The Maternal and Fetal Consequences of Metabolic Dysfunction-Associated Fatty Liver Disease and Gestational Diabetes Mellitus
Abstract
1. Introduction
2. Methodology
3. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)
3.1. Prevalence of MAFLD
3.2. Risk Factors for MAFLD
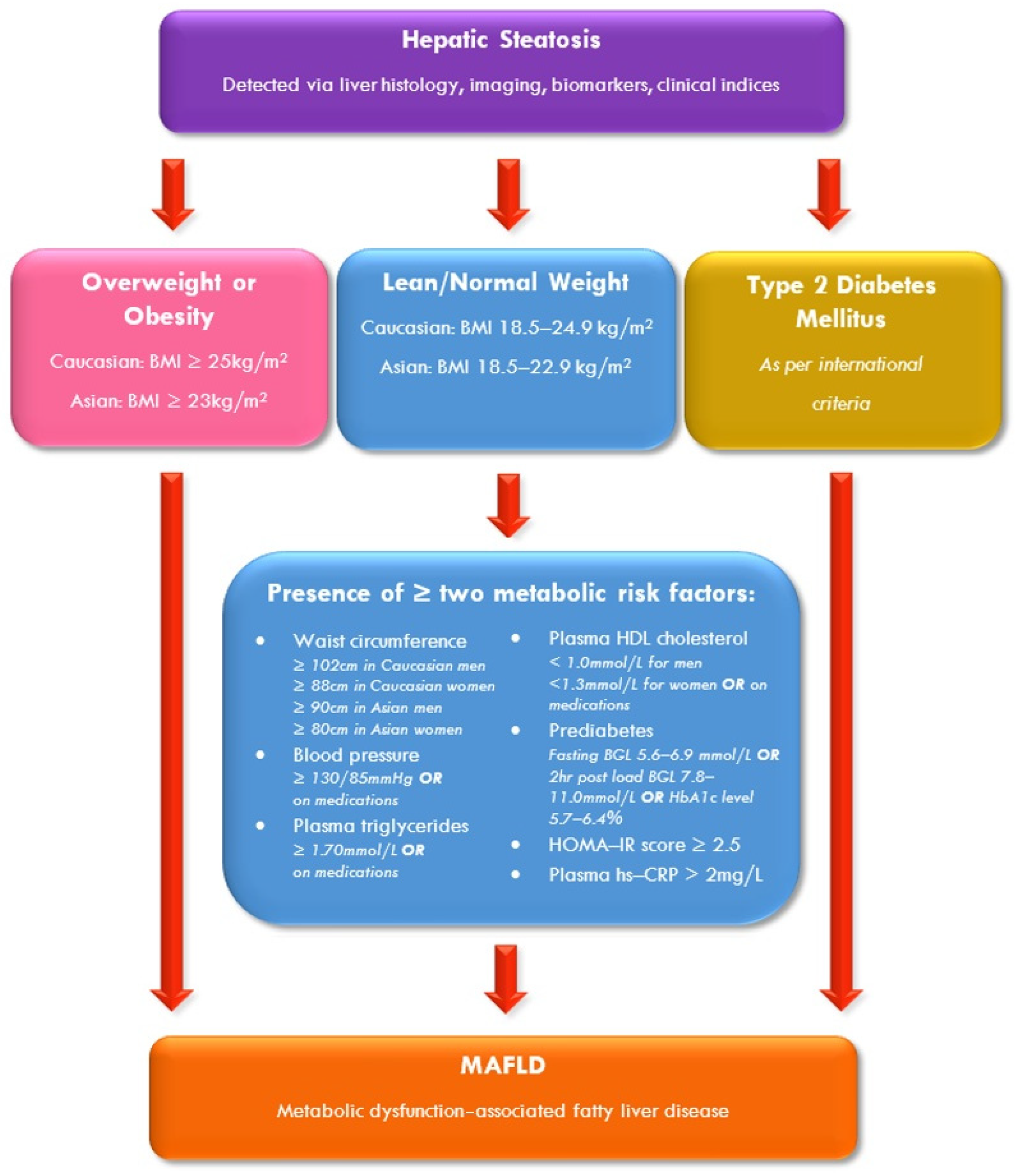
3.3. Diagnosis of MAFLD
3.4. Management of MAFLD
4. Gestational Diabetes Mellitus (GDM)
4.1. Prevalence of GDM
4.2. Risk Factors for GDM
4.3. Diagnosis of GDM
4.4. Management of GDM
5. The Relationship Between Metabolic Dysfunction-Associated Fatty Liver Disease and Gestational Diabetes Mellitus
5.1. A Prior History of GDM Predicts the Postpartum Development of MAFLD
5.2. The Association of MAFLD with the Development of GDM
5.3. Short-Term Adverse Pregnancy Outcomes of MAFLD and GDM
5.4. Long-Term Maternal Consequences of MAFLD and GDM
6. Management of Women with Both Metabolic Dysfunction-Associated Fatty Liver Disease and Gestational Diabetes Mellitus
6.1. Life Course Approach to Management
6.2. Increased Surveillance and Monitoring After Pregnancy Is Required for Women with Both MAFLD and GDM
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| aHR | Adjusted hazard ratio |
| aOR | Adjusted odds ratio |
| aRR | Adjusted risk ratio |
| BGL | Blood glucose level |
| BMI | Body mass index |
| CAP | Controlled attenuation parameter |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| CI | Confidence interval |
| CT | Computed tomography |
| DNA | Deoxyribonucleic acid |
| DPP | Diabetes Prevention Program |
| DSP | Diastolic blood pressure |
| EASD | European Association for the Study of Diabetes |
| EASL | European Association for the Study of Liver |
| EASO | European Association for the Study of Obesity |
| FBGL | Fasting blood glucose level |
| FLI | Fatty Liver Index |
| GCT | Glucose challenge test |
| GDM | Gestational diabetes mellitus |
| GEM-DPP | Gestational Diabetes’ Effects on Moms Diabetes Prevention Program |
| HAPO | Hyperglycaemia and Adverse Pregnancy Outcome |
| HDL-C | High-density lipoprotein cholesterol |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| h | Hour |
| HSI | Hepatic Steatosis Index |
| HTN | Hypertension |
| HU | Hounsfield unit |
| IADPSG | International Association of Diabetes and Pregnancy Study Groups |
| ICD | International Classification of Diseases |
| IDF | International Diabetes Federation |
| IFG | Impaired fasting glucose |
| IGT | Impaired glucose tolerance |
| LDL-C | Low-density lipoprotein cholesterol |
| LGA | Large-for-gestational age |
| LSM | Liver stiffness measurement |
| MAFLD | Metabolic dysfunction-associated fatty liver disease |
| MAGDA-DPP | Mothers after Gestational Diabetes in Australia Diabetes Prevention Program |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NAFLD | Non-alcoholic fatty liver disease |
| NETs | Neutrophil extracellular traps |
| NGT | Normal glucose tolerance |
| NHANES III | Third National Health and Nutrition Examination Survey |
| NIS | National Inpatient Sample |
| OGTT | Oral glucose tolerance test |
| OR | Odds ratio |
| PCOS | Polycystic ovarian syndrome |
| pGDM | Prior history of gestational diabetes mellitus |
| pGIGT | Prior history of gestational impaired glucose tolerance |
| RCT | Randomised controlled trial |
| RR | Relative risk |
| SBP | Systolic blood pressure |
| SGA | Small-for-gestational age |
| SMBG | Self-monitoring of blood glucose |
| T2D | Type 2 diabetes mellitus |
| USA | United States of America |
| USS | Ultrasound |
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Sharma, B.J.; Mostafa, I.; Bugianesi, E.; Wong, V.W.S.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global perspectives on non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Hepatology 2018, 69, 2672–2682. [Google Scholar] [CrossRef]
- Chan, K.E.; Koh, T.J.L.; Tang, A.S.P.; Quek, J.; Yong, J.N.; Tay, P.; Tan, D.J.H.; Lim, W.H.; Lin, S.Y.; Huang, D.; et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: A meta-analysis and systematic review of 10 739 607 individuals. J. Clin. Endocrinol. Metab. 2022, 107, 2691–2700. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Targher, G. Concordance of MAFLD and NAFLD diagnostic criteria in “real-world” data. Liver Int. 2020, 40, 2879–2880. [Google Scholar] [CrossRef]
- Thrift, A.P.; Nguyen, T.H.; Pham, C.; Balakrishnan, M.; Kanwal, F.; Loomba, R.; Duong, H.T.; Ramsey, D.; El-Serag, H.B. The prevalence and determinants of NAFLD and MAFLD and their severity in the VA primary care setting. Clin. Gastroenterol. Hepatol. 2023, 21, 1252–1260.e5. [Google Scholar] [CrossRef]
- Paik, J.M.; Kabbara, K.; Eberly, K.E.; Younossi, Y.; Henry, L.; Younossi, Z.M. Global burden of NAFLD and chronic liver disease among adolescents and young adults. Hepatology 2022, 75, 1204–1217. [Google Scholar] [CrossRef]
- Sarkar, M.; Grab, J.; Dodge, J.L.; Gunderson, E.P.; Rubin, J.; Irani, R.A.; Cedars, M.; Terrault, N. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J. Hepatol. 2020, 73, 516–522. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, B.J.; Koo, J.N.; Norwitz, E.R.; Oh, I.H.; Kim, S.M.; Kim, S.Y.; Kim, G.M.; Kwak, S.H.; Kim, W.; et al. Nonalcoholic fatty liver disease is a risk factor for large-for-gestational-age birthweight. PLoS ONE 2019, 14, e0221400. [Google Scholar] [CrossRef]
- De Souza, L.R.; Berger, H.; Retnakaran, R.; Vlachou, P.A.; Maguire, J.L.; Nathens, A.B.; Connelly, P.W.; Ray, J.G. Non-alcoholic fatty liver disease in early pregnancy predicts dysglycemia in mid-pregnancy: Prospective study. Am. J. Gastroenterol. 2016, 111, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kwak, S.H.; Koo, J.N.; Oh, I.H.; Kwon, J.E.; Kim, B.J.; Kim, S.M.; Kim, S.Y.; Kim, G.M.; Joo, S.K.; et al. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia 2019, 62, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.M.; Lee, S.M.; Hong, S.; Koo, J.N.; Oh, I.H.; Kim, B.J.; Kim, S.M.; Kim, S.Y.; Kim, G.M.; Kyung Joo, S.; et al. The risk of pregnancy-associated hypertension in women with nonalcoholic fatty liver disease. Liver Int. 2020, 40, 2417–2426. [Google Scholar] [CrossRef]
- Lee, S.; Jung, Y.; Choi, E.; Kwak, S.; Koo, J.; Oh, I.; Kim, B.J.; Kim, S.M.; Kim, S.Y.; Kim, G.M.; et al. Metabolic dysfunction-associated fatty liver disease and subsequent development of adverse pregnancy outcomes. Clin. Gastroenterol. Hepatol. 2021, 20, 2542–2550. [Google Scholar] [CrossRef]
- International Diabetes Federation. International Diabetes Federation Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 7 April 2025).
- Cheung, N.W.; Byth, K. Population health significance of gestational diabetes. Diabetes Care 2003, 26, 2005–2009. [Google Scholar] [CrossRef]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Wendland, E.M.; Torloni, M.R.; Falavigna, M.; Trujillo, J.; Dode, M.A.; Campos, M.A.; Duncan, B.B.; Schmidt, M.I. Gestational diabetes and pregnancy outcomes—A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012, 12, 23. [Google Scholar] [CrossRef]
- El Jamaly, H.; Eslick, G.D.; Weltman, M. Systematic review with meta-analysis: Non-alcoholic fatty liver disease and the association with pregnancy outcomes. Clin. Mol. Hepatol. 2022, 28, 52–66. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, G.J.; Wi, W.Y.; Norwitz, E.R.; Koo, B.K.; Lee, J.; Jung, Y.M.; Kwak, S.H.; Park, C.W.; Jun, J.K.; et al. Metabolic dysfunction-associated fatty liver disease as a risk factor for adverse outcomes in subsequent pregnancy: A nationwide cohort study. Hepatol. Int. 2023, 17, 367–376. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Quesenberry, C.P., Jr.; Jacobs, D.R., Jr.; Feng, J.; Lewis, C.E.; Sidney, S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: The CARDIA study. Am. J. Epidemiol. 2010, 172, 1131–1143. [Google Scholar] [CrossRef]
- Marschner, S.; Pant, A.; Henry, A.; Maple-Brown, L.J.; Moran, L.; Cheung, N.W.; Chow, C.K.; Zaman, S. Cardiovascular risk management following gestational diabetes and hypertensive disorders of pregnancy: A narrative review. Med. J. Aust. 2023, 218, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Muzurović, E.; Peng, C.C.; Belanger, M.J.; Sanoudou, D.; Mikhailidis, D.P.; Mantzoros, C.S. Nonalcoholic fatty liver disease and cardiovascular disease: A review of shared cardiometabolic risk factors. Hypertension 2022, 79, 1319–1326. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D. Clinical Review: Nonalcoholic fatty liver disease: A novel cardiometabolic risk factor for type 2 diabetes and its complications. J. Clin. Endocrinol. Metab. 2013, 98, 483–495. [Google Scholar] [CrossRef]
- Zhou, X.D.; Cai, J.; Targher, G.; Byrne, C.D.; Shapiro, M.D.; Sung, K.C.; Somers, V.K.; Chahal, C.A.A.; George, J.; Chen, L.L.; et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc. Diabetol. 2022, 21, 270. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- United Nations; Department of Economic and Social Affairs. Population Division. World Population Ageing 2019: Highlights (ST/ESA/SER.A/430); United Nations: New York, NY, USA, 2019. [Google Scholar]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Fan, J.; Xu, X.; Yang, R.; Nan, Y.; Wei, L.; Jia, J.; Zhuang, H.; Shi, J.; Li, X.; Sun, C.; et al. Guideline for the prevention and treatment of metabolic dysfunction-associated fatty liver disease (version 2024). J. Clin. Transl. Hepatol. 2024, 12, 955–974. [Google Scholar] [CrossRef] [PubMed]
- Stefano, J.; Duarte, S.; Ribeiro Leite Altikes, R.; Oliveira, C. Non-pharmacological management options for MAFLD: A practical guide. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188231160394. [Google Scholar] [CrossRef]
- Mokhtare, M.; Abdi, A.; Sadeghian, A.M.; Sotoudeheian, M.; Namazi, A.; Khalighi Sikaroudi, M. Investigation about the correlation between the severity of metabolic-associated fatty liver disease and adherence to the Mediterranean diet. Clin. Nutr. ESPEN 2023, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Huang, R.; Li, R.; Ning, N.; He, Y.; Zhang, J.; Wang, Y.; Ma, Y.; Jin, L. Low-carbohydrate and low-fat diet with metabolic-dysfunction-associated fatty liver disease. Nutrients 2023, 15, 4763. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A clinical update on gestational diabetes mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef]
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.F.; Immanuel, J.; Meek, C.; Oppermann, M.L.; et al. Epidemiology and management of gestational diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef]
- Hannah, W.; Bhavadharini, B.; Beks, H.; Deepa, M.; Anjana, R.M.; Uma, R.; Martin, E.; McNamara, K.; Versace, V.; Saravanan, P.; et al. Global burden of early pregnancy gestational diabetes mellitus (eGDM): A systematic review. Acta Diabetol. 2022, 59, 403–427. [Google Scholar] [CrossRef]
- Fuchs, F.; Monet, B.; Ducruet, T.; Chaillet, N.; Audibert, F. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS ONE 2018, 13, e0191002. [Google Scholar] [CrossRef]
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186287. [Google Scholar] [CrossRef]
- Solomon, C.G.; Willett, W.C.; Carey, V.J.; Rich-Edwards, J.; Hunter, D.J.; Colditz, G.A.; Stampfer, M.J.; Speizer, F.E.; Spiegelman, D.; Manson, J.E. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997, 278, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Yuen, L.; Wong, V.W. Gestational diabetes mellitus: Challenges for different ethnic groups. World J. Diabetes 2015, 6, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Yuen, L.; Wong, V.W.; Simmons, D. Ethnic disparities in gestational diabetes. Curr. Diabetes Rep. 2018, 18, 68. [Google Scholar] [CrossRef]
- Ferrara, A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care 2007, 30 (Suppl. S2), S141–S146. [Google Scholar] [CrossRef]
- Kim, C.; Berger, D.K.; Chamany, S. Recurrence of gestational diabetes mellitus: A systematic review. Diabetes Care 2007, 30, 1314–1319. [Google Scholar] [CrossRef]
- Kim, C.; Liu, T.; Valdez, R.; Beckles, G.L. Does frank diabetes in first-degree relatives of a pregnant woman affect the likelihood of her developing gestational diabetes mellitus or nongestational diabetes? Am. J. Obstet. Gynecol. 2009, 201, 576.e1–576.e6. [Google Scholar] [CrossRef]
- MacNeill, S.; Dodds, L.; Hamilton, D.C.; Armson, B.A.; VandenHof, M. Rates and risk factors for recurrence of gestational diabetes. Diabetes Care 2001, 24, 659–662. [Google Scholar] [CrossRef]
- Schwartz, N.; Nachum, Z.; Green, M.S. Risk factors of gestational diabetes mellitus recurrence: A meta-analysis. Endocrine 2016, 53, 662–671. [Google Scholar] [CrossRef]
- Giannakou, K.; Evangelou, E.; Yiallouros, P.; Christophi, C.A.; Middleton, N.; Papatheodorou, E.; Papatheodorou, S.I. Risk factors for gestational diabetes: An umbrella review of meta-analyses of observational studies. PLoS ONE 2019, 14, e0215372. [Google Scholar] [CrossRef]
- Rudra, C.B.; Sorensen, T.K.; Leisenring, W.M.; Dashow, E.; Williams, M.A. Weight characteristics and height in relation to risk of gestational diabetes mellitus. Am. J. Epidemiol. 2007, 165, 302–308. [Google Scholar] [CrossRef]
- Chu, S.Y.; Callaghan, W.M.; Kim, S.Y.; Schmid, C.H.; Lau, J.; England, L.J.; Dietz, P.M. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007, 30, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Cheung, N.W.; Moses, R.G. Gestational diabetes mellitus: Is it time to reconsider the diagnostic criteria? Diabetes Care 2018, 41, 1337–1338. [Google Scholar] [CrossRef]
- Long, H. Diagnosing gestational diabetes: Can expert opinions replace scientific evidence? Diabetologia 2011, 54, 2211–2213. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S306–S320. [Google Scholar] [CrossRef]
- Crowther, C.A.; Hiller, J.E.; Moss, J.R.; McPhee, A.J.; Jeffries, W.S.; Robinson, J.S. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 2005, 352, 2477–2486. [Google Scholar] [CrossRef]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M., Jr.; et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N. Engl. J. Med. 2009, 361, 1339–1348. [Google Scholar] [CrossRef]
- Forbes, S.; Taylor-Robinson, S.D.; Patel, N.; Allan, P.; Walker, B.R.; Johnston, D.G. Increased prevalence of non-alcoholic fatty liver disease in European women with a history of gestational diabetes. Diabetologia 2011, 54, 641–647. [Google Scholar] [CrossRef]
- Foghsgaard, S.; Andreasen, C.; Vedtofte, L.; Andersen, E.S.; Bahne, E.; Strandberg, C.; Buhl, T.; Holst, J.J.; Svare, J.A.; Clausen, T.D.; et al. Nonalcoholic fatty liver disease is prevalent in women with prior gestational diabetes mellitus and independently associated with insulin resistance and waist circumference. Diabetes Care 2017, 40, 109–116. [Google Scholar] [CrossRef]
- Mehmood, S.; Margolis, M.; Ye, C.; Maple-Brown, L.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Retnakaran, R. Hepatic fat and glucose tolerance in women with recent gestational diabetes. BMJ Open Diabetes Res. Care 2018, 6, e000549. [Google Scholar] [CrossRef]
- Ajmera, V.H.; Gunderson, E.P.; VanWagner, L.B.; Lewis, C.E.; Carr, J.J.; Terrault, N.A. Gestational diabetes mellitus is strongly associated with non-alcoholic fatty liver disease. Am. J. Gastroenterol. 2016, 111, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Chang, Y.; Ryu, S.; Kim, C.; Wild, S.H.; Byrne, C.D. History of gestational diabetes and incident nonalcoholic fatty liver disease: The Kangbuk Samsung Health Study. Am. J. Gastroenterol. 2023, 118, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Mousa, N.; Abdel-Razik, A.; Shams, M.; Sheta, T.; Zakaria, S.; Shabana, W.; Effat, N.; El-Diasty, M.; Abed, S.; Abd Elsalam, M.; et al. Impact of non-alcoholic fatty liver disease on pregnancy. Br. J. Biomed. Sci. 2018, 75, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; George, J.; Pasupathy, D.; Cheung, N.W. Antenatal FibroScan® assessment for metabolic associated fatty liver in pregnant women at risk of gestational diabetes from a multiethnic population—A pilot study. Intern. Med. J. 2021, 52, 2157–2164. [Google Scholar] [CrossRef]
- Herath, R.P.; Siriwardana, S.R.; Ekanayake, C.D.; Abeysekara, V.; Kodithuwakku, S.U.A.; Herath, H.P. Non-alcoholic fatty liver disease and pregnancy complications among Sri Lankan women: A cross sectional analytical study. PLoS ONE 2019, 14, e0215326. [Google Scholar] [CrossRef]
- Sattari, M.; Bril, F.; Egerman, R.; Kalavalapalli, S.; Cusi, K. Relationship between non-alcoholic fatty liver disease during pregnancy and abnormal glucose metabolism during and after pregnancy. J. Investig. Med. 2020, 68, 743–747. [Google Scholar] [CrossRef]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007, 30 (Suppl. S2), S112–S119. [Google Scholar] [CrossRef]
- Chai, T.Y.; Rajaratnam, R.M.; Deng, D.; George, J.; Pasupathy, D.; Cheung, N.W. The prevalence of gestational diabetes mellitus in women diagnosed with non-alcoholic fatty liver disease during pregnancy: A systematic review and meta-analysis. J. Diabetes Complicat. 2021, 35, 107991. [Google Scholar] [CrossRef]
- Hagström, H.; Höijer, J.; Ludvigsson, J.F.; Bottai, M.; Ekbom, A.; Hultcrantz, R.; Stephansson, O.; Stokkeland, K. Adverse outcomes of pregnancy in women with non-alcoholic fatty liver disease. Liver Int. 2016, 36, 268–274. [Google Scholar] [CrossRef]
- Bartha, J.L.; Martinez-Del-Fresno, P.; Comino-Delgado, R. Gestational diabetes mellitus diagnosed during early pregnancy. Am. J. Obstet. Gynecol. 2000, 182, 346–350. [Google Scholar] [CrossRef]
- Chai, T.Y.; Byth, K.; George, J.; Pasupathy, D.; Cheung, N.W. Elevated Hepatic Steatosis Index is associated with the development of adverse maternal, but not adverse neonatal, outcomes: A retrospective cohort study. Int. J. Women Health 2023, 15, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; George, J.; Pasupathy, D.; Wah Cheung, N. The prevalence of metabolic associated fatty liver detected by FibroScan® in women with gestational diabetes in a multiethnic population. Diabetes Res. Clin. Pract. 2021, 174, 108757. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.Y.; Deng, D.; Byth, K.; George, J.; Pasupathy, D.; Cheung, N.W. The prevalence of metabolic dysfunction-associated fatty liver disease and its association on adverse pregnancy outcomes in women with gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2022, 191, 110038. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Bordoni, T.; Naselli, A.; Nicolosi, G.L.; Grasso, E.; Bianchi, S.; Ferrulli, A.; Lombardo, M.; Ambrosio, G. Influence of gestational diabetes mellitus on subclinical myocardial dysfunction during pregnancy: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 292, 17–24. [Google Scholar] [CrossRef]
- Pathirana, M.M.; Lassi, Z.; Ali, A.; Arstall, M.; Roberts, C.T.; Andraweera, P.H. Cardiovascular risk factors in women with previous gestational diabetes mellitus: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 729–761. [Google Scholar] [CrossRef]
- Xie, W.; Wang, Y.; Xiao, S.; Qiu, L.; Yu, Y.; Zhang, Z. Association of gestational diabetes mellitus with overall and type specific cardiovascular and cerebrovascular diseases: Systematic review and meta-analysis. BMJ 2022, 378, e070244. [Google Scholar] [CrossRef]
- Valero, P.; Cornejo, M.; Fuentes, G.; Wehinger, S.; Toledo, F.; van der Beek, E.M.; Sobrevia, L.; Moore-Carrasco, R. Platelets and endothelial dysfunction in gestational diabetes mellitus. Acta Physiol. 2023, 237, e13940. [Google Scholar] [CrossRef]
- Xiao, W.; Li, J.; Feng, T.; Jin, L. Circulating adipokine concentrations and the risk of venous thromboembolism: A Mendelian randomization and mediation analysis. Front. Genet. 2023, 14, 1113111. [Google Scholar] [CrossRef]
- Shen, D.; Lu, Y.; Li, G.; Hu, M.; Li, S.; Ju, H.; Zhang, M.; Wang, X. Mechanism of neutrophil extracellular traps generation and their role in trophoblasts apoptosis in gestational diabetes mellitus. Cell. Signal. 2021, 88, 110168. [Google Scholar] [CrossRef]
- Duell, P.B.; Welty, F.K.; Miller, M.; Chait, A.; Hammond, G.; Ahmad, Z.; Cohen, D.E.; Horton, J.D.; Pressman, G.S.; Toth, P.P. Nonalcoholic fatty liver disease and cardiovascular risk: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e168–e185. [Google Scholar] [CrossRef]
- Sao, R.; Aronow, W.S. Association of non-alcoholic fatty liver disease with cardiovascular disease and subclinical atherosclerosis. Arch. Med. Sci. 2018, 14, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Al Rifai, M.; Silverman, M.G.; Nasir, K.; Budoff, M.J.; Blankstein, R.; Szklo, M.; Katz, R.; Blumenthal, R.S.; Blaha, M.J. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015, 239, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Giral, P.; Khan, J.F.; Rosenbaum, D.; Housset, C.; Poynard, T.; Ratziu, V. Fatty liver is an independent predictor of early carotid atherosclerosis. J. Hepatol. 2016, 65, 95–102. [Google Scholar] [CrossRef]
- Mahfood Haddad, T.; Hamdeh, S.; Kanmanthareddy, A.; Alla, V.M. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2017, 11 (Suppl. S1), S209–S216. [Google Scholar] [CrossRef]
- Swan, D.; Lisman, T.; Tripodi, A.; Thachil, J. The prothrombotic tendency of metabolic-associated fatty liver disease. J. Thromb. Haemost. 2023, 21, 3045–3055. [Google Scholar] [CrossRef]
- Di Minno, M.N.; Tufano, A.; Rusolillo, A.; Di Minno, G.; Tarantino, G. High prevalence of nonalcoholic fatty liver in patients with idiopathic venous thromboembolism. World J. Gastroenterol. 2010, 16, 6119–6122. [Google Scholar] [CrossRef]
- Cheung, N.W.; Helmink, D. Gestational diabetes: The significance of persistent fasting hyperglycemia for the subsequent development of diabetes mellitus. J. Diabetes Complicat. 2006, 20, 21–25. [Google Scholar] [CrossRef]
- Lee, A.J.; Hiscock, R.J.; Wein, P.; Walker, S.P.; Permezel, M. Gestational diabetes mellitus: Clinical predictors and long-term risk of developing type 2 diabetes: A retrospective cohort study using survival analysis. Diabetes Care 2007, 30, 878–883. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Buchanan, T.A.; Xiang, A.H.; Peters, R.K.; Kjos, S.L. Clinical predictors for a high risk for the development of diabetes mellitus in the early puerperium in women with recent gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2002, 186, 751–756. [Google Scholar] [CrossRef]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.; Kubihal, S.; Kandasamy, D.; Goyal, A.; Goyal, A.; Kalaivani, M.; Tandon, N. Incidence of prediabetes/diabetes among women with prior gestational diabetes and non-alcoholic fatty liver disease: A prospective observational study. Indian J. Endocrinol. Metab. 2023, 27, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Kowalik, O.; Garratt, E.; Godfrey, K.M.; Chan, S.Y.; Teo, A.K.K. Genetics and epigenetics in gestational diabetes contributing to type 2 diabetes. Trends Endocrinol. Metab. 2025. [Google Scholar] [CrossRef]
- Clausen, T.; Mathiesen, E.; Hansen, T.; Pedersen, O.; Jensen, D.; Lauenborg, J.; Damm, P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008, 31, 340–346. [Google Scholar] [CrossRef]
- Clausen, T.; Mathiesen, E.; Hansen, T.; Pedersen, O.; Jensen, D.; Lauenborg, J.; Schmidt, L.; Damm, P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 2464–2470. [Google Scholar] [CrossRef]
- Canouil, M.; Khamis, A.; Keikkala, E.; Hummel, S.; Lobbens, S.; Bonnefond, A.; Delahaye, F.; Tzala, E.; Mustaniemi, S.; Vääräsmäki, M.; et al. Epigenome-wide association study reveals methylation loci associated with offspring gestational diabetes mellitus exposure and maternal methylome. Diabetes Care 2021, 44, 1992–1999. [Google Scholar] [CrossRef]
- Foo, R.; Ma, J.; Du, R.; Goh, G.; Chong, Y.; Zhang, C.; Li, L. Gestational diabetes mellitus and development of intergenerational non-alcoholic fatty liver disease (NAFLD) after delivery: A systematic review and meta-analysis. eClinicalMedicine 2024, 72, 102609. [Google Scholar] [CrossRef]
- Fu, J.; Retnakaran, R. The life course perspective of gestational diabetes: An opportunity for the prevention of diabetes and heart disease in women. eClinicalMedicine 2022, 45, 101294. [Google Scholar] [CrossRef]
- Simmons, D.; Gupta, Y.; Hernandez, T.L.; Levitt, N.; van Poppel, M.; Yang, X.; Zarowsky, C.; Backman, H.; Feghali, M.; Nielsen, K.K. Call to action for a life course approach. Lancet 2024, 404, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Ratner, R.E.; Christophi, C.A.; Metzger, B.E.; Dabelea, D.; Bennett, P.H.; Pi-Sunyer, X.; Fowler, S.; Kahn, S.E. Prevention of diabetes in women with a history of gestational diabetes: Effects of metformin and lifestyle interventions. J. Clin. Endocrinol. Metab. 2008, 93, 4774–4779. [Google Scholar] [CrossRef]
- Aroda, V.R.; Christophi, C.A.; Edelstein, S.L.; Zhang, P.; Herman, W.H.; Barrett-Connor, E.; Delahanty, L.M.; Montez, M.G.; Ackermann, R.T.; Zhuo, X.; et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: The Diabetes Prevention Program outcomes study 10-year follow-up. J. Clin. Endocrinol. Metab. 2015, 100, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.L.; Dunbar, J.A.; Versace, V.; Janus, E.; Best, J.D.; Carter, R.; Oats, J.J.; Skinner, T.; Ackland, M.; Phillips, P.A.; et al. Mothers after gestational diabetes in Australia (MAGDA): A randomised controlled trial of a postnatal diabetes prevention program. PLoS Med. 2016, 13, e1002092. [Google Scholar] [CrossRef]
- Ferrara, A.; Hedderson, M.M.; Brown, S.D.; Albright, C.L.; Ehrlich, S.F.; Tsai, A.L.; Caan, B.J.; Sternfeld, B.; Gordon, N.P.; Schmittdiel, J.A.; et al. The comparative effectiveness of diabetes prevention strategies to reduce postpartum weight retention in women with gestational diabetes mellitus: The Gestational Diabetes’ Effects on Moms (GEM) cluster randomized controlled trial. Diabetes Care 2016, 39, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.C.; Pudwell, J.; Roddy, M.; Cho, C.K.; Smith, G.N. The maternal health clinic: An initiative for cardiovascular risk identification in women with pregnancy-related complications. Am. J. Obstet. Gynecol. 2014, 210, 438.e1–438.e9. [Google Scholar] [CrossRef]
- Janmohamed, R.; Montgomery-Fajic, E.; Sia, W.; Germaine, D.; Wilkie, J.; Khurana, R.; Nerenberg, K.A. Cardiovascular risk reduction and weight management at a hospital-based postpartum preeclampsia clinic. J. Obstet. Gynaecol. Can. 2015, 37, 330–337. [Google Scholar] [CrossRef]
- Celi, A.C.; Seely, E.W.; Wang, P.; Thomas, A.M.; Wilkins-Haug, L.E. Caring for women after hypertensive pregnancies and beyond: Implementation and integration of a postpartum transition clinic. Matern. Child Health J. 2019, 23, 1459–1466. [Google Scholar] [CrossRef]
- Jowell, A.R.; Sarma, A.A.; Gulati, M.; Michos, E.D.; Vaught, A.J.; Natarajan, P.; Powe, C.E.; Honigberg, M.C. Interventions to mitigate risk of cardiovascular disease after adverse pregnancy outcomes: A review. JAMA Cardiol. 2022, 7, 346–355. [Google Scholar] [CrossRef]
- Starfield, B.; Shi, L.; Macinko, J. Contribution of primary care to health systems and health. Milbank Q. 2005, 83, 457–502. [Google Scholar] [CrossRef]
- de Waard, A.M.; Hollander, M.; Korevaar, J.C.; Nielen, M.M.J.; Carlsson, A.C.; Lionis, C.; Seifert, B.; Thilsing, T.; de Wit, N.J.; Schellevis, F.G. Selective prevention of cardiometabolic diseases: Activities and attitudes of general practitioners across Europe. Eur. J. Public Health 2019, 29, 88–93. [Google Scholar] [CrossRef]
| Study (Ref.) | Study Participants (n) | Postpartum Follow-Up (Years) | Modality Used for MAFLD Diagnosis | GDM Diagnostic Criteria (mmol/L) | Risk of MAFLD Development aOR or aHR [95% CI], p-Value | Variables Adjusted for | Other Significant Variables Associated with MAFLD |
|---|---|---|---|---|---|---|---|
| Forbes et al., 2011 [60] |
| 1–10 | • Liver USS | 75 g OGTT:
| aOR 2.77 [1.43–5.37], p = 0.002 | BMI |
|
| Foghsgaard et al., 2017 [61] |
| Median 4.5–4.8 | • Liver USS | 75 g OGTT: • 2 h BGL ≥ 7.8 | Not assessed (MAFLD prevalence 22% (n = 24) in women with a pGDM) | Not assessed |
|
| Mehmood et al., 2018 [62] |
| Mean 4.8 | • Liver USS | 100 g OGTT:
| aOR 3.66 [1.1–12.5], p = 0.04 1 | Age, ethnicity, family history of diabetes, BMI, breast feeding duration, GDM/GIGT, liver fat score ≥ 2 | Not assessed |
| Ajmera et al., 2016 [63] |
| 25 | • Non-contrast CT 2 | Self-report | aOR 2.29 [1.23–4.27], p = 0.01 | Age, parity, baseline BMI, waist circumference, HOMA-IR, HDL-C, triglycerides |
|
| Cho et al., 2023 [64] |
| Median 3.7 | • Liver USS | Self-report | aHR 1.46 [1.33–1.59] (overall MAFLD); p not reported aHR 1.75 [1.25–2.44], (moderate-to-severe MAFLD); p not reported | Current age, age at first birth, hospital centre, BMI, examination year, alcohol consumption, smoking status, physical activity, education level, medication use for hyperlipidaemia, history of hypertension, CVD |
|
| Study (Ref.) | Study Participants (n) | Predominant Ethnicity (n, %) | Modality Used for MAFLD Diagnosis | Gestation (Weeks) of MAFLD Diagnosis | GDM Diagnostic Criteria (mmol/L) | Risk of GDM Development aOR [95% CI], p-Value | Variables Adjusted for |
|---|---|---|---|---|---|---|---|
| De Souza et al., 2016 [11] | Total: 476 MAFLD: 77 | • Caucasian (247, 52) | • Liver USS | 11–14 | 75 g OGTT at 24–28 weeks:
| • 2.2 [1.1–4.3], p not reported | Age, ethnicity, family history of T2D, BMI (pre-gravid), BMI (changed), ≥1 USS feature of hepatic fat |
| Mousa et al., 2018 [65] | Total: 400 MAFLD: 200 | • Egyptian (400, 100) | • Liver USS | 8–12 | 75 g OGTT at 24–28 weeks:
| Not assessed | Not assessed |
| Lee et al., 2019 [12] | Total: 608 MAFLD: 112 | • Korean (608, 100) |
| 10–14 | 50 g OGTT GCT at 24–28 weeks: • 1 h ≥ 7.8 If positive GCT, then 100 g OGTT performed 1:
|
| Age, pGDM, waist circumference, SBP/DBP, HOMA-IR, Selenoprotein P levels > 34.01 μg/mL, Adiponectin levels <1.72 μg/mL |
| Deng et al., 2021 [66] | Total: 50 MAFLD: 6 |
| • Transient elastography (FibroScan®) 2 | <24 | 75 g OGTT at 24–28 weeks:
| Not assessed | Not assessed |
| Herath et al., 2019 [67] | Total: 573 MAFLD: 104 | • Sinhala (542, 95) | • Liver USS | 30+ | FBGL ≥ 5.1 at 24–28 weeks or 75 g OGTT at 24–28 weeks:
| • 1.3 (0.8–2.3), p not reported | Age, BMI, gestational HTN, pre-eclampsia, ≥1 grade steatosis on liver USS |
| Sattari et al., 2020 [68] | Total: 84 MAFLD: 12 | • Caucasian (51, 61) | • Liver USS | 30+ | 75 g OGTT at 24–28 weeks:
| Not assessed | Not assessed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, T.Y.; George, J.; Pasupathy, D.; Cheung, N.W.; Rudland, V.L. The Maternal and Fetal Consequences of Metabolic Dysfunction-Associated Fatty Liver Disease and Gestational Diabetes Mellitus. Nutrients 2025, 17, 1730. https://doi.org/10.3390/nu17101730
Chai TY, George J, Pasupathy D, Cheung NW, Rudland VL. The Maternal and Fetal Consequences of Metabolic Dysfunction-Associated Fatty Liver Disease and Gestational Diabetes Mellitus. Nutrients. 2025; 17(10):1730. https://doi.org/10.3390/nu17101730
Chicago/Turabian StyleChai, Thora Y., Jacob George, Dharmintra Pasupathy, Ngai Wah Cheung, and Victoria L. Rudland. 2025. "The Maternal and Fetal Consequences of Metabolic Dysfunction-Associated Fatty Liver Disease and Gestational Diabetes Mellitus" Nutrients 17, no. 10: 1730. https://doi.org/10.3390/nu17101730
APA StyleChai, T. Y., George, J., Pasupathy, D., Cheung, N. W., & Rudland, V. L. (2025). The Maternal and Fetal Consequences of Metabolic Dysfunction-Associated Fatty Liver Disease and Gestational Diabetes Mellitus. Nutrients, 17(10), 1730. https://doi.org/10.3390/nu17101730






