An Increase of Adropin Can Predict Depression Improvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Clinical and Laboratory Parameters
2.3. Adropin Analysis
2.4. Assessment of Depression Score
2.5. Patient Follow-Up
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt/GSK3β | serine/threonine kinase glycogen synthase kinase 3β |
| BDI II | Beck Depression Inventory-II |
| BMI | body mass index |
| BDNF | brain-derived neurotrophic factor |
| CHD | coronary heart disease |
| CK | creatine kinase |
| CRP | C-reactive protein |
| CNS | central nervous system |
| DDG | depressive disorder group |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| EDTA | Ethylenediaminetetraacetic acid |
| Enho | Energy homeostasis-associated gene |
| eNOS | endothelial nitric oxide synthase |
| ERK ½ | extracellular signal-regulated kinases 1/2 |
| LDL-C | low-density lipoprotein cholesterol |
| NO | nitric oxide |
| PHQ-9 | Patient Health Questionnaire-9 |
| SCID | Structured Clinical Interview for Diagnostic |
| SPSS | Statistical Package for the Social Sciences |
| TIBC | total iron binding capacity |
| TSH | thyroid-stimulating hormone |
| TNFα | tumor Necrosis Factor Alpha |
| UIBC | unsaturated iron binding capacity |
| VEGFR-2 | vascular endothelial growth factor receptor-2 |
References
- Kumar, K.G.; Trevaskis, J.L.; Lam, D.D.; Sutton, G.M.; Koza, R.A.; Chouljenko, V.N.; Kousoulas, K.G.; Rogers, P.M.; Kesterson, R.A.; Thearle, M.; et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008, 8, 468–481. [Google Scholar] [CrossRef]
- Butler, A.A.; Zhang, J.; Price, C.A.; Stevens, J.R.; Graham, J.L.; Stanhope, K.L.; King, S.; Krauss, R.M.; Bremer, A.A.; Havel, P.J. Low plasma adropin concentrations increase risks of weight gain and metabolic dysregulation in response to a high-sugar diet in male nonhuman primates. J. Biol. Chem. 2019, 294, 9706–9719. [Google Scholar] [CrossRef]
- Yolbas, S.; Kara, M.; Kalayci, M.; Yildirim, A.; Gundogdu, B.; Aydin, S.; Koca, S.S. ENHO gene expression and serum adropin level in rheumatoid arthritis and systemic lupus erythematosus. Adv. Clin. Exp. Med. 2018, 27, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A.; Tam, C.S.; Stanhope, K.L.; Wolfe, B.M.; Ali, M.R.; O’Keeffe, M.; St-Onge, M.-P.; Ravussin, E.; Havel, P.J. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J. Clin. Endocrinol. Metab. 2012, 97, 3783–3791. [Google Scholar] [CrossRef]
- Ghoshal, S.; Stevens, J.R.; Billon, C.; Girardet, C.; Sitaula, S.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Rankinen, T.; Bouchard, C.; et al. Adropin: An endocrine link between the biological clock and cholesterol homeostasis. Mol. Metab. 2018, 8, 51–64. [Google Scholar] [CrossRef]
- Gao, S.; McMillan, R.P.; Jacas, J.; Zhu, Q.; Li, X.; Kumar, G.K.; Casals, N.; Hegardt, F.G.; Robbins, P.D.; Lopaschuk, G.D.; et al. Regulation of substrate oxidation preferences in muscle by the peptide hormone adropin. Diabetes 2014, 63, 3242–3252. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhang, C.; Wang, H.; Yang, M.; Guo, Y.; Li, G.; Zhang, H.; Wang, C.; Chen, D.; Geng, C.; et al. Alterations of irisin, adropin, preptin and BDNF concentrations in coronary heart disease patients comorbid with depression. Ann. Transl. Med. 2019, 7, 298. [Google Scholar] [CrossRef]
- Gu, X.; Li, H.; Zhu, X.; Gu, H.; Chen, J.; Wang, L.; Harding, P.; Xu, W. Inverse correlation between plasma adropin and ET-1 levels in essential hypertension: A cross-sectional study. Medicine 2015, 94, e1712. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y.; Zhao, P.; Wu, M.-C.; Liu, J.; Yin, W. Serum adropin levels are decreased in patients with acute myocardial infarction. Regul. Pept. 2014, 190–191, 46–49. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Yang, Y.-J.; Ge, R.-K.; Zhou, M.; Hu, H.; Liu, H.; Cui, J.; Li, L.-L.; Dong, Y.-F.; et al. Aerobic exercise improves endothelial function and serum adropin levels in obese adolescents independent of body weight loss. Sci. Rep. 2017, 7, 17717. [Google Scholar] [CrossRef]
- Andrade, L.; Caraveo-Anduaga, J.J.; Berglund, P.; Bijl, R.V.; De Graaf, R.; Vollebergh, W.; Dragomirecka, E.; Kohn, R.; Keller, M.; Kessler, R.C.; et al. The epidemiology of major depressive episodes: Results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int. J. Methods Psychiatr. Res. 2003, 12, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Bozic, J.; Borovac, J.A.; Galic, T.; Kurir, T.T.; Supe – Domic, D.; Dogas, Z. Adropin and Inflammation Biomarker Levels in Male Patients With Obstructive Sleep Apnea: A Link With Glucose Metabolism and Sleep Parameters. J. Clin. Sleep Med. 2018, 14, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gunraj, R.E.; Yang, C.; Liu, L.; Larochelle, J.; Candelario-Jalil, E. Protective roles of adropin in neurological disease. Am. J. Physiol. Cell. Physiol. 2023, 324, C674–C678. [Google Scholar] [CrossRef]
- Cicek, M.A.; Okutucu, F.T.; Ozturk, N. Irisin, adropin, and preptin as biomarkers of energy dysregulation in depressive disorder. Curr. Med. Res. Opin. 2023, 39, 1263–1270. [Google Scholar] [CrossRef]
- Willner, P.; Belzung, C. Treatment-resistant depression: Are animal models of depression fit for purpose? Psychopharmacology 2015, 232, 3473–3495. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Friston, K.; Mody, M.; Wang, H.; Lu, H.; Hu, D. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci. Ther. 2018, 24, 1004–1019. [Google Scholar] [CrossRef]
- Emamian, E.S.; Hall, D.; Birnbaum, M.J.; Karayiorgou, M.; AGogos, J. Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia. Nat. Genet. 2004, 36, 131–137. [Google Scholar] [CrossRef]
- Jope, R.S. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front. Mol. Neurosci. 2011, 4, 16. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Bosmans, E.; Suy, E.; Vandervorst, C.; DeJonckheere, C.; Raus, J. Depression-related disturbances in mitogen-induced lymphocyte responses and interleukin-1β and soluble interleukin-2 receptor production. Acta Psychiatr. Scand. 1991, 84, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depres-sion. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and endothelial function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A me-ta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef]
- Kofod, J.; Elfving, B.; Nielsen, E.H.; Mors, O.; Köhler-Forsberg, O. Depression and inflammation: Correlation between changes in inflammatory markers with antidepressant response and long-term prognosis. Eur. Neuropsychopharmacol. 2022, 54, 116–125. [Google Scholar] [CrossRef]
- Maes, M.; Bosmans, E.; Suy, E.; Vandervorst, C.; De Jonckheere, C.; Raus, J. Immune disturbances during major depression: Upregulated expression of interleukin-2 receptors. Neuropsychobiology 1990, 24, 115–120. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Zorlu, M.; Şekercï, A.; Tunç, M.; Güler, E.M.; Gülen, B.; Karatoprak, C.; Kiskaç, M.; Çakirca, M. Evaluation of the relationship between vitamin D level and adropin, IL-1ß, IL-6, and oxidative status in women. Turk. J. Med. Sci. 2022, 52, 1197–1206. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Q.; Lin, X.; Chen, M.; Liu, Q. A review of adropin as the medium of dialogue between energy regulation and immune regulation. Oxidative Med. Cell. Longev. 2020, 2020, 3947806. [Google Scholar] [CrossRef]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Al-Omran, M.; Teoh, H.; Verma, S. Adropin is a novel regulator of endothelial function. Circulation 2010, 122, S185–S192. [Google Scholar] [CrossRef]
- Yosaee, S.; Khodadost, M.; Esteghamati, A.; Speakman, J.R.; Shidfar, F.; Nazari, M.N.; Bitarafan, V.; Djafarian, K. Metabolic syndrome patients have lower levels of adropin when compared with healthy overweight/obese and lean subjects. Am. J. Men’s Health 2017, 11, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, J.; Koponen, H.; Kautiainen, H.; Eriksson, J.G.; Kampman, O.; Leiviskä, J.; Männistö, S.; Mäntyselkä, P.; Oksa, H.; Ovaskainen, Y.; et al. Association between vitamin B12 levels and melancholic depressive symptoms: A Finnish population-based study. BMC Psychiatry 2013, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Syed, E.U.; Wasay, M.; Awan, S. Vitamin B12 supplementation in treating major depressive disorder: A randomized controlled trial. Open Neurol. J. 2013, 7, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Baig, M.; Tunio, S.A.; Memon, A.S.; Karmani, H. Neuropsychiatric and neurological problems among vitamin B12 defi-cient young vegetarians. Neurosciences 2017, 22, 228–232. [Google Scholar] [CrossRef]
- Peppard, L.; Oh, K.M.; Gallo, S.; Milligan, R. Risk of depression in pregnant women with low-normal serum vitamin B12. Res. Nurs. Health 2019, 42, 264–272. [Google Scholar] [CrossRef]
| Total | DD Group (N = 54) | Control Group (N = 56) | p | |
|---|---|---|---|---|
| Sex | 0.848 * | |||
| Female | 94 (85) | 47 (87) | 47 (84) | |
| Male | 16 (15) | 7 (13) | 9 (16) | |
| Age (Years) | 45.5 (31–55; 19–65) | 45 (29–57; 19–63) | 45.5 (31–53; 21–65) | 0.634 ** |
| BMI (kg/m2) | 23.7 (22–26; 18–40) | 23.5 (21–26; 18–40) | 23.8 (22–26; 19–31) | 0.460 ** |
| Addictions | 0.349 * | |||
| No | 63 (57.3) | 28 (51.9) | 35 (62.5) | |
| Yes | 47 (42.7) | 26 (48.1) | 21 (37.5) | |
| Smoking | 43 (39.1) | 22 (40.7) | 21 (37.5) | |
| Alcohol Use | 1 (1.09) | 1 (1.9) | 0 | |
| Psychostimulants | 1 (1.09) | 1 (1.9) | 0 | |
| Smoking and Alcohol | 2 (1.8) | 2 (3.7) | 0 | |
| Menopause | 0.383 * | |||
| No | 65 (68) | 29 (63) | 36 (73) | |
| Yes | 30 (32) | 17 (37) | 13 (27) | |
| BDI-II | ||||
| Non-depressed 0 | 56 (50.9) | 0 | 56 (100) | |
| Mild-depressed 1 | 13 (11.8) | 13 (24.1) | 0 | |
| Moderate-depressed 2 | 12 (10.9) | 12 (22.2) | 0 | |
| Severe-depressed 3 | 29 (26.4) | 29 (53.7) | 0 |
| Parameter | DD Group (N = 54) | Control Group (N = 56) | p * |
|---|---|---|---|
| Leukocytes (×109/L) | 6.9 (6–8; 3.7–9.7) | 5.9 (5–8; 3.7–11.4) | 0.848 |
| GUP (mmol/L) | 5.1 (5–6; 2.8–7.2) | 5.1 (5–6; 4–6.7) | 0.281 |
| CK (U/L) | 90 (76–117; 44–481) | 87.5 (57–112; 41–279) | 0.669 |
| CRP (mg/L) | 1.4 (0.7–2; 0.6–26) | 0.9 (0.6–1.5; 0.6–5.9) | 0.061 |
| Total Cholesterol (mmol/L) | 4.9 (4.4–6; 0–8.6) | 5.3 (4.7–6; 3.9–8.3) | 0.683 |
| Triglycerides (mmol/L) | 1.1 (0.6–1.4; 0–3.3) | 1 (0.8–1.4; 0.4–5.8) | 0.497 |
| HDL Cholesterol (mmol/L) | 1.7 (1.3–2.1; 0–2.3) | 1.7 (1.3–1.9; 1–2.4) | 0.880 |
| LDL Cholesterol (mmol/L) | 2.8 (2.4–3.8; 0–6.4) | 3.2 (2.5–3.9; 1.6–5.7) | 0.510 |
| Fe (μmol/L) | 16 (11–21; 5–44) | 18 (14–22; 7–29) | 0.408 |
| UIBC (μmol/L) | 46 (41–54; 24–89) | 39 (34–43; 34–56) | 0.090 |
| TIBC (μmol/L) | 63 (59–69; 44–96) | 57.5 (53–62; 45–72) | 0.246 |
| TS % | 25 (18–34; 6–65) | 32 (26–37; 11–49) | 0.078 |
| Vitamin B12 (pmol/L) | 393 (280–468; 182–760) | 367 (309–447; 168–705) | 0.204 |
| Folic Acid (nmol/L) | 17.4 (14–21; 7.7–45.4) | 16.5 (11–20; 5–51) | 0.088 |
| Vitamin D (nmol/L) | 51.7 (35–65; 18–223) | 49.8 (38–63; 24.8–90) | 0.911 |
| TSH (mIU/L) | 1.17 (0–1.9; 0–4.1) | 1.4 (0.27–1.8; 0–3.6) | 0.314 |
| T4 (nmol/L) | 93 (79–108; 2.3–125) | 95 (89–106; 69–127) | 0.940 |
| T3 (nmol/L) | 1.8 (1.4–2.1; 96–35) | 1.5 (1.4–1.6; 1.1–2.1) | 0.013 |
| Adropin (ng/mL) | 3 (2.5–3.5; 1.4–5.1) | 3.1 (2.7–3.6; 0.85–14.8) | 0.359 |
| BDI-II Baseline | ||||
|---|---|---|---|---|
| Mildly Depressed 1 | Moderately Depressed 2 | Severely Depressed 3 | Total | |
| BDI-II 6-Month Follow-Up | ||||
| Non-depressed 0 | 11 | 6 | 15 | 32 |
| Mildly depressed 1 | 1 | 2 | 6 | 9 |
| Moderately depressed 2 | 1 | 1 | 5 | 7 |
| Severely depressed 3 | 0 | 0 | 2 | 2 |
| Total | 13 | 9 | 28 | 50 |
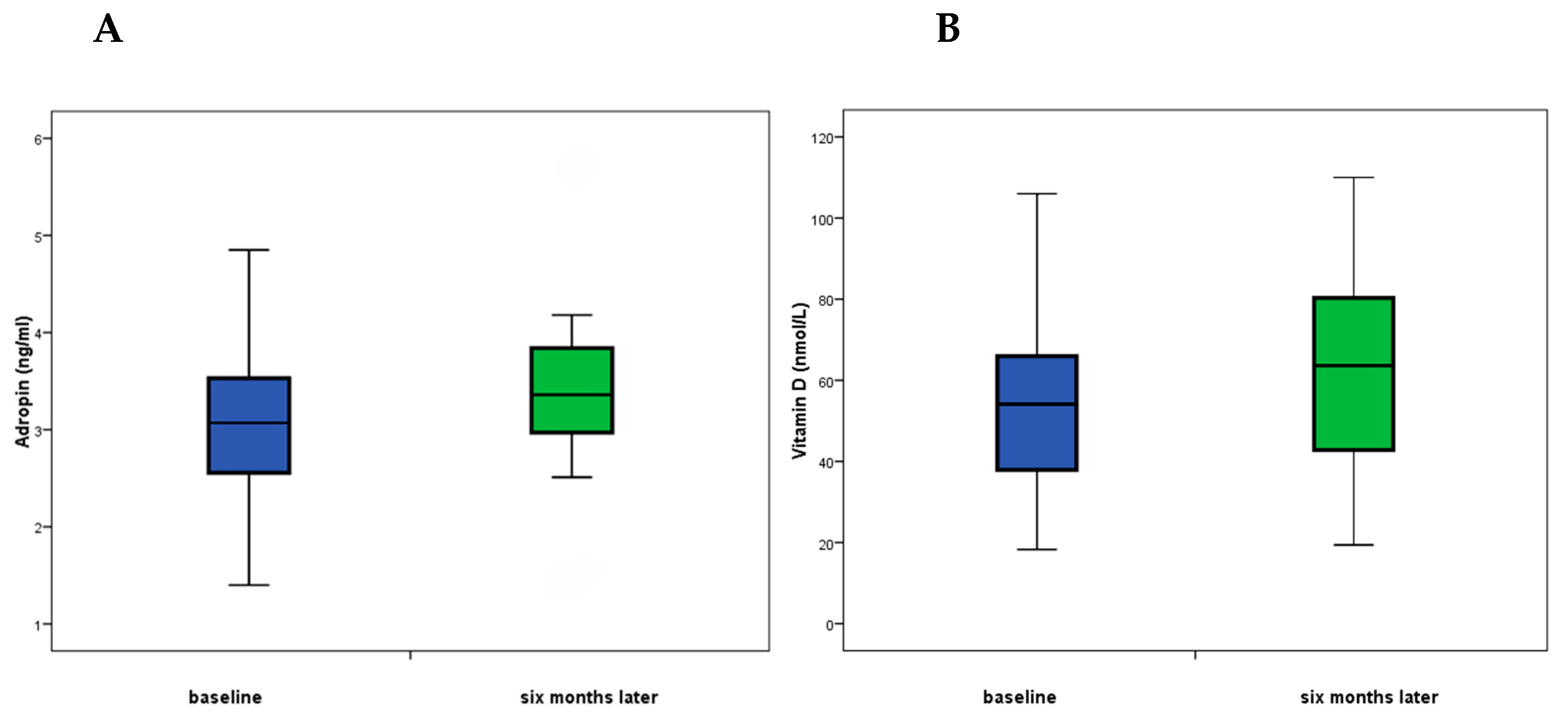
| Baseline | 6-Month Follow-Up | Difference (Baseline—6-Month Follow-Up) | p * | |
|---|---|---|---|---|
| Leukocytes (×109/L) | 6.4 (5.2–8.1; 3.5–9.9) | 6.0 (5.1–7.1; 3.2–10.9) | 0.35 (−0.50 to 1.5; −2.3 to 8.7) | 0.077 |
| GUP (mmol/L) | 5.1 (4.7–5.6; 2.8–8.1) | 4.9 (4.6–5.4; 3.9–11.3) | 0.150 (−0.3 to 0.5; −3.2 to 6.9) | 0.788 |
| CK (U/L) | 86 (73–108; 44–481) | 87.5 (67–110; 34–964) | 5.5 (−11 to 27; −488 to 333) | 0.830 |
| CRP (mg/L) | 1.1 (0.7–2; 0.6–25) | 1.1 (0.6–1.5; 0.6–7.7) | 0.10 (−01 to 0.9; −4.3 to 24) | 0.216 |
| Total Cholesterol (mmol/L) | 4.9 (4.4–6.2; 3.4–8.6) | 5.1 (4.2–6.3; 2.9–8.4) | −0.55 (−4.3 to 0.2; −8.4 to 6.2) | 0.532 |
| Triglycerides (mmol/L) | 0.9 (0.6–1.2; 0.3–3.3) | 1.1 (0.7–1.6; 0.4–4.4) | −0.5 (−1 to 0.1; −4.2 to 1) | 0.147 |
| HDL Cholesterol (mmol/L) | 1.6 (1.3–2.1; 0.9–2.8) | 1.5 (1.3–1.9; 0.7–2.9) | −0.1 (−1.2 to 0.1; −2.1 to 2.8) | 0.362 |
| LDL Cholesterol (mmol/L) | 3 (2.4–3.7; 1.1–6.4) | 2.9 (2,2-4;1.1-5.9) | −0.35 (−2.6 to 0.2; −5 to 3.3) | 0.776 |
| Fe (μmol/L) | 16 (12–20; 5–44) | 16 (12–20; 3–67) | 1.5 (−6 to 8; −38 to 30) | 0.899 |
| UIBC (μmol/L) | 45 (39–49; 18–89) | 42 (35–47; 11–70) | 1.5 (−3 to 10; −21 to 54) | 0.332 |
| TIBC (μmol/L) | 61 (55–65; 44–96) | 58.5 (55–66; 44–96) | 0 (−3 to 7; −12 to 64) | 0.650 |
| TS % | 27 (21–34; 6–65) | 30 (22–33; 4–86) | 0 (−11 to 9; −59 to 47) | 0.754 |
| Vitamin B12 (pmol/L) | 377.5 (272–463; 182–760) | 381 (312–425; 192–760) | −12 (−67 to 52; −151 to 488) | 0.184 |
| Folic Acid (nmol/L) | 16.8 (12.8–22.8; 7.7–45.4) | 17.8 (11–23.2:5–45) | 1.7 (−3.6 to 5.2; −34 to 22) | 0.315 |
| Vitamin D (nmol/L) | 52.3 (39.8–66; 18–223) | 60 (47–69; 19–223) | −7.7 (−20 to 0.12; −61 to 68) | 0.034 |
| TSH (mIU/L) | 1.24 (0–1.96; 0–4) | 1.49 (0.5–2; 0–5.6) | 0 (−0.55 to 0.17; −2.6 to 1.6) | 0.171 |
| T4 (nmol/L) | 95.6 (84–114; 2.3–209) | 97.7 (87–107; 74–150) | 0.85 (−8.9 to 12.8; −95 to 117) | 0.849 |
| T3 (nmol/L) | 1.17 (1.4–2.13; 0.96–35) | 1.6 (1.5–1.9; 0.93–2.56) | 0.09 (−0.16 to 0.3; −0.7 to 33.5) | 0.218 |
| Adropin (ng/mL) | 3.03 (2.6–3.5; 1.4–5.15) | 3.2 (2.7–3.7; 1.5–13.6) | −0.17 (−0.55 to 0.19; −10.7 to 3.1 | 0.018 |
| Responders (N = 32) | p * | Non-Responders (N = 18) | p * | |||
|---|---|---|---|---|---|---|
| Baseline | 6-Month Follow-Up | First Analysis | 6 Months Later Analysis | |||
| Leukocytes (×109/L) | 6.5 (4.7–8.2; 3.7–9.9) | 5.9 (5–7.5; 3.8–11) | 0.266 | 6.4 (6–7.9; 3.5–9.9) | 6.1 (5.4–6.5; 3.2–8.1) | 0.155 |
| GUP (mmol/L) | 5.1 (5.1–4.7; 2.8–8.1) | 5.1 (4.7–5.4; 4–11) | 0.650 | 5 (4.7–5.4; 3.9–7.2) | 4.7 (4.4–5.6; 3.9–7.2) | 0.206 |
| CK (U/L) | 81 (65–94; 44–481) | 90 (70–101; 34–964) | 0.362 | 90 (79–126; 52–288) | 84 (65–117; 47–270) | 0.309 |
| CRP (mg/L) | 1.45 (0.6–2; 0.6–25) | 1.1 (0.6–1.8; 0.6–7.7) | 0.345 | 1.05 (0.7–1.9; 0.6–5.5) | 1.05 (0.6–1.4; 0.6–3.9) | 0.306 |
| Total Cholesterol (mmol/L) | 5.2 (4.7–6.3; 3.5–8.6) | 5.2 (4.2–6.4; 3.4–8.1) | 0.777 | 4.7 (4.4–5.9; 3.4–6.8) | 5.1 (4–5.9; 2.9–8.4) | 0.553 |
| Triglycerides (mmol/L) | 1 (0.5–1.7; 0.5–3.3) | 1 (0.7–1.6; 0.4–4.4) | 0.206 | 0.9 (0.7–1.1; 0.3–1.7) | 1.3 (0.8–1.4; 0.4–3.1) | 0.443 |
| HDL Cholesterol (mmol/L) | 1.6 (1.3–2.1; 0.9–2.2) | 1.5 (1.3–1.8; 0.7–2.4) | 0.328 | 1.6 (1.2–2; 1–2.8) | 1.6 (1.2–2; 0.9–2.9) | 0.951 |
| LDL Cholesterol (mmol/L) | 3 (2.5–3.8; 1.1–6.4) | 3.1 (2.2–4; 1.7–5.9) | 0.856 | 2.9 (2–3.3; 1.2–4.7) | 2.8 (1.8–3.7; 1.1–4.9) | 0.722 |
| Fe (μmol/L) | 16 (12–21; 5–30) | 15.5 (12–20; 3–43) | 0.969 | 16 (11–19; 7–44) | 16.5 (11–20; 7–67) | 0.623 |
| UIBC (μmol/L) | 43.5 (36–49; 18–73) | 40 (33–45; 20–70) | 0.394 | 45 (39–50; 24–89) | 45 (41–51; 11–68) | 0.670 |
| TIBC (μmol/L) | 59.5 (55–64; 44–80) | 57 (52–64; 44–73) | 0.345 | 64 (56–67; 51–96) | 61 (56–69; 49–96) | 0.531 |
| TS % | 27.5 (20–35; 6–62) | 30 (23–32; 4–66) | 0.903 | 25 (21–31; 7–65) | 27 (19–36; 11–86) | 0.641 |
| Vitamin B12 (pmol/L) | 334 (225–415; 204–668) | 372 (311–409; 192–546) | 0.085 | 413 (339–488; 182–760) | 405 (316–433; 206–760) | 0.943 |
| Folic Acid (nmol/L) | 15.5 (12–22; 8–32) | 17.8 (11–22; 6–45) | 0.688 | 17.7 (14–23; 9–45) | 17.8 (12–24; 5–37) | 0.246 |
| Vitamin D (nmol/L) | 54 (38–66; 18–106) | 63.6 (43–80; 19–110) | 0.102 | 50 (43–68; 30–223) | 59 (48–71; 28–223) | 0.227 |
| TSH (mIU/L) | 1.1 (0–1.5; 0–3.9) | 1.5 (0–1.9; 0–5.6) | 0.042 | 1.8 (0.5–2.1; 0–4.1) | 1.8 (1.2–2.3; 0–3.7) | 0.672 |
| T4 (nmol/L) | 95 (84–118; 21–130) | 98 (88–107; 74–150) | 0.969 | 96 (85–108; 23–209) | 97 (87–107; 75–143) | 0.925 |
| T3 (nmol/L) | 1.7 (1.4–2; 0.96–32) | 1.6 (1.5–1.7; 0.93–2.4) | 0.405 | 1.8 (1.6–2.1; 1.1–35) | 1.8 (1.5–2; 1.1–2.5) | 0.301 |
| Adropin (ng/mL) | 3.07 (2.55–3.53; 1.4–4.8) | 3.36 (2.97–3.84; 1.48–5.71) | 0.001 * | 2.86 (2.44–3.34; 1.90–5.15) | 2.88 (2.41–3.36; 2.1–13.67) | 0.903 |
| Differences Between Baseline and 6-Month Follow-Up Median (Q1–Q3; Min–Max) | |||
|---|---|---|---|
| Responders (N = 32) | Non-Responders (N = 18) | p * | |
| Leukocytes (×109/L) | 0.1 (−0.55 to 1; 2.3 to 3.4) | 0.5 (−0.5 to 2.7; −1.3 to 8.7) | 0.192 |
| GUP (mmol/L) | 0.05 (−0.55 to 0.4; −3.2 to 1.6) | 0.25 (0 to 0.9; −1.2 to 6.9) | 0.045 |
| CK (U/L) | −2.5 (−14 to 9; −488 to 333) | 18 (5 to 36; −137 to 126) | 0.027 |
| CRP (mg/L) | 0 (−0.25 to 0.65; −4.3 to 23.9) | 0.25 (0 to 1; −1.3 to 5.5) | 0.273 |
| Total Cholesterol (mmol/L) | −0.6 (−4.3 to 0.15; −6.9 to 2.5) | −0.45 (−4.2 to 0.2; −8.4 to 6.2) | 0.679 |
| Triglycerides (mmol/L) | −0.6 (−0.95 to 0.05; −4.2 to 1) | −0.3 (−1.2 to 0.2; −3.1 to 0.9) | 0.422 |
| HDL Cholesterol (mmol/L) | −0.15 (−1.2 to 0.15; −2.1 to 0.5) | −0.1 (−1.2 to 0.1; −2.1 to 2.8) | 0.778 |
| LDL Cholesterol (mmol/L) | −0.8 (−2.6 to 0.1; −5 to 2) | −0.15 (−1.4 to 0.2; −4.9 to 3.3) | 0.283 |
| Fe (μmol/L) | 2 (−7 to 7; −38 to 14) | 0.5 (−4 to 9; −31 to 30) | 0.503 |
| UIBC (μmol/L) | 2.5 (−4.5 to 8; −21 to 50) | 0.5 (−3 to 13; −14 to 54) | 0.591 |
| TIBC (μmol/L) | 0.5 (−2 to 7; −12 to 12) | 0 (−3 to 6; −10 to 64) | 0.888 |
| TS % | 2.5 (−13 to 9; 159 to 31) | 0 (−5 to 13; −33 to 47) | 0.672 |
| Vitamin B12 (pmol/L) | −32.5 (−70 to 14.4; −135 to 275) | 21 (−48 to 144; −151 to 488) | 0.032 |
| Folic Acid (nmol/L) | 0.88 (−4.5 to 3.9; −34 to 13) | 1.8 (−0.8 to 14; −19.2 to 22.2) | 0.162 |
| Vitamin D (nmol/L) | −3.9 (−29 to 11; −49 to 40) | −5.6 (−13.3 to 26.1; −61 to 68) | 0.303 |
| TSH (mIU/L) | −0.21 (−0.98 to 0.002; −2.6 to 1.2) | 0 (−0.16 to 0.33; −2.4 to 1.6) | 0.097 |
| T4 (nmol/L) | −0.05 (−9.5 to 10; −95 to 31) | 3.5 (−6 to 16.1; −79 to 117) | 0.388 |
| T3 (nmol/L) | 0.085 (−0.18 to 0.25; −0.47 to 31) | 0.19 (−0.11 to 1.25; −0.78 to 33.5) | 0.202 |
| Adropin (ng/mL) | −0.175 (−0.55 to 0.040; −1.58 to 1.29) | 0.21 (−0.43 to 1.05; −10.68 to 3.11) | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krnić, D.; Sablić, S.; Marinović Guić, M.; Budimir Mršić, D.; Krnić, D.; Roje, R.; Domić, D.Š.; Lovrić Kojundžić, S. An Increase of Adropin Can Predict Depression Improvement. Nutrients 2025, 17, 1666. https://doi.org/10.3390/nu17101666
Krnić D, Sablić S, Marinović Guić M, Budimir Mršić D, Krnić D, Roje R, Domić DŠ, Lovrić Kojundžić S. An Increase of Adropin Can Predict Depression Improvement. Nutrients. 2025; 17(10):1666. https://doi.org/10.3390/nu17101666
Chicago/Turabian StyleKrnić, Duška, Sara Sablić, Maja Marinović Guić, Danijela Budimir Mršić, Dragan Krnić, Romilda Roje, Daniela Šupe Domić, and Sanja Lovrić Kojundžić. 2025. "An Increase of Adropin Can Predict Depression Improvement" Nutrients 17, no. 10: 1666. https://doi.org/10.3390/nu17101666
APA StyleKrnić, D., Sablić, S., Marinović Guić, M., Budimir Mršić, D., Krnić, D., Roje, R., Domić, D. Š., & Lovrić Kojundžić, S. (2025). An Increase of Adropin Can Predict Depression Improvement. Nutrients, 17(10), 1666. https://doi.org/10.3390/nu17101666










