The Protective Effects of Pectic Polysaccharides on Dextran Sulfate Sodium-Induced Colitis in Drosophila melanogaster and Their Structure–Function Relationships
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Preparation of PPs from Different Sources
2.3. Structural Modification of PPs
2.3.1. Enzymatic Hydrolysis to Obtain Citrus Pectins with Different Molecular Weights
2.3.2. Ultrasound Treatment of Okra Pectic Polysaccharides to Obtain Different Molecular Weights
2.4. Structural Characterization of PPs
2.4.1. Chemical Composition Analysis
2.4.2. Relative Molecular Weight Measurement
2.4.3. FT-IR Spectra
2.4.4. Monosaccharide Composition Analysis
2.4.5. Methylation Analysis
2.5. DSS-Induced Colitis in Drosophila melanogaster
2.5.1. Drosophila Strains and Rearing
2.5.2. UC Model
- Control group: filter paper containing 5% (w/v) sucrose.
- DSS group: filter paper containing 5% (w/v) sucrose and 5% (w/v) DSS.
- PP-treated groups: filter paper containing 5% (w/v) sucrose, 5% (w/v) DSS, and PPs (0.5, 1, and 2 mg/mL).
2.5.3. Survival Rate Assay
2.5.4. Climbing Assay
2.5.5. Food Consumption Assay
2.5.6. Measurement of Intestinal Length and Body Weight
2.5.7. Smurf Assay
- Control: 2.5% erioglaucin disodium salt + 5% sucrose.
- DSS: 2.5% erioglaucin disodium salt + 5% sucrose + 5% DSS.
- PPs solution: 2.5% erioglaucin disodium salt + 5% sucrose + 5% DSS + 2 mg/mL PPs.
2.5.8. Hematoxylin and Eosin (H&E) Staining
2.5.9. RT-qPCR Analysis
2.6. Statistical Analysis
3. Results
3.1. The Anti-Inflammatory Effects of PPs Were Screened in a Drosophila Model
3.1.1. Structural Features of PP Samples
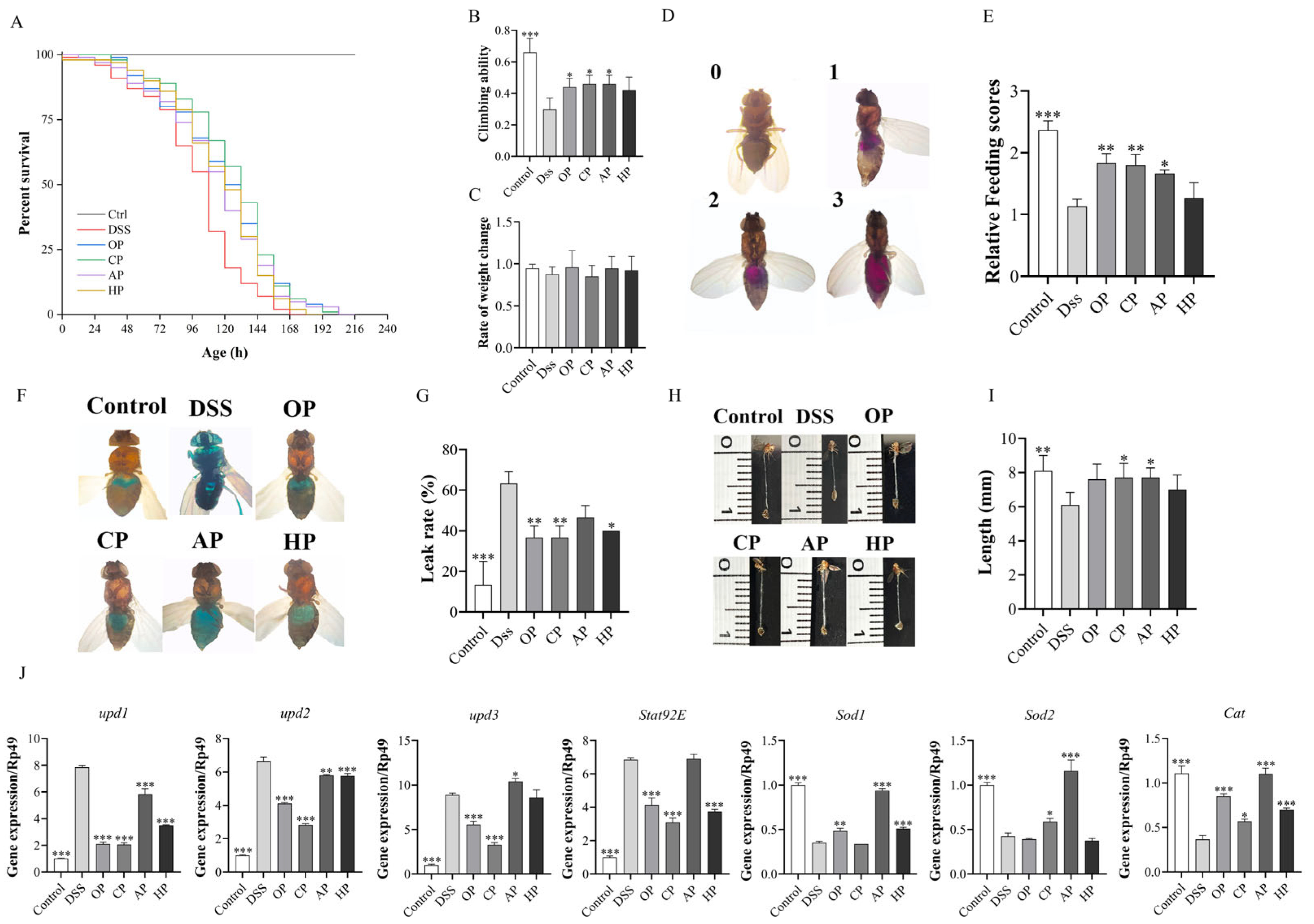
3.1.2. PP Supplementation Increased Survival Rates
3.1.3. PP Supplementation Improved Locomotion and Metabolism Abilities
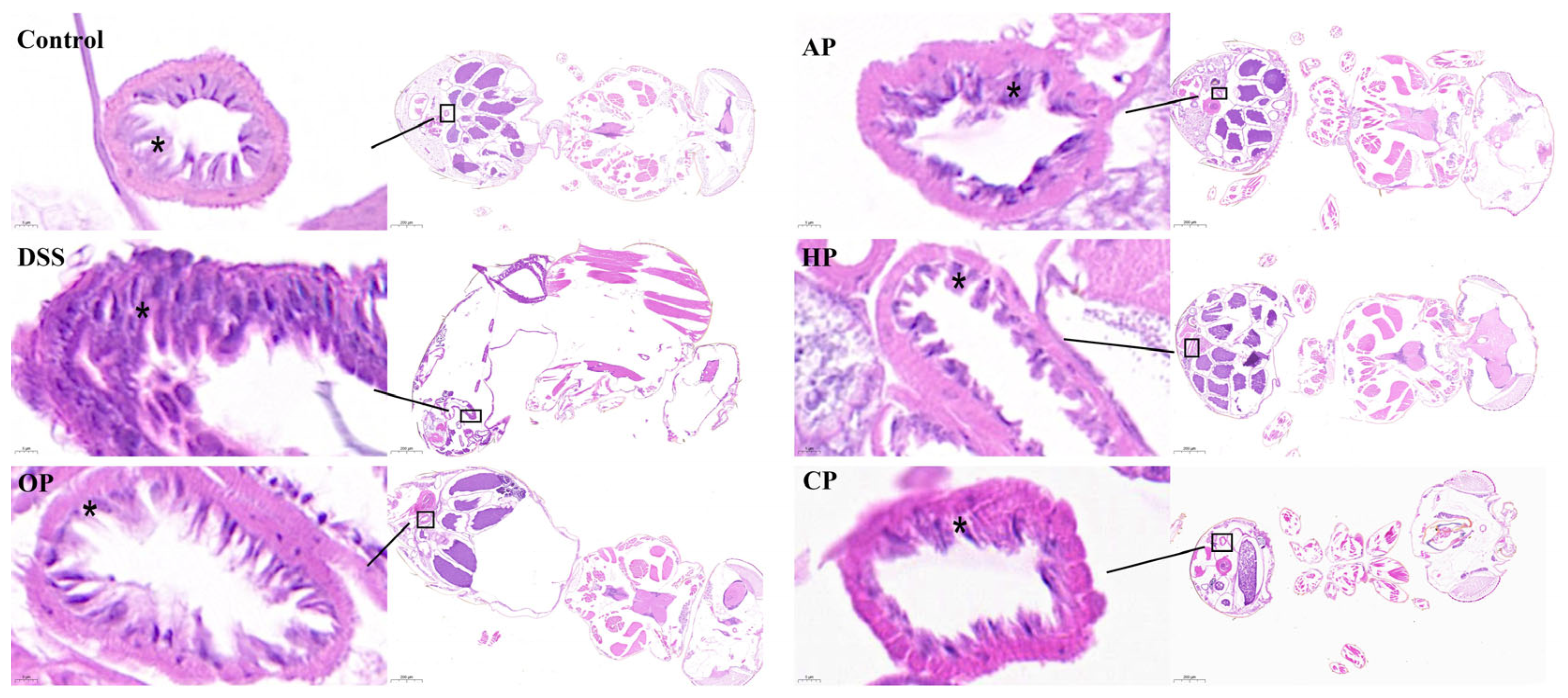
3.1.4. PP Supplementation Protected Intestinal Morphological Integrity
3.1.5. PP Supplementation Alleviates DSS-Induced Intestinal Damage by Regulating Related Signaling Pathways
3.2. Structural Modifications of OP and CP Were Performed, and Their Anti-Inflammatory Effects Were Investigated in a Drosophila Model
3.2.1. Structural Features of Different-Molecular-Weight PP Samples
3.2.2. Effect of Different-Molecular-Weight PP Supplementation on Survival Rate
3.2.3. Effect of Different-Molecular-Weight PP Supplementation on Locomotion and Metabolism Abilities
3.2.4. Effect of Different-Molecular-Weight PP Supplementation on Intestinal Morphological Integrity
3.2.5. Different-Molecular-Weight PP Supplementation Alleviates DSS-Induced Intestinal Damage by Regulating Related Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HG | homogalacturonan |
| RG-I | rhamnogalacturonan I |
| RG-II | rhamnogalacturonan II |
| PPs | pectic polysaccharides |
| UC | ulcerative colitis |
| DSS | dextran sodium sulfate |
| GalA | Galacturonic acid |
| IBD | Inflammatory bowel disease |
| Rha | rhamnose |
| Ara | arabinose |
| Xyl | xylose |
| Gal | galactose |
| Glc | glucose |
| OP | okra pectic polysaccharide |
| CP | citrus pectic polysaccharide |
| AP | apple pectic polysaccharide |
| HP | hawthorn pectic polysaccharide |
| HPSEC | high-performance size exclusion chromatography |
| RID | refractive index detector |
| HPAEC-PAD | high-performance anion-exchange chromatography with pulsed amperometric detection |
| FT-IR | Fourier transform infrared spectroscopy |
| GC-MS | gas chromatography mass spectrometry |
| TFA | trifluoroacetic acid |
| PMAA | partially methylated alditol acetates |
| H&E | hematoxylin and eosin |
| DM | degree of methyl esterification |
References
- Borren, N.Z.; van der Woude, C.J.; Ananthakrishnan, A.N. Fatigue in IBD: Epidemiology, pathophysiology and management. Nat. Rev. Gastro. Hepat. 2019, 16, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Aalto, A.L.; Saadabadi, A.; Lindholm, F.; Kietz, C.; Himmelroos, E.; Marimuthu, P.; Salo-Ahen, O.M.H.; Eklund, P.; Meinander, A. Stilbenoid compounds inhibit NF-κB-mediated inflammatory responses in the Drosophila intestine. Front Immunol. 2023, 14, 1253805. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.; Saeed, R.A.; Khan, M.I.; Faisal, M.N.; Saeed, H.A.; Abdi, G.; Aadil, R.M. Ultrasound-assisted ethanolic extract of Morus nigra fruit alleviates oxidative stress and inflammation via Keap1/Nrf2, dual oxidases, and JAK/STAT pathways. J. Agric. Food Res. 2024, 18, 101378. [Google Scholar] [CrossRef]
- Li, B.T.; Xiu, M.H.; He, L.; Zhou, S.H.; Yi, S.M.; Wang, X.Q.; Cao, W.J.; Liu, Y.Q.; He, J.Z. Protective effect of San Huang Pill and its bioactive compounds against ulcerative colitis in Drosophila via modulation of JAK/STAT, apoptosis, Toll, and Nrf2/Keap1 pathways. J. Ethnopharmacol. 2024, 322, 117578. [Google Scholar] [CrossRef]
- Pereira, M.T.; Malik, M.; Nostro, J.A.; Mahler, G.J.; Musselman, L.P. Effect of dietary additives on intestinal permeability in both Drosophila and a human cell co-culture. Dis. Model. Mech. 2018, 11, dmm034520. [Google Scholar] [CrossRef]
- Howard, A.M.; LaFever, K.S.; Fenix, A.M.; Scurrah, C.R.; Lau, K.S.; Burnette, D.T.; Bhave, G.; Ferrell, N.; Page-McCaw, A. DSS-induced damage to basement membranes is repaired by matrix replacement and crosslinking. J. Cell Sci. 2019, 132, jcs226860. [Google Scholar] [CrossRef]
- Yang, S.P.; Li, X.; Xiu, M.H.; Dai, Y.T.; Wan, S.F.; Shi, Y.; Liu, Y.Q.; He, J.Z. Flos puerariae ameliorates the intestinal inflammation of Drosophila via modulating the Nrf2/Keap1, JAK-STAT and Wnt signaling. Front. Pharmacol. 2022, 13, 893758. [Google Scholar] [CrossRef]
- Lee, S.H.; Goo, T.W.; Yun, E.Y. Allomyrina dichotoma larval extract has protective effects against gut permeability of dextran sulfate sodium-fed Drosophila by E-cadherin and armadillo. J. Ethnopharmacol. 2021, 279, a113786. [Google Scholar] [CrossRef]
- Ke, Y.; Lin, L.Z.; Zhao, M.M. Rhamnogalacturonan I-enriched pectin, flavonoids, and alkaloids from lotus leaf infusion in regulating glycolipid absorption and metabolism: Isolation, in vitro bioactivity verification, and structural characterization. J. Agric. Food Chem. 2023, 71, 8969–8980. [Google Scholar] [CrossRef]
- Shin, H.Y.; Kim, Y.S.; Kim, H.; Lee, K.-H.; Bae, Y.-J.; Moon, S.-K.; Shin, K.-S.; Suh, H.J.; Yu, K.-W. In vitro and in vivo effects of pectin-type polysaccharides isolated from crabapples (Malus prunifolia) on inflammatory colitis models. Int. J. Food. Sci. Tech. 2024, 59, 7334–7345. [Google Scholar] [CrossRef]
- Yu, C.X.; Wu, D.M.; Zhu, K.; Hou, L.J.; Xiao, H.; Ding, T.; Liu, D.H.; Ye, X.Y.; Linhardt, R.J.; Chen, S.G. Challenges of pectic polysaccharides as a prebiotic from the perspective of fermentation characteristics and anti-colitis activity. Carbohyd. Polym. 2021, 270, 118377. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Sahasrabudhe, N.M.; RoSch, C.; Schols, H.A.; Faas, M.M.; De Vos, P. The impact of dietary fibers on dendritic cell responses in vitro is dependent on the differential effects of the fibers on intestinal epithelial cells. Mol. Nutr. Food Res. 2015, 59, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Ju, M.G.; Ma, C.Y.; Li, K.; Cai, S. Immunomodulatory acidic polysaccharide from jujube fruit (Zizyphus jujuba Mill.): Insight into their chemical characteristics and modes of action. J. Agric. Food Chem. 2024, 73, 450–463. [Google Scholar] [CrossRef]
- Sabater, C.; Alberto Molina-Tijeras, J.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal anti-inflammatory effects of artichoke pectin and modified pectin fractions in the dextran sulfate sodium model of mice colitis. Artificial neural network modelling of inflammatory markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef]
- Oh, J.H.; Chung, J.O.; Lee, C.Y.; Yun, Y.C.; Park, M.Y.; Hong, Y.D.; Kim, W.G.; Cha, H.Y.; Shin, K.S.; Hong, G.P.; et al. Characterized polysaccharides from green tea inhibited starch hydrolysis and glucose intestinal uptake by inducing microstructural changes of wheat starch. J. Agric. Food Chem. 2021, 69, 14075–14085. [Google Scholar] [CrossRef]
- Huo, Z.Q.; Li, J.X.; Li, X.F.; Xiao, H.; Lin, Y.; Ma, Y.C.; Li, J.R.; Yang, H.; Zhang, C.J. Functional fractions of Astragalus polysaccharides as a potential prebiotic to alleviate ulcerative colitis. Int. J. Biol. Macromol. 2024, 271, 132580. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hromádková, Z.; Paulsen, B.S.; Polovka, M.; Košťálová, Z.; Ebringerová, A. Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohyd. Polym. 2013, 93, 22–30. [Google Scholar] [CrossRef]
- Zhang, H.X.; Liu, F.F.; Wu, P.; Li, C.; Chen, Q.J.; Wu, H.X.; Qi, X.P. Degradation of (1→3) (1→6)-α-D-dextran by ultrasound: Molecular weight, viscosity and kinetics. Int. J. Biol. Macromol. 2024, 283, 137446. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.F.; Li, C.Y.; Fu, Y.P.; Jiang, Q.X.; Peng, X.; Li, L.X.; Song, X.; Zhao, X.-H.; Li, Y.P.; Chen, X.F.; et al. The comparison of preliminary structure and intestinal anti-inflammatory and anti-oxidative activities of polysaccharides from different root parts of Angelica sinensis (Oliv.) Diels. J. Ethnopharmacol. 2022, 295, 115446. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, W.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Monosaccharide composition analysis of polysaccharides from natural sources: Hydrolysis condition and detection method development. Food Hydrocolloid. 2021, 116, 106641. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohyd. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Remy, N.Q.; Guevarra, J.A.; Vonhoff, F.J. Food supplementation with wheat gluten leads to climbing performance decline in Drosophila melanogaster. MicroPubl. Biol. 2022, 23, 2022. [Google Scholar] [CrossRef]
- Linford, N.J.; Bilgir, C.; Ro, J.; Pletcher, S.D. Measurement of lifespan in Drosophila melanogaster. Jove-J. Vis. Exp. 2013, 71, 50068. [Google Scholar] [CrossRef]
- Zhang, G.C.; Gu, Y.Y.; Dai, X.J. Protective effect of bilberry anthocyanin extracts on dextran sulfate sodium-induced intestinal damage in Drosophila melanogaster. Nutrients 2022, 14, 2875. [Google Scholar] [CrossRef]
- Salem, M.B.; Elzallat, M.; Mostafa Mohammed, D.; Hammam, O.A.; Tamim, A.; Abdel-Wareth, M.; Hassan, M. Helix pomatia mucin alleviates DSS-induced colitis in mice: Unraveling the cross talk between microbiota and intestinal chemokine. Heliyon 2024, 10, e37362. [Google Scholar] [CrossRef]
- Morales, M.A.; Mendoza, B.M.; Lavine, L.C.; Lavine, M.D.; Walsh, D.B.; Zhu, F. Selection of reference genes for expression studies of xenobiotic adaptation in Tetranychus urticae. Int. J. Biol. Sci. 2016, 12, 1129–1139. [Google Scholar] [CrossRef]
- Denman, L.J.; Morris, G.A. An experimental design approach to the chemical characterisation of pectin polysaccharides extracted from Cucumis melo Inodorus. Carbohyd Polym. 2015, 117, 364–369. [Google Scholar] [CrossRef]
- Roman, L.; Guo, M.; Terekhov, A.; Grossutti, M.; Vidal, N.P.; Reuhs, B.L.; Martinez, M.M. Extraction and isolation of pectin rich in homogalacturonan domains from two cultivars of hawthorn berry (Crataegus pinnatifida). Food Hydrocolloid. 2021, 113, 106476. [Google Scholar] [CrossRef]
- Huang, L.L.; Sun, Q.; Li, Q.H.; Li, X. Screening and characterization of an anti-inflammatory pectic polysaccharide from Cucurbita moschata Duch. Int. J. Biol. Macromol. 2024, 264, 130510. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.R.; Sung, S.K.; Jang, M.; Lim, T.-G.; Cho, C.-W.; Han, C.-J.; Hong, H.-D. Enzyme-assisted extraction, chemical characteristics, and immunostimulatory activity of polysaccharides from Korean ginseng (Panax ginseng Meyer). Int. J. Biol. Macromol. 2018, 116, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Z.; Xiu, M.H.; Li, S.; Shi, Y.; Wang, X.Q.; Lin, X.Y.; Cai, H.; Liu, Y.Q.; He, J.Z. Exposure to cytarabine causes side effects on adult development and physiology and induces intestinal damage via apoptosis in Drosophila. Biomed. Pharmacother. 2023, 159, 114265. [Google Scholar] [CrossRef]
- Wu, D.M.; Chen, S.G.; Ye, X.Q.; Ahmadi, S.; Hu, W.W.; Yu, C.X.; Zhu, K.; Cheng, H.; Linhardt, R.J.; He, Q.J. Protective effects of six different pectic polysaccharides on DSS-induced IBD in mice. Food Hydrocolloid. 2022, 127, 107209. [Google Scholar] [CrossRef]
- Zhang, K.E.; Feng, N.X.; Wang, Y.Z.; Li, N.; Qi, X.Y.; Ouyang, X.Y.; Wang, Q.; Liu, M.Q. Exploring the competitive inhibition of α-glucosidase by citrus pectin enzymatic hydrolysate and its mechanism: An integrated experimental and simulation approach. Food Chem. 2025, 464, 141819. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, Y.Y.; Ma, H.L.; Wang, Z.B. Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from Phellinus linteus mycelia. Ultrason. Sonochem. 2016, 29, 251–257. [Google Scholar] [CrossRef]
- Guo, Q.B.; Du, J.H.; Jiang, Y.; Goff, H.D.; Cui, S.W. Pectic polysaccharides from hawthorn: Physicochemical and partial structural characterization. Food Hydrocolloid. 2019, 90, 146–153. [Google Scholar] [CrossRef]
- Hu, J.T.; Mei, Y.X.; Zhang, H.; Li, J.; Zhang, M.; Li, Y.B.; Yang, W.D.; Liu, Y.Y.; Liang, Y.X. Ameliorative effect of an acidic polysaccharide from Phellinus linteus on ulcerative colitis in a DSS-induced mouse model. Int. J. Biol. Macromol. 2024, 265, 130959. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Molina-Tijeras, J.A.; Montilla, A.; Vezza, T.; Sánchez-Milla, M.; Rico-Rodríguez, F.; Villamiel, M. Pectin from sunflower by-products obtained by ultrasound: Chemical characterization and in vivo evaluation of properties in inflammatory bowel disease. Int. J. Biol Macromol. 2023, 246, 125505. [Google Scholar] [CrossRef]
- Wu, D.M.; Chen, S.G.; Ye, X.Q.; Zheng, X.L.; Ahmadi, S.; Hu, W.W.; Yu, C.X.; Cheng, H.; Linhardt, R.J.; Chen, J.L. Enzyme-extracted raspberry pectin exhibits a high-branched structure and enhanced anti-inflammatory properties than hot acid-extracted pectin. Food Chem. 2022, 383, 132387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Liao, J.S.; Qi, J.R. Modulation of Gut Microbiota by Pectin: The critical role of degree of esterification and rhamnogalacturonan-I ratios. Food Biosci. 2024, 64, 105763. [Google Scholar] [CrossRef]
- Jiang, F.; Ding, Y.Y.; Tian, Y.; Yang, R.X.; Quan, M.L.; Tong, Z.Y.; Zhang, X.L.; Luo, D.; Chi, Z.; Liu, C.G. Hydrolyzed low-molecular-weight polysaccharide from Enteromorpha prolifera exhibits high anti-inflammatory activity and promotes wound healing. Biomater. Adv. 2022, 133, 112637. [Google Scholar] [CrossRef] [PubMed]
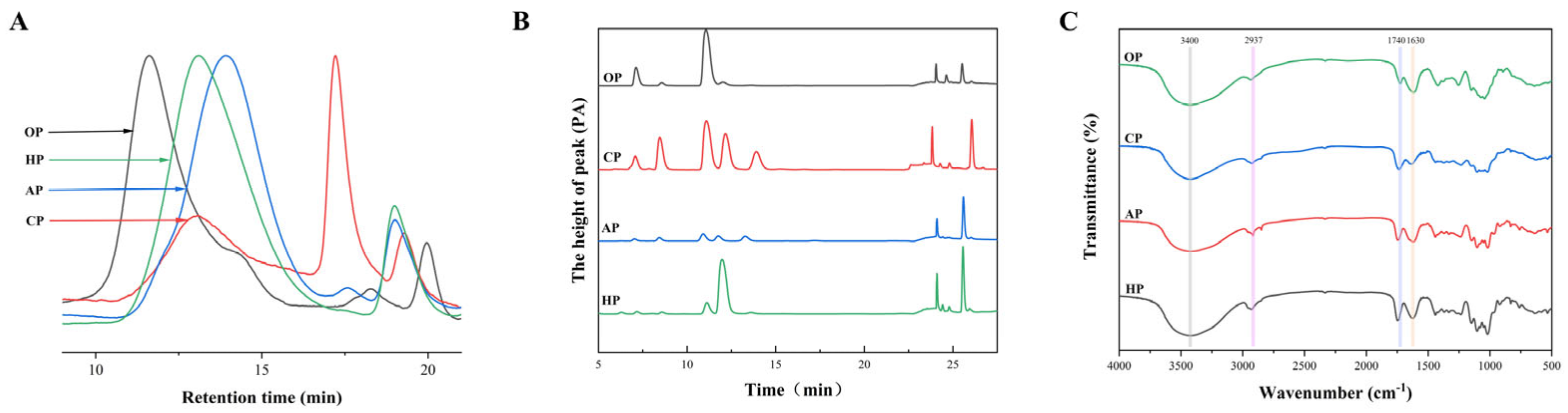


| Chemical Features | OP | CP | AP | HP |
|---|---|---|---|---|
| Rha (%) | 20.89 ± 0.71 a | 7.05 ± 1.67 b | 2.89 ± 0.05 c | 1.76 ± 0.14 c |
| Ara (%) | 3.03 ± 0.66 c | 12.91 ± 1.57 a | 4.91 ± 1.83 b | 1.12 ± 0.33 d |
| Gal (%) | 45.01 ± 5.79 a | 19.90 ± 2.71 b | 8.19 ± 1.55 c | 5.38 ± 0.28 c |
| Glu (%) | 2.42 ± 1.30 c | 13.25 ± 1.74 b | 4.53 ± 0.45 c | 30.93 ± 3.99 a |
| Xyl (%) | - | 6.76 ± 1.18 a | 2.73 ± 1.41 b | 0.72 ± 0.10 c |
| GalA (%) | 28.66 ± 4.63 d | 40.13 ± 6.51 c | 76.74 ± 1.47 a | 60.09 ± 4.64 b |
| % HG = (GalA − Rha) | 7.77 | 33.08 | 73.85 | 58.33 |
| % RG-I = (2Rha + Ara + Gal) | 89.81 | 46.92 | 18.89 | 10.01 |
| Ratio 1 | 0.42 | 1.01 | 4.80 | 7.28 |
| Ratio 2 | 0.73 | 0.18 | 0.04 | 0.03 |
| Ratio 3 | 2.30 | 4.65 | 4.53 | 3.71 |
| Total sugar (wt%) | 45.57 ± 1.81 a | 41.45 ± 2.34 b | 30.87 ± 2.96 c | 40.29 ± 0.80 b |
| Uronic acid (wt%) | 31.87 ± 1.20 d | 41.56 ± 1.33 c | 58.54 ± 4.41 a | 52.83 ± 1.61 b |
| Total phenolic (wt%) | 0.30 ± 0.00 c | 1.36 ± 0.03 a | 1.28 ± 0.09 b | 0.18 ± 0.06 d |
| Protein (wt%) | 4.86 ± 0.19 b | 4.27 ± 0.89 b | 12.71 ± 1.40 a | 4.91 ± 0.57 b |
| Mw (kDa) | 4289.47 | 676.57 | 231.32 | 643.21 |
| Methyl esterification degree (DM) (%) | 19.34 ± 3.04 d | 72.07 ± 3.86 a | 32.11 ± 1.71 c | 38.67 ± 2.75 b |
| Chemical Features | CP | CP1 | CP2 | OP | OP1 | OP2 |
|---|---|---|---|---|---|---|
| Rha (%) | 7.05 ± 1.67 c | 7.17 ± 0.34 c | 5.02 ± 2.40 c | 20.89 ± 0.71 a | 16.01 ± 3.61 b | 21.22 ± 0.83 a |
| Ara (%) | 12.91 ± 1.57 a | 8.00 ± 1.05 b | 8.99 ± 1.82 b | 3.03 ± 0.66 c | 3.22 ± 1.67 c | 2.08 ± 0.90 c |
| Gal (%) | 19.90 ± 2.71 d | 18.76 ± 0.46 d | 24.27 ± 0.45 c | 45.01 ± 5.79 b | 51.11 ± 1.35 a | 48.16 ± 1.63 bc |
| Glu (%) | 13.25 ± 1.74 a | 4.20 ± 1.39 b | 3.97 ± 0.63 b | 2.42 ± 1.30 bc | 1.86 ± 1.77 c | 1.18 ± 1.05 c |
| Xyl (%) | 6.76 ± 1.18 a | 1.10 ± 0.70 b | 1.15 ± 0.27 b | - | - | - |
| GalA (%) | 40.13 ± 6.51 b | 60.77 ± 2.34 a | 56.59 ± 0.49 a | 28.66 ± 4.63 c | 27.81 ± 2.41 c | 27.34 ± 2.65 c |
| % HG = (GalA − Rha) | 33.08 | 53.60 | 51.57 | 7.77 | 7.11 | 6.12 |
| % RG-I = (2 Rha + Ara + Gal) | 46.92 | 41.10 | 43.31 | 89.81 | 92.89 | 92.70 |
| Ratio 1 | 1.01 | 1.79 | 1.48 | 0.42 | 0.33 | 0.38 |
| Ratio 2 | 0.18 | 0.12 | 0.09 | 0.73 | 0.71 | 0.78 |
| Ratio 3 | 4.65 | 3.73 | 6.62 | 2.30 | 3.24 | 2.37 |
| Total sugar (wt%) | 41.45 ± 2.34 b | 43.84 ± 0.67 bc | 43.27 ± 1.12 bc | 45.57 ± 1.81 a | 41.37 ± 1.16 b | 43.05 ± 0.89 bc |
| Uronic acid (wt%) | 41.56 ± 1.33 c | 64.37 ± 2.12 a | 59.64 ± 1.66 b | 31.87 ± 1.20 d | 28.23 ± 0.41 e | 31.47 ± 1.99 d |
| Total phenolic (wt%) | 1.36 ± 0.03 a | 0.33 ± 0.02 b | 0.29 ± 0.01 c | 0.30 ± 0.00 c | 0.34 ± 0.01 b | 0.05 ± 0.00 d |
| Protein (wt%) | 4.27 ± 0.89 a | 1.23 ± 0.31 bc | 0.94 ± 0.02 bc | 4.86 ± 0.19 a | 1.53 ± 0.26 b | 0.73 ± 0.20 c |
| Mw (kDa) | 676.57 | 65.68 | 18.18 | 4289.47 | 1620.59 | 119.12 |
| Methyl esterification degree (DM) (%) | 72.07 ± 3.86 a | 55.04 ± 2.92 b | 55.48 ± 1.23 b | 19.34 ± 3.04 d | 20.44 ± 1.31 d | 24.60 ± 1.37 c |
| Linkage Patterns | Mol Ratios (%) | |||||
|---|---|---|---|---|---|---|
| CP | CP1 | CP2 | OP | OP1 | OP2 | |
| T-Galp | 11.40 | 9.16 | 9.35 | 27.45 | 24.76 | 26.18 |
| 4-Galp(A) | 61.24 | 64.33 | 56.81 | 29.87 | 29.56 | 33.65 |
| 3,4-Galp(A) | 4.90 | 4.66 | 5.21 | 2.87 | 2.50 | 2.98 |
| 4,6-Galp(A) | 3.35 | 2.75 | 2.97 | - | - | - |
| Total galactose/galacturonic acid | 80.88 | 80.90 | 74.34 | 60.19 | 56.82 | 62.81 |
| T-Araf | 2.02 | 2.76 | 4.89 | - | - | - |
| 2-Araf | 1.16 | 0.76 | 1.66 | - | - | - |
| 5-Araf | 0.91 | 1.01 | 5.95 | - | - | - |
| 2,3,4-Arap | - | - | - | 3.25 | 3.35 | 4.17 |
| Total arabinose | 4.09 | 4.52 | 12.50 | 3.25 | 3.35 | 4.17 |
| T-Rhap | - | - | 5.97 | - | - | - |
| 2-Rhap | 2.32 | 3.78 | 2.47 | 6.95 | 8.47 | 4.30 |
| 2,4-Rhap | 2.14 | 2.13 | - | 21.55 | 20.00 | 19.74 |
| 2,3,4-Rhap | - | - | - | 2.45 | 1.64 | 2.11 |
| Total rhamnose | 4.46 | 5.91 | 8.44 | 30.94 | 30.12 | 26.15 |
| T-Glcp | 1.20 | 0.66 | 0.82 | 2.84 | 4.86 | 2.42 |
| 4-Glcp | 9.38 | 7.31 | 2.91 | 2.78 | 4.85 | 3.23 |
| Total glucose | 10.58 | 7.97 | 3.72 | 5.61 | 9.71 | 5.65 |
| T-Xylp | - | 0.70 | 0.99 | - | - | - |
| Total xylose | - | 0.70 | 0.99 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Qi, T.; Cheng, B.; Guo, Y.; Atehli, D.; Cui, S.W.; Kang, J.; Guo, Q. The Protective Effects of Pectic Polysaccharides on Dextran Sulfate Sodium-Induced Colitis in Drosophila melanogaster and Their Structure–Function Relationships. Nutrients 2025, 17, 1738. https://doi.org/10.3390/nu17101738
Sun Z, Qi T, Cheng B, Guo Y, Atehli D, Cui SW, Kang J, Guo Q. The Protective Effects of Pectic Polysaccharides on Dextran Sulfate Sodium-Induced Colitis in Drosophila melanogaster and Their Structure–Function Relationships. Nutrients. 2025; 17(10):1738. https://doi.org/10.3390/nu17101738
Chicago/Turabian StyleSun, Zhenou, Tianyu Qi, Boyu Cheng, Yingxiao Guo, Dima Atehli, Steve W. Cui, Ji Kang, and Qingbin Guo. 2025. "The Protective Effects of Pectic Polysaccharides on Dextran Sulfate Sodium-Induced Colitis in Drosophila melanogaster and Their Structure–Function Relationships" Nutrients 17, no. 10: 1738. https://doi.org/10.3390/nu17101738
APA StyleSun, Z., Qi, T., Cheng, B., Guo, Y., Atehli, D., Cui, S. W., Kang, J., & Guo, Q. (2025). The Protective Effects of Pectic Polysaccharides on Dextran Sulfate Sodium-Induced Colitis in Drosophila melanogaster and Their Structure–Function Relationships. Nutrients, 17(10), 1738. https://doi.org/10.3390/nu17101738






