Zinc-Enriched Bifidobacterium longum subsp. longum CCFM1195 Alleviates Cutibacterium acnes-Induced Skin Lesions in Mice by Mitigating Inflammatory Responses and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Determination of Zn Content
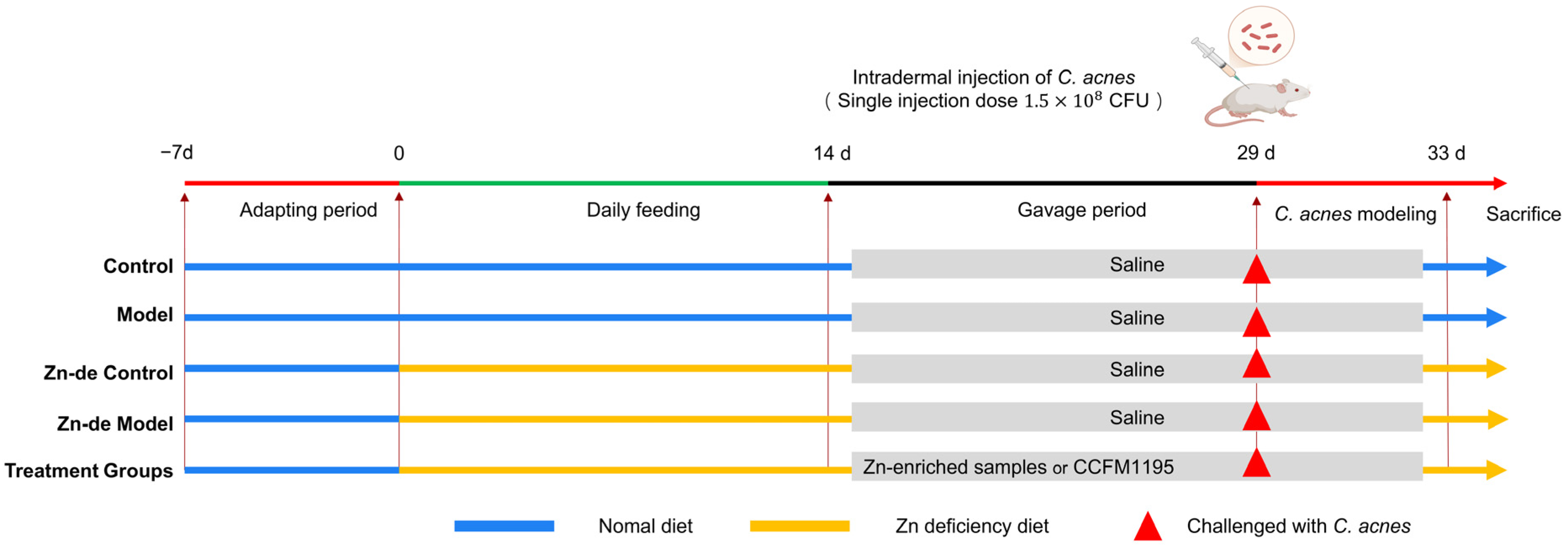
2.3. Animal Experiment Design
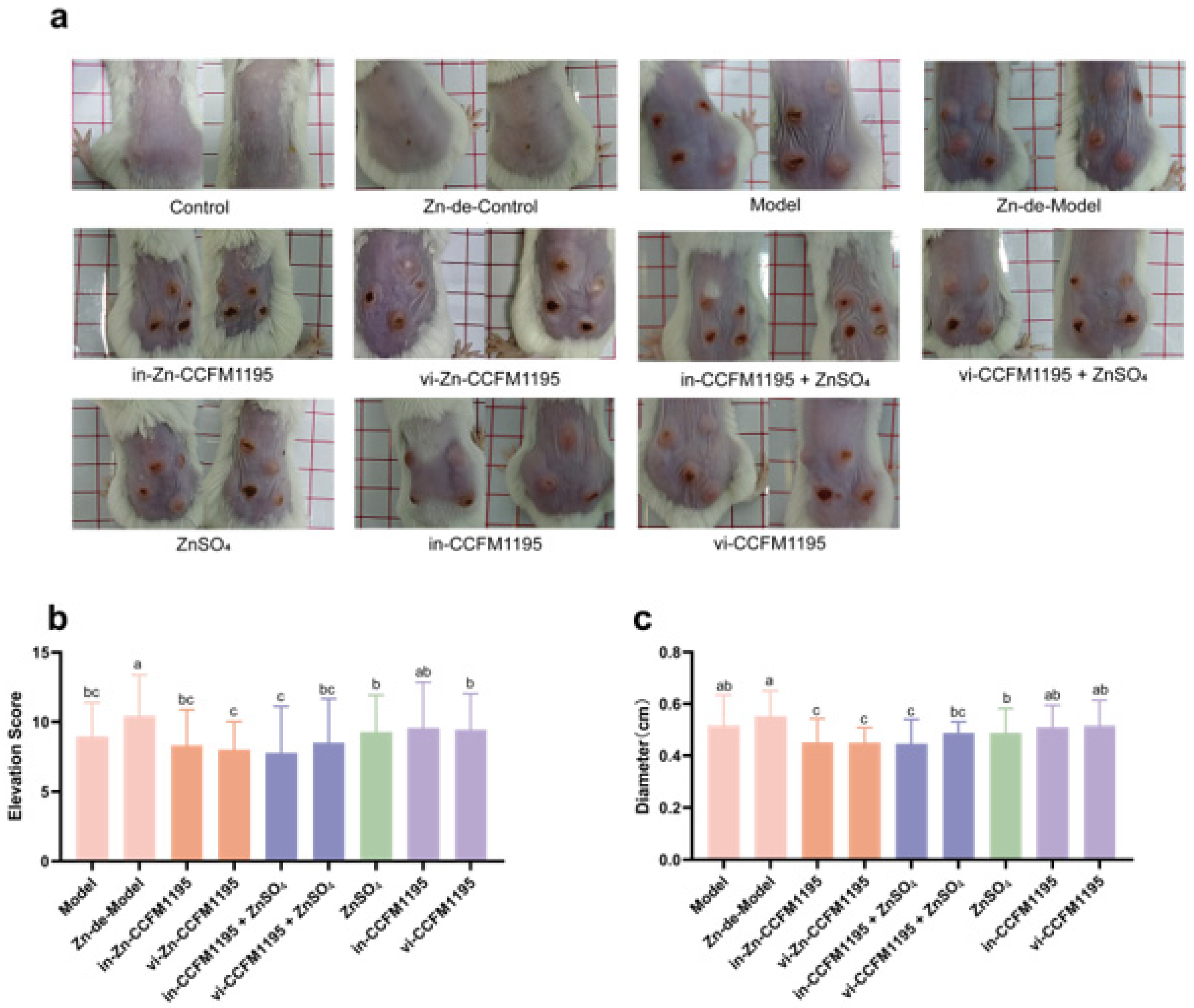
2.4. Assessment of the Severity of Acne Vulgaris
2.5. Biochemical Analysis of Skin Tissue
2.6. Quantitative qPCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Viable Bacteria Count and Zn Content of CCFM1195 Powder
3.2. Zn-Enriched Samples Ameliorate Skin Injury in Mice
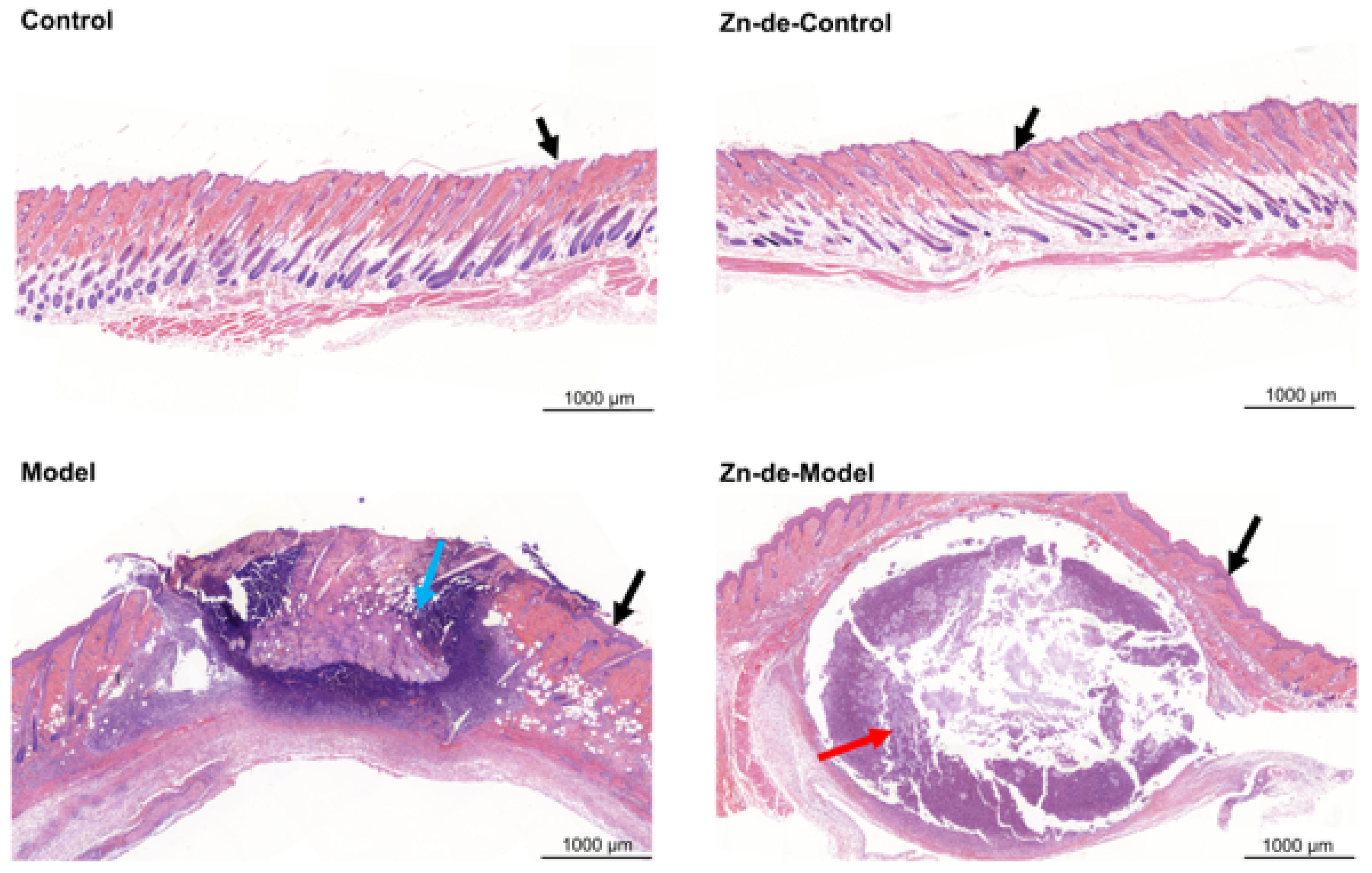
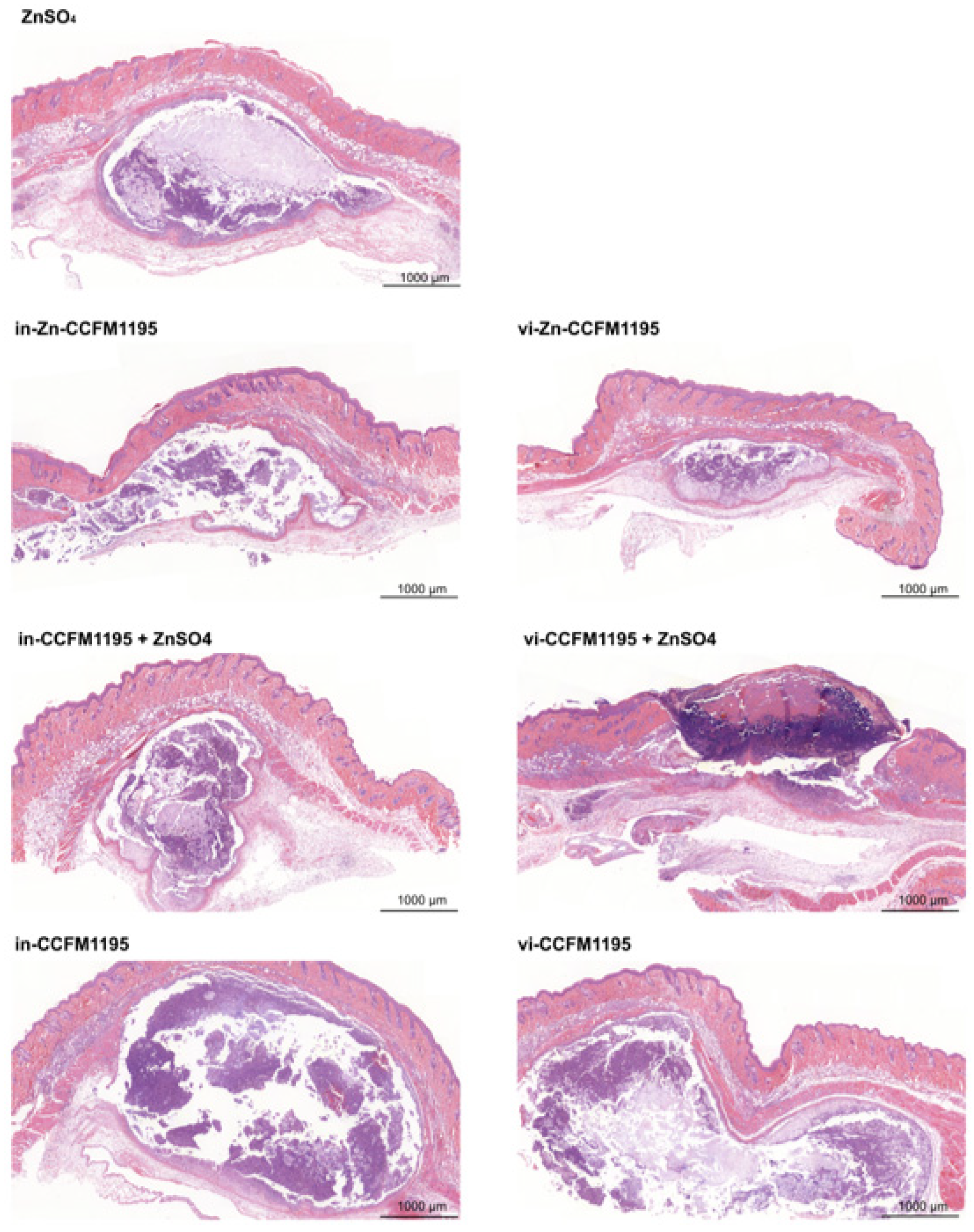
3.3. Zn-Enriched Samples Affect Skin Histopathology
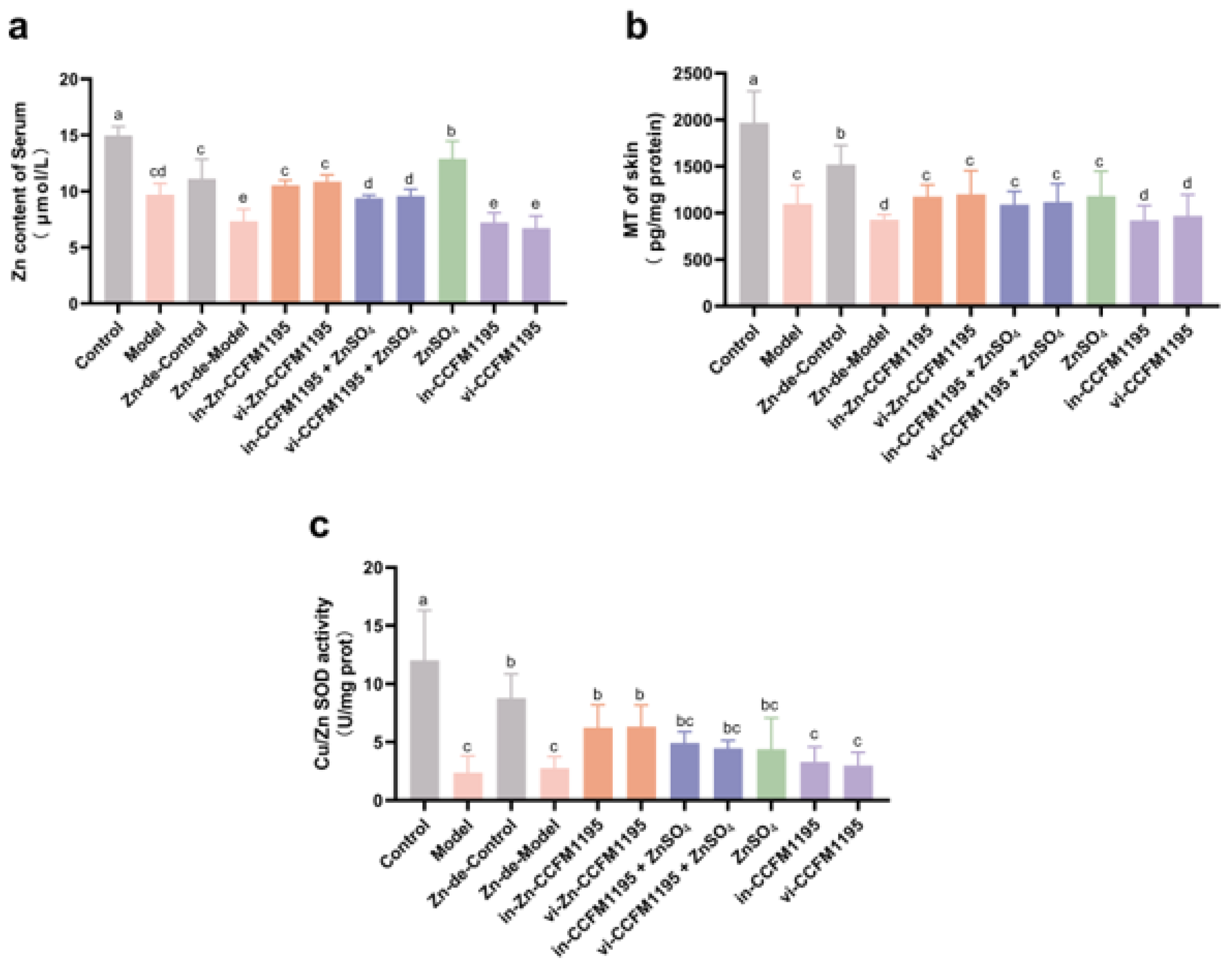
3.4. Analysis of Zn Status Indicators in Serum and Skin After Zn Supplementation
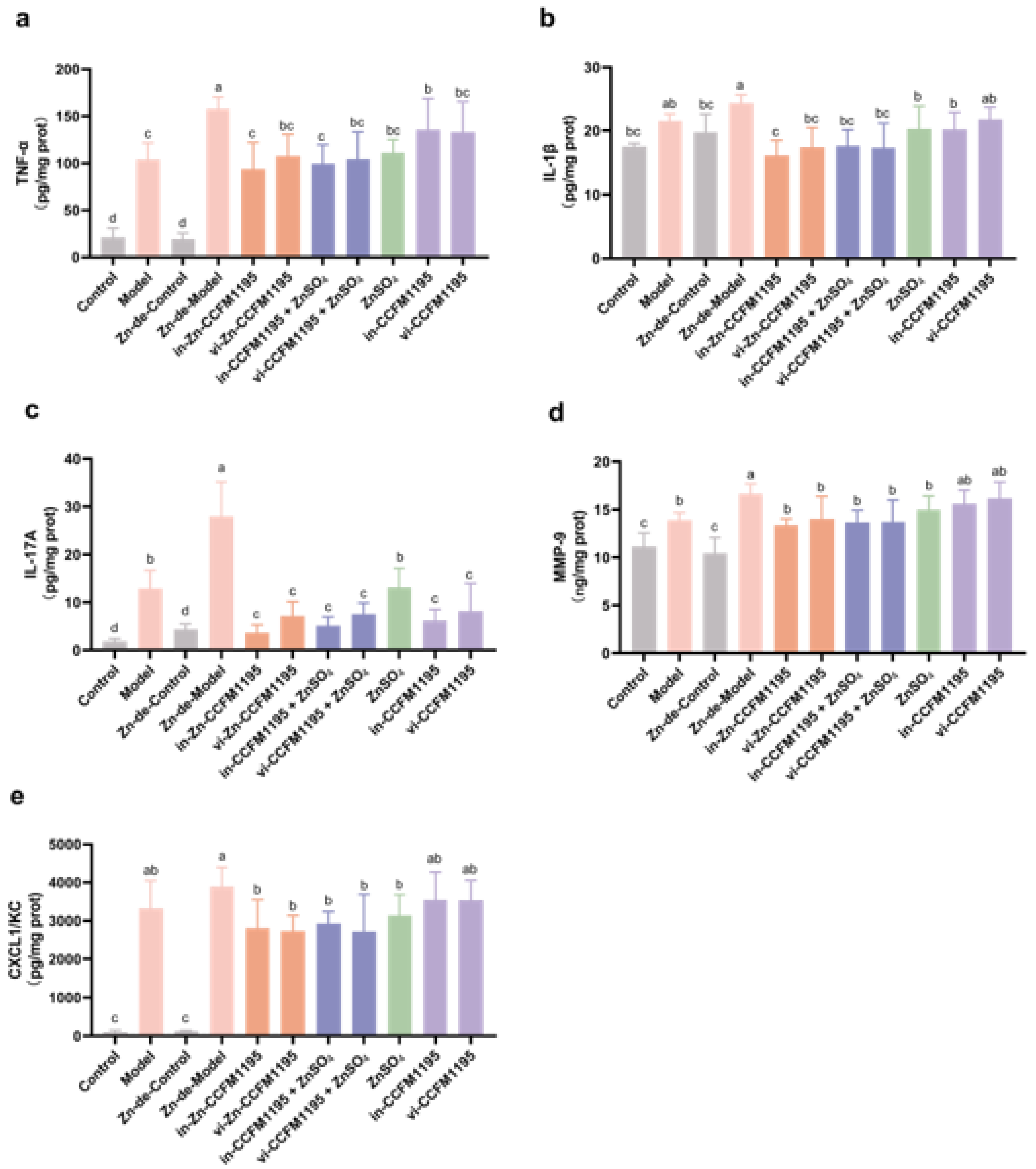
3.5. Zn-Enriched Samples Ameliorate Inflammatory Response of Skin
3.6. Zn-Enriched Samples Improve Oxidative Stress in Skin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Lin, L.; He, Y.; Sun, G.; Dong, D.; Wu, B. BMAL1 Regulates Propionibacterium acnes-Induced Skin Inflammation via REV-ERBα in Mice. Int. J. Biol. Sci. 2022, 18, 2597–2608. [Google Scholar] [CrossRef] [PubMed]
- Tuchayi, S.M.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne Vulgaris. Nat. Rev. Dis. Primers 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.M.; Ravenscroft, J. Adolescent Acne Vulgaris: Current and Emerging Treatments. Lancet Child Adolesc. Health 2023, 7, 136–144. [Google Scholar] [CrossRef]
- Gong, L.; Xu, J.; Guo, M.; Zhao, J.; Xin, X.; Zhang, C.; Ni, X.; Hu, Y.; An, F. Octahydroindolizine Alkaloid Homocrepidine A from Dendrobium Crepidatum Attenuate, P. acnes-Induced Inflammatory in Vitro and in Vivo. J. Ethnopharmacol. 2024, 333, 118455. [Google Scholar] [CrossRef]
- Zhu, T.; Fang, F.; Sun, D.; Yang, S.; Zhang, X.; Yu, X.; Yang, L. Piceatannol Inhibits, P. acnes-Induced Keratinocyte Proliferation and Migration by Downregulating Oxidative Stress and the Inflammatory Response. Inflammation 2020, 43, 347–357. [Google Scholar] [CrossRef]
- Dhaliwal, S.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Chambers, C.J.; Sivamani, R.K. Effects of Zinc Supplementation on Inflammatory Skin Diseases: A Systematic Review of the Clinical Evidence. Am. J. Clin. Dermatol. 2020, 21, 21–39. [Google Scholar] [CrossRef]
- Cervantes, J.; Eber, A.E.; Perper, M.; Nascimento, V.M.; Nouri, K.; Keri, J.E. The Role of Zinc in the Treatment of Acne: A Review of the Literature. Dermatol. Ther. 2018, 31, e12576. [Google Scholar] [CrossRef] [PubMed]
- Al-Momani, H.; Massadeh, M.I.; Almasri, M.; Al Balawi, D.; Aolymat, I.; Hamed, S.; Albiss, B.A.; Ibrahim, L.; Balawi, H.A.; Al Haj Mahmoud, S. Anti-Bacterial Activity of Green Synthesised Silver and Zinc Oxide Nanoparticles against Propionibacterium acnes. Pharmaceuticals 2024, 17, 255. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and Anti-Inflammatory Effects of Zinc. Zinc-Dependent NF-κB Signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Li, L.; Hajam, I.; McGee, J.S.; Tang, Z.; Zhang, Y.; Badey, N.; Mintzer, E.; Zhang, Z.; Liu, G.Y.; Church, G.M.; et al. Comparative Transcriptome Analysis of Acne Vulgaris, Rosacea, and Hidradenitis Suppurativa Supports High-dose Dietary Zinc as a Therapeutic Agent. Exp. Dermatol. 2024, 33, e15145. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, T.; Watanabe, M.; Hatakeyama, S.; Kimura, S.; Nakazono, A.; Honma, A.; Nakamaru, Y.; Vreugde, S.; Homma, A. Role of Intracellular Zinc in Molecular and Cellular Function in Allergic Inflammatory Diseases. Allergol. Int. 2021, 70, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Morikawa, H.; Kamon, H.; Iguchi, M.; Hojyo, S.; Fukada, T.; Yamashita, S.; Kaisho, T.; Akira, S.; Murakami, M.; et al. Toll-like Receptor–Mediated Regulation of Zinc Homeostasis Influences Dendritic Cell Function. Nat. Immunol. 2006, 7, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Meynadier, J. Efficacy and Safety Study of Two Zinc Gluconate Regimens in the Treatment of Inflammatory Acne. Eur. J. Dermatol. 2000, 10, 269–273. [Google Scholar]
- Dreno, B.; Foulc, P.; Reynaud, A.; Moyse, D.; Habert, H.; Richet, H. Effect of Zinc Gluconate on Propionibacterium acnes Resistance to Erythromycin in Patients with Inflammatory Acne: In Vitro and in Vivo Study. Eur. J. Dermatol. 2005, 15, 152–155. [Google Scholar]
- Dreno, B.; Moyse, D.; Alirezai, M.; Amblard, P.; Auffret, N.; Beylot, C.; Bodokh, I.; Chivot, M.; Daniel, F.; Humbert, P.; et al. Multicenter Randomized Comparative Double-Blind Controlled Clinical Trial of the Safety and Efficacy of Zinc Gluconate versus Minocycline Hydrochloride in the Treatment of Inflammatory Acne Vulgaris. Dermatology 2001, 203, 135–140. [Google Scholar] [CrossRef]
- Verma, K.C.; Saini, A.S.; Dhamija, S.K. Oral Zinc Sulphate Therapy in Acne Vulgaris: A Double-Blind Trial. Acta. Derm. Venereol. 1980, 60, 337–340. [Google Scholar] [CrossRef]
- Stiles, L.I.; Ferrao, K.; Mehta, K.J. Role of Zinc in Health and Disease. Clin. Exp. Med. 2024, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Monje, M.; Campos, J.; Alvarez Villamil, E.; Jerez, A.; Dentice Maidana, S.; Elean, M.; Salva, S.; Kitazawa, H.; Villena, J.; García-Cancino, A. Characterization of Weissella viridescens UCO-SMC3 as a Potential Probiotic for the Skin: Its Beneficial Role in the Pathogenesis of Acne Vulgaris. Microorganisms 2021, 9, 1486. [Google Scholar] [CrossRef]
- Kang, S.; Li, R.; Jin, H.; You, H.J.; Ji, G.E. Effects of Selenium- and Zinc-Enriched Lactobacillus Plantarum SeZi on Antioxidant Capacities and Gut Microbiome in an ICR Mouse Model. Antioxidants 2020, 9, 1028. [Google Scholar] [CrossRef]
- Lule, V.K.; Tomar, S.K.; Chawla, P.; Pophaly, S.; Kapila, S.; Arora, S. Bioavailability Assessment of Zinc Enriched Lactobacillus Biomass in a Human Colon Carcinoma Cell Line (Caco-2). Food Chem. 2020, 309, 125583. [Google Scholar] [CrossRef]
- Meng, Y.; Liang, Z.; Yi, M.; Tan, Y.; Li, Z.; Du, P.; Li, A.; Li, C.; Liu, L. Enrichment of Zinc in Lactobacillus Plantarum DNZ-4: Impact on Its Characteristics, Metabolites and Antioxidant Activity. LWT 2022, 153, 112462. [Google Scholar] [CrossRef]
- Zhai, Q.; Zhang, Q.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. The Synergistic Effect of Lactobacillus Plantarum CCFM242 and Zinc on Ulcerative Colitis through Modulating Intestinal Homeostasis. Food Funct. 2019, 10, 6147–6156. [Google Scholar] [CrossRef]
- Han, X.; Liu, F.; Zhang, Q.; Mao, B.; Tang, X.; Huang, J.; Guo, R.; Zhao, J.; Zhang, H.; Cui, S.; et al. Effects of Zn-Enriched Bifidobacterium Longum on the Growth and Reproduction of Rats. Nutrients 2022, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Rimon, A.; Rakov, C.; Lerer, V.; Sheffer-Levi, S.; Oren, S.A.; Shlomov, T.; Shasha, L.; Lubin, R.; Zubeidat, K.; Jaber, N.; et al. Topical Phage Therapy in a Mouse Model of Cutibacterium acnes-Induced Acne-like Lesions. Nat. Commun. 2023, 14, 1005. [Google Scholar] [CrossRef]
- GB 5009.268-2016; National Food Safety Standard-Determination of Multi-Elements in Foods. Standards Press of China: Beijing, China, 2016.
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Luo, J.; He, W.; Li, X.; Ji, X.; Liu, J. Anti-Acne Vulgaris Effects of Chlorogenic Acid by Anti-Inflammatory Activity and Lipogenesis Inhibition. Exp. Dermatol. 2021, 30, 865–871. [Google Scholar] [CrossRef]
- Kolar, S.L.; Tsai, C.-M.; Torres, J.; Fan, X.; Li, H.; Liu, G.-Y. Propionibacterium acnes-Induced Immunopathology Correlates with Health and Disease Association. JCI insight. 2019, 4, e124687. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, P.V. Enzyme-Linked Immunosorbent Assays. Curr. Protoc. Immunol. 2015, 110, 2.1.1–2.1.23. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R., Jr.; Prenzler, P.D. Measurement of Antioxidant Activity with the Thiobarbituric Acid Reactive Substances Assay. Food. Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Ding, M.; Li, B.; Chen, H.; Ross, R.P.; Stanton, C.; Zhao, J.; Chen, W.; Yang, B. Bifidobacterium Longum Subsp. Infantis Promotes IgA Level of Growing Mice in a Strain-Specific and Intestinal Niche-Dependent Manner. Nutrients 2024, 16, 1148. [Google Scholar]
- Zhou, N.; Sun, Y.; Ren, X.; Wang, Y.; Gao, X.; Li, L.; Ma, Y.; Hao, Y.; Wang, Y. Intradermal Injection of Cutibacterium acnes and Staphylococcus: A Pustular Acne-like Murine Model. J. Cosmet. Dermatol. 2024, 23, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, J.; Mao, C.; Zhu, Y.; Wang, C.; Wu, J.; Liu, X.; Wu, S.; Kwan, K.Y.H.; Cheung, K.M.C.; et al. Ultrasound-Triggered Interfacial Engineering-Based Microneedle for Bacterial Infection Acne Treatment. Sci. Adv. 2023, 9, eadf0854. [Google Scholar] [CrossRef]
- Wegmüller, R.; Tay, F.; Zeder, C.; Brnic, M.; Hurrell, R.F. Zinc Absorption by Young Adults from Supplemental Zinc Citrate Is Comparable with That from Zinc Gluconate and Higher than from Zinc Oxide. J. Nutr. 2014, 144, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, T.A.; Renard, N.E.; Kiuchi, A. Clinical Evaluation of the Bioavailability of Zinc-Enriched Yeast and Zinc Gluconate in Healthy Volunteers. Biol. Trace Elem. Res. 2007, 120, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Yuce, K.; Mogulkoc, R. Zinc Metabolism and Metallothioneins. Biol. Trace Elem. Res. 2018, 183, 22–31. [Google Scholar] [CrossRef]
- Yoon, J.S.; Nam, S.Y.; Lee, B.J.; Lee, H.J. Comparative Study on the Effects of Micro- and Nano-Sized Zinc Oxide Supplementation on Zinc-Deficient Mice. J. Vet. Sci. 2023, 24, e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peng, C.; Zhang, Y.; Wang, L.; Yu, L.; Wang, C. Characteristics of Zn Content and Localization, Cu–Zn SOD, and MT Levels in the Tissues of Marginally Zn-Deficient Mice. Biol. Trace Elem. Res. 2023, 201, 262–271. [Google Scholar] [CrossRef]
- Huang, D.; Hu, Q.; Fang, S.; Feng, J. Dosage Effect of Zinc Glycine Chelate on Zinc Metabolism and Gene Expression of Zinc Transporter in Intestinal Segments on Rat. Biol. Trace Elem. Res. 2016, 171, 363–370. [Google Scholar] [CrossRef]
- Kaltenmeier, C.; Wang, R.; Popp, B.; Geller, D.; Tohme, S.; Yazdani, H.O. Role of Immuno-Inflammatory Signals in Liver Ischemia-Reperfusion Injury. Cells 2022, 11, 2222. [Google Scholar] [CrossRef]
- Huang, L.; Yang, S.; Yu, X.; Fang, F.; Zhu, L.; Wang, L.; Zhang, X.; Yang, C.; Qian, Q.; Zhu, T. Association of different cell types and inflammation in early acne vulgaris. Front. Immunol. 2024, 15, 1275269. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.G.A.; Maraee, A.H.; Rifaat Al-Sharaky, D.; Elshaib, M.E.; Kohla, M.S.M.; Shehata, W.A. Tissue Expression of IL-17A and FOXP3 in Acne Vulgaris Patients. J. Cosmet. Dermatol. 2021, 20, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Agak, G.W.; Qin, M.; Nobe, J.; Kim, M.H.; Krutzik, S.R.; Tristan, G.R.; Elashoff, D.; Garbán, H.J.; Kim, J. Propionibacterium acnes Induces an IL-17 Response in Acne Vulgaris That Is Regulated by Vitamin A and Vitamin, D. J. Investig. Dermatol. 2014, 134, 366–373. [Google Scholar] [CrossRef] [PubMed]
- George, M.M.; Subramanian Vignesh, K.; Landero Figueroa, J.A.; Caruso, J.A.; Deepe, G.S.J. Zinc Induces Dendritic Cell Tolerogenic Phenotype and Skews Regulatory T Cell-Th17 Balance. J. Immunol. 2016, 197, 1864–1876. [Google Scholar] [CrossRef]
- Kulik, L.; Maywald, M.; Kloubert, V.; Wessels, I.; Rink, L. Zinc Deficiency Drives Th17 Polarization and Promotes Loss of Treg Cell Function. J. Nutr Biochem. 2019, 63, 11–18. [Google Scholar] [CrossRef]
- Higashimura, Y.; Takagi, T.; Naito, Y.; Uchiyama, K.; Mizushima, K.; Tanaka, M.; Hamaguchi, M.; Itoh, Y. Zinc Deficiency Activates the IL-23/Th17 Axis to Aggravate Experimental Colitis in Mice. J. Crohn’s Colitis 2020, 14, 856–866. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix Metalloproteinases and the Regulation of Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, H. Aberrance of Zinc Metalloenzymes-Induced Human Diseases and Its Potential Mechanisms. Nutrients 2021, 13, 4456. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A Guiding Map for Inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Cao, J.; Xu, M.; Zhu, L.; Xiao, S. Viaminate Inhibits Propionibacterium acnes-Induced Abnormal Proliferation and Keratinization of HaCat Cells by Regulating the S100A8/S100A9-MAPK Cascade. Curr. Drug Targets 2023, 24, 1055–1065. [Google Scholar] [CrossRef]
- Chan, J.K.; Roth, J.; Oppenheim, J.J.; Tracey, K.J.; Vogl, T.; Feldmann, M.; Horwood, N.; Nanchahal, J. Alarmins: Awaiting a Clinical Response. J. Clin. Investig. 2012, 122, 2711–2719. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Song, Y.; He, L. A Review of Skin Immune Processes in Acne. Front. Immunol. 2023, 14, 1324930. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Bungau, A.F.; Radu, A.F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Endres, L.M. Oxidative stress and metabolic syndrome in acne vulgaris: Pathogenetic connections and potential role of dietary supplements and phytochemicals. Biomed. Pharmacother. 2023, 164, 115003. [Google Scholar] [CrossRef]
- Zhan, X.; Li, J.; Zhou, T. Targeting Nrf2-Mediated Oxidative Stress Response Signaling Pathways as New Therapeutic Strategy for Pituitary Adenomas. Front. Pharmacol. 2021, 12, 565748. [Google Scholar] [CrossRef]

| zGene | Primer Sequence (5′-3′) |
|---|---|
| GAPDH | Forward: 5′-GGCAAATTCAACGGCACAGTCAAG-3′ Reverse: 5′-TCGCTCCTGGAAGATGGTGATGG-3′ |
| S100A8 | Forward: 5′-TGCCGTCTGAACTGGAGAAGG-3′ Reverse: 5′-CTTGTAGAGGGCATGGTGATTTCC-3′ |
| S100A9 | Forward: 5′-TGACATCATGGAGGACCTGGACAC-3′ Reverse: 5′-TGGGTTGTTCTCATGCAGCTTCTC-3′ |
| TLR2 | Forward: 5′-CTCCCAGATGCTTCGTTGTTCCC-3′ Reverse: 5′- GTTGTCGCCTGCTTCCAGAGTC-3′ |
| MyD88 | Forward: 5′-AGCAGAACCAGGAGTCCGAGAAG-3′ Reverse: 5′-GGGCAGTAGCAGATAAAGGCATCG-3′ |
| IκBα | Forward: 5′-CTGAAAGCTGGCTGTGATCCTGAG-3′ Reverse: 5′-CTGCGTCAAGACTGCTACACTGG-3′ |
| IKKβ | Forward: 5′-ATAAATTGCTGCTGGCTTGG-3′ Reverse: 5′-AGTGCCATCATCCGCTCTAC-3′ |
| GPX2 | Forward: 5′-AGCCTCAAGTATGTCCGACCTG-3′ Reverse: 5′-GGATGCTCGTTCTGCCCATTG-3′ |
| Nrf2 | Forward: 5′-CCTCACCTCTGCTGCAAGTA-3′ Reverse: 5′-TCAAATCCATGTCCTGCTGGG-3′ |
| Group | Control | Model | Zn-de-Control | Zn-de-Model | in-Zn-CCFM1195 | vi-Zn-CCFM1195 | in-CCFM1195 + ZnSO4 | vi-CCFM1195 + ZnSO4 | ZnSO4 | in-CCFM1195 | vi-CCFM1195 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | ||||||||||||
| S100A8 | 1.12 ± 0.73 e | 159.17 ± 54.92 d | 1.31 ± 0.41 e | 547.61 ± 91.54 a | 196.12 ± 49.64 d | 148.27 ± 38.73 d | 141.22 ± 34.13 d | 134.83 ± 9.32 d | 302.49 ± 77.77 c | 434.55 ± 31.49 b | 469.05 ± 52.17 ab | |
| S100A9 | 0.82 ± 0.25 e | 72.05 ± 12.23 d | 1.17 ± 0.44 e | 308.01 ± 87.48 a | 132.49 ± 6.40 c | 179.22 ± 44.25 bc | 142.74 ± 24.99 c | 129.16 ± 31.33 c | 266.77 ± 25.35 ab | 217.77 ± 15.43 b | 222.28 ± 63.08 b | |
| TLR2 | 1.10 ± 0.48 e | 2.51 ± 0.30 c | 2.27 ± 0.20 c | 4.45 ± 0.32 a | 0.96 ± 0.15 e | 1.92 ± 0.35 d | 2.01 ± 0.58 cd | 1.98 ± 0.20 d | 2.84 ± 0.96 c | 3.06 ± 0.69 bc | 3.75 ± 0.42 b | |
| MyD88 | 1.34 ± 0.70 c | 5.50 ± 1.33 b | 2.75 ± 0.46 c | 8.95 ± 2.01 a | 5.76 ± 1.46 b | 6.43 ± 1.90 b | 7.08 ± 2.13 ab | 8.52 ± 2.51 ab | 6.93 ± 1.10 b | 8.09 ± 1.36 ab | 8.85 ± 1.65 a | |
| IκBα | 1.02 ± 0.12 d | 12.76 ± 3.43 c | 1.89 ± 0.69 d | 31.82 ± 3.33 a | 10.51 ± 1.95 c | 7.87 ± 3.46 c | 13.41 ± 4.22c | 12.10 ± 5.48 c | 20.00 ± 3.13 b | 19.59 ± 6.75 b | 23.04 ± 2.26 b | |
| IKKβ | 1.22 ± 0.55 c | 4.00 ± 0.95 b | 1.61 ± 0.52 c | 6.75 ± 1.94 a | 2.30 ± 1.67 bc | 2.28 ± 0.91 bc | 2.69 ± 1.36 bc | 2.95 ± 1.02 bc | 3.38 ± 1.65 b c | 5.18 ± 1.25 ab | 4.37 ± 1.65 b | |
| MDA (nmol/mg prot) | 0.37 ± 0.1 c | 0.81 ± 0.09 a | 0.45 ± 0.08 bc | 0.81 ± 0.13 a | 0.49 ± 0.03 c | 0.60 ± 0.10 bc | 0.59 ± 0.14 bc | 0.56 ± 0.08 b | 0.67 ± 0.02 a b | 0.68 ± 0.16 ab | 0.77 ± 0.04 a | |
| GSH-Px activity (U/mg prot) | 40.44 ± 3.82 ab | 29.54 ± 5.14 b | 40.85 ± 7.25 a | 23.16 ± 1.93 b | 46.48 ± 3.21 a | 42.44 ± 6.07 a | 39.94 ± 6.80 ab | 43.77 ± 9.27 a | 31.48 ± 1.48 b | 25.61 ± 3.95 b | 27.56 ± 1.75 b | |
| SOD activity (U/mg prot) | 22.68 ± 6.50 a | 9.60 ± 3.67 bc | 21.87 ± 1.50 a | 7.07 ± 1.38 c | 12.35 ± 1.32 b | 11.87 ± 1.82 b | 10.80 ± 1.45 bc | 12.42 ± 3.51 b | 10.29 ± 2.49 bc | 9.43 ± 1.90 bc | 10.91 ± 1.37 bc | |
| Nrf2 | 1.05 ± 0.20 bc | 0.33 ± 0.22 d | 1.24 ± 0.12 ab | 0.61 ± 0.08 c | 1.27 ± 0.16 ab | 1.43 ± 0.18 a | 1.03 ± 0.22 bc | 1.10 ± 0.22 b | 0.80 ± 0.13 c | 0.60 ± 0.05 c d | 0.62 ± 0.09 cd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Wang, B.; Zhang, T.; Zhang, Q.; Mao, B.; Tang, X.; Zhao, J.; Cui, S. Zinc-Enriched Bifidobacterium longum subsp. longum CCFM1195 Alleviates Cutibacterium acnes-Induced Skin Lesions in Mice by Mitigating Inflammatory Responses and Oxidative Stress. Nutrients 2025, 17, 1803. https://doi.org/10.3390/nu17111803
Gu X, Wang B, Zhang T, Zhang Q, Mao B, Tang X, Zhao J, Cui S. Zinc-Enriched Bifidobacterium longum subsp. longum CCFM1195 Alleviates Cutibacterium acnes-Induced Skin Lesions in Mice by Mitigating Inflammatory Responses and Oxidative Stress. Nutrients. 2025; 17(11):1803. https://doi.org/10.3390/nu17111803
Chicago/Turabian StyleGu, Xiangyue, Botao Wang, Tianmeng Zhang, Qiuxiang Zhang, Bingyong Mao, Xin Tang, Jianxin Zhao, and Shumao Cui. 2025. "Zinc-Enriched Bifidobacterium longum subsp. longum CCFM1195 Alleviates Cutibacterium acnes-Induced Skin Lesions in Mice by Mitigating Inflammatory Responses and Oxidative Stress" Nutrients 17, no. 11: 1803. https://doi.org/10.3390/nu17111803
APA StyleGu, X., Wang, B., Zhang, T., Zhang, Q., Mao, B., Tang, X., Zhao, J., & Cui, S. (2025). Zinc-Enriched Bifidobacterium longum subsp. longum CCFM1195 Alleviates Cutibacterium acnes-Induced Skin Lesions in Mice by Mitigating Inflammatory Responses and Oxidative Stress. Nutrients, 17(11), 1803. https://doi.org/10.3390/nu17111803








