Exercise, Diet, and Brain Health: From the Perspective of Gut Microbiota Regulation
Abstract
1. Introduction
2. Study Design
2.1. Databases
2.2. Selection Criteria
2.3. Analysis Approach
3. Moderate Exercise Is a Regulator of Gut Health
3.1. Exercise Regulates Intestinal Barrier Integrity
3.2. Exercise Regulates Gut Microbiota Composition
3.3. Exercise Promotes Beneficial Intestinal Metabolism
4. Effects of Exercise on Brain Health Through the Gut–Brain Axis
4.1. Relieves Neuroinflammation
4.2. Regulates Neurotrophic Factors and Neurotransmitters
4.3. Supports Positive Mood
5. Dietary Strategies Aimed at Regulating the Gut Microbiota
5.1. MD
5.2. DASH Diet
5.3. MIND Diet
5.4. Plant-Based Diet
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barrio, C. The Gut Microbiota-Brain Axis, Psychobiotics and Its Influence on Brain and Behaviour: A Systematic Review. Psychoneuroendocrinology 2022, 137, 105640. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemeş, S.A.; Szabo, K.; Teleky, B.E.; Vodnar, D.-C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association with Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Min, L.; Ablitip, A.; Wang, R.; Luciana, T.; Wei, M.; Ma, X. Effects of Exercise on Gut Microbiota of Adults: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1070. [Google Scholar] [CrossRef] [PubMed]
- Aya, V.; Jimenez, P.; Muñoz, E.; Ramírez, J.D. Effects of Exercise and Physical Activity on Gut Microbiota Composition and Function in Older Adults: A Systematic Review. BMC Geriatr. 2023, 23, 364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Song, Z.; Liu, Y.; Zhang, X. Dietary Strategies to Improve Exercise Performance by Modulating the Gut Microbiota. Foods 2024, 13, 1680. [Google Scholar] [CrossRef]
- Vazquez-Medina, A.; Rodriguez-Trujillo, N.; Ayuso-Rodriguez, K.; Marini-Martinez, F.; Angeli-Morales, R.; Caussade-Silvestrini, G.; Godoy-Vitorino, F.; Chorna, N. Exploring the Interplay between Running Exercises, Microbial Diversity, and Tryptophan Metabolism along the Microbiota-Gut-Brain Axis. Front. Microbiol. 2024, 15, 1326584. [Google Scholar] [CrossRef]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in Gut Microbiota Profile between Women with Active Lifestyle and Sedentary Women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed]
- Keirns, B.H.; Koemel, N.A.; Sciarrillo, C.M.; Anderson, K.L.; Emerson, S.R. Exercise and Intestinal Permeability: Another Form of Exercise-Induced Hormesis? Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G512–G518. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The Role of Short-Chain Fatty Acids in Intestinal Barrier Function, Inflammation, Oxidative Stress, and Colonic Carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Raji, C.A.; Meysami, S.; Hashemi, S.; Garg, S.; Akbari, N.; Ahmed, G.; Chodakiewitz, Y.G.; Nguyen, T.D.; Niotis, K.; Merrill, D.A.; et al. Exercise-Related Physical Activity Relates to Brain Volumes in 10,125 Individuals. J. Alzheimers. Dis. 2024, 97, 829. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Li, J.; Zhou, C.; Li, F.; Yang, X. Physical Activity Can Improve Cognition in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Interv. Aging 2018, 13, 1593–1603. [Google Scholar] [CrossRef]
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.B.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; et al. Diet and Exercise Orthogonally Alter the Gut Microbiome and Reveal Independent Associations with Anxiety and Cognition. Mol. Neurodegener. 2014, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, R.; Chen, L.; Wang, G.; Qin, L.; Yu, Z.; Wan, Z. Microbiota from Exercise Mice Counteracts High-Fat High-Cholesterol Diet-Induced Cognitive Impairment in C57BL/6 Mice. Oxid. Med. Cell. Longev. 2023, 2023, 2766250. [Google Scholar] [CrossRef] [PubMed]
- Gibiino, G.; De Siena, M.; Sbrancia, M.; Binda, C.; Sambri, V.; Gasbarrini, A.; Fabbri, C. Dietary Habits and Gut Microbiota in Healthy Adults: Focusing on the Right Diet. A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6728. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Sidhu, S.R.K.; Kok, C.W.; Kunasegaran, T.; Ramadas, A. Effect of Plant-Based Diets on Gut Microbiota: A Systematic Review of Interventional Studies. Nutrients 2023, 15, 1510. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND Diet Associated with Reduced Incidence of Alzheimer’s Disease. Alzheimers. Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Yang, Y.J. An Overview of Current Physical Activity Recommendations in Primary Care. Korean J. Fam. Med. 2019, 40, 135–142. [Google Scholar] [CrossRef]
- Severo, J.S.; da Silva, A.C.A.; Santos, B.L.B.D.; Reinaldo, T.S.; de Oliveira, A.M.; Lima, R.S.P.; Torres-Leal, F.L.; Santos, A.A.D.; da Silva, M.T.B. Physical Exercise as a Therapeutic Approach in Gastrointestinal Diseases. J. Clin. Med. 2025, 14, 1708. [Google Scholar] [CrossRef]
- Hou, P.; Zhou, X.; Yu, L.; Yao, Y.; Zhang, Y.; Huang, Y.; Chen, M.; Yi, L.; Mi, M. Exhaustive Exercise Induces Gastrointestinal Syndrome through Reduced ILC3 and IL-22 in Mouse Model. Med. Sci. Sports Exerc. 2020, 52, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.N.; Impey, S.G.; Doran, D.A.; Fleming, S.C.; Morton, J.P.; Close, G.L. Acute High-Intensity Interval Running Increases Markers of Gastrointestinal Damage and Permeability but Not Gastrointestinal Symptoms. Appl. Physiol. Nutr. Metab. 2017, 42, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef]
- Luo, B.; Xiang, D.; Nieman, D.C.; Chen, P. The Effects of Moderate Exercise on Chronic Stress-Induced Intestinal Barrier Dysfunction and Antimicrobial Defense. Brain Behav. Immun. 2014, 39, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Cohrs, J.; Salmonson, C.; Fryer, J.D.; Nehra, V.; Hale, V.L.; Kashyap, P.; White, B.A.; Woods, J.A. Exercise Training-Induced Modification of the Gut Microbiota Persists after Microbiota Colonization and Attenuates the Response to Chemically-Induced Colitis in Gnotobiotic Mice. Gut Microbes 2018, 9, 115–130. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Xia, J.; Sun, H. Moderate Treadmill Exercise Modulates Gut Microbiota and Improves Intestinal Barrier in High-Fat-Diet-Induced Obese Mice via the AMPK/CDX2 Signaling Pathway. Diabetes Metab. Syndr. Obes. 2022, 15, 209–223. [Google Scholar] [CrossRef]
- Yu, C.; Liu, S.; Niu, Y.; Fu, L. Exercise Protects Intestinal Epithelial Barrier from High Fat Diet- Induced Permeabilization through SESN2/AMPKα1/HIF-1α Signaling. J. Nutr. Biochem. 2022, 107, 109059. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Assanelli, D.; Testa, C.; Romano, C.; Dioguardi, F.S.; Aquilani, R. Effects of Chronic Exercise on Gut Microbiota and Intestinal Barrier in Human with Type 2 Diabetes. Minerva. Med. 2019, 110, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Hoshino, Y.; Hosokawa, M.; Takeyama, H.; Higuchi, M. Effects of Short-Term Endurance Exercise on Gut Microbiota in Elderly Men. Physiol. Rep. 2018, 6, e13935. [Google Scholar] [CrossRef] [PubMed]
- Rettedal, E.A.; Cree, J.M.E.; Adams, S.E.; Skidmore, P.M.L.; Cameron-Smith, D.; Gant, N.; Blenkiron, C.; Merry, T.L.; MacRae, C. Short-term High-intensity Interval Training Exercise Does Not Affect Gut Bacterial Community Diversity or Composition of Lean and Overweight Men. Exp. Physiol. 2020, 105, 1268–1279. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Morita, E.; Yokoyama, H.; Imai, D.; Takeda, R.; Ota, A.; Kawai, E.; Hisada, T.; Emoto, M.; Suzuki, Y.; Okazaki, K. Aerobic Exercise Training with Brisk Walking Increases Intestinal Bacteroides in Healthy Elderly Women. Nutrients 2019, 11, 868. [Google Scholar] [CrossRef]
- Munukka, E.; Ahtiainen, J.P.; Puigbó, P.; Jalkanen, S.; Pahkala, K.; Keskitalo, A.; Kujala, U.M.; Pietilä, S.; Hollmén, M.; Elo, L.; et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is Not Reflected in Systemic Metabolism in Over-Weight Women. Front. Microbiol. 2018, 9, 2323. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef]
- Mahdieh, M.S.; Maryam, J.; Bita, B.; Neda, F.; Motahare, M.; Mahboobeh, B.; LeBris S, Q.; Kalani Behrooz, S. A Pilot Study on the Relationship between Lactobacillus, Bifidibactrium Counts and Inflammatory Factors Following Exercise Training. Arch. Physiol. Biochem. 2023, 129, 778–787. [Google Scholar] [CrossRef]
- Ortiz-Alvarez, L.; Xu, H.; Martinez-Tellez, B. Influence of Exercise on the Human Gut Microbiota of Healthy Adults: A Systematic Review. Clin. Transl. Gastroenterol. 2020, 11, e00126. [Google Scholar] [CrossRef]
- Huang, L.; Li, T.; Zhou, M.; Deng, M.; Zhang, L.; Yi, L.; Zhu, J.; Zhu, X.; Mi, M. Hypoxia Improves Endurance Performance by Enhancing Short Chain Fatty Acids Production via Gut Microbiota Remodeling. Front. Microbiol. 2021, 12, 820691. [Google Scholar] [CrossRef] [PubMed]
- Magzal, F.; Shochat, T.; Haimov, I.; Tamir, S.; Asraf, K.; Tuchner-Arieli, M.; Even, C.; Agmon, M. Increased Physical Activity Improves Gut Microbiota Composition and Reduces Short-Chain Fatty Acid Concentrations in Older Adults with Insomnia. Sci. Rep. 2022, 12, 2265. [Google Scholar] [CrossRef]
- Aoi, W.; Inoue, R.; Mizushima, K.; Honda, A.; Björnholm, M.; Takagi, T.; Naito, Y. Exercise-Acclimated Microbiota Improves Skeletal Muscle Metabolism via Circulating Bile Acid Deconjugation. iScience 2023, 26, 106251. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Pescador, S.; Porras, D.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Juarez-Fernández, M.; Cuevas, M.J.; Mauriz, J.L.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Beneficial Effects of Exercise on Gut Microbiota Functionality and Barrier Integrity, and Gut-Liver Crosstalk in an in Vivo Model of Early Obesity and Non-Alcoholic Fatty Liver Disease. Dis. Model. Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community Characteristics of the Gut Microbiomes of Competitive Cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-Omic Analysis of Elite Athletes Identifies a Performanceenhancing Microbe That Functions via Lactate Metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Besnier, F.; Labrunée, M.; Pathak, A.; Pavy-Le Traon, A.; Galès, C.; Sénard, J.-M.; Guiraud, T. Exercise Training-Induced Modification in Autonomic Nervous System: An Update for Cardiac Patients. Ann. Phys. Rehabil. Med. 2017, 60, 27–35. [Google Scholar] [CrossRef]
- Lei, W.; Duan, Z. Advances in the Treatment of Cholinergic Anti-Inflammatory Pathways in Gastrointestinal Diseases by Electrical Stimulation of Vagus Nerve. Digestion 2021, 102, 128–138. [Google Scholar] [CrossRef]
- Daniela, M.; Catalina, L.; Ilie, O.; Paula, M.; Daniel-Andrei, I.; Ioana, B. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef]
- Spielman, L.J.; Little, J.P.; Klegeris, A. Physical Activity and Exercise Attenuate Neuroinflammation in Neurological Diseases. Brain Res. Bull. 2016, 125, 19–29. [Google Scholar] [CrossRef]
- Cowan, M.; Petri, W.A. Microglia: Immune Regulators of Neurodevelopment. Front. Immunol. 2018, 9, 2576. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Wright-Jin, E.C.; Gutmann, D.H. Microglia as Dynamic Cellular Mediators of Brain Function. Trends Mol. Med. 2019, 25, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, J.; Colditz, M.J.; Blackmore, D.G.; Ruitenberg, M.J.; Bartlett, P.F. Microglia Modulate Hippocampal Neural Precursor Activity in Response to Exercise and Aging. J. Neurosci. 2012, 32, 6435–6443. [Google Scholar] [CrossRef]
- Kim, D.; Cho, J.; Lee, I.; Jin, Y.; Kang, H. Exercise Attenuates High-Fat Diet-Induced Disease Progression in 3xTg-AD Mice. Med. Sci. Sports Exerc. 2017, 49, 676–686. [Google Scholar] [CrossRef]
- Kohman, R.A.; DeYoung, E.K.; Bhattacharya, T.K.; Peterson, L.N.; Rhodes, J.S. Wheel Running Attenuates Microglia Proliferation and Increases Expression of a Proneurogenic Phenotype in the Hippocampus of Aged Mice. Brain Behav. Immun. 2012, 26, 803–810. [Google Scholar] [CrossRef]
- Casaletto, K.B.; Lindbergh, C.A.; VandeBunte, A.; Neuhaus, J.; Schneider, J.A.; Buchman, A.S.; Honer, W.G.; Bennett, D.A. Microglial Correlates of Late Life Physical Activity: Relationship with Synaptic and Cognitive Aging in Older Adults. J. Neurosci. 2022, 42, 288–298. [Google Scholar] [CrossRef]
- Zhang, X.; He, Q.; Huang, T.; Zhao, N.; Liang, F.; Xu, B.; Chen, X.; Li, T.; Bi, J. Treadmill Exercise Decreases Aβ Deposition and Counteracts Cognitive Decline in APP/PS1 Mice, Possibly via Hippocampal Microglia Modifications. Front. Aging Neurosci. 2019, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.J.; Choi, H.B.; Hines, R.M.; Phillips, A.G.; MacVicar, B.A. Prevention of LPS-Induced Microglia Activation, Cytokine Production and Sickness Behavior with TLR4 Receptor Interfering Peptides. PLoS ONE 2013, 8, e60388. [Google Scholar] [CrossRef] [PubMed]
- Littlefield, A.M.; Setti, S.E.; Priester, C.; Kohman, R.A. Voluntary Exercise Attenuates LPS-Induced Reductions in Neurogenesis and Increases Microglia Expression of a Proneurogenic Phenotype in Aged Mice. J. Neuroinflamm. 2015, 12, 138. [Google Scholar] [CrossRef]
- Choi, J.-W.; Jo, S.-W.; Kim, D.-E.; Paik, I.-Y.; Balakrishnan, R. Aerobic Exercise Attenuates LPS-Induced Cognitive Dysfunction by Reducing Oxidative Stress, Glial Activation, and Neuroinflammation. Redox Biol. 2024, 71, 103101. [Google Scholar] [CrossRef]
- Feng, X.; Uchida, Y.; Koch, L.; Britton, S.; Hu, J.; Lutrin, D.; Maze, M. Exercise Prevents Enhanced Postoperative Neuroinflammation and Cognitive Decline and Rectifies the Gut Microbiome in a Rat Model of Metabolic Syndrome. Front. Immunol. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yang, J.; Jian, Y.; Lei, Y.; Yao, S.; Hu, Z.; Liu, X.; Tang, C.; Liu, W. Treadmill Exercise Modulates Intestinal Microbes and Suppresses LPS Displacement to Alleviate Neuroinflammation in the Brains of APP/PS1 Mice. Nutrients 2022, 14, 4134. [Google Scholar] [CrossRef]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-Chain Fatty Acids (SCFAs) Alone or in Combination Regulate Select Immune Functions of Microglia-like Cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef]
- de Souza, P.B.; de Araujo Borba, L.; Castro de Jesus, L.; Valverde, A.P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise. Int. J. Mol. Sci. 2023, 24, 16870. [Google Scholar] [CrossRef]
- Yu, C.; Liu, S.; Chen, L.; Shen, J.; Niu, Y.; Wang, T.; Zhang, W.; Fu, L. Effect of Exercise and Butyrate Supplementation on Microbiota Composition and Lipid Metabolism. J. Endocrinol. 2019, 243, 125–135. [Google Scholar] [CrossRef]
- Wang, R.; Holsinger, R.M.D. Exercise-Induced Brain-Derived Neurotrophic Factor Expression: Therapeutic Implications for Alzheimer’s Dementia. Ageing Res. Rev. 2018, 48, 109–121. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Vaynman, S.; Ying, Z. Brain-Derived Neurotrophic Factor Functions as a Metabotrophin to Mediate the Effects of Exercise on Cognition. Eur. J. Neurosci. 2008, 28, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A Meta-Analytic Review of the Effects of Exercise on Brain-Derived Neurotrophic Factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctôt, K.L. The Effect of Acute Exercise on Blood Concentrations of Brain-Derived Neurotrophic Factor in Healthy Adults: A Meta-Analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef]
- Ashcroft, S.K.; Ironside, D.D.; Johnson, L.; Kuys, S.S.; Thompson-Butel, A.G. Effect of Exercise on Brain-Derived Neurotrophic Factor in Stroke Survivors: A Systematic Review and Meta-Analysis. Stroke 2022, 53, 3706–3716. [Google Scholar] [CrossRef] [PubMed]
- Molska, M.; Mruczyk, K.; Cisek-Woźniak, A.; Prokopowicz, W.; Szydełko, P.; Jakuszewska, Z.; Marzec, K.; Trocholepsza, M. The Influence of Intestinal Microbiota on BDNF Levels. Nutrients 2024, 16, 2891. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise Influence on the Microbiome-Gut-Brain Axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef]
- Gu, F.; Wu, Y.; Liu, Y.; Dou, M.; Jiang, Y.; Liang, H. Lactobacillus casei Improves Depression-like Behavior in Chronic Unpredictable Mild Stress-Induced Rats by the BDNF-TrkB Signal Pathway and the Intestinal Microbiota. Food Funct. 2020, 11, 6148–6157. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Chen, H.-I.; Kuo, Y.-M.; Yu, L.; Huang, T.-Y.; Chen, S.-J.; Chuang, J.-I.; Wu, F.-S.; Jen, C.J. Chronic Treadmill Running in Normotensive Rats Resets the Resting Blood Pressure to Lower Levels by Upregulating the Hypothalamic GABAergic System. J. Hypertens. 2011, 29, 2339–2348. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020, 31, 77–91.e5. [Google Scholar] [CrossRef]
- Santibañez-Gutierrez, A.; Fernández-Landa, J.; Calleja-González, J.; Delextrat, A.; Mielgo-Ayuso, J. Effects of Probiotic Supplementation on Exercise with Predominance of Aerobic Metabolism in Trained Population: A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2022, 14, 622. [Google Scholar] [CrossRef]
- Dohnalová, L.; Lundgren, P.; Carty, J.R.E.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.-P.; Litichevskiy, L.; Descamps, H.C.; et al. A Microbiome-Dependent Gut-Brain Pathway Regulates Motivation for Exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-Induced Stress Behavior, Gut-Microbiota-Brain Axis and Diet: A Systematic Review for Athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The Microbiota and the Hypothalamus-Pituitary-Adrenocortical (HPA) Axis, Implications for Anxiety and Stress Disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut Microbiota Composition in Male Rat Models under Different Nutritional Status and Physical Activity and Its Association with Serum Leptin and Ghrelin Levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef]
- Mondanelli, G.; Volpi, C. The Double Life of Serotonin Metabolites: In the Mood for Joining Neuronal and Immune Systems. Curr. Opin. Immunol. 2021, 70, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Nishii, A.; Amemiya, S.; Kubota, N.; Nishijima, T.; Kita, I. Effects of Acute Treadmill Running at Different Intensities on Activities of Serotonin and Corticotropin-Releasing Factor Neurons, and Anxiety- and Depressive-like Behaviors in Rats. Behav. Brain Res. 2016, 298, 44–51. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, F.; Liu, S.; Liu, G.; Yang, X.; Gao, W.; Fan, K.; Zhao, H.; Ma, J. Significance of Gastrointestinal Tract in the Therapeutic Mechanisms of Exercise in Depression: Synchronism between Brain and Intestine through GBA. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 103, 109971. [Google Scholar] [CrossRef]
- Gallè, F.; Valeriani, F.; Cattaruzza, M.S.; Gianfranceschi, G.; Liguori, R.; Antinozzi, M.; Mederer, B.; Liguori, G.; Romano Spica, V. Mediterranean Diet, Physical Activity and Gut Microbiome Composition: A Cross-Sectional Study among Healthy Young Italian Adults. Nutrients 2020, 12, 2164. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean Diet and Health: A Comprehensive Overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND Diet Slows Cognitive Decline with Aging. Alzheimers. Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Filippou, C.; Tatakis, F.; Polyzos, D.; Manta, E.; Thomopoulos, C.; Nihoyannopoulos, P.; Tousoulis, D.; Tsioufis, K. Overview of Salt Restriction in the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean Diet for Blood Pressure Reduction. Rev. Cardiovasc. Med. 2022, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The Effects of Plant-Based Diets on the Body and the Brain: A Systematic Review. Transl. Psychiat. 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, J.; Moreno-Indias, I.; Bulló, M.; Lopez, J.V.; Corella, D.; Castañer, O.; Vidal, J.; Atzeni, A.; Fernandez-García, J.C.; Torres-Collado, L.; et al. Effect on Gut Microbiota of a 1-y Lifestyle Intervention with Mediterranean Diet Compared with Energy-Reduced Mediterranean Diet and Physical Activity Promotion: PREDIMED-Plus Study. Am. J. Clin. Nutr. 2021, 114, 1148–1158. [Google Scholar] [CrossRef]
- Gutiérrez-Díaz, I.; Fernández-Navarro, T.; Salazar, N.; Bartolomé, B.; Moreno-Arribas, M.V.; de Andres-Galiana, E.J.; Fernández-Martínez, J.L.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; González, S. Adherence to a Mediterranean Diet Influences the Fecal Metabolic Profile of Microbial-Derived Phenolics in a Spanish Cohort of Middle-Age and Older People. J. Agric. Food Chem. 2017, 65, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean Diet Is Associated with the Gut Microbiota Pattern and Gastrointestinal Characteristics in an Adult Population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front. Pharmacol. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Millman, J.F.; Okamoto, S.; Teruya, T.; Uema, T.; Ikematsu, S.; Shimabukuro, M.; Masuzaki, H. Extra-Virgin Olive Oil and the Gut-Brain Axis: Influence on Gut Microbiota, Mucosal Immunity, and Cardiometabolic and Cognitive Health. Nutr. Rev. 2021, 79, 1362–1374. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; De Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C.; Troncoso, A.M. Phenolic Compounds Characteristic of the Mediterranean Diet in Mitigating Microglia-Mediated Neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Weng, M.-H.; Li, Z.-Y.; Wang, G.-Y.; Yen, G.-C. Protective Effects of Camellia and Olive Oils against Cognitive Impairment via Gut Microbiota-Brain Communication in Rats. Food Funct. 2022, 13, 7168–7180. [Google Scholar] [CrossRef]
- Ghaseminasabparizi, M.; Nazarinia, M.A.; Akhlaghi, M. Adherence to the Dietary Approaches to Stop Hypertension Dietary Pattern and Rheumatoid Arthritis in Iranian Adults. Public Health Nutr. 2021, 24, 6085–6093. [Google Scholar] [CrossRef] [PubMed]
- Jama, H.A.; Beale, A.; Shihata, W.A.; Marques, F.Z. The Effect of Diet on Hypertensive Pathology: Is There a Link via Gut Microbiota-Driven Immunometabolism? Cardiovasc. Res. 2019, 115, 1435–1447. [Google Scholar] [CrossRef]
- Diao, Z.; Molludi, J.; Latef Fateh, H.; Moradi, S. Comparison of the Low-Calorie DASH Diet and a Low-Calorie Diet on Serum TMAO Concentrations and Gut Microbiota Composition of Adults with Overweight/Obesity: A Randomized Control Trial. Int. J. Food Sci. Nutr. 2024, 75, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zuo, H.; Xiang, Y.; Cai, J.; Zhang, N.; Yang, F.; Huang, S.; Zhang, Y.; Chen, H.; Li, S.; et al. Associations of Various Healthy Dietary Patterns with Biological Age Acceleration and the Mediating Role of Gut Microbiota: Results from the China Multi-Ethnic Cohort Study. Br. J. Nutr. 2024, 132, 1490–1502. [Google Scholar] [CrossRef]
- Faraco, G.; Brea, D.; Garcia-Bonilla, L.; Wang, G.; Racchumi, G.; Chang, H.; Buendia, I.; Santisteban, M.M.; Segarra, S.G.; Koizumi, K.; et al. Dietary Salt Promotes Neurovascular and Cognitive Dysfunction through a Gut-Initiated TH17 Response. Nat. Neurosci. 2018, 21, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhu, S.; Peng, X.; Li, K.; Peng, W.; Zhong, Y.; Kang, C.; Cao, X.; Liu, Z.; Zhao, B. High Salt Elicits Brain Inflammation and Cognitive Dysfunction, Accompanied by Alternations in the Gut Microbiota and Decreased SCFA Production. J. Alzheimers. Dis. 2020, 77, 629–640. [Google Scholar] [CrossRef]
- Huang, F.; Marungruang, N.; Martinsson, I.; Camprubí Ferrer, L.; Nguyen, T.D.; Gondo, T.F.; Karlsson, E.N.; Deierborg, T.; Öste, R.; Heyman-Lindén, L. A Mixture of Nordic Berries Improves Cognitive Function, Metabolic Function and Alters the Gut Microbiota in C57Bl/6J Male Mice. Front. Nutr. 2023, 10, 1257472. [Google Scholar] [CrossRef]
- Segers, A.; de Vos, W.M. Mode of Action of Akkermansia Muciniphila in the Intestinal Dialogue: Role of Extracellular Proteins, Metabolites and Cell Envelope Components. Microbiome Res. Rep. 2023, 2, 6. [Google Scholar] [CrossRef]
- Marino, M.; Venturi, S.; Gargari, G.; Del Bo, C.; Martini, D.; Porrini, M.; Guglielmetti, S.; Riso, P. Berries-Gut Microbiota Interaction and Impact on Human Health: A Systematic Review of Randomized Controlled Trials. Food Rev. Int. 2024, 40, 2618–2640. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ho, C.-T.; Zhang, X. Gut Microbiota Mediate the Neuroprotective Effect of Oolong Tea Polyphenols in Cognitive Impairment Induced by Circadian Rhythm Disorder. J. Agric. Food Chem. 2024, 72, 12184–12197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xie, T.; Xi, Y.; Li, L.; Mo, F.; Liu, X.; Liu, Z.; Gao, J.-M.; Yuan, T. Sesamol Attenuates Amyloid Peptide Accumulation and Cognitive Deficits in APP/PS1 Mice: The Mediating Role of the Gut-Brain Axis. J. Agric. Food Chem. 2021, 69, 12717–12729. [Google Scholar] [CrossRef]
- Sekikawa, A.; Wharton, W.; Butts, B.; Veliky, C.V.; Garfein, J.; Li, J.; Goon, S.; Fort, A.; Li, M.; Hughes, T.M. Potential Protective Mechanisms of S-Equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia. Int. J. Mol. Sci. 2022, 23, 11921. [Google Scholar] [CrossRef] [PubMed]
- Kadyan, S.; Park, G.; Hochuli, N.; Miller, K.; Wang, B.; Nagpal, R. Resistant Starches from Dietary Pulses Improve Neurocognitive Health via Gut-Microbiome-Brain Axis in Aged Mice. Front. Nutr. 2024, 11, 1322201. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, L.; Zeng, X.; Zhang, X.; Liu, Y.; Wu, Z.; Weng, P. The Intervention of Unique Plant Polysaccharides—Dietary Fiber on Depression from the Gut-Brain Axis. Int. J. Biol. Macromol. 2021, 170, 336–342. [Google Scholar] [CrossRef]

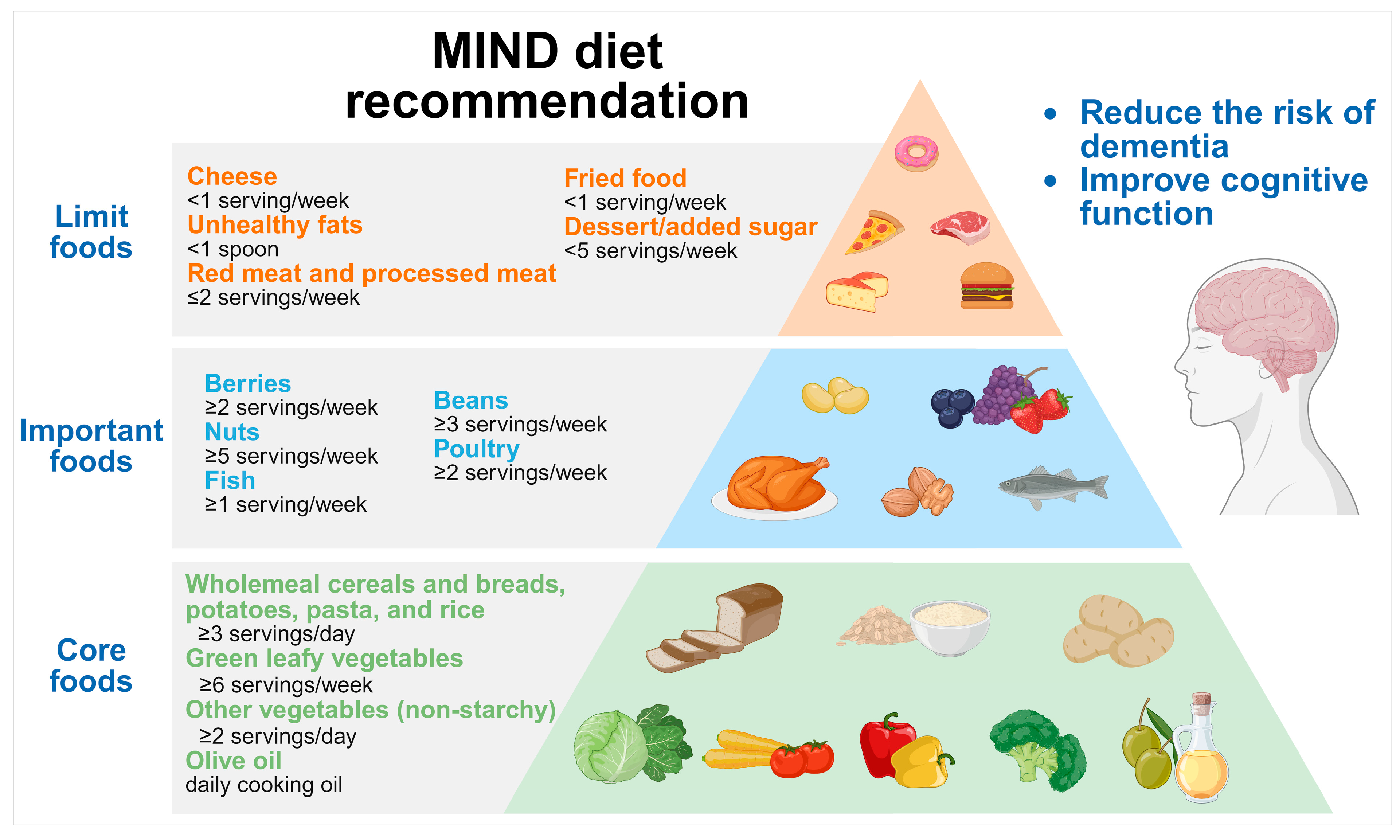
| Dietary Pattern | Core Principles | Main Foods | Characteristics | Reference |
|---|---|---|---|---|
| MD | Based on traditional eating patterns of Mediterranean regions, emphasizing whole foods, healthy fats, and social dining. |
| Focus on lifestyle (e.g., mindful eating, social meals) with olive oil as a staple. | [88,89] |
| MIND diet | Combines Mediterranean and DASH diets, specifically targeting brain health and preventing cognitive decline. |
| Prioritizes brain-boosting foods (berries, greens) and strictly avoids trans fats and processed foods. | [90] |
| DASH diet | Reduces blood pressure through sodium control and balanced nutrition. |
| Strict sodium limits, with emphasis on potassium (bananas, potatoes), calcium (low-fat dairy), and magnesium-rich foods. | [91] |
| Plant-based diet | Primarily plant-derived foods, with minimal or no animal products (e.g., vegan). |
| Highly flexible but requires attention to nutrients (e.g., vitamin B12, iron, Omega-3). | [92,93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, R.; Song, Z.; Zhang, X. Exercise, Diet, and Brain Health: From the Perspective of Gut Microbiota Regulation. Nutrients 2025, 17, 1686. https://doi.org/10.3390/nu17101686
Zhang L, Liu R, Song Z, Zhang X. Exercise, Diet, and Brain Health: From the Perspective of Gut Microbiota Regulation. Nutrients. 2025; 17(10):1686. https://doi.org/10.3390/nu17101686
Chicago/Turabian StyleZhang, Li, Renhe Liu, Zheyi Song, and Xin Zhang. 2025. "Exercise, Diet, and Brain Health: From the Perspective of Gut Microbiota Regulation" Nutrients 17, no. 10: 1686. https://doi.org/10.3390/nu17101686
APA StyleZhang, L., Liu, R., Song, Z., & Zhang, X. (2025). Exercise, Diet, and Brain Health: From the Perspective of Gut Microbiota Regulation. Nutrients, 17(10), 1686. https://doi.org/10.3390/nu17101686














