Abstract
Background/Objectives: The association between magnesium and metabolic syndrome has not been comprehensively examined. We conducted a meta-analysis to quantitatively evaluate the association between intake and blood levels of magnesium and metabolic syndrome. Methods: We searched PubMed, Scopus, and ISI Web of Science databases to identify studies reporting an association between magnesium and metabolic syndrome up to April 2025. To pool the effect sizes on metabolic syndrome according to intake and blood levels of magnesium, a random effects model was used. Results: Twenty-seven publications including 95,933 participants were included in the meta-analysis. The relative risk summary of metabolic syndrome for highest versus lowest intake of magnesium was 0.79 (95% confidence interval [CI]: 0.71–0.88) for prospective cohort studies. In the meta-analysis of cross-sectional studies, magnesium intake was inversely associated with metabolic syndrome (odds ratio = 0.61; 95% CI: 0.39–0.94). High blood levels of magnesium were inversely associated with metabolic syndrome (effect estimate = 0.53; 95% CI: 0.37–0.76). Conclusions: The present meta-analysis indicated that magnesium intake was inversely associated with a risk of metabolic syndrome. Regarding the association between blood levels of magnesium and metabolic syndrome, a significant inverse association was found, but the interpretation was cautious due to the observed high heterogeneity. The association between magnesium status and metabolic syndrome needs to be confirmed with further prospective studies.
1. Introduction
Magnesium is a macromineral contained in green leafy vegetables, legumes, beans, peas, nuts, and whole grains [1]. As the second most abundant cation and fourth most plentiful mineral in the human body, magnesium is involved in more than 600 metabolic reactions [2,3]. Magnesium maintains deoxyribonucleic acid (DNA) stability by forming a structure of DNA and protecting DNA from oxidative stress [3]. Also, magnesium plays a vital role in protein synthesis and acts as a key regulator of cell cycle progression and cell proliferation [3,4]. It is essential to maintain the homeostasis of magnesium in the bones, intestines, and kidneys because magnesium plays a diverse physiological role in the brain, skeletal muscles, and heart [3]. Magnesium deficiency can increase the risk of a variety of chronic diseases by affecting the normal functioning of body functions that depend on magnesium [2].
Metabolic syndrome is the co-occurrence of at least three abnormal levels for waist circumference, fasting blood glucose, blood triglyceride, blood pressure, and blood high-density lipoprotein (HDL) cholesterol [5]. Metabolic syndrome, the leading cause of which is insulin resistance, is positively associated with the risk of many chronic diseases, including cancer, cardiovascular disease (CVD), non-alcoholic steatohepatitis, neurodegenerative disorders, and chronic kidney disease [6]. Worldwide, metabolic syndrome has a high prevalence rate of over 10%, ranging from 12.5% to 31.4% depending on the definition criteria applied [7]. There are many lifestyle risk factors for metabolic syndrome and dietary factors are considered vital among them [8,9,10].
Regarding minerals, accumulating evidence has shown that a high sodium status is associated with an increased risk of metabolic syndrome [11]. Many previous observational studies have explored the risk of metabolic syndrome in relation to the intake [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] or blood levels [27,28,29,30,31,32,33,34,35,36,37,38] of magnesium. Although there have been previous attempts to integrate evidence on the association between magnesium intake and the risk of metabolic syndrome, most of the included studies were cross-sectional [39,40]. As several cohort studies have recently reported the results of studies on magnesium intake and metabolic syndrome [23,24,25], there is a need to integrate the prospective association between magnesium intake and metabolic syndrome. Therefore, we conducted a systematic review and meta-analysis of observational studies to summarize the evidence of the association between intake and blood levels of magnesium and metabolic syndrome.
2. Materials and Methods
2.1. Data Sources and Searches
Electronic databases, including PubMed, Scopus, and ISI Web of Science, were searched to identify articles related to the association between intake or blood levels of magnesium and metabolic syndrome up to April 2025. The search terms used in the database search were as follows: “magnesium” OR “Mg” in combination with “metabolic syndrome”. A manual search was also conducted by reviewing the reference lists of relevant articles to find additional studies. The present meta-analysis was registered at PROSPERO (CRD42024557388).
2.2. Study Selection
Studies were selected for the meta-analysis when they met the following criteria: (1) studies with observational designs (cohort, case-control, or cross-sectional designs); (2) studies where intake or blood levels of magnesium were the exposure of interest; (3) studies where the outcome of interest was metabolic syndrome; and (4) studies where the relative risk (RR) or odds ratio (OR) with 95% confidence intervals (CIs) were provided. The studies whose populations consisted only of patients were excluded. When two different articles were from the same study [33,41], we selected the study results with a larger population [33].
2.3. Data Extraction
Data were collected from the original articles by two independent authors (K.Y. and Y.J.) following the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement [42]. The extracted data were the study design, family name of the first author, publication year, geographic region or country, sex of participants, age, sample size, adjusted covariates, exposure category, and RRs and their 95% CI for each exposure category.
2.4. Quality Assessment
The quality of studies included in the meta-analysis was examined by a single investigator (Y.K.) and checked by the second investigator (Y.J.). For cohort and case-control studies, a maximum score of 9 was assigned based on the Newcastle–Ottawa scale [43]. The scale consisted of the following three domains: selection of population (0–4 points), comparability for controlling confounders (0–2 points), and outcome ascertainment (0–3 points). A modified form of the Newcastle–Ottawa scale was used to assess the cross-sectional studies, referring to previous studies [44]. Studies with a total score ≥ 8 were rated as high quality.
2.5. Statistical Analysis
We used the random effects models of DerSimonian and Laird, which considered both between- and within-study variations [45] to combine RRs from each original study. The RR or OR and its 95% CI were recalculated if an original study did not provide the lowest exposure category as a reference. Statistical heterogeneity and inconsistency among the included studies were assessed using Cochran’s Q test [46] and I2 statistics [47]. A sensitivity analysis excluding one study at a time was performed to examine the extent to which inferences might be affected by a particular study. Subgroup analyses by sex, geographic region, and sample size were conducted when possible. Begg’s [48] and Egger’s tests [49] were used to evaluate publication bias. All analyses were performed using Stata version 17.0 (STATA Corp., College Station, TX, USA). A two-tailed p-value less than 0.05 was considered to be statistically significant.
3. Results
3.1. Study Characteristics
Sixteen papers including 74,106 participants and 20,044 cases were eligible for the association between magnesium intake and metabolic syndrome, and twelve papers involving 21,827 subjects were suitable for the association between blood levels of magnesium and metabolic syndrome [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. The process for the selection of studies for the meta-analysis is presented in Figure 1. Among the studies included in this meta-analysis, four had a cohort design [14,23,24,25], four had a case-control design [28,29,30,35], eighteen had a cross-sectional design [12,13,15,16,17,18,19,20,21,22,26,27,32,33,34,36,37,38], and one study reported both cohort and cross-sectional results [31]. By region of study, seven were performed in the United States [12,14,15,16,17,20,23], eleven in Asia [21,22,24,25,30,32,34,36,37,38], two in Europe [13,31], five in the Middle East [18,26,27,33,36], and two in Mexico [28,29]. The age range of the participants was 18 or older and the number of subjects in each study ranged from 150 to 9887. All studies were controlled for age, and most studies were adjusted for smoking (n = 17), alcohol (n = 15), and energy intake (n = 14). Most studies defined metabolic syndrome based on the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) [50], International Diabetes Foundation (IDF) [51], and harmonized [5] definitions. The characteristics of the studies included in the meta-analysis of the association between metabolic syndrome and magnesium intake or blood levels are presented in Table 1, Table 2 and Table 3. As a result of the quality assessment, 24 out of 27 studies scored 8 points or higher; the remaining 3 scored 7 points, showing relatively high quality.
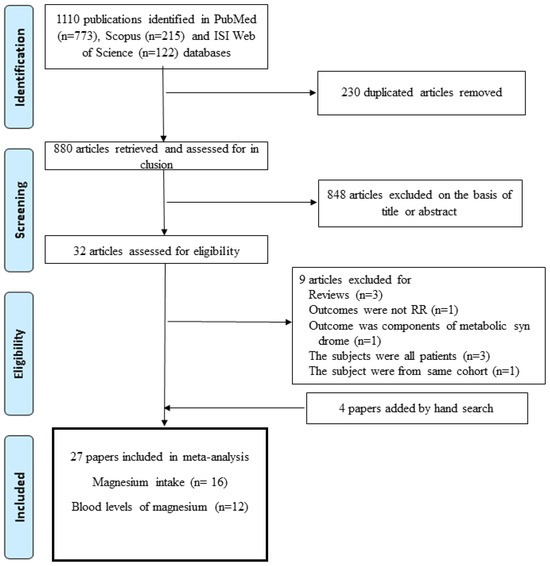
Figure 1.
Flowchart for study selection procedure.

Table 1.
Characteristics of prospective cohort studies included in the meta-analysis of magnesium intake and metabolic syndrome.

Table 2.
Characteristics of cross-sectional studies included in the meta-analysis of magnesium intake and metabolic syndrome.

Table 3.
Characteristics of observational studies included in the meta-analysis of blood levels of magnesium and metabolic syndrome.
3.2. Magnesium and Metabolic Syndrome
The results of the meta-analysis regarding the association between magnesium intake and metabolic syndrome by study design are shown in Figure 2 and Figure 3. The pooled RR for the prospective cohort studies was 0.79 (95% CI: 0.71–0.88), with no heterogeneity (I2 = 0.0%; p = 0.40) (Figure 2). For the cross-sectional studies, the pooled OR was 0.61 (95% CI: 0.39–0.94), and a significant heterogeneity was observed among the studies (I2 = 96.6%; p < 0.001) (Figure 3). The observed heterogeneity slightly decreased (I2 = 71.2%; p < 0.001) after excluding a study with the strongest inverse association between dietary intake and metabolic syndrome [21]. The subgroup analysis results for the cross-sectional studies are presented in Table 4. When we examined by sex, a significant inverse association between dietary magnesium and metabolic syndrome was found, but only for women (OR = 0.69; 95% CI: 0.58–0.83); men showed a non-significant inverse association (OR = 0.77; 95% CI: 0.59–1.01). However, the difference by sex was not significant (p for difference = 0.55). By geographic region, an inverse association between dietary magnesium and metabolic syndrome was significant in the United States (OR = 0.71; 95% CI: 0.60–0.84), but the difference by region was not significant (p for difference > 0.6 for all comparisons). There was no significant difference for the sample size (p for difference = 0.73) or adjustment factor (p for difference > 0.4 for all comparisons).
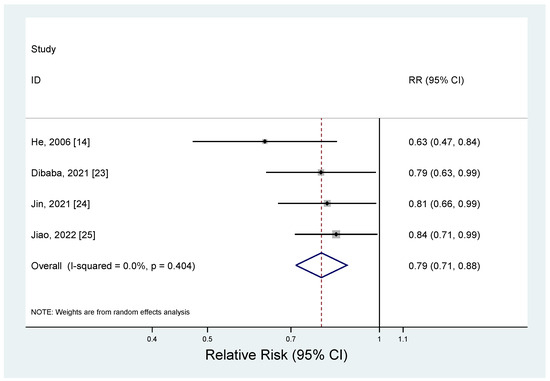
Figure 2.
Forest plots of the prospective cohort studies of metabolic syndrome for high versus low magnesium intake [14,23,24,25].
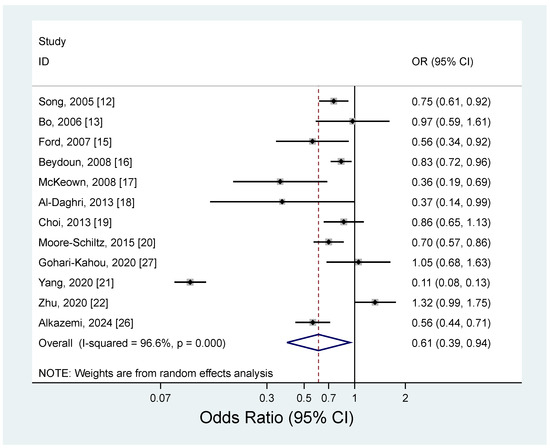
Figure 3.
Forest plots of the cross-sectional studies of metabolic syndrome for high versus low magnesium intake [12,13,15,16,17,18,19,20,21,22,26,27].

Table 4.
Summary of adjusted odds ratios (ORs) of metabolic syndrome for magnesium intake.
The pooled estimate (ES) of metabolic syndrome for the highest versus lowest blood levels of magnesium is presented in Figure 4 (ES = 0.53; 95% CI: 0.37–0.76). There was a significant heterogeneity among the studies (I2 = 95.6%; p < 0.001).
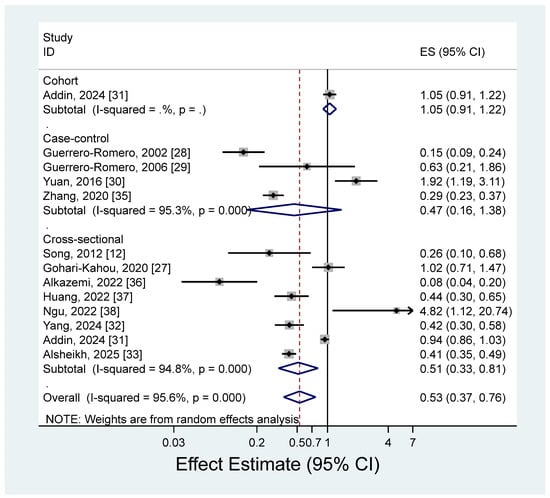
Figure 4.
Forest plots of the observational studies of metabolic syndrome for high versus low blood magnesium levels [12,27,28,29,30,31,32,33,35,36,37,38].
3.3. Publication Bias
There was no significant evidence of publication bias in the prospective cohort studies examining the association between dietary magnesium and metabolic syndrome in Begg’s test (p = 0.09), but some evidence of publication bias was observed in Egger’s test (p = 0.04). We found no significant evidence of publication bias in the cross-sectional studies investigating the association between dietary magnesium and metabolic syndrome (Begg’s test, p = 0.24; Egger’s test, p = 0.96) or for studies reporting the association between blood levels of magnesium and metabolic syndrome (Begg’s test, p = 0.86; Egger’s test, p = 0.20).
4. Discussion
The current meta-analysis summarized the association between magnesium intake and metabolic syndrome from prospective cohort and cross-sectional studies. A high magnesium intake was associated with a 21% lower incidence of metabolic syndrome compared with a low magnesium intake in the meta-analysis of prospective cohort studies. The results of the meta-analysis of cross-sectional studies showed that people with a high magnesium intake had 39% lower odds of metabolic syndrome compared with people with a low magnesium intake.
The observed inverse association between magnesium intake and metabolic syndrome in our analysis was in line with previous results. A meta-analysis published in 2014, including six cross-sectional studies, suggested that a high magnesium intake was associated with 31% lower odds of metabolic syndrome than a low magnesium intake [39]. In addition, the results of a meta-analysis of two cohort and seven cross-sectional studies indicated that high consumption of magnesium was associated with 27% lower odds of metabolic syndrome [40]. Not only observational studies but also experimental studies have suggested an inverse association between magnesium intake and metabolic syndrome. The summary results of twelve randomized controlled trials that examined the effect of magnesium supplementation on insulin resistance in humans showed a reduction in the homeostasis model assessment of insulin resistance (HOMA-IR) and fasting glucose levels with a 50 mg increment in dietary magnesium [52]. A pooled analysis of eight randomized controlled trials demonstrated that magnesium supplementation contributed to reducing the systolic and diastolic blood pressure of type 2 diabetes patients [53]. A meta-analysis of twelve randomized controlled trials suggested that longer than twelve weeks of magnesium supplementation reduced serum total cholesterol, and magnesium supplementation less than 300 mg decreased serum low-density lipoprotein (LDL) cholesterol levels [54]. In addition, accumulating evidence from twenty-five randomized controlled trials has reported an increase in HDL cholesterol with magnesium supplementation [55]. Regarding obesity, a meta-analysis of thirty-two randomized controlled trials examining obesity measures found a significant reduction in BMI following magnesium supplementation [56]. These protective effects of magnesium intake on components of metabolic syndrome observed through randomized controlled trials may have contributed to the inverse association between magnesium consumption and metabolic syndrome.
Several potential mechanisms can explain the beneficial effect of magnesium on metabolic syndrome. Firstly, magnesium controls the insulin signaling pathway by enhancing tyrosine kinase activity by increasing the insulin receptor’s affinity for adenosine triphosphate [57]. In the downstream pathways, magnesium regulates the membrane transport of the glucose receptor GLUT4 in muscles, acts as an essential regulator of gluconeogenic enzymes (including glucose-6-phosphatase and phosphoenolpyruvate carboxykinase in the liver), and acts as an anti-inflammatory factor that reduces the secretion of interleukin 1 and tumor necrosis factor-α in adipose tissue [58]. As magnesium possesses these functions, hypomagnesemia may lead to increased insulin resistance, which has been confirmed in animal studies [59]. Secondly, magnesium acts as an antagonist of calcium, which acts as a vasoconstrictor molecule while being involved in the secretion of catecholamines from the adrenal glands [60,61]. In the coronary endothelium, magnesium stimulates the production and release of vasodilation molecules, including nitric oxide and prostacyclin [61,62]. Thirdly, magnesium modulates the activity of enzymes such as lipoprotein lipase (LPL), desaturase, and lecithin–cholesterol acyltransferase (LCAT) [63]. LCAT plays an important role in maintaining the lipoprotein balance in the body by lowering LDL cholesterol and triglyceride and raising HDL cholesterol [63,64]. An impairment in LPL and LCAT activity results in increases in triglycerides, LDL cholesterol, and the saturated-to-unsaturated fatty acid ratio as well as decreases in HDL cholesterol [63,65]. Although the mechanisms of an inverse association between magnesium consumption and obesity are unclear, magnesium can reduce the absorption of fat by forming soaps with fatty acids [66]. Magnesium also is related to the secretion of epinephrine, which plays a role in obesity development [67].
Although not included in our meta-analysis, there were observational studies that reported an association between magnesium intake and metabolic syndrome in patients with specific diseases. A prospective cohort study in Iran found a non-significant inverse association between magnesium intake and metabolic syndrome (OR for tertile3 vs. tertile1 = 0.80; 95% CI: 0.16–3.86) among 160 renal-transplant recipients after a 1 year follow-up [68]. A cross-sectional study from Taiwan analyzed the association between magnesium intake and metabolic parameters, and observed a positive association with HDL cholesterol and an inverse association with obesity [69]. However, they failed to find a significant association with metabolic syndrome incidence [69].
We found a significant inverse association between blood levels of magnesium and metabolic syndrome, but there was high heterogeneity among the studies. Although most of the studies reported estimates that were lower than 1, indicating an inverse association between blood magnesium levels and metabolic syndrome, two studies reported a significant positive association, showing estimates that were higher than 1 [30,38]. One study in China showed a positive association between blood magnesium levels and metabolic syndrome (OR for tertile3 vs. tertile1 = 1.92; 95% CI: 1.19–3.11) [30]. This study adjusted for age and sex only, and metabolic syndrome was defined according to Chinese Diabetes Society criteria. Another study from Taiwan showed an estimate greater than 1 (OR for tertile3 vs. tertile1 = 4.82; 95% CI: 1.12–20.73), but had a relatively small sample of 150 subjects [38]. Although the magnesium level in blood is the fastest and most commonly used clinical indicator of magnesium nutritional status, the actual proportion in blood is less than 1% of the total magnesium in the human body [70]. Given the heterogeneity of our study results and the lack of representativeness of serum magnesium levels in assessing magnesium nutritional status, the observed inverse association between serum magnesium levels and metabolic syndrome should be cautiously interpreted. We additionally performed a subgroup analysis based on the median of high blood magnesium levels (0.88 mmol/L) to ascertain if the results varied by level of magnesium intake. However, no significant differences were observed (ES = 0.51, 95% CI: 0.32–0.80 in <median; ES = 0.55, 95% CI: 0.36–0.85 in ≥median).
To the best of our knowledge, this is the first meta-analysis to examine the prospective association between magnesium intake and metabolic syndrome. Prospective design studies have a lower risk of recall bias than retrospective designs, such as case-control and cross-sectional studies, and are relevant to examine for a causal relationship. In addition to a meta-analysis of prospective cohort studies, we also performed a meta-analysis of cross-sectional studies. Additionally, a meta-analysis of blood magnesium levels and metabolic syndrome was conducted to comprehensively understand the effects of magnesium intake and blood levels on metabolic syndrome. Despite these strengths, there are several limitations of this study that should be carefully considered. Firstly, a subgroup analysis of cohort studies for a more in-depth exploration could not be conducted due to the insufficient number of studies. Secondly, measurement errors could have occurred when assessing magnesium intake. However, errors tend to be non-differential; thus, there was little concern that the results were exaggerated. Lastly, most studies in the meta-analysis adjusted for potential confounders, including energy intake, smoking, and alcohol. However, residual confounders should always be considered when understanding the results of observational studies.
5. Conclusions
In conclusion, a high magnesium intake was associated with a lower risk of metabolic syndrome in a meta-analysis of prospective cohort studies. This inverse association was also found in the results of cross-sectional studies. A meta-analysis of observational studies analyzing blood mg levels and metabolic syndrome also showed an inverse association. However, the heterogeneity among studies was high, so caution is needed when interpreting the results. The present results from our analysis require further confirmation by future prospective cohort studies and randomized controlled trials.
Author Contributions
The authors’ responsibilities were as follows: Y.K. and Y.J.: study concept and design; Y.K.: data collection and statistical analysis; Y.K.: writing—original draft; Y.J.: writing—review and editing; Y.J.: study supervision; Y.K. and Y.J.: interpretation of the data, critical revision of the paper for important intellectual content, and approval of the final paper for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF), grant funded by the Korean government (MSIT) (RS-2025-00562173). The NRF had no role in the study design, data collection, analysis, interpretation, manuscript preparation, or the decision to publish.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Acknowledgments
We thank all authors of the studies included in the meta-analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DNA | Deoxyribonucleic acid |
| HDL | High-density lipoprotein |
| CVD | Cardiovascular disease |
| RR | Relative risk |
| OR | Odds ratio |
| ES | Estimate |
| CI | Confidence interval |
| BMI | Body mass index |
| LDL | Low-density lipoprotein |
| LPL | Lipoprotein lipase |
| LCAT | Lecithin–cholesterol acyltransferase |
References
- Tarleton, E.K. Factors influencing magnesium consumption among adults in the United States. Nutr. Rev. 2018, 76, 526–538. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef] [PubMed]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Rubin, H. The membrane, magnesium, mitosis (MMM) model of cell proliferation control. Magnes. Res. 2005, 18, 268–274. [Google Scholar] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef]
- Di Marzo, V.; Silvestri, C. Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef]
- Codazzi, V.; Frontino, G.; Galimberti, L.; Giustina, A.; Petrelli, A. Mechanisms and risk factors of metabolic syndrome in children and adolescents. Endocrine 2024, 84, 16–28. [Google Scholar] [CrossRef]
- Yamaoka, K.; Tango, T. Effects of lifestyle modification on metabolic syndrome: A systematic review and meta-analysis. BMC Med. 2012, 10, 138. [Google Scholar] [CrossRef]
- Soltani, S.; Kolahdouz Mohammadi, R.; Shab-Bidar, S.; Vafa, M.; Salehi-Abargouei, A. Sodium status and the metabolic syndrome: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ridker, P.M.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 2005, 28, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Durazzo, M.; Guidi, S.; Carello, M.; Sacerdote, C.; Silli, B.; Rosato, R.; Cassader, M.; Gentile, L.; Pagano, G. Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am. J. Clin. Nutr. 2006, 84, 1062–1069. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Morris, S.J.; Loria, C.M.; Van Horn, L.; Jacobs, D.R., Jr.; Savage, P.J. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006, 113, 1675–1682. [Google Scholar] [CrossRef]
- Ford, E.S.; Li, C.; McGuire, L.C.; Mokdad, A.H.; Liu, S. Intake of dietary magnesium and the prevalence of the metabolic syndrome among U.S. adults. Obesity 2007, 15, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Gary, T.L.; Caballero, B.H.; Lawrence, R.S.; Cheskin, L.J.; Wang, Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1914–1925. [Google Scholar] [CrossRef]
- McKeown, N.M.; Jacques, P.F.; Zhang, X.L.; Juan, W.; Sahyoun, N.R. Dietary magnesium intake is related to metabolic syndrome in older Americans. Eur. J. Nutr. 2008, 47, 210–216. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Khan, N.; Alkharfy, K.M.; Al-Attas, O.S.; Alokail, M.S.; Alfawaz, H.A.; Alothman, A.; Vanhoutte, P.M. Selected dietary nutrients and the prevalence of metabolic syndrome in adult males and females in Saudi Arabia: A pilot study. Nutrients 2013, 5, 4587–4604. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Bae, Y.J. Relationship between dietary magnesium, manganese, and copper and metabolic syndrome risk in Korean adults: The Korea National Health and Nutrition Examination Survey (2007–2008). Biol. Trace Elem. Res. 2013, 156, 56–66. [Google Scholar] [CrossRef]
- Moore-Schiltz, L.; Albert, J.M.; Singer, M.E.; Swain, J.; Nock, N.L. Dietary intake of calcium and magnesium and the metabolic syndrome in the National Health and Nutrition Examination (NHANES) 2001–2010 data. Br. J. Nutr. 2015, 114, 924–935. [Google Scholar] [CrossRef]
- Yang, N.; He, L.; Li, Y.; Xu, L.; Ping, F.; Li, W.; Zhang, H. Reduced Insulin Resistance Partly Mediated the Association of High Dietary Magnesium Intake with Less Metabolic Syndrome in a Large Chinese Population. Diabetes Metab. Syndr. Obes. 2020, 13, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; He, Y.; Wu, F.; Zhao, L.; Wu, C.; Lu, Y.; Zang, J.; Wang, Z.; Sun, J.; Huang, J.; et al. The Associations of Dietary Iron, Zinc and Magnesium with Metabolic Syndrome in China’s Mega Cities. Nutrients 2020, 12, 659. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Chen, C.; Lu, L.; Bidulescu, A.; Fly, A.D.; Xun, P.; Judd, S.E.; Cushman, M.; Kahe, K. Magnesium intake is inversely associated with the risk of metabolic syndrome in the REasons for geographic and racial differences in stroke (REGARDS) cohort study. Clin. Nutr. 2021, 40, 2337–2342. [Google Scholar] [CrossRef]
- Jin, S.; Liu, J.; Jia, Y.; Han, T.; Zhao, X.; Sun, C.; Na, L. The association of dietary flavonoids, magnesium and their interactions with the metabolic syndrome in Chinese adults: A prospective cohort study. Br. J. Nutr. 2021, 126, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, W.; Wang, L.; Jiang, H.; Wang, S.; Jia, X.; Wang, Z.; Wang, H.; Zhang, B.; Ding, G. Relationship between Dietary Magnesium Intake and Metabolic Syndrome. Nutrients 2022, 14, 2013. [Google Scholar] [CrossRef]
- Alkazemi, D.U.Z.; Zafar, T.A.; Alsouri, N.Y.; Aljahdali, A.A.; Kubow, S. Low dietary magnesium and fiber intakes among women with metabolic syndrome in Kuwait. Front. Nutr. 2024, 11, 1451220. [Google Scholar] [CrossRef]
- Gohari-Kahou, M.; Darroudi, S.; Saberi-Karimian, M.; Parizadeh, S.; Asadi, Z.; Javandoost, A.; Safarian, M.; Mouhebati, M.; Ebrahimi, M.; Ferns, G.A.; et al. The association between serum and dietary magnesium with cardiovascular disease risk factors in Iranian adults with metabolic syndrome. Transl. Metab. Syndr. Res. 2020, 3, 42–48. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Low serum magnesium levels and metabolic syndrome. Acta Diabetol. 2002, 39, 209–213. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodriguez-Moran, M. Hypomagnesemia, oxidative stress, inflammation, and metabolic syndrome. Diabetes Metab. Res. Rev. 2006, 22, 471–476. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, C.; Tian, Y.; Zhang, X.; Ye, H.; Jin, L.; Ruan, L.; Sun, Z.; Zhu, Y. Higher Levels of Magnesium and Lower Levels of Calcium in Whole Blood Are Positively Correlated with the Metabolic Syndrome in a Chinese Population: A Case-Control Study. Ann. Nutr. Metab. 2016, 69, 125–134. [Google Scholar] [CrossRef]
- Addin, N.S.; Niedermayer, F.; Thorand, B.; Linseisen, J.; Seissler, J.; Peters, A.; Rospleszcz, S. Association of serum magnesium with metabolic syndrome and the role of chronic kidney disease: A population-based cohort study with Mendelian randomization. Diabetes Obes. Metab. 2024, 26, 1808–1820. [Google Scholar] [CrossRef]
- Yang, J.; Cao, Y.; Zhang, H.; Hu, Y.; Lu, J.; Wang, R.; Feng, J.; Yang, L. Association and dose-response relationship of plasma magnesium with metabolic syndrome in Chinese adults older than 45 years. Front. Nutr. 2024, 11, 1346825. [Google Scholar] [CrossRef]
- Alsheikh, R.; Aldulaimi, H.; Hinawi, R.; Al-Sadi, F.; Al-Baker, A.; Alkuwari, A.; Sameer, M.; Al-Abdulla, G.; Shi, Z.; Rathnaiah Babu, G. Association of serum magnesium and calcium with metabolic syndrome: A cross-sectional study from the Qatar-biobank. Nutr. Metab. 2025, 22, 8. [Google Scholar] [CrossRef]
- Song, I.J.; Park, C.; Uh, W.C.; Yang, J.Y.; Lee, J.; Lee, S.; Ga, H. The Relationship Between Serum Magnesium Levels and Metabolic Syndrome in Korean Adults. J. Obes. Metab. Syndr. 2012, 21, 11–17. [Google Scholar] [CrossRef]
- Zhang, W.; Du, J.; Li, H.; Yang, Y.; Cai, C.; Gao, Q.; Xing, Y.; Shao, B.; Li, G. Multiple-element exposure and metabolic syndrome in Chinese adults: A case-control study based on the Beijing population health cohort. Environ. Int. 2020, 143, 105959. [Google Scholar] [CrossRef] [PubMed]
- Alkazemi, D.; Alsouri, N.; Zafar, T.; Kubow, S. Hypomagnesemia and the Metabolic Syndrome among Apparently Healthy Kuwaiti Adults: A Cross-Sectional Study. Nutrients 2022, 14, 5257. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhong, D.; Lv, Z.; Cheng, J.; Zou, X.; Wang, T.; Wen, Y.; Wang, C.; Yu, S.; Huang, H.; et al. Associations of multiple plasma metals with the risk of metabolic syndrome: A cross-sectional study in the mid-aged and older population of China. Ecotoxicol. Environ. Saf. 2022, 231, 113183. [Google Scholar] [CrossRef]
- Ngu, Y.J.; Skalny, A.V.; Tinkov, A.A.; Tsai, C.S.; Chang, C.C.; Chuang, Y.K.; Nikolenko, V.N.; Zotkin, D.A.; Chiu, C.F.; Chang, J.S. Association Between Essential and Non-essential Metals, Body Composition, and Metabolic Syndrome in Adults. Biol. Trace Elem. Res. 2022, 200, 4903–4915. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; Fly, A.D.; Yokota, K.; He, K. Dietary magnesium intake and risk of metabolic syndrome: A meta-analysis. Diabet. Med. 2014, 31, 1301–1309. [Google Scholar] [CrossRef]
- Sarrafzadegan, N.; Khosravi-Boroujeni, H.; Lotfizadeh, M.; Pourmogaddas, A.; Salehi-Abargouei, A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition 2016, 32, 409–417. [Google Scholar] [CrossRef]
- Al Shammaa, A.; Al-Thani, A.; Al-Kaabi, M.; Al-Saeed, K.; Alanazi, M.; Shi, Z. Serum Magnesium is Inversely Associated with Body Composition and Metabolic Syndrome. Diabetes Metab. Syndr. Obes. 2023, 16, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 May 2025).
- Herzog, R.; Alvarez-Pasquin, M.J.; Diaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Cleeman, J.I. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; de Alencar, G.R.R.; de Oliveira, A.R.S.; Cruz, K.J.C.; Marreiro, D.D.N.; Freitas, B.; de Carvalho, C.M.R.; Martins, M.; Frota, K.M.G. Effect of magnesium supplementation on insulin resistance in humans: A systematic review. Nutrition 2017, 38, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, X.; Wang, X.; Xu, M. Effects of magnesium supplementation on improving hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes: A pooled analysis of 24 randomized controlled trials. Front. Nutr. 2022, 9, 1020327. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Moradi, S.; Nezamoleslami, S.; Moosavian, S.P.; Hojjati Kermani, M.A.; Lazaridi, A.V.; Miraghajani, M. The Effects of Magnesium Supplementation on Lipid Profile Among Type 2 Diabetes Patients: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Biol. Trace Elem. Res. 2021, 199, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Sohrabi, M.; Gholami, A. The effect of magnesium supplementation on serum concentration of lipid profile: An updated systematic review and dose-response meta-analysis on randomized controlled trials. Nutr. J. 2025, 24, 24. [Google Scholar] [CrossRef]
- Askari, M.; Mozaffari, H.; Jafari, A.; Ghanbari, M.; Darooghegi Mofrad, M. The effects of magnesium supplementation on obesity measures in adults: A systematic review and dose-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 2921–2937. [Google Scholar] [CrossRef]
- Vinals, F.; Camps, M.; Testar, X.; Palacin, M.; Zorzano, A. Effect of cations on the tyrosine kinase activity of the insulin receptor: Inhibition by fluoride is magnesium dependent. Mol. Cell Biochem. 1997, 171, 69–73. [Google Scholar] [CrossRef]
- Gommers, L.M.; Hoenderop, J.G.; Bindels, R.J.; de Baaij, J.H. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef]
- Suarez, A.; Pulido, N.; Casla, A.; Casanova, B.; Arrieta, F.J.; Rovira, A. Impaired tyrosine-kinase activity of muscle insulin receptors from hypomagnesaemic rats. Diabetologia 1995, 38, 1262–1270. [Google Scholar] [CrossRef]
- Louvet, L.; Bazin, D.; Buchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z.A. Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium. PLoS ONE 2015, 10, e0115342. [Google Scholar] [CrossRef]
- Dominguez, L.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2020, 13, 139. [Google Scholar] [CrossRef]
- Satake, K.; Lee, J.D.; Shimizu, H.; Uzui, H.; Mitsuke, Y.; Yue, H.; Ueda, T. Effects of magnesium on prostacyclin synthesis and intracellular free calcium concentration in vascular cells. Magnes. Res. 2004, 17, 20–27. [Google Scholar]
- Verma, H.; Garg, R. Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A.; Seelig, M.S. Comparison of mechanism and functional effects of magnesium and statin pharmaceuticals. J. Am. Coll. Nutr. 2004, 23, 501S–505S. [Google Scholar] [CrossRef]
- Viljoen, A.; Wierzbicki, A.S. Dyslipidemia: Diabetes Lipidtherapies. In Textbook of Diabetes; Holt Rig, C.C., Flyvbjerg, A., Goldstein, B.J., Eds.; Blackwell Science: Boston, MA, USA, 2010. [Google Scholar]
- Shamnani, G.R.C.; Gupta, V.; Singh, S.; Tiwari, S.; Bhartiy, S.S.; Sharma, P. Serum magnesium in relation with obesity. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 1074–1077. [Google Scholar] [CrossRef]
- Kazuko, M.; Gavin, W.L. Epinephrine and its Role in the Development of Obesity and Hypertension. Curr. Hypertens. Rev. 2011, 7, 144–152. [Google Scholar] [CrossRef]
- Noori, N.; Nafar, M.; Poorrezagholi, F.; Ahmadpoor, P.; Samadian, F.; Firouzan, A.; Einollahi, B. Dietary intakes of fiber and magnesium and incidence of metabolic syndrome in first year after renal transplantation. J. Ren. Nutr. 2010, 20, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Lu, Y.F.; Cheng, F.C.; Lee, J.N.; Tsai, L.C. Correlation of magnesium intake with metabolic parameters, depression and physical activity in elderly type 2 diabetes patients: A cross-sectional study. Nutr. J. 2012, 11, 41. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of magnesium status for diagnosis and therapy. Magnes. Res. 2010, 23, S194–S198. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).