Teenagers with Obesity at the Gym: Recommendations for Physical Activity, Diet, and Supplementation—A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
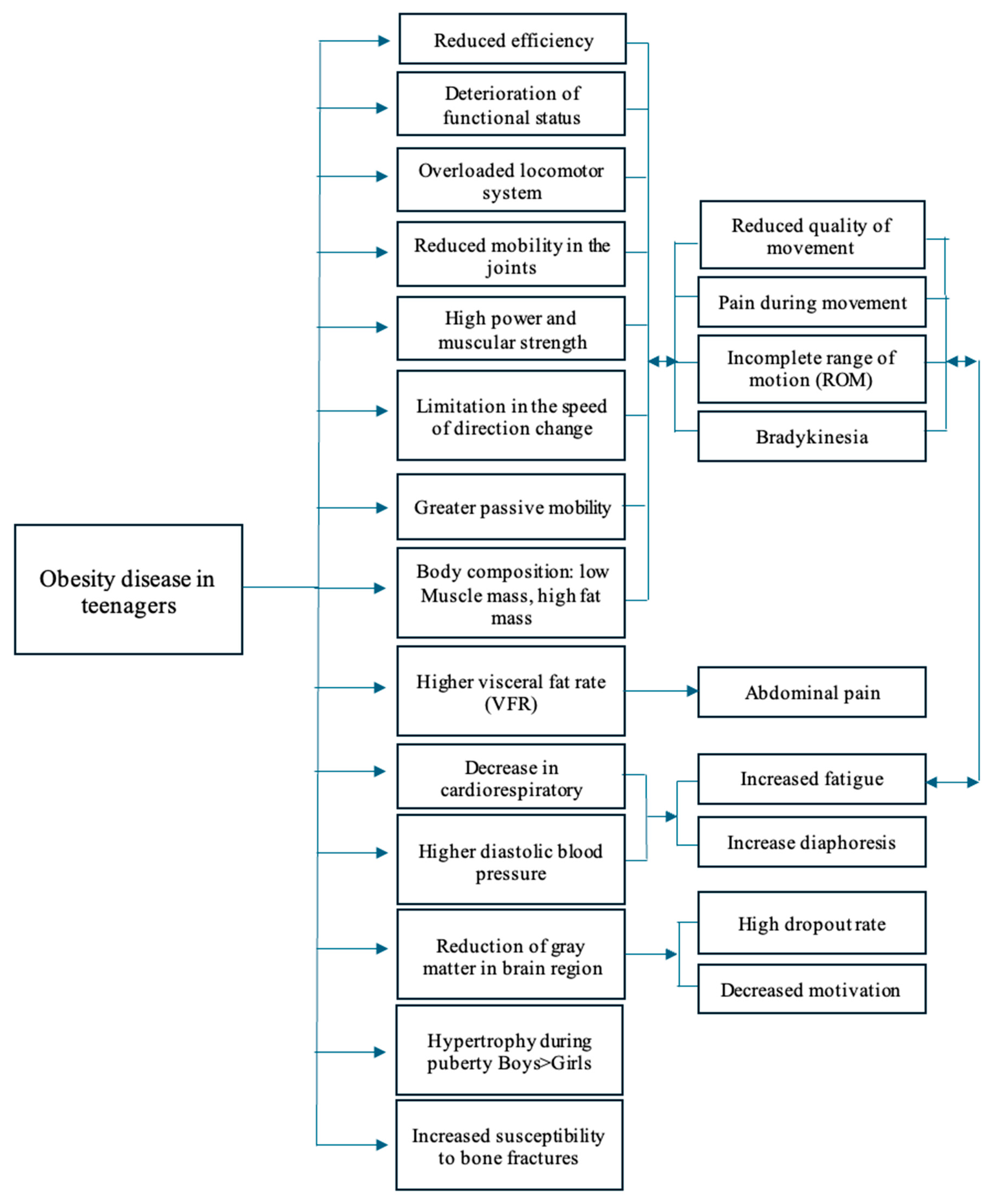
3. Medical Aspects of Gym Training in Adolescence
4. Recommendations for Physical Activity
5. Diet and Supplementation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight: Fact Sheet. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 March 2025).
- Chaput, J.P.; Willumsen, J.; Bull, F.; Chou, R.; Ekelund, U.; Firth, J.; Jago, R.; Ortega, F.B.; Katzmarzyk, P.T. 2020 WHO Guidelines on Physical Activity and Sedentary Behaviour for Children and Adolescents Aged 5–17 Years: Summary of the Evidence. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 141. [Google Scholar] [CrossRef] [PubMed]
- Committee on Fitness Measures and Health Outcomes in Youth; Food and Nutrition Board; Institute of Medicine. Fitness Measures and Health Outcomes in Youth; Pate, R., Oria, M., Pillsbury, L., Eds.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Porter, A.K.; Matthews, K.J.; Salvo, D.; Kohl, H.W., III. Associations of Physical Activity, Sedentary Time, and Screen Time with Cardiovascular Fitness in United States Adolescents: Results from the NHANES National Youth Fitness Survey. J. Phys. Act. Health 2017, 14, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Cordero, M.J.; León Ríos, X.A.; Rojas-Carvajal, A.M.; Latorre-García, J.; Expósito-Ruiz, M.; Sánchez-López, A.M. Effects of Physical Activity on Quality of Life in Overweight and Obese Children. Nutr. Hosp. 2021, 38, 736–741. [Google Scholar] [CrossRef]
- Ramos-Arellano, L.E.; Matia-Garcia, I.; Marino-Ortega, L.A.; Castro-Alarcón, N.; Muñoz-Valle, J.F.; Salgado-Goytia, L.; Salgado-Bernabé, A.B.; Parra-Rojas, I. Obesity, Dyslipidemia, and High Blood Pressure Are Associated with Cardiovascular Risk, Determined Using High-Sensitivity C-Reactive Protein Concentration, in Young Adults. J. Int. Med. Res. 2020, 48, 300060520980596. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Global, Regional, and Country Estimates of Metabolic Syndrome Burden in Children and Adolescents in 2020: A Systematic Review and Modelling Analysis. Lancet Child Adolesc. Health 2022, 6, 158–170. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, J.; Zhao, Y.; Wang, N.; Jin, M.; Mao, W.; Zhu, G.; Wang, D.; Liang, J.; Shen, B.; et al. The Associations of Insulin Resistance, Obesity, and Lifestyle with the Risk of Developing Hyperuricaemia in Adolescents. BMC Endocr. Disord. 2024, 24, 220. [Google Scholar] [CrossRef]
- Altavilla, G.; Aliberti, S.; D’Elia, F. Assessment of Motor Performance and Self-Perceived Psychophysical Well-Being in Relation to Body Mass Index in Italian Adolescents. Children 2024, 11, 1119. [Google Scholar] [CrossRef]
- Manneville, F.; Legrand, K.; Omorou, A.Y.; Rydberg, J.A.; Langlois, J.; Böhme, P.; Saez, L.; Lecomte, E.; Briançon, S. Lifestyle Behaviors and Psychological Health in Adolescents with Overweight or Obesity: Cross-Sectional Associations with Weight Underestimation. Int. J. Behav. Med. 2024. [Google Scholar] [CrossRef]
- Chagas, D.D.V.; Joia, M.C. Motor Competence as a Protection Factor Against Pediatric Obesity: The Bidirectional Relationship Between Motor Competence and Weight Status. Res. Q. Exerc. Sport 2025, 96, 126–132. [Google Scholar] [CrossRef]
- Brzęk, A.; Sołtys, J.; Gallert-Kopyto, W.; Gwizdek, K.; Plinta, R. Body Posture in Children with Obesity—The Relationship to Physical Activity (PA). Pediatr. Endocrinol. Diabetes Metab. 2016, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.H.; Min, J.Y.; Seo, K.; Min, K.B. Association of Sedentary Lifestyle with Skeletal Muscle Strength and Mass in US Adolescents: Results from the National Health and Nutrition Examination Survey (2011–2014). J. Prev. Med. Public Health 2025. [Google Scholar] [CrossRef] [PubMed]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Global Trends in Insufficient Physical Activity Among Adolescents: A Pooled Analysis of 298 Population-Based Surveys with 1.6 Million Participants. Lancet Child Adolesc. Health 2020, 4, 23–35. [Google Scholar] [CrossRef]
- Watts, K.; Jones, T.W.; Davis, E.A.; Green, D. Exercise Training in Obese Children and Adolescents: Current Concepts. Sports Med. 2005, 35, 375. [Google Scholar] [CrossRef]
- Sarwan, G.; Daley, S.F.; Rehman, A. Management of Weight Loss Plateau. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Stricker, P.R.; Faigenbaum, A.D.; McCambridge, T.M.; Council on Sports Medicine and Fitness. Resistance Training for Children and Adolescents. Pediatrics 2020, 145, e20201011. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.C.; Torode, M.E.; Fiatarone Singh, M.A. The Effect of High-Intensity Progressive Resistance Training on Adiposity in Children: A Randomized Controlled Trial. Int. J. Obes. 2008, 32, 1016. [Google Scholar] [CrossRef]
- Watts, K.; Beye, P.; Siafarikas, A.; Davis, E.A.; Jones, T.W.; O’Driscoll, G.; Green, D.J. Exercise Training Normalizes Vascular Dysfunction and Improves Central Adiposity in Obese Adolescents. J. Am. Coll. Cardiol. 2004, 43, 1823. [Google Scholar] [CrossRef]
- Hansen, H.S.; Froberg, K.; Hyldebrandt, N.; Nielsen, J.R. A Controlled Study of Eight Months of Physical Training and Reduction of Blood Pressure in Children: The Odense Schoolchild Study. Br. Med. J. 1991, 303, 682. [Google Scholar] [CrossRef]
- Sung, R.Y.; Yu, C.W.; Chang, S.K.; Mo, S.W.; Woo, K.S.; Lam, C.W.K. Effects of Dietary Intervention and Strength Training on Blood Lipid Level in Obese Children. Arch. Dis. Child. 2002, 86, 407. [Google Scholar] [CrossRef]
- American College of Sports Medicine. The Prevention of Sport Injuries of Children and Adolescents. Med. Sci. Sports Exerc. 1993, 25, 1. [Google Scholar]
- Webb, D.R. Strength Training in Children and Adolescents. Pediatr. Clin. N. Am. 1990, 37, 1187. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, T.; Funato, K.; Ikegawa, S. The Effects of Resistance Training on Muscle Area and Strength in Prepubescent Age. Ann. Physiol. Anthropol. 1992, 11, 357. [Google Scholar] [CrossRef]
- Ozmun, J.C.; Mikesky, A.E.; Surburg, P.R. Neuromuscular Adaptations Following Prepubescent Strength Training. Med. Sci. Sports Exerc. 1994, 26, 510. [Google Scholar] [CrossRef]
- Schafer, J.P. Prepubescent and Adolescent Weight Training: Is It Safe? Is It Beneficial? J. Strength Cond. Res. 1991, 13, 39. [Google Scholar]
- Faigenbaum, A.D.; Myer, G.D.; Naclerio, F.; Casas, A. Injury Trends and Prevention in Youth Resistance Training. Strength Cond. J. 2011, 33, 36. [Google Scholar] [CrossRef]
- Myer, G.D.; Quatman, C.E.; Khoury, J.; Wall, E.J.; Hewett, T.E. Youth Versus Adult “Weightlifting” Injuries Presenting to United States Emergency Rooms: Accidental Versus Nonaccidental Injury Mechanisms. J. Strength Cond. Res. 2009, 23, 2054. [Google Scholar] [CrossRef]
- Faigenbaum, A.D.; Kraemer, W.J.; Blimkie, C.J.; Jeffreys, I.; Micheli, L.J.; Nitka, M.; Rowland, T.W. Youth Resistance Training: Updated Position Statement Paper from the National Strength and Conditioning Association. J. Strength Cond. Res. 2009, 23 (Suppl. S5), S60–S79. [Google Scholar] [CrossRef] [PubMed]
- McKelvie, K.J.; Khan, K.M.; McKay, H.A. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? A systematic review. Br. J. Sports Med. 2002, 36, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Alrushud, A.S. A cross-sectional study of musculoskeletal injuries related to exercise among gym members in Saudi Arabia in 2022: Prevalence, common types, and predictor factors. BMC Musculoskelet. Disord. 2024, 25, 621. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Woo, J.G.; Sinaiko, A.R.; Daniels, S.R.; Ikonen, J.; Juonala, M.; Kartiosuo, N.; Lehtimäki, T.; Magnussen, C.G.; Viikari, J.S.A.; et al. Childhood Cardiovascular Risk Factors and Adult Cardiovascular Events. N. Engl. J. Med. 2022, 386, 1877–1888. [Google Scholar] [CrossRef]
- Lurbe, E.; Mancia, G.; Calpe, J.; Drożdż, D.; Erdine, S.; Fernandez-Aranda, F.; Hadjipanayis, A.; Hoyer, P.F.; Jankauskiene, A.; Jiménez-Murcia, S.; et al. Joint Statement for Assessing and Managing High Blood Pressure in Children and Adolescents: Chapter 1. How to Correctly Measure Blood Pressure in Children and Adolescents. Front. Pediatr. 2023, 11, 1140357. [Google Scholar] [CrossRef] [PubMed]
- Wühl, E.; Calpe, J.; Drożdż, D.; Erdine, S.; Fernandez-Aranda, F.; Hadjipanayis, A.; Hoyer, P.F.; Jankauskiene, A.; Jiménez-Murcia, S.; Litwin, M.; et al. Joint Statement for Assessing and Managing High Blood Pressure in Children and Adolescents: Chapter 2. How to Manage High Blood Pressure in Children and Adolescents. Front. Pediatr. 2023, 11, 1140617. [Google Scholar] [CrossRef]
- Bülbül, S. Exercise in the treatment of childhood obesity. Turk. Pediatri. Ars. 2020, 55, 2–10. [Google Scholar]
- Davis, C.L.; Pollock, N.K.; Waller, J.L.; Allison, J.D.; Dennis, B.A.; Bassali, R.; Meléndez, A.; Boyle, C.A.; Gower, B.A. Exercise dose and diabetes risk in overweight and obese children: A rando ized controlled trial. JAMA 2012, 308, 1103–1112. [Google Scholar] [CrossRef]
- Dâmaso, A.R.; da Silveira Campos, R.M.; Caranti, D.A.; de Piano, A.; Fisberg, M.; Foschini, D.; de Lima Sanches, P.; Tock, L.; Lederman, H.M.; Tufik, S.; et al. Aerobic plus resistance training was more effective in improving the visceral adiposity, metabolic profile and inflammatory markers than aerobic training in obese adolescents. J. Sports Sci. 2014, 32, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Alberga, A.S.; Prud’homme, D.; Kenny, G.P.; Goldfield, G.S.; Hadjiyannakis, S.; Gougeon, R.; Phillips, P.; Malcolm, J.; Wells, G.; Doucette, S.; et al. Effects of aerobic and resistance training on abdominal fat, apolipoproteins and high-sensitivity C-reactive protein in adolescents with obesity: The HEARTY randomized clinical trial. Int. J. Obes. 2015, 39, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Goldfield, G.S.; Kenny, G.P.; Prud’homme, D.; Holcik, M.; Alberga, A.S.; Fahnestock, M.; Cameron, J.D.; Doucette, S.; Hadjiyannakis, S.; Tulloch, H.; et al. Effects of aerobic training, resistance training, or both on brain-derived neurotrophic factor in adolescents with obesity: The hearty randomized controlled trial. Physiol. Behav. 2018, 191, 138–145, Corrected in: Physiol. Behav. 2019, 198, 161. [Google Scholar] [CrossRef]
- Dias, K.A.; Ingul, C.B.; Tjønna, A.E.; Keating, S.E.; Gomersall, S.R.; Follestad, T.; Hosseini, M.S.; Hollekim-Strand, S.M.; Ro, T.B.; Haram, M.; et al. Effect of High-Intensity Interval Training on Fitness, Fat Mass and Cardiometabolic Biomarkers in Children with Obesity: A Randomised Controlled Trial. Sports Med. 2018, 48, 733–746. [Google Scholar] [CrossRef]
- Ingul, C.B.; Dias, K.A.; Tjonna, A.E.; Follestad, T.; Hosseini, M.S.; Timilsina, A.S.; Hollekim-Strand, S.M.; Ro, T.B.; Davies, P.S.W.; Cain, P.A.; et al. Effect of High Intensity Interval Training on Cardiac Function in Children with Obesity: A Randomised Controlled Trial. Prog. Cardiovasc. Dis. 2018, 61, 214–221. [Google Scholar] [CrossRef]
- Lee, J. Influences of exercise interventions on overweight and obesity in children and adolescents. Public Health Nurs. 2021, 38, 502–516. [Google Scholar] [CrossRef]
- Shao, X.; Tan, L.H.; He, L. Physical Activity and Exercise Alter Cognitive Abilities, and Brain Structure and Activity in Obese Children. Front. Neurosci. 2022, 16, 1019129. [Google Scholar] [CrossRef] [PubMed]
- Jullien, S.; Carai, S.; Weber, M.W. Addressing the Growing Burden of Obesity, Diabetes and Asthma in Children and Adolescents: The Role of Primary Health Care and the WHO Pocket Book in Europe for a Healthy Future. Glob. Pediatr. 2024, 9, 100186. [Google Scholar] [CrossRef]
- Dykstra, B.J.; Griffith, G.J.; Renfrow, M.S.; Mahon, A.D.; Harber, M.P. Cardiorespiratory and Muscular Fitness in Children and Adolescents with Obesity. Curr. Cardiol. Rep. 2024, 26, 349–357. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Queralt, M.; Vicente, M.A.; González, M.; Portillo, M.P. Metabolically Healthy Obesity and Metabolically Obese Normal Weight: A Review. J. Physiol. Biochem. 2021, 77, 175–189. [Google Scholar] [CrossRef]
- Ou, X.; Andres, A.; Pivik, R.T.; Cleves, M.A.; Badger, T.M. Brain Gray and White Matter Differences in Healthy Normal Weight and Obese Children. J. Magn. Reson. Imaging 2015, 42, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.C.; Moreno, B.; González-Santos, L.; Concha, L.; Barquera, S.; Barrios, F.A. Child Overweight and Obesity Are Associated with Reduced Executive Cognitive Performance and Brain Alterations: A Magnetic Resonance Imaging Study in Mexican Children. Pediatr. Obes. 2015, 10, 196–204. [Google Scholar] [CrossRef]
- Jiang, F.; Li, G.; Ji, W.; Zhang, Y.; Wu, F.; Hu, Y.; Zhang, W.; Manza, P.; Tomasi, D.; Volkow, N.D.; et al. Obesity Is Associated with Decreased Gray Matter Volume in Children: A Longitudinal Study. Cereb. Cortex 2023, 33, 3674–3682. [Google Scholar] [CrossRef] [PubMed]
- Fintini, D.; Cianfarani, S.; Cofini, M.; Andreoletti, A.; Ubertini, G.M.; Cappa, M.; Manco, M. The Bones of Children with Obesity. Front. Endocrinol. 2020, 11, 200. [Google Scholar] [CrossRef]
- Oliveira, A.; Monteiro, Â.; Jácome, C.; Afreixo, V.; Marques, A. Effects of Group Sports on Health-Related Physical Fitness of Overweight Youth: A Systematic Review and Meta-Analysis. Scand. J. Med. Sci. Sports 2017, 27, 604–611. [Google Scholar] [CrossRef]
- Chen, T.; Lin, J.; Lin, Y.; Xu, L.; Lu, D.; Li, F.; Hou, L.; Yu, C.C.W. Effects of Aerobic Exercise and Resistance Exercise on Physical Indexes and Cardiovascular Risk Factors in Obese and Overweight School-Age Children: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0257150. [Google Scholar] [CrossRef]
- Goldfield, G.S.; Kenny, G.P.; Alberga, A.S.; Prud’homme, D.; Hadjiyannakis, S.; Gougeon, R.; Phillips, P.; Tulloch, H.; Malcolm, J.; Doucette, S.; et al. Effects of Aerobic Training, Resistance Training, or Both on Psychological Health in Adolescents with Obesity: The HEARTY Randomized Controlled Trial. J. Consult. Clin. Psychol. 2015, 83, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Council on Sports Medicine and Fitness. Strength Training by Children and Adolescents. Pediatrics 2008, 121, 835–840. [Google Scholar] [CrossRef]
- Faigenbaum, A.; Pérez, T.; Naclerio Ayllón, F. Resistance Training for Overweight Youth. Kronos 2011, 10, 5–14. [Google Scholar]
- Franklin, B.A.; Rusia, A.; Haskin-Popp, C.; Tawney, A. Chronic Stress, Exercise and Cardiovascular Disease: Placing the Benefits and Risks of Physical Activity into Perspective. Int. J. Environ. Res. Public Health 2021, 18, 9922. [Google Scholar] [CrossRef]
- Faigenbaum, A.D.; McFarland, J. Resistance Training for Kids: Right from the Start. ACSM’s Health Fit. J. 2016, 20, 16–22. [Google Scholar] [CrossRef]
- Harries, S.K.; Lubans, D.R.; Callister, R. Resistance Training to Improve Power and Sports Performance in Adolescent Athletes: A Systematic Review and Meta-Analysis. J. Sci. Med. Sport 2012, 15, 532–540. [Google Scholar] [CrossRef]
- Woo, K.S.; Chook, P.; Yu, C.W.; Sung, R.Y.; Qiao, M.; Leung, S.S.; Lam, C.W.; Metreweli, C.; Celermajer, D.S. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation 2004, 109, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.A.; Chen, K.Y.; Lira, F.S.; Saraiva, B.T.; Antunes, B.M.; Campos, E.Z.; Freitas, I.F., Jr. Concurrent and aerobic exercise training promote similar benefits in body composition and metabolic profiles in obese adolescents. Lipids Health Dis. 2015, 14, 153. [Google Scholar] [CrossRef]
- Zehsaz, F.; Farhangi, N.; Ghahramani, M. The response of circulating omentin-1 concentration to 16-week exercise training in male children with obesity. Phys. Sportsmed. 2016, 44, 355–361. [Google Scholar] [CrossRef]
- Williams, C.F.; Bustamante, E.E.; Waller, J.L.; Davis, C.L. Exercise effects on quality of life, mood, and self-worth in overweight children: The SMART randomized controlled trial. Transl. Behav. Med. 2019, 9, 451–459. [Google Scholar] [CrossRef]
- Behringer, M.; Vom Heede, A.; Matthews, M.; Mester, J. Effects of Strength Training on Motor Performance Skills in Children and Adolescents: A Meta-Analysis. Pediatr. Exerc. Sci. 2011, 23, 186–206. [Google Scholar] [CrossRef] [PubMed]
- Serra-Paya, N.; Ensenyat, A.; Castro-Viñuales, I.; Real, J.; Sinfreu-Bergués, X.; Zapata, A.; Mur, J.M.; Galindo-Ortego, G.; Solé-Mir, E.; Teixido, C. Effectiveness of a Multi-Component Intervention for Overweight and Obese Children (Nereu Program): A Randomized Controlled Trial. PLoS ONE 2015, 10, e0144502. [Google Scholar] [CrossRef]
- Murphy, E.C.; Carson, L.; Neal, W.; Baylis, C.; Donley, D.; Yeater, R. Effects of an exercise intervention using Dance Dance Revolution on endothelial function and other risk factors in overweight children. Int. J. Pediatr. Obes. 2009, 4, 205–214. [Google Scholar] [CrossRef]
- Cohen, T.R.; Hazell, T.J.; Vanstone, C.A.; Rodd, C.; Weiler, H.A. Bone Health Is Maintained, While Fat Mass Is Reduced in Pre-Pubertal Children with Obesity Participating in a 1-Year Family-Centered Lifestyle Intervention. Calcif. Tissue Int. 2017, 101, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Fredericson, M. Influence of Sports Participation on Bone Health in the Young Athlete: A Review of the Literature. PM&R 2011, 3, 861–867. [Google Scholar]
- Eiholzer, U.; Meinhardt, U.; Petrò, R.; Witassek, F.; Gutzwiller, F.; Gasser, T. High-Intensity Training Increases Spontaneous Physical Activity in Children: A Randomized Controlled Study. J. Pediatr. 2010, 156, 242–246. [Google Scholar] [CrossRef]
- Smithey, J.; Debevec, C.; Jha, A.; Gillon, W.; Dillon, T.; Forrest, A.; Middleton, A.; Bhanat, E.; Nehete, P.; Melancon, D.P.; et al. The Relationship Between Obesity and Lateral Condyle Fracture Healing: A Pilot Study. J. Pediatr. Orthop. 2025, 45, e207–e211. [Google Scholar] [CrossRef] [PubMed]
- Fornari, E.D.; Suszter, M.; Roocroft, J.; Bastrom, T.; Edmonds, E.W.; Schlechter, J. Childhood obesity as a risk factor for lateral condyle fractures over supracondylar humerus fractures pediatrics. Clin. Orthop. Relat. Res. 2013, 471, 1193–1198. [Google Scholar] [CrossRef]
- Schranz, N.; Tomkinson, G.; Olds, T. What is the effect of resistance training on the strength, body composition and psychosocial status of overweight and obese children and adolescents? A Systematic review and meta-analysis. Sports Med. 2013, 43, 893–907. [Google Scholar] [CrossRef]
- Marson, E.C.; Delevatti, R.S.; Prado, A.K.; Netto, N.; Kruel, L.F.M. Effects of aerobic, resistance, and combined exercise training on in-sulin resistance markers in overweight or obese children and adoles-cents: A systematic review and meta-analysis. Prev. Med. 2016, 93, 211–218. [Google Scholar] [CrossRef]
- Tan, S.; Yang, C.; Wang, J. Physical training of 9- to 10-year-old children with obesity to lactate threshold intensity. Pediatr. Exerc. Sci. 2010, 22, 477–485. [Google Scholar] [CrossRef]
- Lloyd, R.S.; Faigenbaum, A.D.; Stone, M.H.; Oliver, J.L.; Jeffreys, I.; Moody, J.A.; Brewer, C.; Pierce, K.C.; McCambridge, T.M.; Howard, R.; et al. Position Statement on Youth Resistance Training: The 2014 International Consensus. Br. J. Sports Med. 2014, 48, 498–505. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, M.R.; Tatasciore, M.; Newton, R.U.; Pettigrew, S. Eight Weeks of Resistance Training Can Significantly Alter Body Composition in Children Who Are Overweight or Obese. J. Strength Cond. Res. 2009, 23, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Sgro, M.; McGuigan, M.R.; Pettigrew, S.; Newton, R.U. The Effect of Duration of Resistance Training Interventions in Children Who Are Overweight or Obese. J. Strength Cond. Res. 2009, 23, 1263–1270. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Lu, F.; Zhu, D. Effects of Aerobic Exercise Combined with Resistance Training on Body Composition and Metabolic Health in Children and Adolescents with Overweight or Obesity: Systematic Review and Meta-Analysis. Front. Public Health 2024, 12, 1409660. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.E.; Carrion, V.M.; Bhammar, D.M.; Howard, J.B.; Armstrong, S.C. A Randomized Trial of Inspiratory Training in Children and Adolescents with Obesity. Child Obes. 2024, 20, 517–525. [Google Scholar] [CrossRef]
- Mazur, A.; Zachurzok, A.; Baran, J.; Dereń, K.; Łuszczki, E.; Weres, A.; Wyszyńska, J.; Dylczyk, J.; Szczudlik, E.; Drożdż, D.; et al. Childhood Obesity: Position Statement of Polish Society of Pediatrics, Polish Society for Pediatric Obesity, Polish Society of Pediatric Endocrinology and Diabetes, the College of Family Physicians in Poland and Polish Association for Study on Obesity. Nutrients 2022, 14, 3806. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Davidson, C.R.; Wingard, E.E.; Wilcox, S.; Frongillo, E.A. Comparative effectiveness of plant-based diets for weight loss: A randomized controlled trial of five different diets. Nutrition 2015, 31, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Klementova, M.; Herynek, V.; Skoch, A.; Herynek, S.; Hill, M.; Mari, A.; Pelikanova, T. The Effect of a Vegetarian vs Conventional Hypocaloric Diabetic Diet on Thigh Adipose Tissue Distribution in Subjects with Type 2 Diabetes: A Randomized Study. J. Am. Coll. Nutr. 2017, 36, 364–369. [Google Scholar] [CrossRef]
- Szczygieł, B. Malnutrition: Incidence, Causes, Consequences, Diagnosis and Treatment. Clin. Chem. Line 2007, 2, 3–10. [Google Scholar]
- Pound, C.M.; Blair, B. Energy and Sports Drinks in Children and Adolescents. Paediatr. Child Health 2017, 22, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Desbrow, B.; McCormack, J.; Burke, L.M.; Cox, G.R.; Fallon, K.; Hislop, M.; Logan, R.; Marino, N.; Sawyer, S.M.; Shaw, G.; et al. Sports Dietitians Australia Position Statement: Sports Nutrition for the Adolescent Athlete. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 570–584. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Shao, A.; Inoue, T.; Kreider, R.B. Analysis of the Efficacy, Safety, and Regulatory Status of Novel Forms of Creatine. Amino Acids 2011, 40, 1369–1383. [Google Scholar] [CrossRef]
- Jagim, A.R.; Kerksick, C.M.; Barbieri, E. Creatine supplementation in children and adolescents. Nutrients 2021, 13, 664. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Ziegenhagen, R.; Braun, H.; Carlsohn, A.; Großhauser, M.; Heseker, H.; König, D.; Mosler, S.; Nieß, A.; Oberritter, H.; Schäbethal, K.; et al. Safety aspects of dietary supplements in sports. Ernahr. Umsch. 2020, 67, 42–50. [Google Scholar] [CrossRef]
- American Academy of Pediatrics, Committee on Nutrition. Pediatric nutrition. In Sports Nutrition; Kleinman, R.E., Greer, F.R., Eds.; American Academy of Pediatrics: Itasca, IL, USA, 2019; pp. 321–365. [Google Scholar]
- Gardiner, P.; Buettner, C.; Davis, R.B.; Phillips, R.S.; Kemper, K.J. Factors and Common Conditions Associated with Adolescent Dietary Supplement Use: An Analysis of the National Health and Nutrition Examination Survey (NHANES). BMC Complement. Altern. Med. 2008, 8, 9. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Sasaki, S.; Mezawa, H.; Konishi, M.; Igarashi, M.; Yamamoto-Hanada, K.; Nakayama, S.F.; Ohya, Y. Dietary Supplement Use in Elementary School Children: A Japanese Web-Based Survey. Environ. Health Prev. Med. 2021, 26, 63. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Demmelmair, H.; Grote, V.; Prell, C.; Weber, M. High Protein Intake in Young Children and Increased Weight Gain and Obesity Risk. Am. J. Clin. Nutr. 2016, 103, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Arnesen, E.K.; Thorisdottir, B.; Lamberg-Allardt, C.; Bärebring, L.; Nwaru, B.; Dierkes, J.; Ramel, A.; Åkesson, A. Protein intake in children and growth and risk of overweight or obesity: A systematic review and meta-analysis. Food Nutr. Res. 2022, 66, 10–29219. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; DiMarco, N.M.; Langley, S.; American Dietetic Association; Dietitians of Canada; American College of Sports Medicine. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Am. Diet. Assoc. 2009, 109, 509–527. Available online: https://europepmc.org/article/med/19278045 (accessed on 20 May 2025).
- Majewska, K.A.; Kobylinska, M.M.; Tchorzewska-Skrobich, M.; Korcz-Iżykowska, M.; Kędzia, A. Modifications of energy balance in the treatment of obesity in children. Piel Pol. 2020, 75, 57–63. [Google Scholar] [CrossRef]
- Walentukiewicz, A.; Wilk, B. Pre-training supplementation in top athletes in rhythmic gymnastics and men’s gymnastics. Żyw Człow Metab. 2009, XXXVI, 112–117. [Google Scholar]
- Bell, A.; Dorsch, K.D.; McCreary, D.R.; Hovey, R. A look at nutritional supplement use in adolescents. J. Adolesc. Health 2004, 34, 508–516. [Google Scholar] [CrossRef]
- Greenwood, M.; Kalman, D.S.; Antonio, J. Nutritional Supplements in Sports and Exercise; Humana Press: Totowa, NJ, USA, 2008. [Google Scholar]
- Desbrow, B. Youth athlete development and nutrition. Sports Med. 2021, 51, 3–12. [Google Scholar] [CrossRef]
- Arnaoutis, G.; Kavouras, S.A.; Angelopoulou, A. Fluid Balance During Training in Elite Young Athletes of Different Sports. J. Strength Cond. Res. 2015, 29, 3447–3452. [Google Scholar] [CrossRef]
- Cordrey, K.; Keim, S.A.; Milanaik, R. Adolescent consumption of sports drinks. Pediatrics 2018, 141, e20172784. [Google Scholar] [CrossRef]
- Suppiah, H.T.; Ling Ng, E.; Wee, J. Hydration status and fluid replacement strategies of high-performance adolescent athletes: An application of machine learning to distinguish hydration characteristics. Nutrients 2021, 13, 4073. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Risi, R.; Masi, D.; Caputi, A.; Balena, A.; Rossini, G.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Manfrini, S.; et al. Current evidence to propose different food supplements for weight loss: A comprehensive review. Nutrients 2020, 12, 2873. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Nutrition Standards for the Polish Population and Their Application. National Institute of Public Health-National Institute of Hygiene; National Institute of Public Health-National Institute of Hygiene: Warsaw, Poland, 2020. [Google Scholar]
- Bai, J.; Hu, Y.; Bruner, D.W. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7–18 years old children from the American Gut Project. Pediatr. Obes. 2019, 14, e12480. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, Y.J.; Kim, M.; Kim, M.; Kwak, J.H.; Lee, J.W.; Ahn, Y.T.; Sim, J.H.; Lee, J.H. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduced body adiposity and Lp-PLA2 activity in overweight subjects. J. Funct. Foods 2015, 19, 744–752. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Wiedmer, E.B.; Herter-Aeberli, I. The potential of prebiotic and probiotic supplementation during obese pregnancy to improve maternal and offspring’s metabolic health and reduce obesity risk—A narrative review. Front. Nutr. 2022, 9, 819882. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Piza, A.; Lee, S.J. Effects of dietary fibers and prebiotics in adiposity regulation via modulation of gut microbiota. Appl. Biol. Chem. 2020, 63, 2. [Google Scholar] [CrossRef]
- Park, D.Y.; Ahn, Y.T.; Park, S.H.; Huh, C.S.; Yoo, S.R.; Yu, R.; Sung, M.K.; McGregor, R.A.; Choi, M.S. Supplementation of lactobacillus curvatus KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS ONE 2013, 8, e59426. [Google Scholar] [CrossRef]
- Solito, A.; Bozzi Cionci, N.; Calgaro, M.; Caputo, M.; Vannini, L.; Hasballa, I.; Archero, F.; Giglione, E.; Ricotti, R.; Walker, G.E.; et al. Supplementation with Bifidobacterium Breve BR03 and B632 Strains Improved Insulin Sensitivity in Children and Adolescents with Obesity in a Cross-over, Randomized Double-Blind Placebo-Controlled Trial. Clin. Nutr. 2021, 40, 4585–4594. [Google Scholar] [CrossRef]
- Indiani, C.M.D.S.P.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T.M. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child. Obes. Print 2018, 14, 501–509. [Google Scholar] [CrossRef]
- Stenman, L.K.; Lehtinen, M.J.; Meland, N.; Christensen, J.E.; Yeung, N.; Saarinen, M.T.; Courtney, M.; Burcelin, R.; Lähdeaho, M.L.; Linros, J.; et al. Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults—Randomized controlled trial. EBioMedicine 2016, 13, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Michael, D.R.; Davies, T.S.; Jack, A.A.; Masetti, G.; Marchesi, J.R.; Wang, D.; Mullish, B.H.; Plummer, S.F. Daily supplementation with the Lab4P probiotic consortium induces significant weight loss in overweight adults. Sci. Rep. 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Farajian, S.; Kelishadi, R.; Mirlohi, M.; Hashemipour, M. The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: A randomized triple-masked controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Miccheli, A.; Capuani, G.; Marini, F.; Tomassini, A.; Praticò, G.; Ceccarelli, S.; Gnani, D.; Baviera, G.; Alisi, A.; Putignani, L.; et al. Urinary 1 H-NMR-based metabolic profiling of children with NAFLD undergoing VSL#3 treatment. Int. J. Obes. 2015, 39, 1118–1125. [Google Scholar]
- Chen, R.; Ai, Z.; Yang, X.; Zhang, Y.; Yuan, X. Effect of probiotics intake on obese children. Horm. Res. Paediatr. 2019, 91, 222–223. [Google Scholar]
- Gombert, M.; Sanchis Chorda, J.; Gomez Del Pulgar, E.; Benitez, P.A.; Martin, C.V.; Carrasco, L.J.; Codoner, F.P. Improvement of chronic inflammation in obese children consecutive to probiotic intake. Obes. Facts 2019, 12, 119. [Google Scholar]
- Alisi, A.; Bedogni, G.; Baviera, G.; Giorgio, V.; Porro, E.; Paris, C.; Giammaria, P.; Reali, L.; Anania, F.; Nobili, V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2014, 39, 1276–1285. [Google Scholar]
- Goyal, P.; Thapa, B.R.; Sharma, N.R.; Bhatia, A. Probiotic and lifestyle modification in obese pediatrics with non-alcoholic fatty liver disease. Indian J. Community Health 2019, 31, 50–56. [Google Scholar] [CrossRef]
| Part of Training | Intensity/Time | Recommendations | Contraindications |
|---|---|---|---|
| Warm-up |
|
|
|
| Proper training |
|
|
|
| Cool-down |
|
|
|
| General guidelines |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozioł-Kozakowska, A.; Wójcik, M.; Mazur-Kurach, P.; Drożdż, D.; Brzęk, A. Teenagers with Obesity at the Gym: Recommendations for Physical Activity, Diet, and Supplementation—A Narrative Review. Nutrients 2025, 17, 1798. https://doi.org/10.3390/nu17111798
Kozioł-Kozakowska A, Wójcik M, Mazur-Kurach P, Drożdż D, Brzęk A. Teenagers with Obesity at the Gym: Recommendations for Physical Activity, Diet, and Supplementation—A Narrative Review. Nutrients. 2025; 17(11):1798. https://doi.org/10.3390/nu17111798
Chicago/Turabian StyleKozioł-Kozakowska, Agnieszka, Małgorzata Wójcik, Paulina Mazur-Kurach, Dorota Drożdż, and Anna Brzęk. 2025. "Teenagers with Obesity at the Gym: Recommendations for Physical Activity, Diet, and Supplementation—A Narrative Review" Nutrients 17, no. 11: 1798. https://doi.org/10.3390/nu17111798
APA StyleKozioł-Kozakowska, A., Wójcik, M., Mazur-Kurach, P., Drożdż, D., & Brzęk, A. (2025). Teenagers with Obesity at the Gym: Recommendations for Physical Activity, Diet, and Supplementation—A Narrative Review. Nutrients, 17(11), 1798. https://doi.org/10.3390/nu17111798







