Probiotic Supplements Effect on Feeding Tolerance, Growth and Neonatal Morbidity in Extremely Preterm Infants: A Systematic Review and Meta-Analysis
Highlights
- Probiotic supplementation exhibited a beneficial trend in reducing feeding intolerance but had little to no effect on postnatal growth.
- A significant risk reduction in NEC and all-cause mortality was observed in infants supplemented with probiotics.
- Evidence of the effect of probiotic supplementation in extremely preterm infants remains limited and warrants further investigation. Large RCTs focused on neonatal morbidity combined with feeding intolerance and growth outcomes should be prioritized.
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search and Information Sources
2.3. Search Strategy
2.4. Data Collection and Selection Process
2.5. Data Extraction
2.6. Data Items
2.6.1. Primary Outcome Measures
- Feeding tolerance, with the following outcome measures:
- ○
- Average enteral volume (first 28 days);
- ○
- Duration of parenteral nutrition;
- ○
- Interruptions of enteral feeds (number of days/episodes);
- ○
- Number of gastric residuals per week;
- ○
- Time to full enteral feeds (120–160 mL/kg/day);
- ○
- Time to 100 mL/kg/day enteral feeds;
- ○
- Time to 50 mL/kg/day enteral feeds.
- Growth, with the following outcome measures:
- ○
- Growth velocity—weight gain, length gain, increase in head circumference during neonatal period;
- ○
- Weight at end of neonatal period (gestational week 36–40);
- ○
- Length at end of neonatal period (gestational week 36–40);
- ○
- Head circumference at end of neonatal period (gestational week 36–40);
- ○
- Standard deviation score during neonatal period and at end of neonatal period (gestational week 36–40);
- ○
- Weight > 10 percentile at gestational week 34;
- ○
- Growth failure (other definition).
2.6.2. Secondary Outcome Measures
- Necrotizing enterocolitis Bell Stage II–III;
- Sepsis (late-onset sepsis and culture-proven late-onset sepsis);
- All-cause mortality (during hospitalization);
- Length of hospitalization;
- Adverse events—any reported adverse events related to the intervention of probiotic supplements.
2.7. Missing Data
2.8. Effect Measures
2.9. Synthesis Methods
2.9.1. Heterogeneity
2.9.2. Subgroup Analysis
2.9.3. Sensitivity Analysis
2.10. Risk of Bias Assessment
2.11. Certainty Assessment
3. Results
3.1. Results of the Search
3.2. Study Design

3.3. Study Population
3.4. Study Interventions
3.5. Risk of Bias in Included Studies
3.6. Certainty of the Evidence
3.7. Main Results
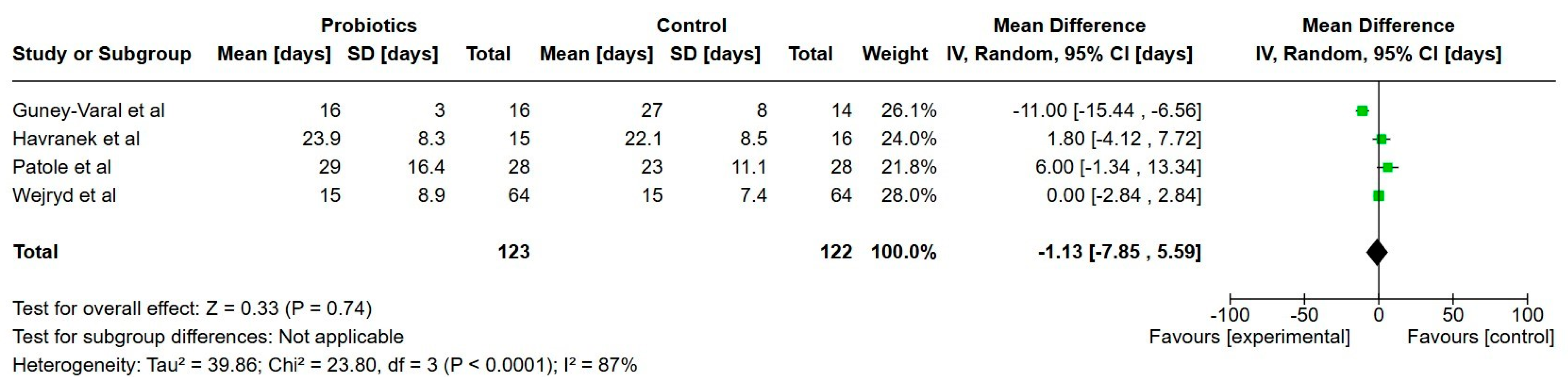

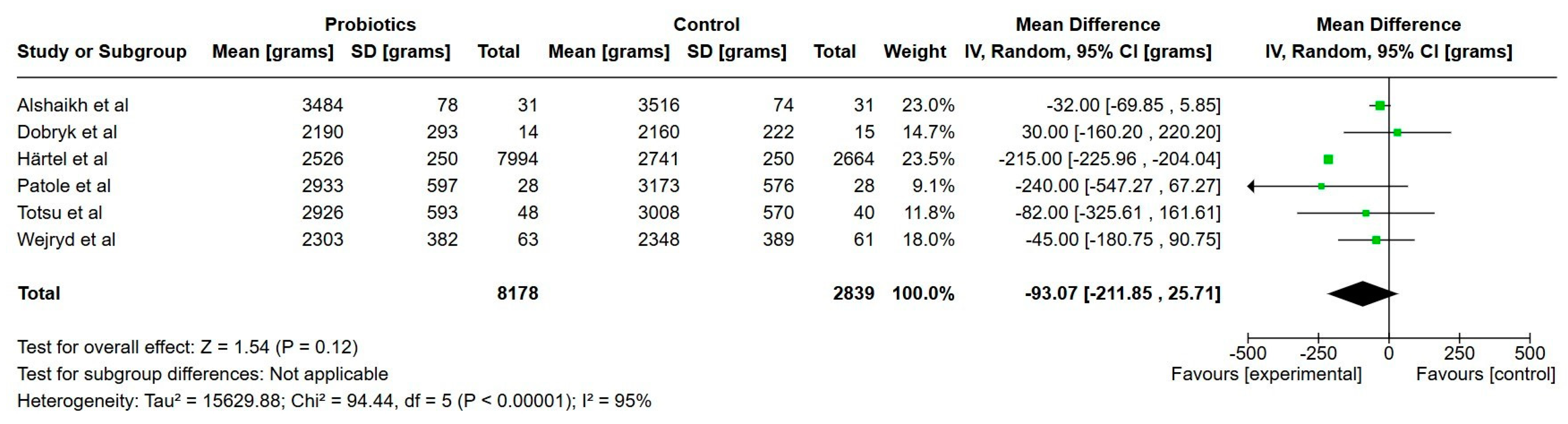

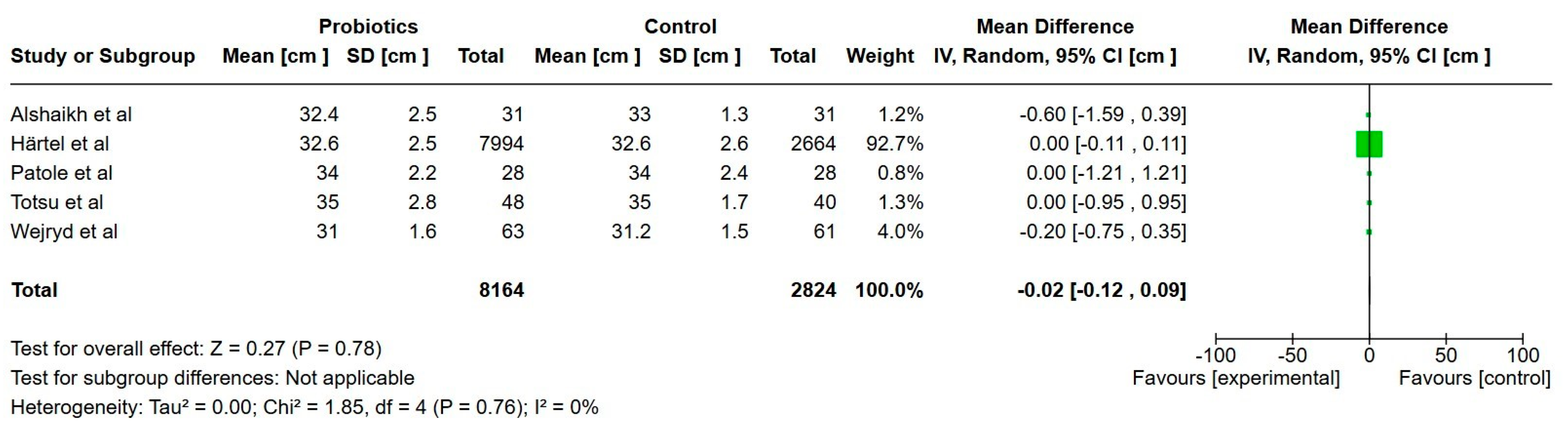
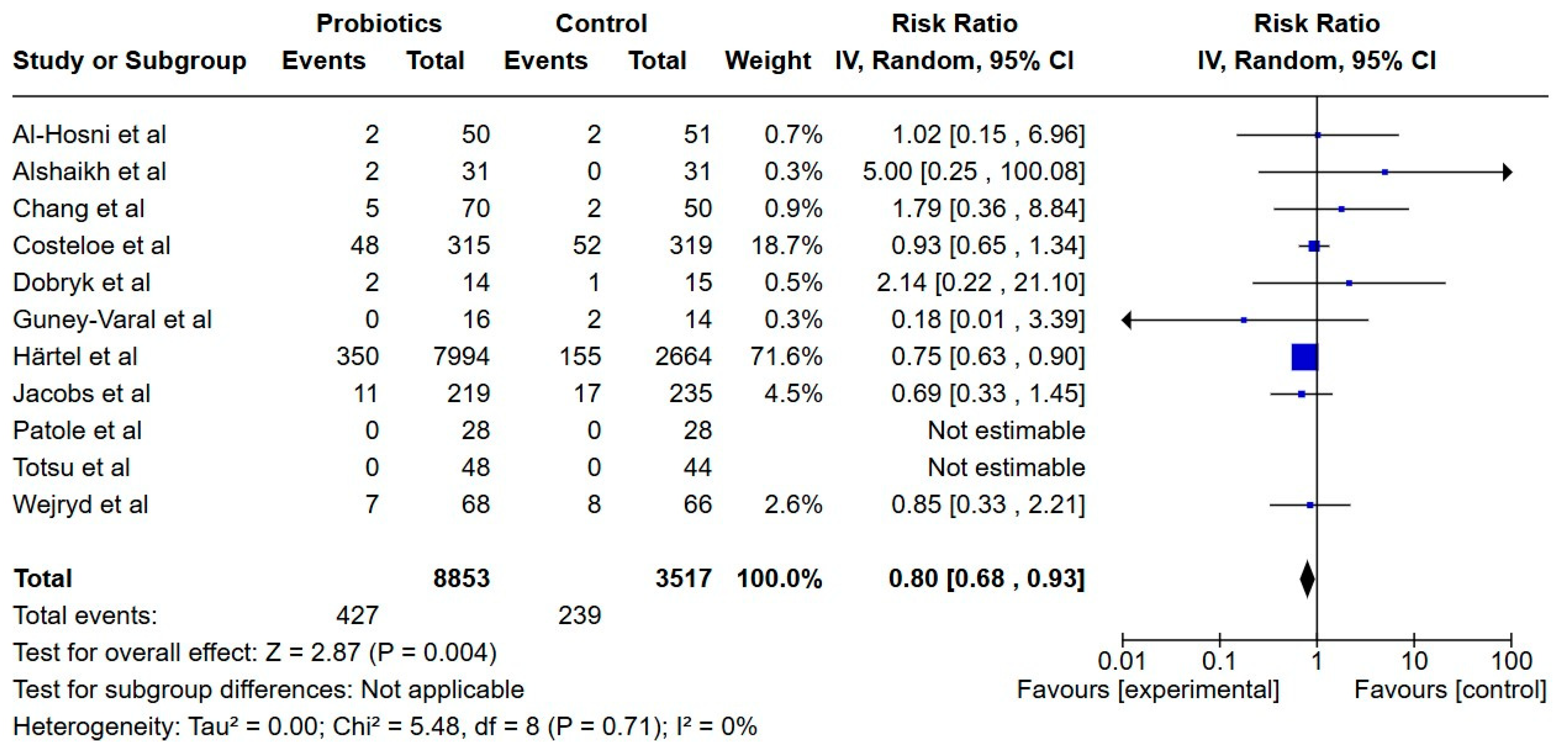
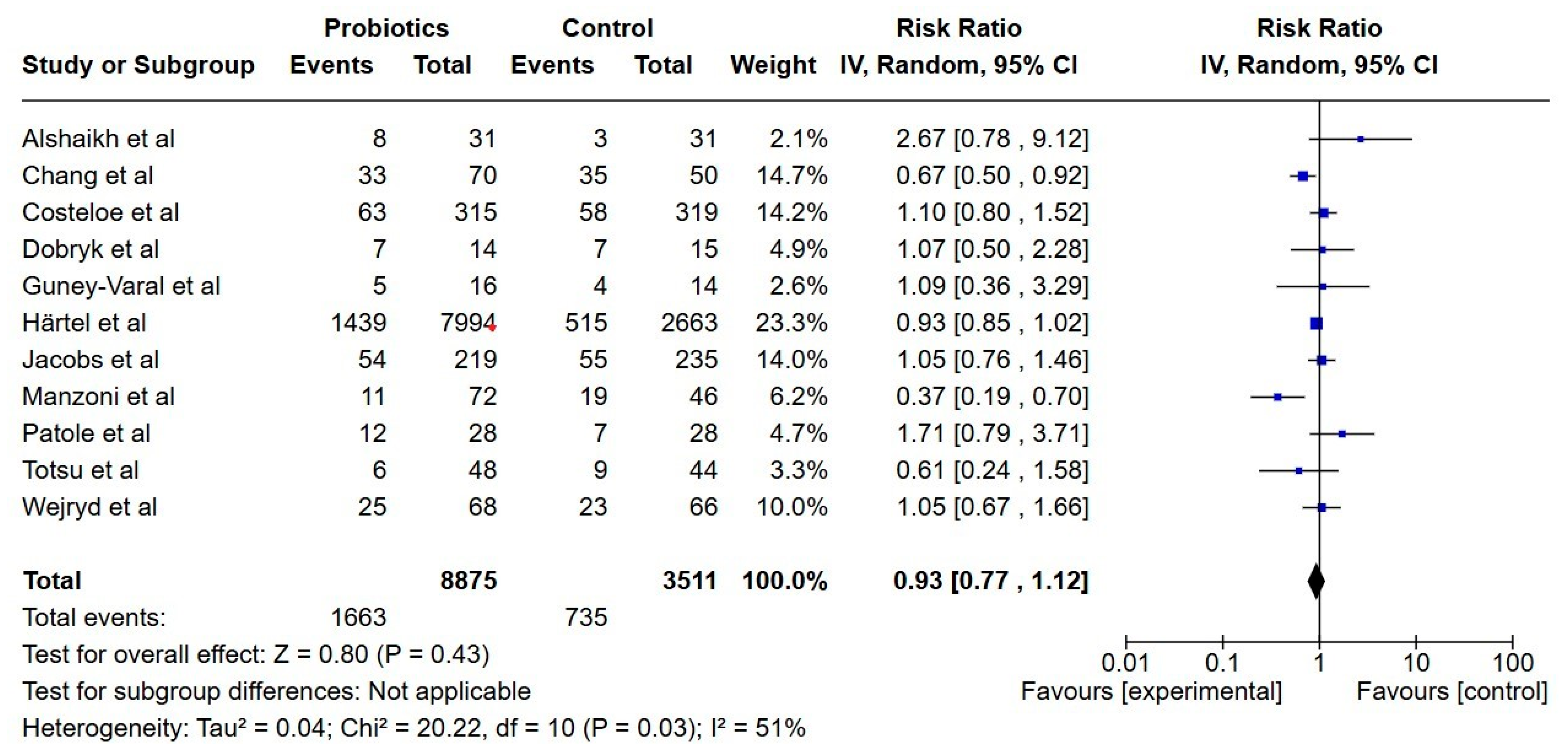

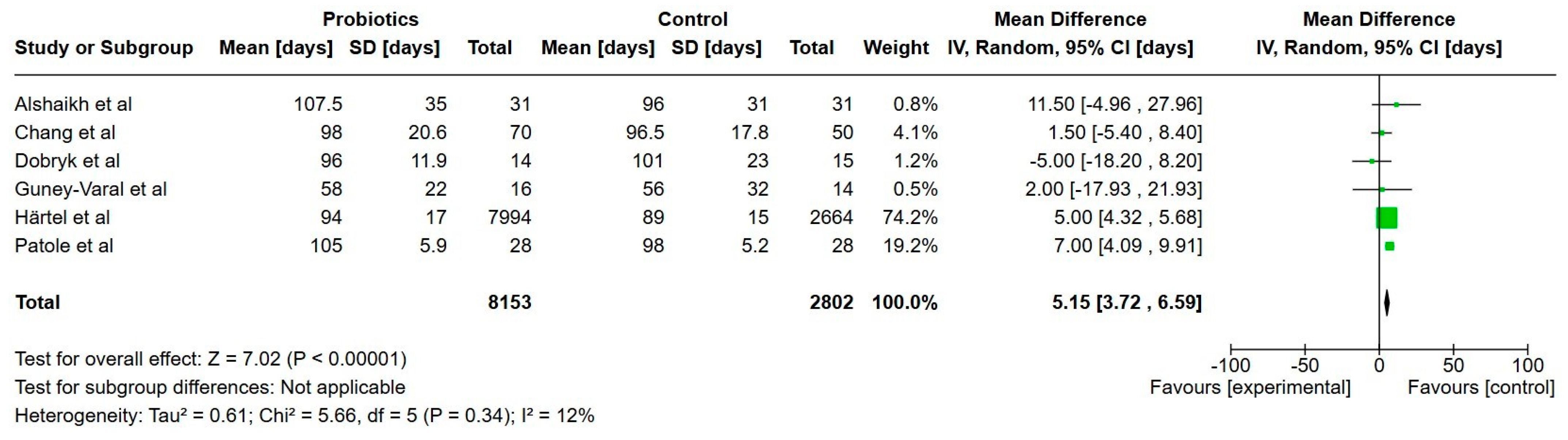
3.7.1. Primary Outcomes
Feeding Tolerance
Growth
3.7.2. Secondary Outcomes
Necrotizing Enterocolitis
Late-Onset Sepsis
All-Cause Mortality
Hospitalization
Adverse Events
Results of Subgroup and Sensitivity Analysis
4. Discussion
4.1. Summary of Main Results
4.2. Quality of the Evidence
4.3. Agreements and Disagreements with Other Reviews
4.4. Implication for Daily Clinical Care
4.5. Implication for Research
4.6. Limitations of This Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GA | Gestational age |
| GRADE | Grading of recommendations, assessment, development and evaluations |
| LOS | Late-onset sepsis |
| NEC | Necrotizing enterocolitis |
| RoB | Risk of bias |
References
- Beharry, K.D.; Latkowska, M.; Valencia, A.M.; Allana, A.; Soto, J.; Cai, C.L.; Golombek, S.; Hand, I.; Aranda, J.V. Factors Influencing Neonatal Gut Microbiome and Health with a Focus on Necrotizing Enterocolitis. Microorganisms 2023, 11, 2528. [Google Scholar] [CrossRef]
- Duchon, J.; Barbian, M.E.; Denning, P.W. Necrotizing Enterocolitis. Clin. Perinatol. 2021, 48, 229–250. [Google Scholar] [PubMed]
- Dong, Y.; Speer, C.P. Late-onset neonatal sepsis: Recent developments. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F257–F263. [Google Scholar] [CrossRef] [PubMed]
- Raiten, D.J.; Steiber, A.L.; Carlson, S.E.; Griffin, I.; Anderson, D.; Hay, W.W., Jr.; Robins, S.; Neu, J.; Georgieff, M.K.; Groh-Wargo, S.; et al. Working group reports: Evaluation of the evidence to support practice guidelines for nutritional care of preterm infants—The Pre-B Project. Am. J. Clin. Nutr. 2016, 103, 648S–678S. [Google Scholar] [CrossRef]
- Pammi, M.E.; Weisman, L.E. Late-onset sepsis in preterm infants: Update on strategies for therapy and prevention. Expert Rev. Anti-Infect. Ther. 2015, 13, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.; Bizzarri, B.; Giampietro, S.; De Curtis, M. Feeding intolerance in preterm infants. How to understand the warning signs. J. Matern. Fetal. Neonatal Med. 2011, 24 (Suppl. 1), 72–74. [Google Scholar] [CrossRef]
- Oddie, S.J.; Young, L.; McGuire, W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst. Rev. 2021, 8, CD001241. [Google Scholar]
- Viswanathan, S.; McNelis, K.; Super, D.; Einstadter, D.; Groh-Wargo, S.; Collin, M. Standardized Slow Enteral Feeding Protocol and the Incidence of Necrotizing Enterocolitis in Extremely Low Birth Weight Infants. JPEN J. Parenter. Enter. Nutr. 2015, 39, 644–654. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, S.H.; Cho, H.; Shin, S.H.; Kim, S.H.; Song, I.G.; Kim, E.-K.; Kim, H.-S. Extrauterine growth restriction in extremely preterm infants based on the Intergrowth-21st Project Preterm Postnatal Follow-up Study growth charts and the Fenton growth charts. Eur. J. Pediatr. 2021, 180, 817–824. [Google Scholar] [CrossRef]
- Ottolini, K.M.; Andescavage, N.; Keller, S.; Limperopoulos, C. Nutrition and the developing brain: The road to optimizing early neurodevelopment: A systematic review. Pediatr. Res. 2020, 87, 194–201. [Google Scholar] [CrossRef]
- Saenz de Pipaon, M.; Nelin, L.D.; Gehred, A.; Rossholt, M.E.; Moltu, S.; Van den Akker, C.; van Kaam, A.H.; Sánchez, A.; Khashu, M.; Roehr, C.C.; et al. The role of nutritional interventions in the prevention and treatment of chronic lung disease of prematurity. Pediatr. Res. 2024, 18, 6245. [Google Scholar] [CrossRef]
- Strobel, K.M.; Wood, T.R.; Valentine, G.C.; German, K.R.; Gogcu, S.; Hendrixson, D.T.; Kolnik, S.E.; Law, J.B.; Mayock, D.E.; Comstock, B.A.; et al. Contemporary definitions of infant growth failure and neurodevelopmental and behavioral outcomes in extremely premature infants at two years of age. J. Perinatol. 2024, 44, 811–818. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Patel, R.M.; Underwood, M.A. Probiotics and necrotizing enterocolitis. Semin. Pediatr Surg. 2018, 27, 39–46. [Google Scholar] [CrossRef]
- Sharif, S.; Meader, N.; Oddie, S.J.; Rojas-Reyes, M.X.; McGuire, W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst. Rev. 2023, 7, Cd005496. [Google Scholar]
- Wang, Y.; Florez, I.D.; Morgan, R.L.; Foroutan, F.; Chang, Y.; Crandon, H.N.; Couban, R.; Foroutan, F.; Florez, I.D. Probiotics, Prebiotics, Lactoferrin, and Combination Products for Prevention of Mortality and Morbidity in Preterm Infants: A Systematic Review and Network Meta-Analysis. JAMA Pediatr. 2023, 177, 1158–1167. [Google Scholar]
- Deshmukh, M.; Patole, S. Prophylactic Probiotic Supplementation for Preterm Neonates—A Systematic Review and Meta-Analysis of Nonrandomized Studies. Adv. Nutr. Int. Rev. J. 2021, 12, 1411–1423. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Xing, Y.; Wang, H.; Fu, B.; Long, M.; Cao, J. Clinical efficacy of probiotics on feeding intolerance in preterm infants: A systematic review and meta-analysis. Transl. Pediatr. 2022, 11, 229–238. [Google Scholar] [CrossRef]
- Panchal, H.; Athalye-Jape, G.; Rao, S.; Patole, S. Growth and neuro-developmental outcomes of probiotic supplemented preterm infants—A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2023, 77, 855–871. [Google Scholar] [CrossRef]
- Clark, J.M.; Sanders, S.; Carter, M.; Honeyman, D.; Cleo, G.; Auld, Y.; Booth, D.; Condron, P.; Dalais, C.; Bateup, S.; et al. Improving the translation of search strategies using the Polyglot Search Translator: A randomized controlled trial. J. Med. Libr. Assoc. 2020, 108, 195–207. [Google Scholar] [CrossRef]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar]
- Güney-Varal, I.; Köksal, N.; Özkan, H.; Bağcı, O.; Doğan, P. The effect of early administration of combined multi-strain and multi-species probiotics on gastrointestinal morbidities and mortality in preterm infants: A randomized controlled trial in a tertiary care unit. Turk. J. Pediatr. 2017, 59, 13–19. [Google Scholar] [CrossRef]
- Härtel, C.; Pagel, J.; Spiegler, J.; Buma, J.; Henneke, P.; Zemlin, M.; Viemann, D.; Gille, C.; Gehring, S.; Frommhold, D.; et al. Lactobacillus acidophilus/Bifidobacterium infantis probiotics are associated with increased growth of VLBWI among those exposed to antibiotics. Sci. Rep. 2017, 7, 5633. [Google Scholar] [CrossRef]
- Patole, S.; Keil, A.D.; Chang, A.; Nathan, E.; Doherty, D.; Simmer, K.; Esvaran, M.; Conway, P. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates—A randomised double blind placebo controlled trial. PLoS ONE 2014, 9, e89511. [Google Scholar] [CrossRef]
- Totsu, S.; Yamasaki, C.; Terahara, M.; Uchiyama, A.; Kusuda, S. Bifidobacterium and enteral feeding in preterm infants: Clus-ter-randomized trial. Pediatr. Int. Official. J. Jpn. Pediatr. Soc. 2014, 56, 714–749. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar]
- Al-Hosni, M.; Duenas, M.; Hawk, M.; Stewart, L.A.; Borghese, R.A.; Cahoon, M.; Atwood, L.; Howard, D.; Ferrelli, K.; Soll, R. Probiotics-supplemented feeding in extremely low-birth-weight infants. J. Perinatol. 2012, 32, 253–259. [Google Scholar] [CrossRef]
- Alshaikh, B.; Samara, J.; Moossavi, S.; Ferdous, T.; Soraisham, A.; Dersch-Mills, D.; Arrieta, M.-C.; Amin, H. Multi-strain probiotics for extremely preterm infants: A randomized controlled trial. Pediatr. Res. 2022, 92, 1663–1670. [Google Scholar] [CrossRef]
- Chang, C.M.; Tsai, M.H.; Liao, W.C.; Yang, P.H.; Li, S.W.; Chu, S.M.; Huang, H.R.; Chiang, M.C.; Hsu, J.F. Effects of Probiotics on Gut Microbiomes of Extremely Preterm Infants in the Neonatal Intensive Care Unit: A Prospective Cohort Study. Nutrients 2022, 14, 3239. [Google Scholar] [CrossRef]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R. Probiotics in Preterm Infants Study Collaborative G. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2016, 387, 649–660. [Google Scholar]
- Dobryk, D.S.; Dobryanskyy, D. Probiotics, gut microbiota, and diseases associated with the immaturity of the digestive tract in very preterm infants. Mod. Pediatr. 2023, 3, 22–30. [Google Scholar]
- Havranek, T.; Al-Hosni, M.; Armbrecht, E. Probiotics supplementation increases intestinal blood flow velocity in extremely low birth weight preterm infants. J. Perinatol. 2013, 33, 40–44. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Tobin, J.M.; Opie, G.F.; Donath, S.; Tabrizi, S.N.; Pirotta, M.; Morley, C.J.; Garland, S.M. Probiotic effects on late-onset sepsis in very preterm infants: A randomized controlled trial. Pediatrics 2013, 132, 1055–1062. [Google Scholar] [CrossRef]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA 2009, 302, 1421–1428. [Google Scholar]
- Singh, B.; Shah, P.S.; Afifi, J.; Simpson, C.D.; Mitra, S.; Dow, K.; El-Naggar, W. Probiotics for preterm infants: A National Retrospective Cohort Study. J. Perinatol. 2019, 39, 533–539. [Google Scholar] [CrossRef]
- Wejryd, E.; Marchini, G.; Frimmel, V.; Jonsson, B.; Abrahamsson, T. Probiotics promoted head growth in extremely low birthweight infants in a double-blind placebo-controlled trial. Acta Paediatr. 2019, 108, 62–69. [Google Scholar] [CrossRef]
- Benders, M. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN96620855 (accessed on 1 December 2024).
- Bhandresh, R.V. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2024/01/062023 (accessed on 1 December 2024).
- Bowornkitiwong, W. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20200101001 (accessed on 1 December 2024).
- Elfving, K. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=PACTR202309763146420 (accessed on 1 December 2024).
- Infant Bacterial Therapeutics AB (IBT). World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2018-000754-22-GB (accessed on 1 December 2024).
- Masood, I. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2300071089 (accessed on 1 December 2024).
- Mukthar, G. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2018/04/013401 (accessed on 1 December 2024).
- Pandey, P. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/03/031724 (accessed on 1 December 2024).
- Rakow, A. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05604846 (accessed on 1 December 2024).
- Taslimi Taleghani, N. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20220505054746N1 (accessed on 1 December 2024).
- Tunpowpong, P. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20220829005 (accessed on 1 December 2024).
- van Wyk, L. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Tri-al2.aspx?TrialID=PACTR202011513390736 (accessed on 1 December 2024).
- Vaswani, N. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2023/04/051455 (accessed on 1 December 2024).
- Wang, H. World Health Organization: International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2400079546 (accessed on 1 December 2024).
- Chi, C.; Li, C.; Buys, N.; Wang, W.; Yin, C.; Sun, J. Effects of Probiotics in Preterm Infants: A Network Meta-analysis. Pediatrics 2021, 147, e20200706. [Google Scholar] [CrossRef]
- van den Akker, C.H.P.; van Goudoever, J.B.; Szajewska, H.; Embleton, N.D.; Hojsak, I.; Reid, D.; Shamir, R. Probiotics for Preterm Infants: A Strain-Specific Systematic Review and Network Meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 103–122. [Google Scholar]
- Sun, J.; Marwah, G.; Westgarth, M.; Buys, N.; Ellwood, D.; Gray, P.H. Effects of Probiotics on Necrotizing Enterocolitis, Sepsis, Intraventricular Hemorrhage, Mortality, Length of Hospital Stay, and Weight Gain in Very Preterm Infants: A Meta-Analysis. Adv. Nutr. Int. Rev. J. 2017, 8, 749–763. [Google Scholar] [CrossRef]
| Study | Country/Setting | Funding/Conflict of Interest | Study Design | Intervention and Comparator | Extremely Preterm Infants (n) | Gestational Age and Body Weight of Extremely Preterm Infants, Mean (SD) |
|---|---|---|---|---|---|---|
| Al-Hosni, 2012 [29] | USA, multicenter | N/A * | Prospective, double-blinded RCT (no placebo) | Probiotics: Lactobacillus rhamnosus GG and Bifidobacterium infantis Control: No supplement | Total: 101 Probiotics: 50 No probiotics: 51 | Probiotics Gestational age: 25.7 (1.4) Birth weight: 778 (138) No probiotics Gestational age: 25.7 (1.4) Birth weight: 779 (126) |
| Alshaikh, 2022 [30] | Canada, single center | N/A | Prospective, open-label RCT (no placebo) | Probiotics: Bifidobacterium breve HA-129, Bifidobacterium bifidum HA-132, Bifidobacterium longum subsp. infantis HA-116, Bifidobacterium longum subsp. longum HA-135 and Lacticaseibacillus (formerly Lactobacillus) rhamnosus HA-111 Control: No supplement | Total: 62 Probiotics: 31 No probiotics: 31 | Probiotics Gestational age: 25.8 (1.5) No probiotics Gestational age: 25.6 (1.3) Birth weight: 751 (132) |
| Chang, 2022 [31] | Taiwan, single center | Ministry of Science and Technology under the grants MOST 108-2314-B-182-064-MY3, and the Chang Gung Memorial Hospital under the grants NMRPD1J1192. | Prospective cohort study | Probiotics: Lactobacillus acidophilus and Bifidobacterium bifidum Control: No supplement | Total: 120 Probiotics: 70 No probiotics: 50 | Probiotics Gestational age: 26.0 (25.0–27.0) ** Birth weight: 780.0 (689.3–915.0) ** No probiotics Gestational age: 26.0 (25.0–27.0) ** Birth weight: 815.0 (757.5–920.0) ** |
| Costeloe, 2016 [32] | England, multicenter | UK National Institute for Health Research Health Technology Assessment programme | Prospective placebo-controlled, double-blinded RCT | Probiotics: B breve BBG-001 Control: Corn starch | Total: 634 Probiotics: 315 No probiotics: 319 | Gestational age: N/A Birth weight: N/A |
| Dobryk, 2023 [33] | Ukraine, multicenter | N/A | Prospective, open-label RCT (no placebo) | Probiotics: Lactobacillus reuteri Control: No supplement | Total: 29 Probiotics: 14 No probiotics: 15 | Probiotics Gestational age: N/A Birth weight: N/A No probiotics Gestational age: N/A Birth weight: N/A |
| Guney-Varal, 2017 [22] | Turkey, single center | N/A | Prospective RCT (no information about blinding) | Probiotics: Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus plantorum, Bifidobacterium animalis Control: No supplement | Total: 30 * Probiotics: 16 No probiotics: 14 | Probiotics Gestational age: 26 (24–27) ** Birth weight: 978 (248) No probiotics Gestational age: 27 (25–27) ** Birth weight: 1080 (240) |
| Härtel, 2017 [23] | Germany, multicenter | N/A | Prospective cohort study | Probiotics: Lactobacillus Acidophilus and Bifidobacterium infantis Control: No supplement | Total: 10,658 Probiotics: 7994 No probiotics: 2664 | Probiotics Gestational age: N/A Birth weight: 782 (230) No probiotics Gestational age: N/A Birth weight: 767 (240) |
| Havranek, 2013 [34] | US, single center | N/A | Prospective, double-blinded RCT (no placebo) | Probiotics: Lactobacillus rhamnosus GG and Bifidobacterium infantis Control: No supplement | Total: 31 Probiotics: 15 No probiotics: 16 | Probiotics Gestational age: 25.9 (1.3) Birth weight: 856 (105) No probiotics Gestational age: 25.9 (1.5) Birth weight: 789 (129) |
| Jacobs, 2013 [35] | Australia and New Zealand, multicenter | National Health and Research Medical Council of Australia (project grant 454629), The Royal Women’s Hospital Foundation, Melbourne, Australia, and The Angior Family Foundation, Melbourne, Australia | Prospective placebo-controlled, double-blinded RCT | Probiotics: Bifidobacterium infantis, S thermophilus and Bifidobacterium lactis Control: placebo (maltodextrin) | Total: 454 Probiotics: 219 No probiotics: 235 | Probiotics Gestational age: N/A Birth weight: N/A No probiotics Gestational age: N/A Birth weight: N/A |
| Manzoni, 2009 [36] | Italy, multicenter | Dicofarm SpA supported this study with a grant and supplied the drugs and placebo used in the study | Prospective placebo-controlled, double-blinded RCT | Probiotics: 1. Bovine lactoferrin 2. Bovine lactoferrin + Lactobacillus rhamnosus GG Control: placebo (2 mL of a 5% glucose solution) | Total: 114 Probiotics: 54 No probiotics: 60 | Probiotics Gestational age: N/A Birth weight: N/A No probiotics Gestational age: N/A Birth weight: N/A |
| Patole, 2014 [24] | Australia, single center | Telethon Channel 7 Trust, Western Australia | Prospective placebo-controlled, double-blinded RCT | Probiotics: B. breve M-16V Control: placebo (dextrin) | Total: 57 Probiotics: 28 No probiotics: 29 | Probiotics Gestational age: 25.5 (1.3) Birth weight: 742 (191) No probiotics Gestational age: 25.9 (1.4) Birth weight: 826 (190) |
| Totsu, 2014 [25] | Japan, multicenter | No funding source. Study product was provided by Meiji Dairies Corporation, Odawara, Japan | Prospective, placebo-controlled, double-blinded RCT | Probiotics: Bifidobacterium bifidum OLB6378 Control: placebo (not specified) | Total: 92 Probiotics: 48 No probiotics: 44 | Probiotics Gestational age: 26 (1.1) Birth weight: 838 (190) No probiotics Gestational age: 25 (1.4) Birth weight: 783 (191) |
| Wejryd, 2019 [38] | Sweden, multicenter | Swedish Research Council (grant number 921.2014-7060), the Swedish Society for Medical Research, the Swedish Society of Medicine, the Research Council for the South-East Sweden, ALF Grants, Region Ostergotland, the Ekhaga Foundation, and BioGaia AB. | Prospective placebo-controlled, double-blinded RCT | Probiotics: L. reuteri DSM 17938 Control: placebo (maltodextrin) | Total: 134 Probiotics: 68 No probiotics: 66 | Probiotics Gestational age: 25.5 (1.2) Birth weight: 731 (129) No probiotics Gestational age: 25.5 (1.3) Birth weight: 740 (148) |
| Corresponding Author, Email | Registration Title | Intervention and Comparator | Outcomes | Registration Date | Trial Status |
|---|---|---|---|---|---|
| Benders [39], M.Benders@umcutrecht.nl | NutriBrain: protocol for a randomised, double-blind, controlled trial to evaluate the effects of a nutritional product on brain integrity in preterm infants | Intervention: a mixture of probiotics, prebiotics and free amino acid Control: maltodextrin, casein and whey protein hydrolysates | Adverse events, growth, number of days with parenteral, days to full enteral nutrition | 17 March 2021 | Ongoing |
| Bhadresh [40], bhadreshrvyas@yahoo.co.uk | To study the effect of probiotics preparation Infloran® supplementation in on morbidities in Indian preterm neonates (PrISM)—PrISM | Intervention: Infloran®; Lactobacillus Acidophilus, Bifidobacterium Bifidum Control: standard of care | Necrotizing enterocolitis, sepsis, mortality rate, time to full enteral nutrition | 30 January 2024 | Not Yet Recruiting |
| Bowornkitiwong [41], walbj@hotmail.com | Effect of probiotics on the incidence of necrotizing enterocolitis in preterm | Intervention: L. acidophilus, L. casei, B. bifidum, B. infantis, B. longum, Lactococcus lactis Control: only breastmilk or formula | Necrotizing enterocolitis, sepsis, mortality rate, time to full enteral nutrition | 1 January 2020 | Recruiting |
| Elfving [42], kristina.elfving@gu.se | Probiotic treatment versus placebo to low birth weight neonates for prevention of mortality or undernutrition: two-arm multi-center superiority randomized controlled trial | Intervention: Bifidobacterium infantis Bb-02 (DSM 33361) 300 million, Bifidobacterium lactis (BB-12®) 350 million, and Streptococcus thermophilus (TH-4®) 350 million Control: placebo (maltodextrin) | All-cause mortality, sepsis, growth | 26 April 2023 | Pending |
| Infant Bacterial Therapeutics AB (IBT) [43], clinical@ibtherapeutics.com | Study on the prevention of necrotizing enterocolitis (a severe inflammation and death go intestines) in premature infants | Intervention: Lactobaciullus reuteri Control: N/A * | Necrotizing enterocolitis, all-cause mortality, duration of hospitalization, growth and feeding tolerance | 21 March 2019 | Authorized recruitment may be ongoing or finished |
| Masood [44], drimranmasood@iub.edu.pk | Evaluation of the clinical and growth-related significance of probiotics in preterm infants | Intervention: 1: Lactobacillus rhamnosus 2: Bifidobacterium BB-12, Lactobacillus paracasei, L casei-431, Streptococcus thermophilus TH-4 (Amybact) Control: Dextrose water 10% | Morbidity (general), growth, feeding patterns | 4 May 2023 | Recruiting |
| Mukthar [45], drmushtaq816@gmail.com | Role of prophylactic microbial supplements in prevention of blood stream infection and intestinal tract injury in premature neonates | Intervention: Mixture of lactobacillus acidophilus, lactobacillus rhamnosus, bifidobacterium longum and streptomyces boulardii Control: only breastmilk/formula | Necrotizing enterocolitis, hospital stay, time to establish full feeds, episodes of feeding intolerance, nosocomial sepsis, all-cause mortality | 20 April 2018 | Open to recruitment |
| Pandey [46], sshahdoc@gmail.com | Lactobacillus Rhamnosus GG to reduce NEC, sepsis and mortality in VLBW infants –A Randomised Controlled Trial | Interevention: Lactobacillus Rhamnosus GG (liquid form) Control: only mothers breastmilk or donor milk | Necrotizing enterocolitis, sepsis, all-cause mortality, time to full feeds, duration of parental nutrition, hospital stay, growth | 4 March 2021 | Closed to recruitment |
| Rakow [47], alexander.rakow@regionstockholm.se | Probiotic Supplementation in Extremely Preterm Infants in Scandinavia (PEPS) | Intervention: Bifidobacterium infantis Bb-02 (DSM 33361) 300 million, Bifidobacterium lactis (BB-12®) 350 million, and Streptococcus thermophilus (TH-4®) 350 million Control: placebo (maltodextrin) | Necrotizing enterocolitis, sepsis, all-cause mortality, time to full feeds, duration of parenteral nutrition, hospital stay, use of antibiotics, growth, feeding tolerance | 16 October 2023 | Recruiting |
| Taslimi Taleghani [48], naeemetaslimi@yahoo.com | The effect of early oral probiotics prescription on feeding intolerance, regain birth weight and secondary outcomes in very low birth weight | Intervention: Bifidobacterium lactis, Bifidobacterium infantis and Streptococcus thermophilus 3 Control: placebo | Feeding tolerance, sepsis, necrotizing enterocolitis, time to full enteral nutrition, hospital stay, days to attain birth weight | 4 August 2022 | Recruiting |
| Tunpowpong [49], tuinoi1@gmail.com | Randomized controlled trial comparing gut microbiota of preterm neonates receiving Infloran versus not receiving Infloran | Intervention: B. bifidum and L. acidophilus Control: standard feeding protocol | Necrotizing enterocolitis, gut microbiome alteration | 28 September 2022 | Recruiting |
| van Wyk [50], lizelle@sun.ac.za | The role of a multi-strain probiotic in very low birth weight infants. | Intervention: Labinic probiotic Control: placebo | Rectal colonization, postnatal growth, bloodstream infections, incidence of necrotizing enterocolitis and severity, time to full feeds | 4 April 2020 | Pending |
| Vaswani [51], ndvaswani@hotmail.com | To study the effect of supplementation of probiotics on enteral feed tolerance in very low birth weight neonates—Randomized Control Trial | Intervention: N/A Control: formula milk as placebo | Time to full enteral nutrition, feeding tolerance, necrotizing enterocolitis, sepsis, length of hospital stay | 4 October 2023 | Not Yet Recruiting |
| Wang [52], 568241134@qq.com | Clinical study on the effect of probiotics on intestinal metabolites and serum inflammatory factors on the outcome of premature infants | Intervention: probiotics (not specified) Control: Dextrose 5% | Age at return to birth weight, time to central cannulation, weight gain (g/day), length of hospitalization, late onset sepsis, incidence of feeding intolerance, necrotizing enterocolitis | 5 January 2024 | Completed, no publication |
| Corresponding Author, Email | Title | Intervention and Comparator | Outcomes | Publication Date | Reason for Awaiting Classification |
|---|---|---|---|---|---|
| Singh [37], balpreet-singh@iwk.nshealth.ca | Probiotics for preterm infants: A National Retrospective Cohort Study | Intervention: 1. Bifidobacterium species (B. breve, B. bifidum, B. infantis, and B. longum) and Lactobacillus rhamnosus GG 2. Lactobacillus reuteri Control: no probiotics | Days with parenteral nutrition, mortality, NEC, late-onset sepsis, length of hospital stay | 28 January 2019 | Study included babies < 29 gestational weeks. Awaiting data for babies born <28 gestational weeks |
| Study | Outcomes (as Mentioned by Study Authors for Infants < 28 weeks GA *) | Primary Outcomes for This Systematic Review (Feeding Tolerance, Growth) | Secondary Outcomes for This Systematic Review (Death, NEC, Sepsis, Hospitalization, ABs) | Risk of Bias |
|---|---|---|---|---|
| Al-Hosni, 2012[29] | Primary Feeding volume Growth velocity Weight gain per day Weight < 10 percentile at 34 GW Secondary Antimicrobial days Antibacterial days Antifungal days Death at 34 GW NEC ** Bell Stage I–III NEC surgery Sepsis (bacterial and fungal) Weight 34 GW | Feeding volume (average mL/kg/day) first 28 days Intervention: 59 Control: 71 Growth velocity (g/kg/day) Intervention: 14.9 Control: 12.6 Weight < 10 percentile at 34 GW Intervention: 27/47 Control: 28/47 Weight at 34 GW (grams) Intervention: 1656 Control: 1599 | Death Intervention: 3/50 Control: 4/51 NEC Bell Stage II-III Intervention: 2/50 Control: 2/51 Sepsis (bacterial and fungal) Intervention: 13/50 Control: 16/51 | Low |
| Alshaikh, 2022 [30] | Primary Changes in fecal microbiota Secondary Duration parenteral nutrition Enteral nutrition interrupted Change in weight z-score between birth and 40 GW Growth velocity day 7–36 GW Head circumference at 40 GW Head circumference Z-score at 40 GW Hospitalization Late-onset sepsis Length at 40 GW Length Z-score at 40 GW NEC Bell Stage IIIII Time to full enteral nutrition Weight at 40 GW Weigth Z-score at 40 GW Weight < 10 percentile at 40 GW | Days full enteral nutrition (120 mL/kg/day) Intervention: 12 Control: 13 Duration parenteral nutrition (days) Intervention: 20 Control: 17 Enteral nutrition interrupted (>24 h), episodes (n) Intervention: 3 Control: 4 Change in weight z-score between birth and 40 GW Intervention: −1.2 Control: −1.2 Growth velocity day 7–36 GW (g/kg/day) Intervention: 17.4 Control: 18.5 Head circumference at 40 GW (cm) Intervention: 32.4 Control: 33 Head circumference Z-score at 40 GW Intervention: −1.8 Control: −1.4 Length at 40 GW (cm) Intervention: 48 Control: 48.1 Length Z-score at 40 GW Intervention: −2.5 Control: −2.5 Weight at 40 GW (grams) Intervention: 3484 Control: 3516 Weight Z-score at 40 GW Intervention: −1.7 Control: −1.6 Weight < 10 percentile at 40 GW Intervention: 15/31 Control: 17/31 | Hospitalization (median days) Intervention: 107.5 Control: 96 NEC Intervention: 2/31 Control: 0/31 Late-onset sepsis Intervention: 8/31 Control: 3/31 | Moderate |
| Chang, 2022 [31] | Primary Fecal microbiota Secondary Duration parenteral nutrition Hospitalization In-hospital mortality NEC Bell Stage II-III Late-onset sepsis | Duration of parenteral nutrition (days) Intervention: 29 Control: 35.5 | Hospitalization (median days) Intervention: 96.5 Control: 98 In-hospital mortality Intervention: 3/70 Control: 3/50 NEC Bell Stage II-III Intervention: 5/70 Control: 2/50 Late-onset sepsis Intervention: 33/70 Control: 35/50 | Serious |
| Costeloe, 2016 [32] | Primary Culture-proven sepsis > 72 h after birth In-hospital mortality NEC Bell Stage II-III | N/A *** | Culture-proven sepsis Intervention: 63/315 Control: 58/319 In-hospital mortality Intervention: 42/315 Control: 53/319 NEC Bell Stage II-III Intervention: 48/315 Control: 52/319 | Serious |
| Dobryk, 2023 [33] | Primary NEC Bell Stage II-III Secondary Antibiotic use Days to full enteral feeds Hospitalization Late-onset sepsis Mortality Reduced feeding tolerance Weight at 36 GW | Days to full enteral feeds (160 mL/kg/day) Intervention: 37 Control: 40 Reduced feeding tolerance (mean number of episodes) Intervention: 1 Control: 3 Weight at 36 GW (grams) Intervention: 2190 Control: 2160 | Hospitalization (mean days) Intervention: 96 Control: 101 Late-onset sepsis Intervention: 7/14 Control: 7/15 Mortality Intervention: 1/14 Control: 2/15 NEC Bell Stage II-III Intervention: 2/15 Control: 1/14 | Moderate |
| Guney-Varal, 2017 [22] | Primary NEC Bell Stage II-III Secondary Days to 100 mL/kg/day Days to 150 mL/kg/day Duration parenteral nutrition Feeding intolerance episodes ≥ 3 times Hospitalization Mortality Late-onset sepsis | Days to 100 mL/kg/day Intervention: 13 Control: 22 Days to 150 mL/kg/day Intervention: 16 Control: 27 Duration parenteral nutrition (mean days) Intervention: 16 Control: 29 Feeding intolerance episodes ≥3 times (defined as abdominal distension, gastric residue or vomiting) Intervention: 9 Control: 14 | Hospitalization (mean days) Intervention: 58 Control: 56 Mortality Intervention: 1/16 Control: 7/14 NEC Bell Stage II-III Intervention: 0/16 Control: 2/14 Late-onset sepsis Intervention: 5/16 Control: 4/14 | Moderate |
| Härtel, 2017 [23] | Primary Growth velocity (grams/day) Head circumference at discharge Length at discharge Weight at discharge Secondary Hospitalization In hospital mortality NEC Bell Stage II-III Late-onset sepsis | Growth velocity (grams/day) Intervention: 21 Control: 20 Head circumference at discharge (cm) Intervention: 32.6 Control: 32.6 Length at discharge (cm) Intervention: 46.2 Control: 44.6 Weight at discharge (grams) Intervention: 2526 Control: 2741 | Hospitalization (mean days) Intervention: 94 Control: 89 In hospital mortality Intervention: 405/7994 Control: 360/2663 NEC Bell Stage II-III Intervention: 350/7993 Control: 155/2664 Late-onset sepsis Intervention: 1439/7994 Control: 515/2663 | Moderate |
| Havranek, 2013 [34] | Primary Blood flow velocity Secondary Days to full enteral nutrition | Time to full enteral nutrition (150 mL/kg/day) (days) Intervention: 23.9 Control: 22.1 | N/A Sub-study of Al-Hosni. Primary and secondary outcomes are already presented and are not reported again. | Moderate |
| Jacobs, 2013 [35] | Primary Late-onset sepsis Secondary NEC Bell Stage II-III | N/A | NEC Bell Stage II-III Intervention: 11/219 Control: 17/235 Late-onset sepsis Intervention: 54/219 Control: 55/235 | Low |
| Manzoni, 2009 [36] | Primary First onset of late-onset sepsis Secondary Late-onset sepsis | N/A | Late-onset sepsis (bacterial and fungal) Intervention: 6/37 Control: 19/46 | Low |
| Patole, 2014 [24] | Primary Fecal microbiota Secondary Duration parenteral nutrition Growth retardation at discharge Head circumference at discharge Hospitalization Mortality at discharge NEC Bell Stage II-III Late-onset sepsis Time to 50 mL/kg/day enteral nutrition Time to 100 mL/kg/day enteral nutrition Time to 150 mL/kg/day enteral nutrition Weight at discharge Length at discharge | Duration parenteral nutrition (mean days) Intervention: 20 Control: 17 Growth retardation at discharge (no clear definition) Intervention: 19/28 Control:10/28 Head circumference at discharge (mean cm) Intervention: 34 Control: 34 Length at discharge (mean cm) Intervention: 48/18 Control: 51/14 Time to 50 mL/kg/day enteral nutrition (days) Intervention: 14 Control: 13 Time to 100 mL/kg/day enteral nutrition (days) Intervention: 18 Control: 16 Time to 150 mL/kg/day enteral nutrition (days) Intervention: 29 Control: 23 Weight at discharge (mean grams) Intervention: 2933 Control: 3173 | Hospitalization (mean days) Intervention: 105 Control: 98 Late-onset sepsis Intervention: 12/28 Control: 7/28 Mortality at discharge Intervention: 0/28 Control: 0/28 NEC Bell Stage II-III Intervention: 0/28 Control: 0/28 | Low |
| Totsu, 2014 [25] | Primary Established enteral nutrition (>100 mL/kg/day) Secondary Head circumference at discharge Late-onset sepsis **** Mortality at discharge NEC Bell Stage II-III Weight at discharge | Time to 100 mL/kg/day enteral nutrition (days) Intervention: 13 Control: 13 Head circumference at discharge (mean cm) Intervention: 35/48 Control: 35/40 Weight at discharge (mean grams) Intervention: 2926/48 Control: 3008/48 | Late-onset sepsis **** Intervention: 6/48 Control: 9/44 Mortality at discharge Intervention: 0/48 Control: 0/44 NEC Bell Stage II-III Intervention: 0/48 Control: 0/44 | Moderate |
| Wejryd, 2019 [38] | Primary Time to full enteral nutrition Secondary Culture-proven sepsis Duration parenteral nutrition Gastric residuals Head circumference day 14 (cm) Head circumference day 28 (cm) Head circumference 36 GW (cm) Interrupted feeding Length day 14 (cm) Length day 28 (cm) Length 36 GW (cm) Mortality NEC Bell Stage II-III Stools per week Weight day 7 (g) Weight day 14 (g) Weight day 21 (g) Weight day 28 (g) Weight 36 GW (g) | Duration parenteral nutrition (mean days) Intervention: 24.1 Control: 23.1 Gastric residuals (larger than 2 mL/kg and exceed volume of previous meal, mean n) Intervention: 3 Control: 3.8 Head circumference day 14 (mean cm) Intervention: 23.2 Control: 23.5 Head circumference day 28 (mean cm) Intervention: 25.2 Control: 24.9 Head circumference 36 GW (mean cm) Intervention: 31.2 Control: 31 Interrupted feeding (mean days) Intervention: 5.5 Control: 6 Length day 14 (mean cm) Intervention: 33.9 Control: 34.3 Length day 28 (mean cm) Intervention: 35.9 Control: 35.5 Length 36 GW (mean cm) Intervention: 43 Control: 43.4 Time to full enteral nutrition 150 mL/kg/day (median days) Intervention: 15 Control: 15 Weight day 7 (mean grams) Intervention: 767 Control: 761 Weight day 14 (mean grams) Intervention: 867 Control: 889 Weight day 21 (mean grams) Intervention: 975 Control: 972 Weight day 28 (mean grams) Intervention: 1075 Control: 1074 Weight 36 GW (mean grams) Intervention: 2303 Control: 2348 | Culture-proven sepsis Intervention: 25/68 Control: 23/66 Mortality Intervention: 5/68 Control: 5/66 NEC Bell Stage II-III Intervention: 7/68 Control: 8/66 | Low |
| Outcomes | Anticipated Effect | Mean Difference/Risk Ratio (95% CI) | Number of Participants | Certainty of Evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with Control Group | Risk with Probiotics Group | ||||
| Feeding intolerance | |||||
| Days to full enteral nutrition (150 mL/kg/day) | 21.8 | 21 | 1.1 days lower (7.83 lower to 5.56 higher) | 251 | Very low |
| Duration of parenteral nutrition (days) | 24.3 | 22 | 2.4 days lower (7.44 lower to 2.58 higher) | 402 | Very low |
| Growth | |||||
| Weight at discharge (grams) | 2824 | 2736 | 88 g lower (205 lower to 30 higher) | 10,988 | Very low |
| Length at discharge (cm) | 46.8 | 47.1 | 0.3 cm higher (1.1 lower to 1.8 higher) | 11,068 | Very low |
| Head circumference at discharge (cm) | 33.2 | 33 | 0.02 cm lower (0.12 lower to 0.09 higher) | 10,876 | Low |
| Necrotizing enterocolitis | 7 per 100 | 5 per 100 | RR 0.80 (0.68 to 0.93) | 12,369 | Low |
| All-cause mortality (during hospital stay) | 13 per 100 | 7 per 100 | RR 0.56 (0.33 to 0.93) | 11,853 | Very low |
| Late-onset sepsis | 21 per 100 | 20 per 100 | RR 0.95 (0.79 to 1.11) | 12,452 | Very low |
| Hospitalization (days) | 88 | 93 | 5 days higher (3.7 higher to 6.6 higher) | 10,887 | Very low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Söderquist Kruth, S.; Persad, E.; Rakow, A. Probiotic Supplements Effect on Feeding Tolerance, Growth and Neonatal Morbidity in Extremely Preterm Infants: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 1228. https://doi.org/10.3390/nu17071228
Söderquist Kruth S, Persad E, Rakow A. Probiotic Supplements Effect on Feeding Tolerance, Growth and Neonatal Morbidity in Extremely Preterm Infants: A Systematic Review and Meta-Analysis. Nutrients. 2025; 17(7):1228. https://doi.org/10.3390/nu17071228
Chicago/Turabian StyleSöderquist Kruth, Sofia, Emma Persad, and Alexander Rakow. 2025. "Probiotic Supplements Effect on Feeding Tolerance, Growth and Neonatal Morbidity in Extremely Preterm Infants: A Systematic Review and Meta-Analysis" Nutrients 17, no. 7: 1228. https://doi.org/10.3390/nu17071228
APA StyleSöderquist Kruth, S., Persad, E., & Rakow, A. (2025). Probiotic Supplements Effect on Feeding Tolerance, Growth and Neonatal Morbidity in Extremely Preterm Infants: A Systematic Review and Meta-Analysis. Nutrients, 17(7), 1228. https://doi.org/10.3390/nu17071228






