Boosting Immunity Through Nutrition and Gut Health: A Narrative Review on Managing Allergies and Multimorbidity
Abstract
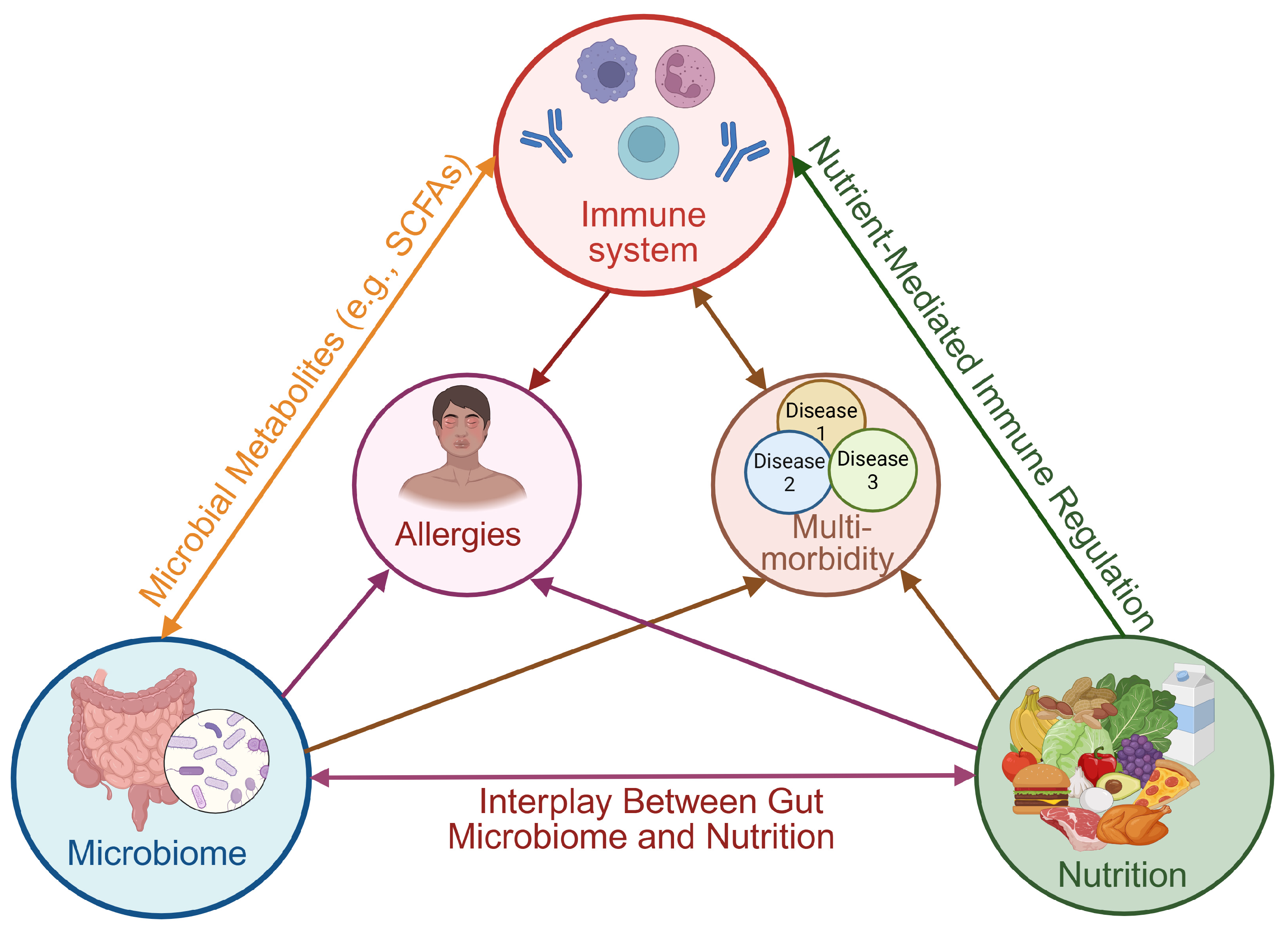
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection Process
2.4. Quality Appraisal
2.5. Data Synthesis
3. Fundamentals of Immune Function and Regulation
3.1. Innate and Adaptive Immunity.: Two Sides of the Same Coin
3.2. Allergies: A Battle of Balance in the Immune System
3.3. The Overlapping Puzzle: Understanding Multimorbidity and Immune Dysregulation
Multimorbidity and Immune Aging: The Chronic Loop
- Immunosenescence—loss of immune surveillance: Aging is accompanied by an accumulation of senescent immune cells, particularly CD8+ T cells and memory T cells. These cells exhibit reduced proliferative capacity and actively secrete pro-inflammatory mediators known collectively as the senescence-associated secretory phenotype (SASP). The accumulation of these cells not only exacerbates systemic inflammation but also disrupts tissue repair, weakens tolerance mechanisms, and increases susceptibility to autoimmune disorders [66].
- Neuroimmune crosstalk and cognitive decline: Recent evidence highlights neuroimmune interactions as crucial to aging-related diseases [67]. For example, activated microglia and elevated levels of inflammatory cytokines, such as IL-1β and IL-6, impair synaptic plasticity, contributing to cognitive impairment and conditions like Alzheimer’s disease and depression. Notably, these neurological conditions often co-occur with cardiometabolic disorders, underscoring their interconnected pathogenesis in aging populations [68].
- Metaflammation and mitochondrial dysfunction: Metaflammation—a chronic, low-grade inflammatory response triggered by metabolic overload and overnutrition—differs fundamentally from classical inflammation. Metabolic stress induces mitochondrial dysfunction, increasing reactive oxygen species (ROS) generation and mitochondrial DNA release, subsequently activating inflammasomes such as NLRP3. This process contributes significantly to metabolic syndrome and insulin resistance, two key components of multimorbidity [69].
- Epigenetic aging and biological clocks: Biological aging, measured by DNA methylation clocks (e.g., the Horvath clock), correlates more closely with multimorbidity risk than chronological age alone. Epigenetic drift accelerates under conditions of chronic inflammation, lifestyle factors, and microbiota alterations, forming a mechanistic bridge linking immune aging with metabolic and cardiovascular diseases [70].
4. Microbial Guardians: How the Gut Shapes Our Immunity
Allergies and Gut Health: The Microbiome–Immune Connection
5. Nutrition, Immunity, and Microbiota: The Triad of Resilience
5.1. Nutrition and Gut Health Hacks for Managing Allergies and Multimorbidity
| Nutrient | Immune Benefits | Dietary Sources |
|---|---|---|
| Vitamin D | Regulates T cell responses, enhances antimicrobial peptides, and reduces autoimmune activity. | Sunlight exposure, fatty fish, fortified dairy, eggs |
| Vitamin A | Supports mucosal immunity, influences Treg cells, enhances gut barrier defense. | Liver, carrots, sweet potatoes, dark leafy greens |
| Vitamin C | Antioxidant, crucial for neutrophil function, cytokine production, and oxidative stress reduction. | Citrus fruits, bell peppers, strawberries, broccoli |
| Vitamin E | Reduces oxidative stress, supports T cell function, enhances NK cell activity. | Nuts, seeds, spinach, sunflower oil |
| B Vitamins (B6, B12, Folate) | Essential for immune cell proliferation, DNA synthesis, and homocysteine regulation. | Whole grains, legumes, eggs, leafy greens, animal proteins |
| Zinc | Critical for T cell activation, antioxidant function, and mucosal immunity. | Shellfish, red meat, pumpkin seeds, legumes |
| Selenium | Supports glutathione peroxidase, reduces inflammation, and oxidative stress. | Brazil nuts, seafood, whole grains, eggs |
| Iron | Required for immune cell function, but excess can promote oxidative stress and microbial growth. | Red meat, lentils, spinach, fortified cereals |
| Magnesium | Regulates inflammation, stress response, and mitochondrial function. | Nuts, seeds, whole grains, leafy greens |
| Omega-3 Fatty Acids (EPA and DHA) | Modulates inflammatory responses, supports gut microbiota diversity, suppresses Th2-driven allergic inflammation. | Fatty fish (salmon, sardines), flaxseeds, chia seeds, walnuts |
| Polyphenols | Modulates gut microbiota, suppresses oxidative stress, enhances anti-inflammatory pathways. | Berries, tea, dark chocolate, turmeric, grapes |
| Short-Chain Fatty Acids (SCFAs) | Regulate Treg cells, reduce inflammation, and support intestinal homeostasis. | Fermented fiber-rich foods (oats, legumes, green bananas, resistant starch sources) |
5.2. Optimizing Immune Function Through Nutrient-Rich Diets
5.2.1. The Role of Vitamins in Immune Function
| Vitamin | Primary Immune Functions | Deficiency Effects | Recommended Intake | Dietary Sources | Refs |
|---|---|---|---|---|---|
| A |
|
|
|
| [142,143] |
| C |
|
|
|
| [144] |
| D |
|
|
|
| [145,146,147] |
| E |
|
|
|
| [148,149] |
| B6 |
|
|
|
| [150,151] |
| B12 |
|
|
|
| [150,151] |
| Folate (B9) |
|
|
|
| [150,151] |
5.2.2. The Mediterranean Diet and Allergies: A Protective Role?
- Anti-inflammatory properties: The MD is abundant in anti-inflammatory compounds, including polyphenols, flavonoids, and omega-3 fatty acids, which help regulate immune responses and suppress chronic inflammation. Since allergic diseases are characterized by excessive Th2-driven immune activation and inflammation, the MD’s ability to modulate cytokine production and inhibit oxidative stress may contribute to reduced allergic symptoms [155].
- Gut microbiota modulation: A well-balanced gut microbiota is essential for immune homeostasis and allergic tolerance [158]. The MD, which is rich in fiber, fermented foods, and plant-based prebiotics, promotes gut microbial diversity and the production of SCFAs. These microbial metabolites enhance Treg cell activity, reduce intestinal permeability, and mitigate systemic inflammation, thereby lowering allergy susceptibility.
- Antioxidant defense against allergic reactions: Many MD components, including fruits, vegetables, olive oil, and nuts, are rich in antioxidants such as vitamins C and E, carotenoids, and polyphenols. These compounds help protect immune cells from oxidative stress-induced damage, which is a key factor in allergic inflammation and airway hyperreactivity. By neutralizing reactive oxygen species (ROS), these antioxidants may reduce mast cell degranulation and histamine release, thereby lessening the severity of allergic reactions [159].
- Polyunsaturated fatty acids (PUFAs) and immune modulation: The MD is rich in long-chain omega-3 fatty acids (EPA and DHA) from fish, olive oil, and nuts, which exert immune-modulating effects. PUFAs influence eicosanoid synthesis, leading to the production of anti-inflammatory mediators that help balance Th1/Th2 immune responses [160]. Several studies have shown that higher omega-3 intake is associated with lower asthma prevalence and improved lung function, supporting the hypothesis that the MD may be particularly beneficial in respiratory allergies [161].
- Maternal nutrition and early immune programming: Maternal diet plays a critical role in fetal immune system development [162]. Studies suggest that adherence to the MD during pregnancy may reduce the risk of allergic sensitization in offspring, possibly through epigenetic modifications, altered gut microbiota transmission, and early exposure to immune-regulating nutrients [163]. However, findings remain inconsistent, emphasizing the need for longitudinal studies tracking maternal and child dietary patterns.
5.2.3. Breastfeeding and Allergies: The Role of Early Nutrition in Immune Development
5.2.4. Personalized Nutrition in Food Allergy: From Diagnostics to Dietary Management
5.3. Breaking the Cycle: How Nutrition Can Combat Immune Dysregulation in Multimorbidity
Obesity, Aging, and Multimorbidity: The Role of Precision Nutrition
- Conducting comprehensive individualized nutritional assessments incorporating genetic, microbiome, and advanced dietary monitoring data.
- Integrating AI-driven tools, wearables, and mobile health applications into standard clinical practice.
- Fostering interdisciplinary collaboration among nutritionists, geneticists, microbiologists, healthcare professionals, and technologists.
- Prioritizing patient-centered care by respecting individual preferences, cultural factors, lifestyle considerations, and accessibility concerns in personalized dietary interventions.
6. Technological Advances in Nutritional Assessment and Monitoring
- Enhancing AI Accuracy and Usability: Refine AI algorithms, improve machine learning models, and enhance user interfaces to optimize accuracy, reliability, and user experience.
- Interdisciplinary Collaboration: Foster collaboration among nutritionists, clinicians, software developers, data scientists, and policymakers to design integrative and user-centered nutritional monitoring platforms.
- Reducing Health Disparities: Develop affordable and accessible technologies, ensuring their applicability across diverse socioeconomic groups and resource-limited settings.
- Ensuring Ethical and Transparent Data Management: Strengthen regulatory frameworks and ethical guidelines to protect user privacy, data security, and informed consent.
- Validation Studies and Real-world Testing: Conduct robust validation studies across various populations and settings, including longitudinal assessments, to verify reliability, effectiveness, and clinical utility of these technologies.
7. Conclusions, Recommendations, and Perspectives
- Integration of personalized nutrition into clinical care: Adopt personalized dietary recommendations informed by patient phenotype, microbiome profiles, genetic background, lifestyle, cultural preferences, and local dietary patterns. Such precision nutrition plans should aim to balance nutrient adequacy, allergen avoidance, and quality of life.
- Leverage technological innovations: Incorporate AI-based tools, wearable sensors, mobile applications, and real-time dietary monitoring technologies into clinical practice and public health programs. This integration can enhance accuracy in dietary assessment, adherence to nutritional guidance, and support continuous patient engagement.
- Promotion of anti-inflammatory dietary patterns: Prioritize dietary patterns such as the Mediterranean diet for their proven capacity to enhance immune resilience, modulate microbiota composition, and reduce chronic inflammation. Public health policies should support widespread education and accessibility of such dietary models.
- Life-course approach to nutrition and immune health: Nutritional interventions should begin prenatally, continue through childhood, adulthood, and into older age, to build long-term immune resilience and delay the onset or progression of chronic diseases.
- Ensuring equitable access to personalized care: Develop inclusive and culturally sensitive nutrition programs and digital health solutions to ensure that personalized nutritional strategies are accessible and affordable across diverse socioeconomic and geographic populations, preventing further disparities in healthcare.
- Long-term data and interventional studies: There is a clear need for robust, long-term randomized controlled trials and cohort studies to assess the sustained impacts of personalized nutrition and microbiome-based interventions on multimorbidity and allergic disease progression.
- Standardization of methodologies and data integration: Heterogeneity in dietary assessment methods, microbiome analyses, and nutritional biomarker interpretation remains a challenge. Efforts to standardize methodologies and integrate datasets will enhance comparability and clinical translation.
- Ethical, privacy, and accessibility issues: Data privacy, security, and ethical considerations related to digital health tools must be addressed. Additionally, ensuring equitable access to advanced nutritional technologies and personalized care remains a major public health challenge.
- Integration of multi-omics approaches: Combining genomics, transcriptomics, proteomics, metabolomics, and microbiome data will enhance the precision of dietary interventions and improve our understanding of individual responses to nutritional changes.
- Enhanced role of digital health in nutritional management: Future innovations in AI, deep learning, and wearable technologies are expected to refine dietary assessment, providing personalized feedback in real-time and improving dietary adherence and outcomes.
- Microbiome-based therapeutics and precision probiotics: The advancement of precision probiotics and microbiome-targeted therapies, informed by individual microbiota profiles and immune status, presents an exciting frontier for personalized nutritional interventions.
- Policy and public health innovations: Public health strategies should evolve to support personalized nutrition initiatives. This includes investing in community education programs, supporting food system reforms, and developing regulatory frameworks for digital health interventions.
8. Limitations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and immune function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Tomar, N.; De, R.K. A brief outline of the immune system. Methods Mol. Biol. 2014, 1184, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Kipnis, J. Self and non-self discrimination is needed for the existence rather than deletion of autoimmunity: The role of regulatory T cells in protective autoimmunity. Cell Mol. Life Sci. 2004, 61, 2285–2289. [Google Scholar] [CrossRef] [PubMed]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Warrington, R.; Watson, W.; Kim, H.L.; Antonetti, F.R. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2011, 7 (Suppl. S1), S1. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef]
- Jo, E.-K. Interplay between host and pathogen: Immune defense and beyond. Exp. Mol. Med. 2019, 51, 1–3. [Google Scholar] [CrossRef]
- Shin, Y.H.; Hwang, J.; Kwon, R.; Lee, S.W.; Kim, M.S.; Shin, J.I.; Yon, D.K. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Allergy 2023, 78, 2232–2254. [Google Scholar] [CrossRef] [PubMed]
- Woodfolk, J.A.; Commins, S.P.; Schuyler, A.J.; Erwin, E.A.; Platts-Mills, T.A. Allergens, sources, particles, and molecules: Why do we make IgE responses? Allergol. Int. 2015, 64, 295–303. [Google Scholar] [CrossRef]
- Gautier, C.; Charpin, D. Environmental triggers and avoidance in the management of asthma. J. Asthma Allergy 2017, 10, 47–56. [Google Scholar] [CrossRef]
- Spacova, I.; Ceuppens, J.L.; Seys, S.F.; Petrova, M.I.; Lebeer, S. Probiotics against airway allergy: Host factors to consider. Dis. Model. Mech. 2018, 11, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Gu, J.Q.; Li, L.S.; Wang, X.Y.; Wang, H.T.; Wang, Y.; Chang, C.; Sun, J.L. The association between intestinal bacteria and allergic diseases-cause or consequence? Front. Cell Infect. Microbiol. 2021, 11, 650893. [Google Scholar] [CrossRef]
- Head, A.; Fleming, K.; Kypridemos, C.; Pearson-Stuttard, J.; O’Flaherty, M. Multimorbidity: The case for prevention. J. Epidemiol. Community Health 2021, 75, 242–244. [Google Scholar] [CrossRef]
- Vogeli, C.; Shields, A.E.; Lee, T.A.; Gibson, T.B.; Marder, W.D.; Weiss, K.B.; Blumenthal, D. Multiple chronic conditions: Prevalence, health consequences, and implications for quality, care management, and costs. J. Gen. Intern. Med. 2007, 22 (Suppl. S3), 391–395. [Google Scholar] [CrossRef]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Lewis, E.D.; Wu, D.; Meydani, S.N. Age-associated alterations in immune function and inflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 118, 110576. [Google Scholar] [CrossRef]
- Calderón-Larrañaga, A.; Vetrano, D.L.; Ferrucci, L.; Mercer, S.W.; Marengoni, A.; Onder, G.; Eriksdotter, M.; Fratiglioni, L. Multimorbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways. J. Intern. Med. 2019, 285, 255–271. [Google Scholar] [CrossRef]
- Munteanu, C.; Schwartz, B. The relationship between nutrition and the immune system. Front. Nutr. 2022, 9, 1082500. [Google Scholar] [CrossRef]
- Belli, M.; Barone, L.; Longo, S.; Prandi, F.R.; Lecis, D.; Mollace, R.; Margonato, D.; Muscoli, S.; Sergi, D.; Federici, M.; et al. Gut microbiota composition and cardiovascular disease: A potential new therapeutic target? Int. J. Mol. Sci. 2023, 24, 11971. [Google Scholar] [CrossRef] [PubMed]
- Golshany, H.; Helmy, S.A.; Morsy, N.F.S.; Kamal, A.; Yu, Q.; Fan, L. The gut microbiome across the lifespan: How diet modulates our microbial ecosystem from infancy to the elderly. Int. J. Food Sci. Nutr. 2025, 76, 95–121. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, C.-H.; Lei, M.; Zeng, Q.; Li, L.; Tang, H.; Zhang, N. Metabolic regulation of the immune system in health and diseases: Mechanisms and interventions. Signal Transduct. Target. Ther. 2024, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Pu, H.; Voss, M. Overview of anti-inflammatory diets and their promising effects on non-communicable diseases. Br. J. Nutr. 2024, 132, 898–918. [Google Scholar] [CrossRef]
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef]
- Vitte, J.; Vibhushan, S.; Bratti, M.; Montero-Hernandez, J.E.; Blank, U. Allergy, anaphylaxis, and nonallergic hypersensitivity: IgE, mast cells, and beyond. Med. Princ. Pract. 2022, 31, 501–515. [Google Scholar] [CrossRef]
- Cifuentes, M.; Verdejo, H.E.; Castro, P.F.; Corvalan, A.H.; Ferreccio, C.; Quest, A.F.G.; Kogan, M.J.; Lavandero, S. Low-grade chronic inflammation: A shared mechanism for chronic diseases. Physiology 2025, 40, 4–25. [Google Scholar] [CrossRef]
- Daley, D. The evolution of the hygiene hypothesis: The role of early-life exposures to viruses and microbes and their relationship to asthma and allergic diseases. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 390–396. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Pepper, M.; Thomas, P.G. Principles and therapeutic applications of adaptive immunity. Cell 2024, 187, 2052–2078. [Google Scholar] [CrossRef] [PubMed]
- Parnham, M.J.; Rossi, A.G. Innate Immunity: Phagocytes, Natural Killer Cells, and the Complement System. In Nijkamp and Parnham’s Principles of Immunopharmacology; Parnham, M.J., Nijkamp, F.P., Rossi, A.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 117–137. [Google Scholar]
- Nicholson, L.B. The immune system. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef]
- Abós, B.; Bailey, C.; Tafalla, C. Adaptive immunity. In Principles of Fish Immunology: From Cells and Molecules to Host Protection; Buchmann, K., Secombes, C.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 105–140. [Google Scholar]
- Cooper, M.D. The early history of B cells. Nat. Rev. Immunol. 2015, 15, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Audiger, C.; Rahman, M.J.; Yun, T.J.; Tarbell, K.V.; Lesage, S. The importance of dendritic cells in maintaining immune tolerance. J. Immunol. 2017, 198, 2223–2231. [Google Scholar] [CrossRef]
- Balato, A.; Unutmaz, D.; Gaspari, A.A. Natural killer T cells: An unconventional T-cell subset with diverse effector and regulatory functions. J. Investig. Dermatol. 2009, 129, 1628–1642. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Soyer, O.U.; Akdis, M.; Ring, J.; Behrendt, H.; Crameri, R.; Lauener, R.; Akdis, C.A. Mechanisms of peripheral tolerance to allergens. Allergy 2013, 68, 161–170. [Google Scholar] [CrossRef]
- Igea, J.M. The history of the idea of allergy. Allergy 2013, 68, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.S.; Mistry, K.J.; Kakade, A.M.; Niphadkar, P.V. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India 2010, 27, 66–71. [Google Scholar] [CrossRef]
- Shamji, M.H.; Valenta, R.; Jardetzky, T.; Verhasselt, V.; Durham, S.R.; Würtzen, P.A.; van Neerven, R.J.J. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy 2021, 76, 3627–3641. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Mikami, N.; Kawakami, R.; Sakaguchi, S. New Treg cell-based therapies of autoimmune diseases: Towards antigen-specific immune suppression. Curr. Opin. Immunol. 2020, 67, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Calzada, D.; Baos, S.; Cremades-Jimeno, L.; Cárdaba, B. Immunological mechanisms in allergic diseases and allergen tolerance: The role of Treg cells. J. Immunol. Res. 2018, 2018, 6012053. [Google Scholar] [CrossRef]
- Broide, D.H. Molecular and cellular mechanisms of allergic disease. J. Allergy Clin. Immunol. 2001, 108, S65–S71. [Google Scholar] [CrossRef]
- Lv, J.J.; Kong, X.M.; Zhao, Y.; Li, X.Y.; Guo, Z.L.; Zhang, Y.J.; Cheng, Z.H. Global, regional and national epidemiology of allergic disorders in children from 1990 to 2019: Findings from the Global Burden of Disease study 2019. BMJ Open 2024, 14, e080612. [Google Scholar] [CrossRef]
- Falcon, R.M.G.; Caoili, S.E.C. Immunologic, genetic, and ecological interplay of factors involved in allergic diseases. Front. Allergy 2023, 4, 1215616. [Google Scholar] [CrossRef]
- Navickas, R.; Petric, V.K.; Feigl, A.B.; Seychell, M. Multimorbidity: What do we know? What should we do? J. Comorb. 2016, 6, 4–11. [Google Scholar] [CrossRef]
- Langenberg, C.; Hingorani, A.D.; Whitty, C.J.M. Biological and functional multimorbidity—From mechanisms to management. Nat. Med. 2023, 29, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Liu, L.; Miura, K.; Fujiyoshi, A.; Kadota, A.; Miyagawa, N.; Nakamura, Y.; Ohkubo, T.; Okayama, A.; Okamura, T.; Ueshima, H. Impact of metabolic syndrome on the risk of cardiovascular disease mortality in the United States and in Japan. Am. J. Cardiol. 2014, 113, 84–89. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Akbaraly, T.N.; Hamer, M.; Ferrie, J.E.; Lowe, G.; Batty, G.D.; Hagger-Johnson, G.; Singh-Manoux, A.; Shipley, M.J.; Kivimäki, M. Chronic inflammation as a determinant of future aging phenotypes. Can. Med. Assoc. J. 2013, 185, E763–E770. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Ferrucci, L.; Newman, A.B.; Aroda, V.R.; Bahnson, J.L.; Divers, J.; Espeland, M.A.; Marcovina, S.; Pollak, M.N.; Kritchevsky, S.B.; et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: Report from the TAME Biomarkers Workgroup. Geroscience 2018, 40, 419–436. [Google Scholar] [CrossRef]
- St Sauver, J.; Rocca, W.; LeBrasseur, N.; Chamberlain, A.; Olson, J.; Jacobson, D.; McGree, M.; Mielke, M. Inflammatory biomarkers, multi-morbidity, and biologic aging. J. Int. Med. Res. 2022, 50, 3000605221109393. [Google Scholar] [CrossRef]
- Chen, Y.-L.; You, J.; Guo, Y.; Zhang, Y.; Yao, B.-R.; Wang, J.-J.; Chen, S.-D.; Ge, Y.-J.; Yang, L.; Wu, X.-R.; et al. Identifying proteins and pathways associated with multimorbidity in 53,026 adults. Metabolism 2025, 164, 156126. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Netea, M.G.; Chavakis, T. Trained immunity in chronic inflammatory diseases and cancer. Nat. Rev. Immunol. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Barnes, P.J. Mechanisms of development of multimorbidity in the elderly. Eur. Respir. J. 2015, 45, 790–806. [Google Scholar] [CrossRef]
- Friedman, E.M.; Christ, S.L.; Mroczek, D.K. Inflammation partially mediates the association of multimorbidity and functional limitations in a national sample of middle-aged and older adults: The MIDUS study. J. Aging. Health 2015, 27, 843–863. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; An, Y.; Zoli, M.; Simonsick, E.M.; Guralnik, J.M.; Bandinelli, S.; Boyd, C.M.; Ferrucci, L. Aging and the burden of multimorbidity: Associations with inflammatory and anabolic hormonal biomarkers. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 63–70. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef]
- Chen, B.-R.; Wu, T.; Chen, T.-H.; Wang, Y. Neuroimmune interactions and their roles in neurodegenerative diseases. Fundam. Res. 2024, 4, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Dilger, R.N.; Johnson, R.W. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J. Leukoc. Biol. 2008, 84, 932–939. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Foundations of immunometabolism and implications for metabolic health and disease. Immunity 2017, 47, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Riiser, A. The human microbiome, asthma, and allergy. Allergy Asthma Clin. Immunol. 2015, 11, 35. [Google Scholar] [CrossRef]
- Rastall, R.A. Bacteria in the gut: Friends and foes and how to alter the balance. J. Nutr. 2004, 134, 2022s–2026s. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food (2002). Available online: https://www.foodinprogress.com/wp-content/uploads/2019/04/Guidelines-for-the-Evaluation-of-Probiotics-in-Food.pdf (accessed on 30 March 2025).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Hirata, S.I.; Kunisawa, J. Gut microbiome, metabolome, and allergic diseases. Allergol. Int. 2017, 66, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, J.G.; Longman, R.S. Innate lymphoid cells link gut microbes with mucosal T cell immunity. Gut Microbes 2020, 11, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Hegazy, A.N.; West, N.R.; Stubbington, M.J.T.; Wendt, E.; Suijker, K.I.M.; Datsi, A.; This, S.; Danne, C.; Campion, S.; Duncan, S.H.; et al. Circulating and tissue-resident CD4(+) T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology 2017, 153, 1320–1337.e1316. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef]
- Zhang, X.; Irajizad, E.; Hoffman, K.L.; Fahrmann, J.F.; Li, F.; Seo, Y.D.; Browman, G.J.; Dennison, J.B.; Vykoukal, J.; Luna, P.N.; et al. Modulating a prebiotic food source influences inflammation and immune-regulating gut microbes and metabolites: Insights from the BE GONE trial. eBioMedicine 2023, 98, 104873. [Google Scholar] [CrossRef]
- Lazar, V.; Ditu, L.M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Abt, M.C.; Artis, D. The dynamic influence of commensal bacteria on the immune response to pathogens. Curr. Opin. Microbiol. 2013, 16, 4–9. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Artis, D. Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 2013, 14, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Augustine, T.; Kumar, M.; Al Khodor, S.; van Panhuys, N. Microbial dysbiosis tunes the immune response towards allergic disease outcomes. Clin. Rev. Allergy Immunol. 2023, 65, 43–71. [Google Scholar] [CrossRef]
- Akdis, C.A. Allergy and hypersensitivity: Mechanisms of allergic disease. Curr. Opin. Immunol. 2006, 18, 718–726. [Google Scholar] [CrossRef]
- Veldhoen, M.; Uyttenhove, C.; van Snick, J.; Helmby, H.; Westendorf, A.; Buer, J.; Martin, B.; Wilhelm, C.; Stockinger, B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008, 9, 1341–1346. [Google Scholar] [CrossRef]
- Blaser, M.J.; Falkow, S. What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 2009, 7, 887–894. [Google Scholar] [CrossRef]
- Montecchiani, V.; Fanos, V. Human microbiome and allergy. Pediatr. Allergy Immunol. 2020, 31 (Suppl. S26), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional diversity of the gastrointestinal microbiome. Cell Host Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Arifuzzaman, M.; Won, T.H.; Li, T.-T.; Yano, H.; Digumarthi, S.; Heras, A.F.; Zhang, W.; Parkhurst, C.N.; Kashyap, S.; Jin, W.-B.; et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature 2022, 611, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.; Hughes, M.R.; Li, Y.; Cait, A.; Hirst, M.; Mohn, W.W.; McNagny, K.M. Butyrate shapes immune cell fate and function in allergic asthma. Front. Immunol. 2021, 12, 628453. [Google Scholar] [CrossRef]
- Pessa-Morikawa, T.; Husso, A.; Kärkkäinen, O.; Koistinen, V.; Hanhineva, K.; Iivanainen, A.; Niku, M. Maternal microbiota-derived metabolic profile in fetal murine intestine, brain and placenta. BMC Microbiol. 2022, 22, 46. [Google Scholar] [CrossRef]
- Wu, K.; Guo, B.; Guo, Y.; Han, M.; Xu, H.; Luo, R.; Hong, Z.; Zhang, B.; Dong, K.; Wu, J.; et al. Association between residential greenness and gut microbiota in Chinese adults. Environ. Int. 2022, 163, 107216. [Google Scholar] [CrossRef]
- Ege, M.J.; Mayer, M.; Normand, A.C.; Genuneit, J.; Cookson, W.O.; Braun-Fahrländer, C.; Heederik, D.; Piarroux, R.; von Mutius, E. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef]
- Haahtela, T.; Holgate, S.; Pawankar, R.; Akdis, C.A.; Benjaponpitak, S.; Caraballo, L.; Demain, J.; Portnoy, J.; von Hertzen, L. The biodiversity hypothesis and allergic disease: World allergy organization position statement. World Allergy Organ. J. 2013, 6, 3. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; van den Brandt, P.A.; Kummeling, I.; Snijders, B.; Stelma, F.; Adams, H.; van Ree, R.; Stobberingh, E.E. Gut microbiota composition and development of atopic manifestations in infancy: The KOALA Birth Cohort Study. Gut 2007, 56, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- López, P.; González-Rodríguez, I.; Gueimonde, M.; Margolles, A.; Suárez, A. Immune response to Bifidobacterium bifidum strains support Treg/Th17 plasticity. PLoS ONE 2011, 6, e24776. [Google Scholar] [CrossRef]
- Arnold, I.C.; Dehzad, N.; Reuter, S.; Martin, H.; Becher, B.; Taube, C.; Müller, A. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J. Clin. Investig. 2011, 121, 3088–3093. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, K.M.; Konstantinou, G.N.; Pilapil, M.; Arrieta, M.C.; Noone, S.; Sampson, H.A.; Meddings, J.; Nowak-Węgrzyn, A. Intestinal permeability in children with food allergy on specific elimination diets. Pediatr. Allergy Immunol. 2013, 24, 589–595. [Google Scholar] [CrossRef]
- Barthow, C.; Wickens, K.; Stanley, T.; Mitchell, E.A.; Maude, R.; Abels, P.; Purdie, G.; Murphy, R.; Stone, P.; Kang, J.; et al. The Probiotics in Pregnancy Study (PiP Study): Rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth 2016, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.; O’Mahony, D.; O’Brien, F.; MacSharry, J.; Sheil, B.; Ceddia, M.; Russell, W.M.; Forsythe, P.; Bienenstock, J.; Kiely, B.; et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin. Exp. Allergy 2010, 40, 811–819. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef]
- Chua, M.C.; Ben-Amor, K.; Lay, C.; Neo, A.G.E.; Chiang, W.C.; Rao, R.; Chew, C.; Chaithongwongwatthana, S.; Khemapech, N.; Knol, J.; et al. Effect of synbiotic on the gut microbiota of cesarean delivered infants: A randomized, double-blind, multicenter study. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 102–106. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary fibre modulates the gut microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Zhang, P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Newsholme, P. Cellular and metabolic mechanisms of nutrient actions in immune function. Nutr. Diabetes 2021, 11, 22. [Google Scholar] [CrossRef]
- Dawson, S.L.; Todd, E.; Ward, A.C. The interplay of nutrition, the gut microbiota and immunity and its contribution to human disease. Biomedicines 2025, 13, 329. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.L.; Yap, Y.A.; McLeod, K.H.; Mackay, C.R.; Mariño, E. Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clin. Transl. Immunol. 2016, 5, e82. [Google Scholar] [CrossRef] [PubMed]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean diet as a tool to combat inflammation and chronic diseases. An overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Aamir, A.B.; Kumari, R.; Latif, R.; Ahmad, S.; Rafique, N.; Salem, A.M.; Alasoom, L.I.; Alsunni, A.; Alabdulhadi, A.S.; Chander, S. Effects of intermittent fasting and caloric restriction on inflammatory biomarkers in individuals with obesity/overweight: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2025, 26, e13838. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Calder, P.C. Foods to deliver immune-supporting nutrients. Curr. Opin. Food Sci. 2022, 43, 136–145. [Google Scholar] [CrossRef]
- Aldakheel, F.M. Allergic diseases: A comprehensive review on risk factors, immunological mechanisms, link with COVID-19, potential treatments, and role of allergen bioinformatics. Int. J. Environ. Res. Public Health 2021, 18, 12105. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Godos, J.; Giampieri, F.; Micek, A.; Battino, M.; Forbes-Hernández, T.Y.; Quiles, J.L.; Paladino, N.; Falzone, L.; Grosso, G. Effect of Brazil nuts on selenium status, blood lipids, and biomarkers of oxidative stress and inflammation: A systematic review and meta-analysis of randomized clinical trials. Antioxidants 2022, 11, 403. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D sources, metabolism, and deficiency: Available compounds and guidelines for its treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Kirby, T.O.; Sapp, P.A.; Gonzalez, A.M.; Marshall, T.M.; Esposito, R. Nutrient synergy: Definition, evidence, and future directions. Front. Nutr. 2023, 10, 1279925. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of vitamin A in the immune system. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s effect on immune function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Han, S.N.; Wu, D. Vitamin E and immune response in the aged: Molecular mechanisms and clinical implications. Immunol. Rev. 2005, 205, 269–284. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B vitamins and their role in immune regulation and cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune function and micronutrient requirements change over the life course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Estruch, R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. EMID-DT 2014, 14, 245–254. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Panagiotou, E.; Andreou, E.; Nicolaou, S.A. The effect of dietary components of the Mediterranean diet on food allergies: A systematic review. Nutrients 2023, 15, 3295. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Guibas, G.V.; Papadopoulos, N.G. Mediterranean-type diets as a protective factor for asthma and atopy. Nutrients 2022, 14, 1825. [Google Scholar] [CrossRef]
- Koumpagioti, D.; Boutopoulou, B.; Moriki, D.; Priftis, K.N.; Douros, K. Does adherence to the mediterranean diet have a protective effect against asthma and allergies in children? A systematic review. Nutrients 2022, 14, 1618. [Google Scholar] [CrossRef]
- Castro-Rodriguez, J.A.; Garcia-Marcos, L. What are the effects of a Mediterranean diet on allergies and asthma in children? Front. Pediatr. 2017, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Artis, D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 2010, 28, 623–667. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; La Vecchia, C.; Bamia, C. Mediterranean diet and its components in relation to all-cause mortality: Meta-analysis. Br. J. Nutr. 2018, 120, 1081–1097. [Google Scholar] [CrossRef]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi Sözener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The influence of dietary fatty acids on immune responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Nelson, B.N.; Friedman, J.E. Developmental programming of the fetal Immune dystem by maternal western-style diet: Mechanisms and implications for disease pathways in the offspring. Int. J. Mol. Sci. 2024, 25, 5951. [Google Scholar] [CrossRef]
- Zaragoza-Martí, A.; Ruiz-Ródenas, N.; Herranz-Chofre, I.; Sánchez-SanSegundo, M.; Serrano Delgado, V.C.; Hurtado-Sánchez, J.A. Adherence to the Mediterranean diet in pregnancy and its benefits on maternal-fetal health: A systematic review of the literature. Front. Nutr. 2022, 9, 813942. [Google Scholar] [CrossRef]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean diet: An update of the clinical trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Wang, D.Y. Risk factors of allergic rhinitis: Genetic or environmental? Ther. Clin. Risk Manag. 2005, 1, 115–123. [Google Scholar] [CrossRef]
- Shamir, R. The benefits of breast feeding. Nestle Nutr. Inst. Workshop Ser. 2016, 86, 67–76. [Google Scholar] [CrossRef]
- Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Oddy, W.H. Breastfeeding, childhood asthma, and allergic disease. Ann. Nutr. Metab. 2017, 70 (Suppl. S2), 26–36. [Google Scholar] [CrossRef]
- Høst, A.; Halken, S.; Muraro, A.; Dreborg, S.; Niggemann, B.; Aalberse, R.; Arshad, S.H.; von Berg, A.; Carlsen, K.H.; Duschén, K.; et al. Dietary prevention of allergic diseases in infants and small children. Pediatr. Allergy Immunol. 2008, 19, 1–4. [Google Scholar] [CrossRef]
- Gdalevich, M.; Mimouni, D.; Mimouni, M. Breast-feeding and the risk of bronchial asthma in childhood: A systematic review with meta-analysis of prospective studies. J. Pediatr. 2001, 139, 261–266. [Google Scholar] [CrossRef]
- Wright, A.L.; Holberg, C.J.; Taussig, L.M.; Martinez, F.D. Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax 2001, 56, 192–197. [Google Scholar] [CrossRef]
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The Relationship between the infant gut microbiota and allergy. The role of Bifidobacterium breve and prebiotic oligosaccharides in the activation of anti-allergic mechanisms in early life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef]
- Gabbianelli, R.; Bordoni, L.; Morano, S.; Calleja-Agius, J.; Lalor, J.G. Nutri-epigenetics and gut microbiota: How birth care, bonding and breastfeeding can influence and be influenced? Int. J. Mol. Sci. 2020, 21, 5032. [Google Scholar] [CrossRef]
- Koukou, Z.; Papadopoulou, E.; Panteris, E.; Papadopoulou, S.; Skordou, A.; Karamaliki, M.; Diamanti, E. The effect of breastfeeding on food allergies in newborns and infants. Children 2023, 10, 1046. [Google Scholar] [CrossRef]
- Deschildre, A.; Lejeune, S.; Cap, M.; Flammarion, S.; Jouannic, L.; Amat, F.; Just, J. Food allergy phenotypes: The key to personalized therapy. Clin. Exp. Allergy 2017, 47, 1125–1137. [Google Scholar] [CrossRef]
- D’Auria, E.; Abrahams, M.; Zuccotti, G.V.; Venter, C. Personalized nutrition approach in food allergy: Is it prime time yet? Nutrients 2019, 11, 359. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Lopes, J.P.; Sicherer, S. Food allergy: Epidemiology, pathogenesis, diagnosis, prevention, and treatment. Curr. Opin. Immunol. 2020, 66, 57–64. [Google Scholar] [CrossRef]
- Bellanti, J.A. IgE and non-IgE food allergy: A review of immunological mechanisms. J. Food Allergy 2024, 6, 37–46. [Google Scholar] [CrossRef]
- Van Gool, F.; Nguyen, M.L.T.; Mumbach, M.R.; Satpathy, A.T.; Rosenthal, W.L.; Giacometti, S.; Le, D.T.; Liu, W.; Brusko, T.M.; Anderson, M.S.; et al. A mutation in the transcription factor Foxp3 drives T helper 2 effector function in regulatory T cells. Immunity 2019, 50, 362–377.e366. [Google Scholar] [CrossRef]
- Smith, M.; Tourigny, M.R.; Noakes, P.; Thornton, C.A.; Tulic, M.K.; Prescott, S.L. Children with egg allergy have evidence of reduced neonatal CD4(+)CD25(+)CD127(lo/-) regulatory T cell function. J. Allergy Clin. Immunol. 2008, 121, 1460–1466.e7. [Google Scholar] [CrossRef]
- Lee, M.P.; Saffari, S.E.; Loh, W.; Goh, S.H.; Goh, A.; Chiang, W.C.; Chong, K.W. A 5-year retrospective review of children with peanut allergy in the largest paediatric hospital in Singapore. Asia Pac. Allergy 2020, 10, e6. [Google Scholar] [CrossRef]
- Lee, A.J.; Thalayasingam, M.; Lee, B.W. Food allergy in Asia: How does it compare? Asia Pac. Allergy 2013, 3, 3–14. [Google Scholar] [CrossRef]
- Suther, C.; Moore, M.D.; Beigelman, A.; Zhou, Y. The gut microbiome and the big eight. Nutrients 2020, 12, 3728. [Google Scholar] [CrossRef]
- Moffat, K.; Mercer, S.W. Challenges of managing people with multimorbidity in today’s healthcare systems. BMC Fam. Pract. 2015, 16, 129. [Google Scholar] [CrossRef]
- Dugravot, A.; Fayosse, A.; Dumurgier, J.; Bouillon, K.; Rayana, T.B.; Schnitzler, A.; Kivimaki, M.; Sabia, S.; Singh-Manoux, A. Social inequalities in multimorbidity, frailty, disability, and transitions to mortality: A 24-year follow-up of the Whitehall II cohort study. Lancet Public Health 2020, 5, e42–e50. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.; Marengoni, A.; Forjaz, M.J.; Jureviciene, E.; Laatikainen, T.; Mammarella, F.; Muth, C.; Navickas, R.; Prados-Torres, A.; Rijken, M.; et al. Multimorbidity care model: Recommendations from the consensus meeting of the Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle (JA-CHRODIS). Health Policy 2018, 122, 4–11. [Google Scholar] [CrossRef]
- Friedman, E.; Shorey, C. Inflammation in multimorbidity and disability: An integrative review. Health Psychol. 2019, 38, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Weyand, C.M. Immune aging and autoimmunity. Cell Mol. Life Sci. 2012, 69, 1615–1623. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Beck, M.A.; Alwarawrah, Y.; MacIver, N.J. Emerging mechanisms of obesity-associated immune dysfunction. Nat. Rev. Endocrinol. 2024, 20, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mehdi, M.M. Oxidative stress, inflammation and hormesis: The role of dietary and lifestyle modifications on aging. Neurochem. Int. 2023, 164, 105490. [Google Scholar] [CrossRef]
- Song, G.; Li, W.; Ma, Y.; Xian, Y.; Liao, X.; Yang, X.; Zhang, H.; Cade, J.E. Nutrient intake and risk of multimorbidity: A prospective cohort study of 25,389 women. BMC Public Health 2024, 24, 696. [Google Scholar] [CrossRef]
- Ruel, G.; Shi, Z.; Zhen, S.; Zuo, H.; Kröger, E.; Sirois, C.; Lévesque, J.F.; Taylor, A.W. Association between nutrition and the evolution of multimorbidity: Τhe importance of fruits and vegetables and whole grain products. Clin. Nutr. 2014, 33, 513–520. [Google Scholar] [CrossRef]
- Dekker, L.H.; de Borst, M.H.; Meems, L.M.G.; de Boer, R.A.; Bakker, S.J.L.; Navis, G.J. The association of multimorbidity within cardio-metabolic disease domains with dietary patterns: A cross-sectional study in 129 369 men and women from the Lifelines cohort. PLoS ONE 2019, 14, e0220368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Carrillo-Larco, R.M.; Lim, C.C.W.; Mishra, S.R.; Yuan, C.; Xu, X. Association of dietary patterns and food groups intake with multimorbidity: A prospective cohort study. Clin. Nutr. ESPEN 2022, 51, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.G.A.; Wilmot, C.; Griffiths, H.R. Personalising nutrition for older adults: The InCluSilver project. Nutr. Bull. 2018, 43, 442–455. [Google Scholar] [CrossRef]
- Kyprianidou, M.; Panagiotakos, D.; Faka, A.; Kambanaros, M.; Makris, K.C.; Christophi, C.A. Adherence to the Mediterranean diet in Cyprus and its relationship to multi-morbidity: An epidemiological study. Public Health Nutr. 2021, 24, 4546–4555. [Google Scholar] [CrossRef]
- Vicinanza, R.; Bersani, F.S.; D’Ottavio, E.; Murphy, M.; Bernardini, S.; Crisciotti, F.; Frizza, A.; Mazza, V.; Biondi, M.; Troisi, G.; et al. Adherence to Mediterranean diet moderates the association between multimorbidity and depressive symptoms in older adults. Arch. Gerontol. Geriatr. 2020, 88, 104022. [Google Scholar] [CrossRef]
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019, 70, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.W.; Kullgren, J.T.; Malani, P.N.; Singer, D.C.; Kirch, M.; Solway, E.; Wolfson, J.A. Food insecurity is associated with multiple chronic conditions and physical health status among older US adults. Prev. Med. Rep. 2020, 20, 101211. [Google Scholar] [CrossRef] [PubMed]
- Marsman, D.; Belsky, D.W.; Gregori, D.; Johnson, M.A.; Low Dog, T.; Meydani, S.; Pigat, S.; Sadana, R.; Shao, A.; Griffiths, J.C. Healthy ageing: The natural consequences of good nutrition-a conference report. Eur. J. Nutr. 2018, 57, 15–34. [Google Scholar] [CrossRef]
- Jeelani, I.; Nawaz, A.; Asif, H.M.; Ahmad, I.; Gattu, A.K. Editorial: Recent advances in immunometabolism. Front. Pharmacol. 2024, 15, 1422816. [Google Scholar] [CrossRef]
- Downer, S.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food is medicine: Actions to integrate food and nutrition into healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef]
- Fabbri, E.; Zoli, M.; Gonzalez-Freire, M.; Salive, M.E.; Studenski, S.A.; Ferrucci, L. Aging and multimorbidity: New tasks, priorities, and frontiers for integrated gerontological and clinical research. J. Am. Med. Dir. Assoc. 2015, 16, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, C.S.; Kirchberger, I.; Heier, M.; Zimmermann, A.K.; Peters, A.; Döring, A.; Thorand, B. Physical activity is inversely associated with multimorbidity in elderly men: Results from the KORA-Age Augsburg Study. Prev. Med. 2013, 57, 17–19. [Google Scholar] [CrossRef]
- Booth, H.P.; Prevost, A.T.; Gulliford, M.C. Impact of body mass index on prevalence of multimorbidity in primary care: Cohort study. Fam. Pract. 2014, 31, 38–43. [Google Scholar] [CrossRef]
- Nagel, G.; Peter, R.; Braig, S.; Hermann, S.; Rohrmann, S.; Linseisen, J. The impact of education on risk factors and the occurrence of multimorbidity in the EPIC-Heidelberg cohort. BMC Public Health 2008, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.N.; de Camargo Cancela, M.; de Souza, D.L.B. Lifestyle factors and high body mass index are associated with different multimorbidity clusters in the Brazilian population. PLoS ONE 2018, 13, e0207649. [Google Scholar] [CrossRef]
- Daniel, S.; Soleymani, T.; Garvey, W.T. A complications-based clinical staging of obesity to guide treatment modality and intensity. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K. Current and emerging medications for overweight or obesity in people with comorbidities. Diabetes Obes. Metab. 2015, 17, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Ngangue, P.; Bouhali, T.; Ryan, B.L.; Stewart, M.; Fortin, M. Social vulnerability in patients with multimorbidity: A cross-sectional analysis. Int. J. Environ. Res. Public Health 2019, 16, 1244. [Google Scholar] [CrossRef]
- Leahy, S.; Cassarino, M.; MD, O.C.; Glynn, L.; Galvin, R. Dynapaenic obesity and its association with health outcomes in older adult populations: Protocol for a systematic review. BMJ Open 2019, 9, e027728. [Google Scholar] [CrossRef]
- Hassapidou, M.; Vlassopoulos, A.; Kalliostra, M.; Govers, E.; Mulrooney, H.; Ells, L.; Salas, X.R.; Muscogiuri, G.; Darleska, T.H.; Busetto, L.; et al. European Association for the Study of Obesity Position Statement on Medical Nutrition Therapy for the Management of Overweight and Obesity in Adults Developed in Collaboration with the European Federation of the Associations of Dietitians. Obes. Facts 2023, 16, 11–28. [Google Scholar] [CrossRef]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-derived antioxidants and their role in inflammation, obesity and gut microbiota modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Shyam, S.; Lee, K.X.; Tan, A.S.W.; Khoo, T.A.; Harikrishnan, S.; Lalani, S.A.; Ramadas, A. Effect of personalized nutrition on dietary, physical activity, and health outcomes: A systematic review of randomized trials. Nutrients 2022, 14, 4104. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef]
- Singar, S.; Nagpal, R.; Arjmandi, B.H.; Akhavan, N.S. Personalized nutrition: Tailoring dietary recommendations through genetic insights. Nutrients 2024, 16, 2673. [Google Scholar] [CrossRef]
- Abeltino, A.; Hatem, D.; Serantoni, C.; Riente, A.; De Giulio, M.M.; De Spirito, M.; De Maio, F.; Maulucci, G. Unraveling the gut microbiota: Implications for precision nutrition and personalized medicine. Nutrients 2024, 16, 3806. [Google Scholar] [CrossRef]
- Gibbons, S.M.; Gurry, T.; Lampe, J.W.; Chakrabarti, A.; Dam, V.; Everard, A.; Goas, A.; Gross, G.; Kleerebezem, M.; Lane, J.; et al. Perspective: Leveraging the gut microbiota to predict personalized responses to dietary, prebiotic, and probiotic interventions. Adv. Nutr. 2022, 13, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Kassem, H.; Beevi, A.A.; Basheer, S.; Lutfi, G.; Cheikh Ismail, L.; Papandreou, D. Investigation and assessment of AI’s role in nutrition-An updated narrative review of the evidence. Nutrients 2025, 17, 190. [Google Scholar] [CrossRef]
- Donovan, S.M.; Abrahams, M.; Anthony, J.C.; Bergia, R.; Blander, G.; Brisbois, T.D.; Keck, A.-S.; Moore, E.G.; Morck, T.A.; Nieman, K.M.; et al. Perspective: Challenges for personalized nutrition in the current United States regulatory framework and future opportunities. Adv. Nutr. 2025, 16, 100382. [Google Scholar] [CrossRef]
- Ring, M.; Ai, D.; Maker-Clark, G.; Sarazen, R. Cooking up change: DEIB principles as key ingredients in nutrition and culinary medicine education. Nutrients 2023, 15, 4257. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.; Shen, J.; An, R. Artificial intelligence applications to measure food and nutrient intakes: Scoping review. J. Med. Internet Res. 2024, 26, e54557. [Google Scholar] [CrossRef]
- Das, S.K.; Miki, A.J.; Blanchard, C.M.; Sazonov, E.; Gilhooly, C.H.; Dey, S.; Wolk, C.B.; Khoo, C.S.H.; Hill, J.O.; Shook, R.P. Perspective: Opportunities and challenges of technology tools in dietary and activity assessment: Bridging stakeholder viewpoints. Adv. Nutr. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gelli, A.; Nwabuikwu, O.; Bannerman, B.; Ador, G.; Atadze, V.; Asante, M.; Bempong, S.; McCloskey, P.; Nguyen, P.H.; Hughes, D.; et al. Computer vision-assisted dietary assessment through mobile phones in female youth in urban Ghana: Validity against weighed records and comparison with 24-h recalls. Am. J. Clin. Nutr. 2024, 120, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Heydarian, H.; Adam, M.; Burrows, T.; Collins, C.; Rollo, M.E. Assessing eating behaviour using upper limb mounted motion sensors: A Systematic review. Nutrients 2019, 11, 1168. [Google Scholar] [CrossRef]
- Shonkoff, E.; Cara, K.C.; Pei, X.A.; Chung, M.; Kamath, S.; Panetta, K.; Hennessy, E. AI-based digital image dietary assessment methods compared to humans and ground truth: A systematic review. Ann. Med. 2023, 55, 2273497. [Google Scholar] [CrossRef] [PubMed]
- Godevithana, J.; Wijesinghe, C.J.; Wijesinghe, M.S.D. Paper-based and mobile application-based self-monitoring tool for healthy dietary intake, development and applicability: A non-randomized trial. BMC Digit. Health 2024, 2, 53. [Google Scholar] [CrossRef]
- Sjöblom, L.; Hantikainen, E.; Dahlgren, A.; Trolle Lagerros, Y.; Bonn, S.E. The effect of an app-based dietary education on dietary intake and cardiometabolic risk markers in people with type 2 diabetes: Results from a randomized controlled trial. Nutr. J. 2025, 24, 2. [Google Scholar] [CrossRef]
- Huang, X.; Gu, L.; Sun, J.; Eils, R. Bridging the gaps: Overcoming challenges of implementing AI in healthcare. Med 2025, 6, 100666. [Google Scholar] [CrossRef]
- Kapsokefalou, M.; Roe, M.; Turrini, A.; Costa, H.S.; Martinez-Victoria, E.; Marletta, L.; Berry, R.; Finglas, P. Food composition at present: New challenges. Nutrients 2019, 11, 1174. [Google Scholar] [CrossRef]
| Feature | Innate Immunity | Adaptive Immunity |
|---|---|---|
| Feature | Innate immunity | Adaptive immunity |
| Response Time | Immediate (mins to hours) | Delayed (days to weeks) |
| Specificity | Non-specific, recognizes conserved patterns (PAMPs) | Highly specific, targets unique antigens |
| Memory | No memory | Generates immunologic memory |
| Key Cells | Macrophages, neutrophils, NK cells, dendritic cells | B cells, T cells (CD4+, CD8+) |
| Soluble Factors | Complement, cytokines, and antimicrobial peptides | Antibodies, cytokines |
| Component | Role in Allergic Response |
|---|---|
| Antigen-Presenting Cells (APCs) | Capture and present allergens to naïve T cells, initiating adaptive immune responses. |
| T-helper 2 (Th2) Cells | Drive allergic inflammation by secreting IL-4, IL-5, and IL-13, enhancing IgE production and eosinophil activity. |
| Regulatory T Cells (Tregs) | Suppress excessive immune responses; impaired Treg activity contributes to allergic sensitization. |
| B Cells and Plasma Cells | Produce allergen-specific IgE antibodies that sensitize mast cells and basophils to allergens. |
| Mast Cells | Store histamine and inflammatory mediators release them upon IgE cross-linking, triggering allergic symptoms. |
| Basophils | Circulating cells amplify allergic inflammation through the release of histamine, leukotrienes, and IL-4. |
| Eosinophils | Mediate late-phase allergic responses; release cytotoxic granules, causing chronic inflammation and tissue damage. |
| Cytokines |
|
| Histamine and Leukotrienes | Released from mast cells and basophils; mediate vasodilation, bronchoconstriction, and mucus production. |
| Gut Microbiota | Essential for immune tolerance; microbial dysbiosis increases susceptibility to allergic diseases. |
| Mediterranean Diet Component | Immune and Allergy-Related Effects | Dietary Sources |
|---|---|---|
| Olive Oil | Rich in polyphenols; anti-inflammatory; supports gut microbiota diversity and barrier integrity. | Extra virgin olive oil |
| Fruits and Vegetables | High in antioxidants (vitamins C, E, carotenoids); reduces oxidative stress and inflammation. | Leafy greens, berries, citrus fruits, tomatoes, bell peppers, carrots |
| Whole Grains | Prebiotic fiber supports gut microbiota; enhances SCFA production for immune modulation. | Whole wheat, brown rice, quinoa, barley, oats |
| Legumes | High fiber and protein content; contributes to gut microbiota balance and Treg activation. | Lentils, chickpeas, black beans, kidney beans |
| Nuts and Seeds | Source of polyphenols and healthy fats; modulates inflammatory and immune responses. | Almonds, walnuts, flaxseeds, chia seeds, sunflower seeds |
| Fish and Seafood | High in omega-3 fatty acids (EPA and DHA); reduces airway inflammation and asthma risk. | Salmon, sardines, mackerel, tuna, shrimp |
| Dairy (Moderate Consumption) | Source of probiotics may support gut microbiota and immune tolerance. | Yogurt, cheese, kefir |
| Red Wine (Moderate Consumption) | Contains polyphenols (resveratrol); potential immunomodulatory and anti-inflammatory effects. | Red wine (in moderation, as part of a balanced diet) |
| Recommendation | Description |
|---|---|
| Integrate clinical phenotype and diagnostic tools | Use skin prick tests (SPTs), specific IgE levels, component-resolved diagnostics (CRD), and oral food challenges (OFCs) to define allergy phenotype and assess severity. |
| Use epigenetic biomarkers where available | Incorporate emerging biomarkers such as FOXP3 and PGM3 methylation status to inform immune tolerance capacity and personalize intervention strategies. |
| Account for regional allergen profiles | Tailor dietary advice based on geographic allergen prevalence (e.g., cashew allergy in Southeast Asia vs. peanut allergy in Western countries), food availability, and cultural dietary norms. |
| Tailor allergen avoidance based on risk | Adapt the strictness of avoidance diets according to reaction threshold, allergen form (raw vs. baked), and cross-reactivity risk. |
| Monitor and support nutritional adequacy | Ensure diets do not lead to micronutrient deficiencies, especially in children. Monitor growth and dietary intake regularly. |
| Incorporate microbiome insights | Consider microbiome composition and diversity (e.g., SCFA production, Bifidobacterium abundance) when planning interventions that support immune tolerance. |
| Apply shared decision-making and cultural sensitivity | Engage patients in personalized planning, accounting for food preferences, religious or cultural practices, and psychosocial factors to improve adherence. |
| Involve registered dietitians in care plans | Dietitians should provide individualized guidance on allergen avoidance, nutrient adequacy, recipe substitutions, and label reading. |
| Strategy | Mechanism | Potential Benefits |
|---|---|---|
| High-Fiber Diet (↑ 1 SCFAs) | Modulates Th1/Th2 balance, enhances Tregs | ↓ 2 Allergy risk, ↓ Inflammation |
| Mediterranean Diet | Rich in polyphenols, omega-3s, and fiber | ↓ Asthma, eczema, CVD, depression |
| Breastfeeding | Promotes beneficial microbes (e.g., Bifidobacterium), provides IgA | ↓ Early-life allergy susceptibility |
| Probiotics and Prebiotics | Restores microbial diversity, enhances gut barrier | Adjunct therapy in allergy and metabolic diseases |
| Targeted Micronutrient Supplementation | Corrects deficiencies that impair immune signaling | Immune rejuvenation in aging, ↓ Multimorbidity |
| Anti-inflammatory Compounds (e.g., turmeric, flavonoids) | Suppress NF-κB and other inflammatory pathways | Supports immune tolerance, prevents escalation of chronic disease |
| Intermittent Fasting/Caloric Restriction | Enhances immune cell renewal, ↓ oxidative stress | May slow immune aging and disease accumulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreou, E.; Papaneophytou, C. Boosting Immunity Through Nutrition and Gut Health: A Narrative Review on Managing Allergies and Multimorbidity. Nutrients 2025, 17, 1685. https://doi.org/10.3390/nu17101685
Andreou E, Papaneophytou C. Boosting Immunity Through Nutrition and Gut Health: A Narrative Review on Managing Allergies and Multimorbidity. Nutrients. 2025; 17(10):1685. https://doi.org/10.3390/nu17101685
Chicago/Turabian StyleAndreou, Eleni, and Christos Papaneophytou. 2025. "Boosting Immunity Through Nutrition and Gut Health: A Narrative Review on Managing Allergies and Multimorbidity" Nutrients 17, no. 10: 1685. https://doi.org/10.3390/nu17101685
APA StyleAndreou, E., & Papaneophytou, C. (2025). Boosting Immunity Through Nutrition and Gut Health: A Narrative Review on Managing Allergies and Multimorbidity. Nutrients, 17(10), 1685. https://doi.org/10.3390/nu17101685








