Evaluation of Bovine Lactoferrin for Prevention of Late-Onset Sepsis in Low-Birth-Weight Infants: A Double-Blind Randomized Controlled Trial
Abstract
1. Introduction
2. Methods
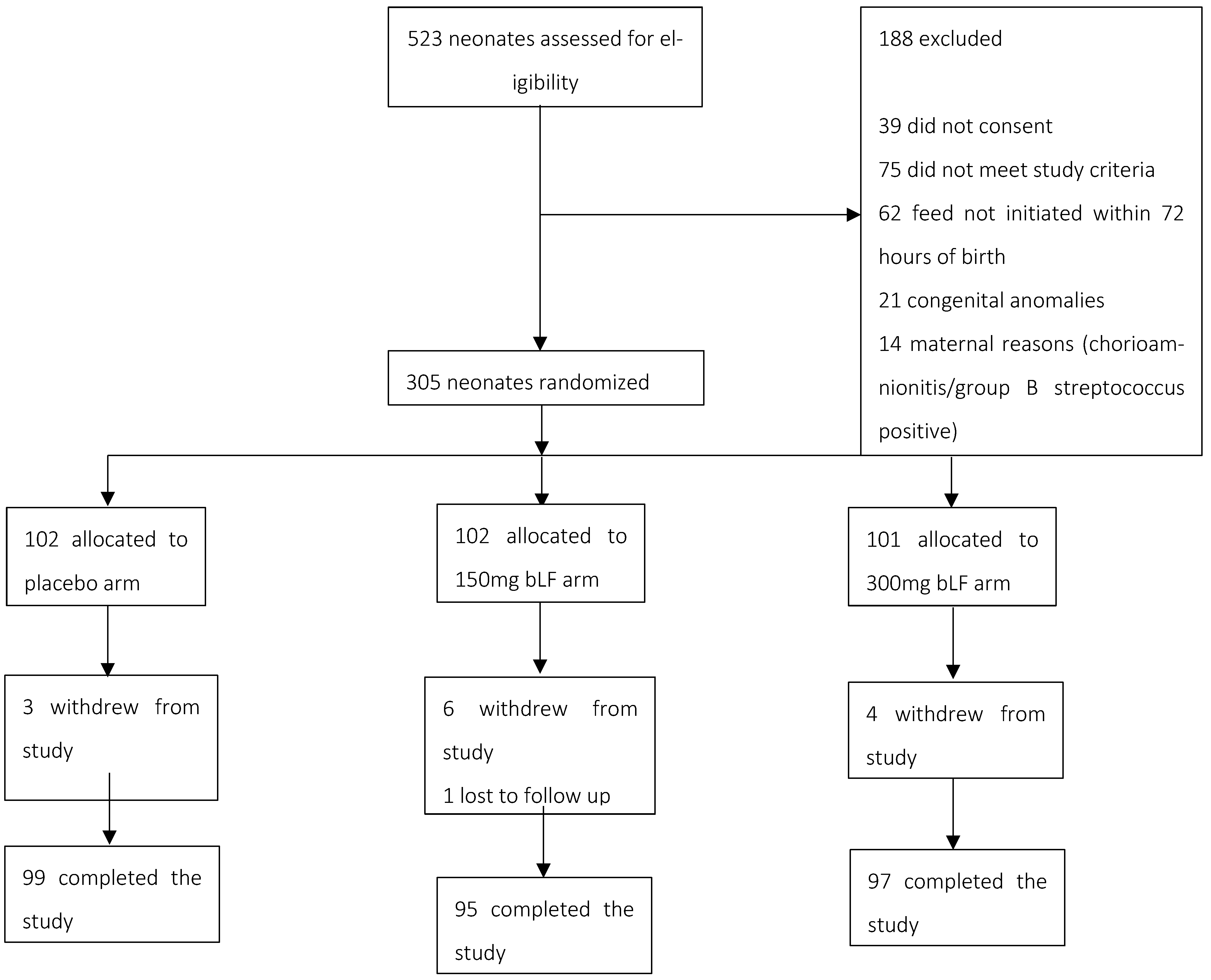
2.1. Trial Design, Setting, and Participants
2.2. Randomization and Masking
2.3. Procedures
2.4. Primary Outcome
2.5. Secondary Outcomes
2.6. Data Collection
2.7. Sample Size
2.8. Statistical Analysis
2.9. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Briggs, A.M. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Kampmann, B.; Mazmanian, S.K.; Marchant, A.; Levy, O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 2017, 46, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Tonk, E.C.M.; Piersma, A.H.; Van Loveren, H. 5.13—Reproductive and Developmental Immunology. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 249–269. [Google Scholar]
- Wynn, J.L.; Wong, H.R. 152—Pathophysiology of Neonatal Sepsis. In Fetal and Neonatal Physiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1536–1552.e10. [Google Scholar] [CrossRef]
- McGuire, W.; Clerihew, L.; Fowlie, P.W. Infection in the preterm infant. BMJ 2004, 329, 1277–1280. [Google Scholar] [CrossRef]
- Pace, E.; Yanowitz, T. Infections in the NICU: Neonatal sepsis. Semin. Pediatr. Surg. 2022, 31, 151200. [Google Scholar] [CrossRef]
- Savioli, K.; Rouse, C.; Susi, A.; Gorman, G.; Hisle-Gorman, E. Suspected or known neonatal sepsis and neurodevelopmental delay by 5 years. J. Perinatol. 2018, 38, 1573–1580. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Sizonenko, S.V. Lactoferrin and prematurity: A promising milk protein? Biochem. Cell Biol. 2017, 95, 22–30. [Google Scholar] [CrossRef]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Mendoza, K.; Carcamo, C.; Zegarra, J.; Bellomo, S.; Jacobs, J.; Cossey, V. Is Mother’s Own Milk Lactoferrin Intake Associated with Reduced Neonatal Sepsis, Necrotizing Enterocolitis, and Death? Neonatology 2020, 117, 167–174. [Google Scholar] [CrossRef]
- Tarnow-Mordi, W.O.; Abdel-Latif, M.E.; Martin, A.; Pammi, M.; Robledo, K.; Manzoni, P.; Osborn, D.; Lui, K.; Keech, A.; Hague, W.; et al. The effect of lactoferrin supplementation on death or major morbidity in very low birthweight infants (LIFT): A multicentre, double-blind, randomised controlled trial. Lancet Child Adolesc. Health 2020, 4, 444–454. [Google Scholar] [CrossRef]
- Griffiths, J.; Jenkins, P.; Vargova, M.; Bowler, U.; Juszczak, E.; King, A.; Manjunatha, C.M. Enteral lactoferrin supplementation for very preterm infants: A randomised placebo-controlled trial. Lancet 2019, 393, 423–433. [Google Scholar] [CrossRef]
- Ochoa, T.J. Is lactoferrin still a treatment option to reduce neonatal sepsis? Lancet Child Adolesc. Health 2020, 4, 411–412. [Google Scholar] [CrossRef]
- Ariff, S.; Soofi, S.; Aamir, A.; D’Almeida, M.; Aziz Ali, A.; Alam, A.; Dibley, M. Bovine Lactoferrin to Prevent Neonatal Infections in Low-Birth-Weight Newborns in Pakistan: Protocol for a Three-Arm Double-Blind Randomized Controlled Trial. JMIR Res. Protoc. 2021, 10, e23994. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2020, 3, Cd007137. [Google Scholar] [CrossRef]
- Berrington, J.E.; McGuire, W.; Embleton, N.D. ELFIN, the United Kingdom preterm lactoferrin trial: Interpretation and future questions. Biochem. Cell Biol. 2021, 99, 1–6. [Google Scholar] [CrossRef]
- Kaur, G.; Gathwala, G. Efficacy of Bovine Lactoferrin Supplementation in Preventing Late-onset Sepsis in low Birth Weight Neonates: A Randomized Placebo-Controlled Clinical Trial. J. Trop. Pediatr. 2015, 61, 370–376. [Google Scholar] [CrossRef]
- Fleischmann, C.; Reichert, F.; Cassini, A.; Horner, R.; Harder, T.; Markwart, R.; Tröndle, M.; Savova, Y.; Kissoon, N.; Schlattmann, P.; et al. Global incidence and mortality of neonatal sepsis: A systematic review and meta-analysis. Arch. Dis. Child. 2021, 106, 745–752. [Google Scholar] [CrossRef]
- Nwankwor, O.C.; McKelvie, B.; Frizzola, M.; Hunter, K.; Kabara, H.S.; Oduwole, A.; Oguonu, T.; Kissoon, N. A National Survey of Resources to Address Sepsis in Children in Tertiary Care Centers in Nigeria. Front. Pediatr. 2019, 7, 234. [Google Scholar] [CrossRef]
- Murthy, S.; Godinho, M.A.; Guddattu, V.; Lewis, L.E.S.; Nair, N.S. Risk factors of neonatal sepsis in India: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0215683. [Google Scholar] [CrossRef]
- Adatara, P.; Afaya, A.; Salia, S.M.; Afaya, R.A.; Konlan, K.D.; Agyabeng-Fandoh, E.; Agbinku, E.; Ayandayo, E.A.; Boahene, I.G. Risk Factors Associated with Neonatal Sepsis: A Case Study at a Specialist Hospital in Ghana. Sci. World J. 2019, 2019, 9369051. [Google Scholar] [CrossRef] [PubMed]
- Bohanon, F.J.; Nunez Lopez, O.; Adhikari, D.; Mehta, H.B.; Rojas-Khalil, Y.; Bowen-Jallow, K.A.; Radhakrishnan, R.S. Race, Income and Insurance Status Affect Neonatal Sepsis Mortality and Healthcare Resource Utilization. Pediatr. Infect. Dis. J. 2018, 37, e178–e184. [Google Scholar] [CrossRef] [PubMed]
- Ullah, O.; Khan, A.; Ambreen, A.; Ahmad, I.; Akhtar, T.; Gandapor, A.J.; Khan, A.M. Antibiotic Sensitivity pattern of Bacterial Isolates of Neonatal Septicemia in Peshawar, Pakistan. Arch. Iran. Med. 2016, 19, 866–869. [Google Scholar]
- Taylor, A.W.; Blau, D.M.; Bassat, Q.; Onyango, D.; Kotloff, K.L.; Arifeen, S.E.; Mandomando, I.; Chawana, R.; Baillie, V.L.; Akelo, V.; et al. Initial findings from a novel population-based child mortality surveillance approach: A descriptive study. Lancet Glob. Health 2020, 8, e909–e919. [Google Scholar] [CrossRef]
- Rahman, S.; Hameed, A.; Roghani, M.T.; Ullah, Z. Multidrug resistant neonatal sepsis in Peshawar, Pakistan. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, F52–F54. [Google Scholar] [CrossRef]
- Ochoa, T.; Loli, S.; Mendoza, K.; Carcamo, C.; Bellomo, S.; Cam, L.; Castaneda, A.; Campos, M.; Jacobs, J.; Cossey, V.; et al. Effect of bovine lactoferrin on prevention of late-onset sepsis in infants <1500 g: A pooled analysis of individual patient data from two randomized controlled trials. Biochem. Cell Biol. 2021, 99, 14–19. [Google Scholar]
- Liu, J.; Zhu, H.; Li, B.; Robinson, S.C.; Lee, C.; O’Connell, J.S.; Pierro, A. Lactoferrin Reduces Necrotizing Enterocolitis Severity by Upregulating Intestinal Epithelial Proliferation. Eur. J. Pediatr. Surg. 2020, 30, 90–95. [Google Scholar] [CrossRef]
- Robblee, E.D.; Erickson, P.S.; Whitehouse, N.L.; McLaughlin, A.M.; Schwab, C.G.; Rejman, J.J.; Rompala, R. Supplemental lactoferrin improves health and growth of Holstein calves during the preweaning phase. J. Dairy Sci. 2003, 86, 1458–1464. [Google Scholar] [CrossRef]
- Johnston, W.H.; Ashley, C.; Yeiser, M.; Harris, C.L.; Stolz, S.I.; Wampler, J.L.; Wittke, A.; Cooper, T.R. Growth and tolerance of formula with lactoferrin in infants through one year of age: Double-blind, randomized, controlled trial. BMC Pediatr. 2015, 15, 173. [Google Scholar] [CrossRef]
| Characteristics | Placebo | 150 mg bLF | 300 mg bLF |
|---|---|---|---|
| n = 102 | n = 102 | n = 101 | |
| Gestational age (weeks) | 34.0 (32.0–35.0) | 35.0 (33.0–36.0) | 34.0 (32.0–35.3) |
| Birth weight (grams) | 1885.0 (1520.0–2100.0) | 1960.0 (1700.0–2100.0) | 1900.0 (1600.0–2100.0) |
| Length (cms) | 43.5 (41.1–45.2) | 44.0 (42.3–45.3) | 43.8 (41.2–45.5) |
| Head circumference (cms) | 30.8 (29.5–32.0) | 31.1 (29.8–32.3) | 31.0 (29.6–32.0) |
| Sex of the child | |||
| Male | 46 (45.1%) | 44 (43.1%) | 49 (48.5%) |
| Antibiotics received | 32 (31.4%) | 28 (27.5%) | 30 (29.7%) |
| Mode of delivery | |||
| Spontaneous vaginal delivery | 17 (16.7%) | 18 (17.6%) | 10 (9.9%) |
| Caesarean section | 85 (83.3%) | 84 (82.4%) | 91 (90.1%) |
| Length of stay (days) | 5.5 (3.0–8.0) | 3.0 (2.0–6.0) | 4.0 (2.0–8.0) |
| Surfactant received | 3 (2.9%) | 5 (4.9%) | 6 (5.9%) |
| Oxygen received | 61 (59.8%) | 48 (47.1%) | 52 (51.5%) |
| Type of oxygen | |||
| CPAP | 19 (31.1%) | 15 (31.3%) | 20 (38.5%) |
| Mechanical ventilator | 2 (3.3%) | 3 (6.3%) | 2 (3.8%) |
| Nasal prongs | 40 (65.6%) | 30 (62.5%) | 30 (57.7%) |
| Age at recruitment (days) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) |
| Feeding method | |||
| Exclusive breastfeeding | 48/99 (48.5%) | 46/95 (48.4%) | 56/97 (57.7%) |
| Mixed feeding | 50/99 (50.5%) | 49/95 (51.6%) | 41/97 (42.3%) |
| Formula milk feeding | 1/99 (1.0%) | 0/95 (0.0%) | 0/97 (0.0%) |
| Outcome Measure | Arm | Placebo vs. 150 mg bLF p-Value | Placebo vs. 300 mg bLF p-Value | ||
|---|---|---|---|---|---|
| Placebo n = 99 | 150 mg bLF n = 95 | 300 mg bLF n = 99 | |||
| Culture-proven sepsis, n (%) | 8 (8%) | 1 (1%) | 5 (5%) | 0.020 | 0.390 |
| Presumed sepsis, n (%) | 3 (3%) | 4 (4%) | 3 (3%) | 0.660 | >0.999 |
| Presumed and culture-proven sepsis, n (%) | 11 (11%) | 5 (5%) | 8 (8%) | 0.140 | 0.470 |
| NEC, n (%) | 3 (3%) | 0 (0%) | 2 (2%) | 0.087 | 0.650 |
| * Pre-NEC, n (%) | 4 (4%) | 0 (0%) | 3 (3%) | 0.048 | 0.700 |
| Mortality | 0 | 0 | 2 | - | - |
| Fungal | Gram-Negative | Gram-Positive |
|---|---|---|
| Aspergillus Fumigatus | Bacilli | Corynebacterium species |
| Citrobacter Freundi | Micrococcus Species | |
| Escherichia Coli | Staphylococcus Aureus | |
| Klebsiella Pneumonia | Staphylococcus Species |
| Placebo | 150 mg bLF | 300 mg bLF | Placebo vs. 150 mg bLF p-Value | Placebo vs. 300 mg bLF p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | |||
| Weight (gm) ᵠ | ||||||||
| At enrollment | 1885 (1520.0–2100.0) | 102 | 1960 (1700.0–2100.0) | 102 | 1900 (1600.0–2100.0) | 101 | 0.120 | 0.880 |
| 14th day of life | 2155 (1750.0–2480.0) | 96 | 2200 (1985.0–2490.0) | 96 | 2032.5 (1780.0–2440.0) | 98 | 0.270 | 0.680 |
| 28th day of life | 2537.5 (2060.0–2900.0) | 94 | 2515 (2220.0–2900.0) | 94 | 2405 (2070.0–2800.0) | 96 | 0.380 | 0.550 |
| Average weight gain (gm) ᵠ | ||||||||
| Enrollment on the 28th day of life | 655.0 (420.0–830.0) | 94 | 620.0 (450.0–805.0) | 94 | 580.0 (420.0–770.0) | 96 | 0.800 | 0.220 |
| Arm | Placebo vs. 150 mg bLF p-Value | Placebo vs. 300 mg bLF p-Value | |||
|---|---|---|---|---|---|
| Placebo n = 99 | 150 mg bLF n = 95 | 300 mg bLF n = 97 | |||
| Children who took all doses, n (%) | 79 (79.8) | 78 (82.1) | 82 (84.5) | ||
| Compliance (sachets used/30 days) * | 95.7% | 98.3% | 98.6% | 0.093 | 0.055 |
| Children hospitalized, n (%) | 13 (13.1) | 9 (9.5) | 16 (16.5) | ||
| Length of stay in days ᵠ, median (IQR) | 14 (4.0–22.0) | 7 (4.0–12.0) | 6 (3.0–10.5) | 0.260 | 0.120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariff, S.; Soofi, S.B.; Jiwani, U.; Aamir, A.; Ansari, U.; Rizvi, A.; D’Almeida, M.; Alam, A.; Dibley, M. Evaluation of Bovine Lactoferrin for Prevention of Late-Onset Sepsis in Low-Birth-Weight Infants: A Double-Blind Randomized Controlled Trial. Nutrients 2025, 17, 1774. https://doi.org/10.3390/nu17111774
Ariff S, Soofi SB, Jiwani U, Aamir A, Ansari U, Rizvi A, D’Almeida M, Alam A, Dibley M. Evaluation of Bovine Lactoferrin for Prevention of Late-Onset Sepsis in Low-Birth-Weight Infants: A Double-Blind Randomized Controlled Trial. Nutrients. 2025; 17(11):1774. https://doi.org/10.3390/nu17111774
Chicago/Turabian StyleAriff, Shabina, Sajid Bashir Soofi, Uswa Jiwani, Almas Aamir, Uzair Ansari, Arjumand Rizvi, Michelle D’Almeida, Ashraful Alam, and Michael Dibley. 2025. "Evaluation of Bovine Lactoferrin for Prevention of Late-Onset Sepsis in Low-Birth-Weight Infants: A Double-Blind Randomized Controlled Trial" Nutrients 17, no. 11: 1774. https://doi.org/10.3390/nu17111774
APA StyleAriff, S., Soofi, S. B., Jiwani, U., Aamir, A., Ansari, U., Rizvi, A., D’Almeida, M., Alam, A., & Dibley, M. (2025). Evaluation of Bovine Lactoferrin for Prevention of Late-Onset Sepsis in Low-Birth-Weight Infants: A Double-Blind Randomized Controlled Trial. Nutrients, 17(11), 1774. https://doi.org/10.3390/nu17111774






