46–51. [Google Scholar] [CrossRef]
- Disha, T.; Haque, F. Prevalence and risk factors of vulvovaginal candidosis during pregnancy: A review. Infect. Dis. Obstet. Gynecol. 2022, 2022, 6195712. [Google Scholar] [CrossRef]
- Vasudevan, R. Urinary tract infection: An overview of the infection and the associated risk factors. J. Microbiol. Exp. 2014, 1, 42–54. [Google Scholar] [CrossRef]
- Kuperman, A.A.; Koren, O. Antibiotic use during pregnancy: How bad is it? BMC Med. 2016, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Gan, X.-P.; Li, F.-F.; Zhang, D.-Y.; Chen, L.; Cao, Y.-N.; Qiu, H.-H.; Cheng, D.-C.; Zu, J.-F.; Liu, W.-Y. Effect of exposure to antibiotics on the gut microbiome and biochemical indexes of pregnant women. BMJ Open Diabetes Res. Care 2021, 9, e002321. [Google Scholar] [CrossRef]
- Wegienka, G.; Havstad, S.; Zoratti, E.M.; Kim, H.; Ownby, D.R.; Johnson, C.C. Combined effects of prenatal medication use and delivery type are associated with eczema at age 2 years. Clin. Exp. Allergy 2015, 45, 660–668. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Chen, C.; Fang, H.; Zhao, Y.; Fei, W.; Chen, F.; Zheng, C. The role of microbiomes in pregnant women and offspring: Research progress of recent years. Front. Pharmacol. 2020, 11, 643. [Google Scholar] [CrossRef]
- Ma, G.; Shi, Y.; Meng, L.; Fan, H.; Tang, X.; Luo, H.; Wang, D.; Zhou, J.; Xiao, X. Factors affecting the early establishment of neonatal intestinal flora and its intervention measures. Front. Cell. Infect. Microbiol. 2023, 13, 1295111. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Ortiz-Andrellucchi, A.; Sánchez-Villegas, A.; Rodríguez-Gallego, C.; Lemes, A.; Molero, T.; Soria, A.; Pena-Quintana, L.; Santana, M.; Ramírez, O.; García, J. Immunomodulatory effects of the intake of fermented milk with Lactobacillus casei DN114001 in lactating mothers and their children. Br. J. Nutr. 2008, 100, 834–845. [Google Scholar] [CrossRef]
- Trifkovič, K.Č.; Mičetić-Turk, D.; Kmetec, S.; Strauss, M.; Dahlen, H.G.; Foster, J.P.; Fijan, S. Efficacy of direct or indirect use of probiotics for the improvement of maternal depression during pregnancy and in the postnatal period: A systematic review and meta-analysis. Healthcare 2022, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Reznichenko, H.; Henyk, N.; Maliuk, V.; Khyzhnyak, T.; Tynna, Y.; Filipiuk, I.; Veresniuk, N.; Zubrytska, L.; Quintens, J.; Richir, K. Oral intake of lactobacilli can Be helpful in symptomatic bacterial vaginosis: A randomized clinical study. J. Low. Genit. Tract Dis. 2020, 24, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Bagheri, N.; Mohammad-Alizadeh-Charandabi, S.; Kashani, N.; Mobaraki-Asl, N.; Mirghafurvand, M.; Asgharian, H.; Ansari, F.; Pourjafar, H. Prevention of gestational diabetes mellitus (GDM) and probiotics: Mechanism of action: A review. Curr. Diabetes Rev. 2020, 16, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Myhre, R.; Haugen, M.; Myking, S.; Sengpiel, V.; Magnus, P.; Jacobsson, B.; Meltzer, H.M. Intake of probiotic food and risk of preeclampsia in primiparous women: The Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 2011, 174, 807–815. [Google Scholar] [CrossRef]
- Nordqvist, M.; Jacobsson, B.; Brantsæter, A.-L.; Myhre, R.; Nilsson, S.; Sengpiel, V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: A prospective observational cohort study in Norway. BMJ Open 2018, 8, e018021. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, F. Microbial transmission, colonisation and succession: From pregnancy to infancy. Gut 2023, 72, 772–786. [Google Scholar] [CrossRef]
- Isolauri, E.; Rautava, S.; Collado, M.; Salminen, S. Role of probiotics in reducing the risk of gestational diabetes. Diabetes Obes. Metab. 2015, 17, 713–719. [Google Scholar] [CrossRef]
- Reid, G.; Kumar, H.; Khan, A.; Rautava, S.; Tobin, J.; Salminen, S. The case in favour of probiotics before, during and after pregnancy: Insights from the first 1,500 days. Benef. Microbes 2016, 7, 353–362. [Google Scholar] [CrossRef]
- Zaidi, A.Z.; Moore, S.E.; Okala, S.G. Impact of Maternal Nutritional Supplementation during Pregnancy and Lactation on the Infant Gut or Breastmilk Microbiota: A Systematic Review. Nutrients 2021, 13, 1137. [Google Scholar] [CrossRef]
- Navarro-Tapia, E.; Sebastiani, G.; Sailer, S.; Almeida Toledano, L.; Serra-Delgado, M.; García-Algar, Ó.; Andreu-Fernández, V. Probiotic Supplementation during the Perinatal and Infant Period: Effects on gut Dysbiosis and Disease. Nutrients 2020, 12, 2243. [Google Scholar] [CrossRef] [PubMed]
- Cuinat, C.; Stinson, S.E.; Ward, W.E.; Comelli, E.M. Maternal intake of probiotics to program offspring health. Curr. Nutr. Rep. 2022, 11, 537–562. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, L.; Xia, J.; Cheng, L.; Chen, G.; Wang, J.; Raghavan, V. Probiotics supplementation during pregnancy or infancy on multiple food allergies and gut microbiota: A systematic review and meta-analysis. Nutr. Rev. 2025, 83, e25–e41. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J. Pharmacol. Pharmacother. 2010, 1, 100–107. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782. [Google Scholar] [CrossRef]
- Bekkali, N.; Hamers, S.L.; Reitsma, J.B.; Van Toledo, L.; Benninga, M.A. Infant Stool Form Scale: Development and Results. J. Pediatr. 2009, 154, 521–526.e521. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, R.; Birolo, G.; James, S.A.; Telatin, A. Dadaist2: A Toolkit to Automate and Simplify Statistical Analysis and Plotting of Metabarcoding Experiments. Int. J. Mol. Sci. 2021, 22, 5309. [Google Scholar] [CrossRef]
- Wilks, M.; Wiggins, R.; Whiley, A.; Hennessy, E.; Warwick, S.; Porter, H.; Corfield, A.; Millar, M. Identification and H2O2 Production of Vaginal Lactobacilli from Pregnant Women at High Risk of Preterm Birth and Relation with Outcome. J. Clin. Microbiol. 2004, 42, 713–717. [Google Scholar] [CrossRef]
- Langkamp-Henken, B.; Rowe, C.C.; Ford, A.L.; Christman, M.C.; Nieves, C.; Khouri, L.; Specht, G.J.; Girard, S.A.; Spaiser, S.J.; Dahl, W.J. Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 113, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Anukam, K.C.; Osazuwa, E.; Osemene, G.I.; Ehigiagbe, F.; Bruce, A.W.; Reid, G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect./Inst. Pasteur 2006, 8, 2772–2776. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Bastani, P.; Ziyadi, S.; Mohammad-Alizadeh-Charandabi, S.; Ghalibaf, M.; Mortazavian, A.M.; Mehrabany, E.V. Effects of probiotics on the recurrence of bacterial vaginosis: A review. J. Low. Genit. Tract Dis. 2014, 18, 79–86. [Google Scholar] [CrossRef]
- Hilton, E.; Isenberg, H.D.; Alperstein, P.; France, K.; Borenstein, M.T. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann. Intern. Med. 1992, 116, 353–357. [Google Scholar] [CrossRef]
- Mortensen, M.S.; Rasmussen, M.A.; Stokholm, J.; Brejnrod, A.D.; Balle, C.; Thorsen, J.; Krogfelt, K.A.; Bisgaard, H.; Sørensen, S.J. Modeling transfer of vaginal microbiota from mother to infant in early life. eLife 2021, 10, e57051. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, Z.D.; Müller, C.L.; Miraldi, E.R.; Littman, D.R.; Blaser, M.J.; Bonneau, R.A. Sparse and Compositionally Robust Inference of Microbial Ecological Networks. PLoS Comput. Biol. 2015, 11, e1004226. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Bozzi Cionci, N.; Baffoni, L.; Gaggìa, F.; Di Gioia, D. Therapeutic Microbiology: The Role of Bifidobacterium breve as Food Supplement for the Prevention/Treatment of Paediatric Diseases. Nutrients 2018, 10, 1723. [Google Scholar] [CrossRef]
- Bae, W.-Y.; Lee, Y.J.; Jung, W.-H.; Shin, S.L.; Kim, T.-R.; Sohn, M. Draft genome sequence and probiotic functional property analysis of Lactobacillus gasseri LM1065 for food industry applications. Sci. Rep. 2023, 13, 12212. [Google Scholar] [CrossRef]
- Barrons, R.; Tassone, D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: A review. Clin. Ther. 2008, 30, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Disease-a-Month 2003, 49, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Lamas, A.; Del Carmen Mondragón, A.; Cardelle-Cobas, A.; Regal, P.; Rodriguez-Avila, J.A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Probiotic Effects against Virus Infections: New Weapons for an Old War. Foods 2021, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, K. Prevention of infections by probiotics. J. Biosci. Bioeng. 2005, 100, 583–592. [Google Scholar] [CrossRef]
- Ho, M.; Chang, Y.-Y.; Chang, W.-C.; Lin, H.-C.; Wang, M.-H.; Lin, W.-C.; Chiu, T.-H. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial. Taiwan. J. Obstet. Gynecol. 2016, 55, 515–518. [Google Scholar] [CrossRef]
- Martín, V.; Cárdenas, N.; Ocaña, S.; Marín, M.; Arroyo, R.; Beltrán, D.; Badiola, C.; Fernández, L.; Rodríguez, J.M. Rectal and vaginal eradication of Streptococcus agalactiae (GBS) in pregnant women by using Lactobacillus salivarius CECT 9145, a target-specific probiotic strain. Nutrients 2019, 11, 810. [Google Scholar] [CrossRef]
- Ang, X.Y.; Mageswaran, U.M.; Chung, Y.L.F.; Lee, B.K.; Azhar, S.N.A.; Roslan, N.S.; Saufian, I.F.B.; Mustaffa, N.S.; Kalam, E.M.; Ibrahim, A.F. Probiotics reduce vaginal candidiasis in pregnant women via modulating abundance of Candida and Lactobacillus in vaginal and cervicovaginal regions. Microorganisms 2022, 10, 285. [Google Scholar] [CrossRef]
- Freitas, A.C.; Chaban, B.; Bocking, A.; Rocco, M.; Yang, S.; Hill, J.E.; Money, D.M.; Hemmingsen, S.; Reid, G.; Dumonceaux, T.; et al. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Sci. Rep. 2017, 7, 9212. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.-A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Van Den Veyver, I.; Milosavljevic, A.; et al. A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef]
- Macintyre, D.A.; Chandiramani, M.; Lee, Y.S.; Kindinger, L.; Smith, A.; Angelopoulos, N.; Lehne, B.; Arulkumaran, S.; Brown, R.; Teoh, T.G.; et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 2015, 5, 8988. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, K.; Cao, T.; Duan, Z. Characterization of a Lactobacillus gasseri strain as a probiotic for female vaginitis. Sci. Rep. 2024, 14, 14426. [Google Scholar] [CrossRef]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef] [PubMed]
- Begum, J.; Buyamayum, B.; Lingaraju, M.C.; Dev, K.; Biswas, A. Probiotics: Role in immunomodulation and consequent effects: Probiotics and immunity. Lett. Anim. Biol. 2021, 1, 1–6. [Google Scholar] [CrossRef]
- Godlewska, U.; Bulanda, E.; Wypych, T.P. Bile acids in immunity: Bidirectional mediators between the host and the microbiota. Front. Immunol. 2022, 13, 949033. [Google Scholar] [CrossRef]
- Maia, L.P.; Levi, Y.L.d.A.S.; do Prado, R.L.; dos Santos Santinoni, C.; Marsicano, J.A. Effects of probiotic therapy on serum inflammatory markers: A systematic review and meta-analysis. J. Funct. Foods 2019, 54, 466–478. [Google Scholar] [CrossRef]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics importance and their immunomodulatory properties. J. Cell. Physiol. 2019, 234, 8008–8018. [Google Scholar] [CrossRef]
- Kazemi, A.; Soltani, S.; Nasri, F.; Clark, C.C.; Kolahdouz-Mohammadi, R. The effect of probiotics, parabiotics, synbiotics, fermented foods and other microbial forms on immunoglobulin production: A systematic review and meta-analysis of clinical trials. Int. J. Food Sci. Nutr. 2021, 72, 632–649. [Google Scholar] [CrossRef]
- Prescott, S.; Wickens, K.; Westcott, L.; Jung, W.; Currie, H.; Black, P.; Stanley, T.; Mitchell, E.; Fitzharris, P.; Siebers, R. Supplementation with Lactobacillus rhamnosus or Bifidobacterium lactis probiotics in pregnancy increases cord blood interferon-γ and breast milk transforming growth factor-β and immunoglobin A detection. Clin. Exp. Allergy 2008, 38, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.E.; Di Mauro, A.; Mastromarino, P.; Fanelli, M.; Martinelli, D.; Urbano, F.; Capobianco, D.; Laforgia, N. Administration of a multi-strain probiotic product to women in the perinatal period differentially affects the breast milk cytokine profile and may have beneficial effects on neonatal gastrointestinal functional symptoms. A randomized clinical trial. Nutrients 2016, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, M.F.; Abrahamsson, T.R.; Fredriksson, M.; Jakobsson, T.; Björkstén, B. Low breast milk TGF-β2 is induced by Lactobacillus reuteri supplementation and associates with reduced risk of sensitization during infancy. Pediatr. Allergy Immunol. 2008, 19, 497–504. [Google Scholar] [CrossRef]
- Schultz, M.; Göttl, C.; Young, R.J.; Iwen, P.; Vanderhoof, J.A. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 293–297. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sakata, S.; Kalliomäki, M.; Isolauri, E.; Benno, Y.; Salminen, S. Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 166–170. [Google Scholar] [CrossRef]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef]
- Bertelsen, R.J.; Jensen, E.T.; Ringel-Kulka, T. Use of probiotics and prebiotics in infant feeding. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 39–48. [Google Scholar] [CrossRef]
- Tannock, G.W. Building robust assemblages of bacteria in the human gut in early life. Appl. Environ. Microbiol. 2021, 87, e01449-21. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Dinsmoor, A.M.; Wang, M.; Donovan, S.M. Microbiome composition in pediatric populations from birth to adolescence: Impact of diet and prebiotic and probiotic interventions. Dig. Dis. Sci. 2020, 65, 706–722. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Alvarado-Jasso, G.M.; Salazar, N.; De Los Reyes-Gavilán, C.G.; Gueimonde, M.; Arboleya, S. Role of Bifidobacteria on Infant Health. Microorganisms 2021, 9, 2415. [Google Scholar] [CrossRef]
- Sandall, J.; Tribe, R.M.; Avery, L.; Mola, G.; Visser, G.H.A.; Homer, C.S.E.; Gibbons, D.; Kelly, N.M.; Kennedy, H.P.; Kidanto, H.; et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 2018, 392, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Angolile, C.M.; Max, B.L.; Mushemba, J.; Mashauri, H.L. Global increased cesarean section rates and public health implications: A call to action. Health Sci. Rep. 2023, 6, e1274. [Google Scholar] [CrossRef]
- Samara, J.; Moossavi, S.; Alshaikh, B.; Ortega, V.A.; Pettersen, V.K.; Ferdous, T.; Hoops, S.L.; Soraisham, A.; Vayalumkal, J.; Dersch-Mills, D.; et al. Supplementation with a probiotic mixture accelerates gut microbiome maturation and reduces intestinal inflammation in extremely preterm infants. Cell Host Microbe 2022, 30, 696–711.e695. [Google Scholar] [CrossRef]
- Faust, K.; Sathirapongsasuti, J.F.; Izard, J.; Segata, N.; Gevers, D.; Raes, J.; Huttenhower, C. Microbial Co-occurrence Relationships in the Human Microbiome. PLoS Comput. Biol. 2012, 8, e1002606. [Google Scholar] [CrossRef]
- Yefet, E.; Bejerano, A.; Iskander, R.; Zilberman Kimhi, T.; Nachum, Z. The Association between Gestational Diabetes Mellitus and Infections in Pregnancy—Systematic Review and Meta-Analysis. Microorganisms 2023, 11, 1956. [Google Scholar] [CrossRef]





| Placebo | Probiotics | p-Value | |
|---|---|---|---|
| Women (n) | 90 | 90 | |
| Age in years (Mean ± SD) | 30.4 ± 4.0 | 31.2 ± 4.0 | 0.26 |
| Height in cm (Mean ± SD) | 163.9 ± 6.0 | 163.5 ± 7.0 | 0.63 |
| Weight in kg (Mean ± SD) | 78.1 ± 15.0 | 78.3 ± 13.0 | 0.62 |
| Infants (n) | 71 | 69 | |
| Gestational age at birth in weeks (Mean ± SD) | 39.8 ± 1.3 | 39.7 ± 1.2 | 0.78 |
| Weight at birth in kg (Mean ± SD) | 3.4 ± 0.4 | 3.4 ± 0.5 | 0.93 |
| Length at birth in cm (Mean ± SD) a | 50.3 ± 2.4 | 50.2 ± 2.4 | 0.88 |
| Head circumference at birth in cm (Mean ± SD) b | 34.5 ± 1.4 | 34.7 ± 1.5 | 0.92 |
| Born by C-section | 17 | 15 | 0.84 |
| Placebo | Probiotics | p-Value | Effect Size | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| n = 90 | n = 90 | Lower | Upper | |||
| All Diagnosed Infections (Mean ± SD) | 0.20 ± 0.45 | 0.09 ± 0.32 | 0.07 | 0.278 | −0.052 | 1.673 |
| Diagnosed BV (Mean ± SD) | 0.13 ± 0.37 | 0.07 ± 0.29 | 0.19 | 0.226 | −0.344 | 1.731 |
| Days with Infections (Mean ± SD) | 14.83 ± 7.93 | 13.75 ± 9.07 | 0.76 | 0.14 | −0.428 | 0.58 |
| Placebo | Probiotics | p-Value | Effect Size | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| n = 71 | n = 69 | Lower | Upper | |||
| Days with Infections (Mean ± SD) | 10.5 ± 5.57 | 4.7 ± 2.43 | 0.03 | 1.8 | 0.12 | 1.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binda, S.; Chow-Shi-Yée, M.; El Salti, S.; Auclair-Ouellet, N.; Oula, M.-L.; Carton, T.; Leuillet, S.; Tomassi, D.; Hemmings, R.; Kadoch, I.-J. The Effect of Probiotics on Health in Pregnancy and Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2025, 17, 1825. https://doi.org/10.3390/nu17111825
Binda S, Chow-Shi-Yée M, El Salti S, Auclair-Ouellet N, Oula M-L, Carton T, Leuillet S, Tomassi D, Hemmings R, Kadoch I-J. The Effect of Probiotics on Health in Pregnancy and Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2025; 17(11):1825. https://doi.org/10.3390/nu17111825
Chicago/Turabian StyleBinda, Sylvie, Mélanie Chow-Shi-Yée, Saly El Salti, Noémie Auclair-Ouellet, Marie-Laure Oula, Thomas Carton, Sébastien Leuillet, Diego Tomassi, Robert Hemmings, and Isaac-Jacques Kadoch. 2025. "The Effect of Probiotics on Health in Pregnancy and Infants: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 17, no. 11: 1825. https://doi.org/10.3390/nu17111825
APA StyleBinda, S., Chow-Shi-Yée, M., El Salti, S., Auclair-Ouellet, N., Oula, M.-L., Carton, T., Leuillet, S., Tomassi, D., Hemmings, R., & Kadoch, I.-J. (2025). The Effect of Probiotics on Health in Pregnancy and Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 17(11), 1825. https://doi.org/10.3390/nu17111825






