Protective Effects of a Standardized Water Extract from the Stem of Ipomoea batatas L. Against High-Fat Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. WIB Preparation, Identification, and Standardization
2.3. Animals and Experimental Design
2.4. Body Fat Composition Analysis
2.5. H&E Staining Analysis
2.6. Protein Extraction and Western Blot Analysis
2.7. Biochemical Examination of Blood Plasma
2.8. Genomic DNA (gDNA) Extraction and Microbiome Profiling
2.9. Statistical Analysis
3. Results
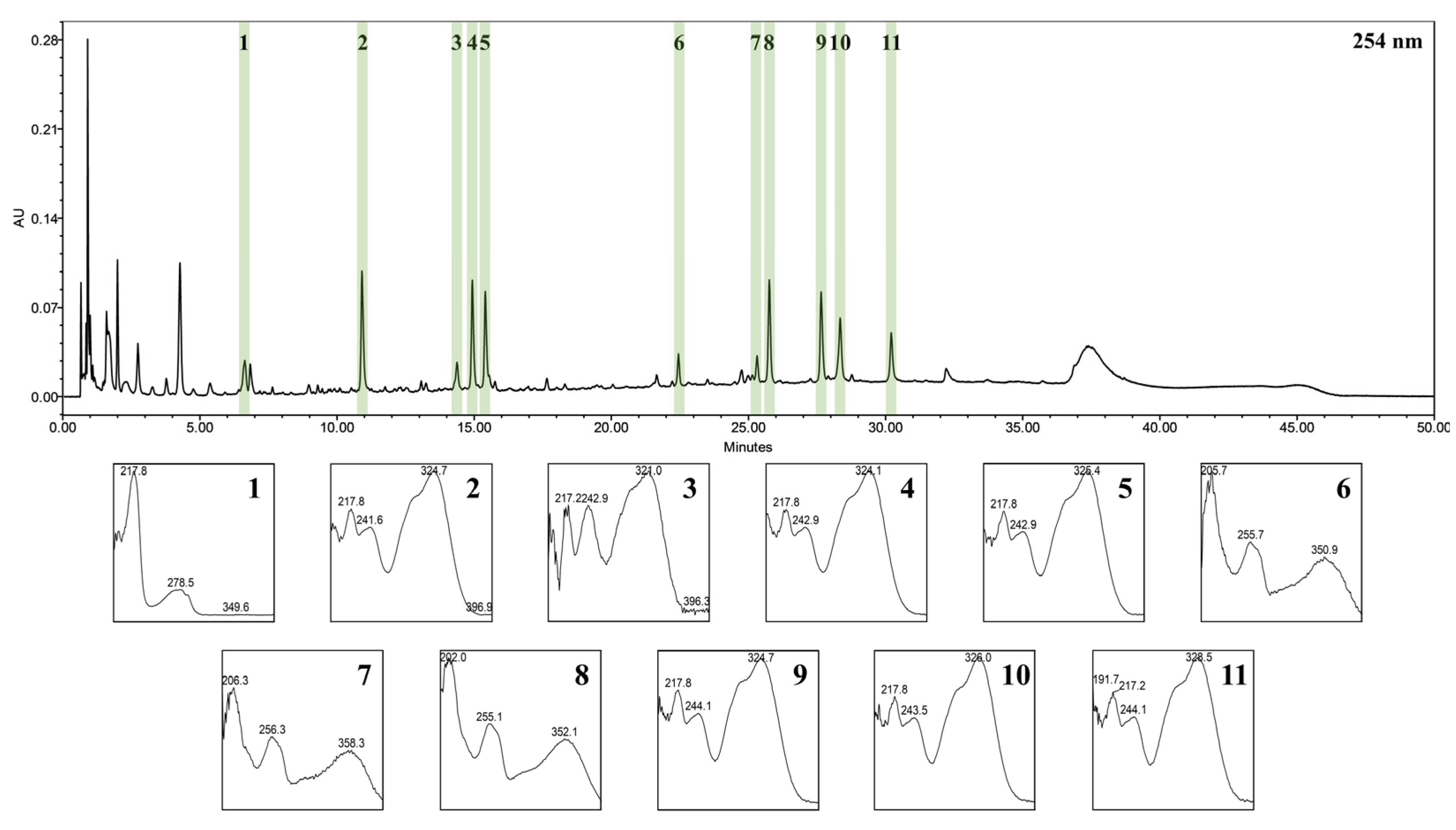
3.1. UPLC-PDA-ESI-TOF-MS for Identification of WIB Constituents
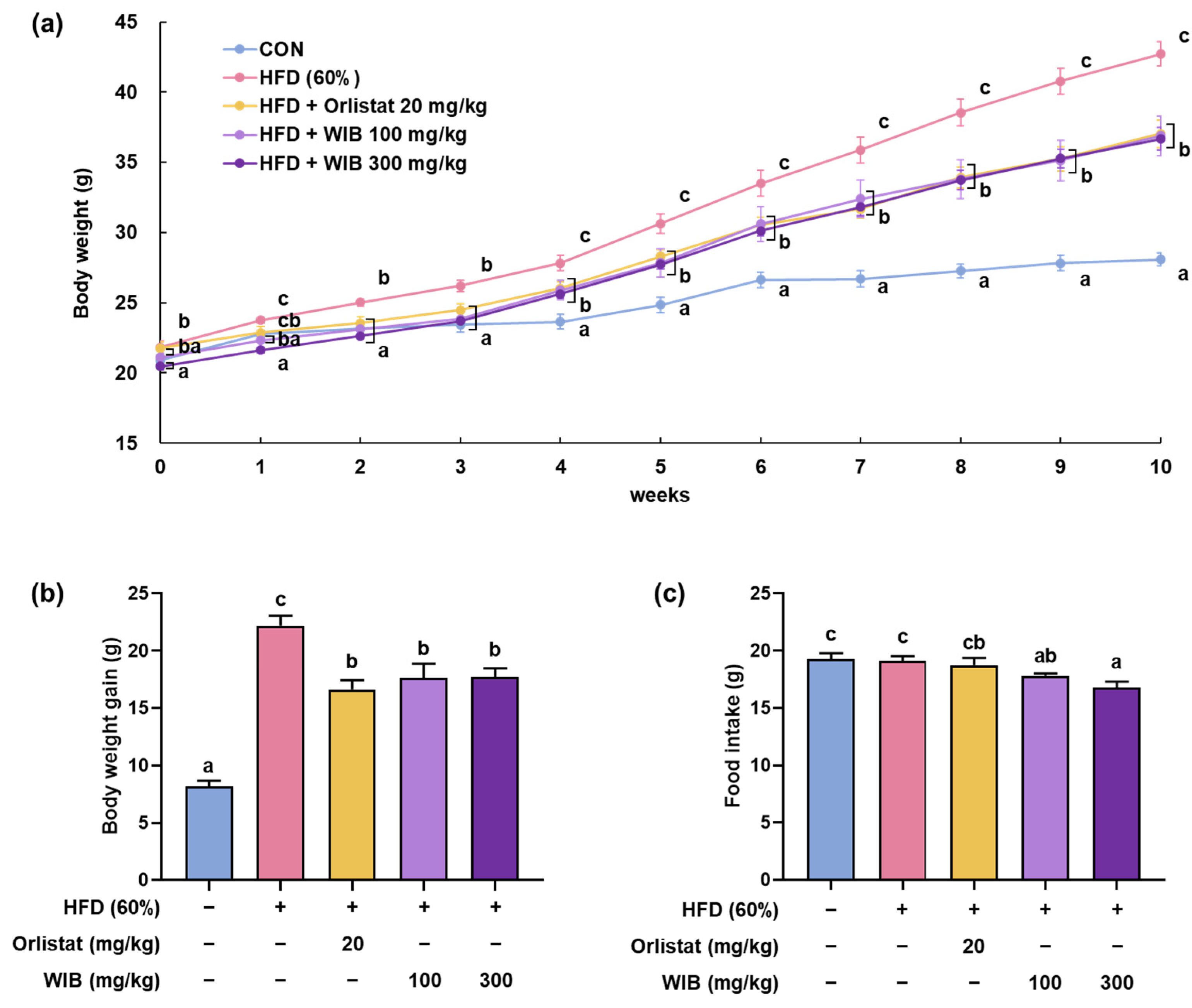
3.2. WIB Lessens the Body Weight Gain in HFD-Induced Mice
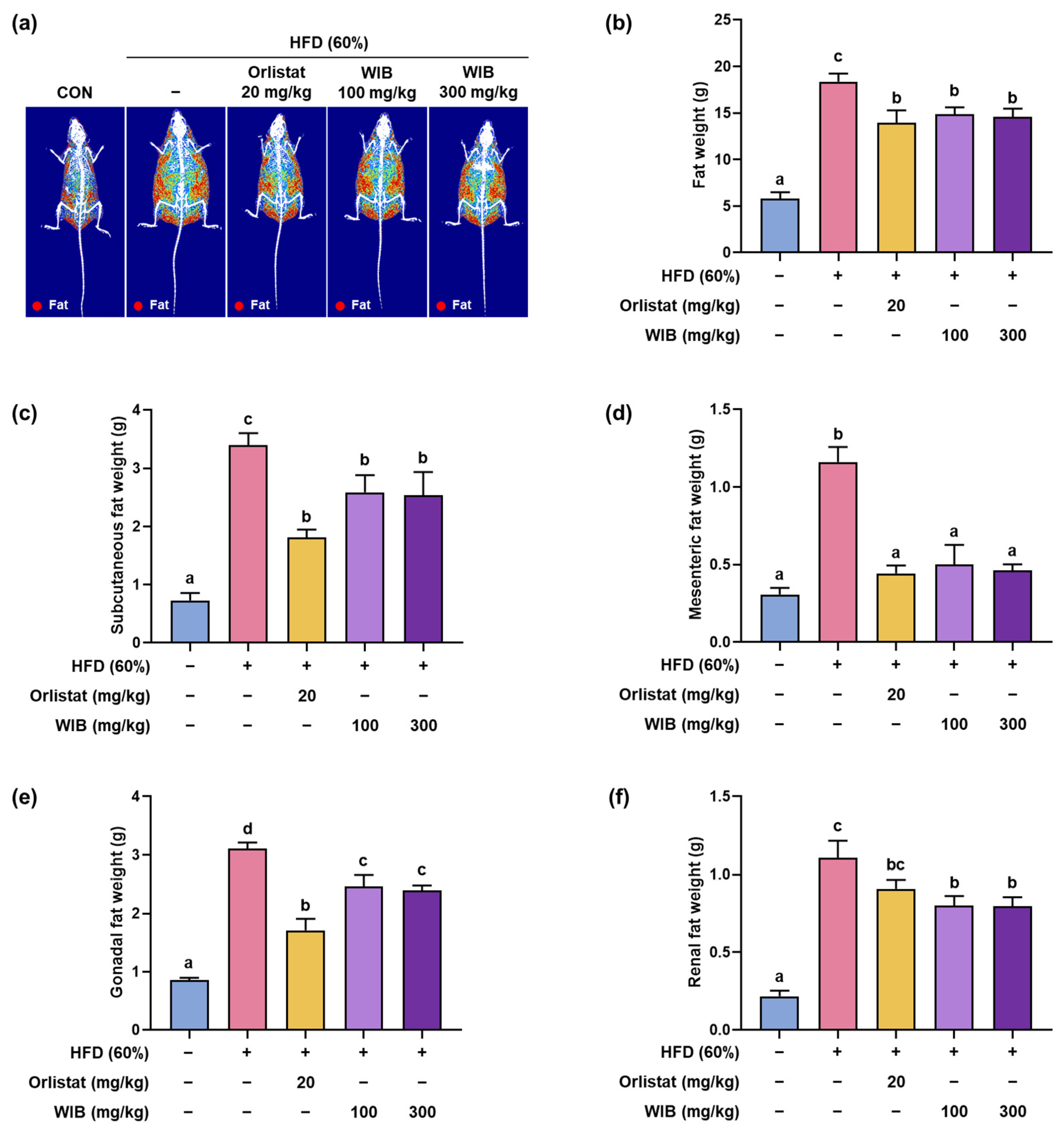
3.3. WIB Modified the Fat Composition in HFD-Induced Mice
3.4. WIB Ameliorates the Lipid Parameters in Blood Plasma of HFD-Induced Mice
3.5. WIB Refined the Obesity-Related Hormones in Blood Plasma of HFD-Induced Mice
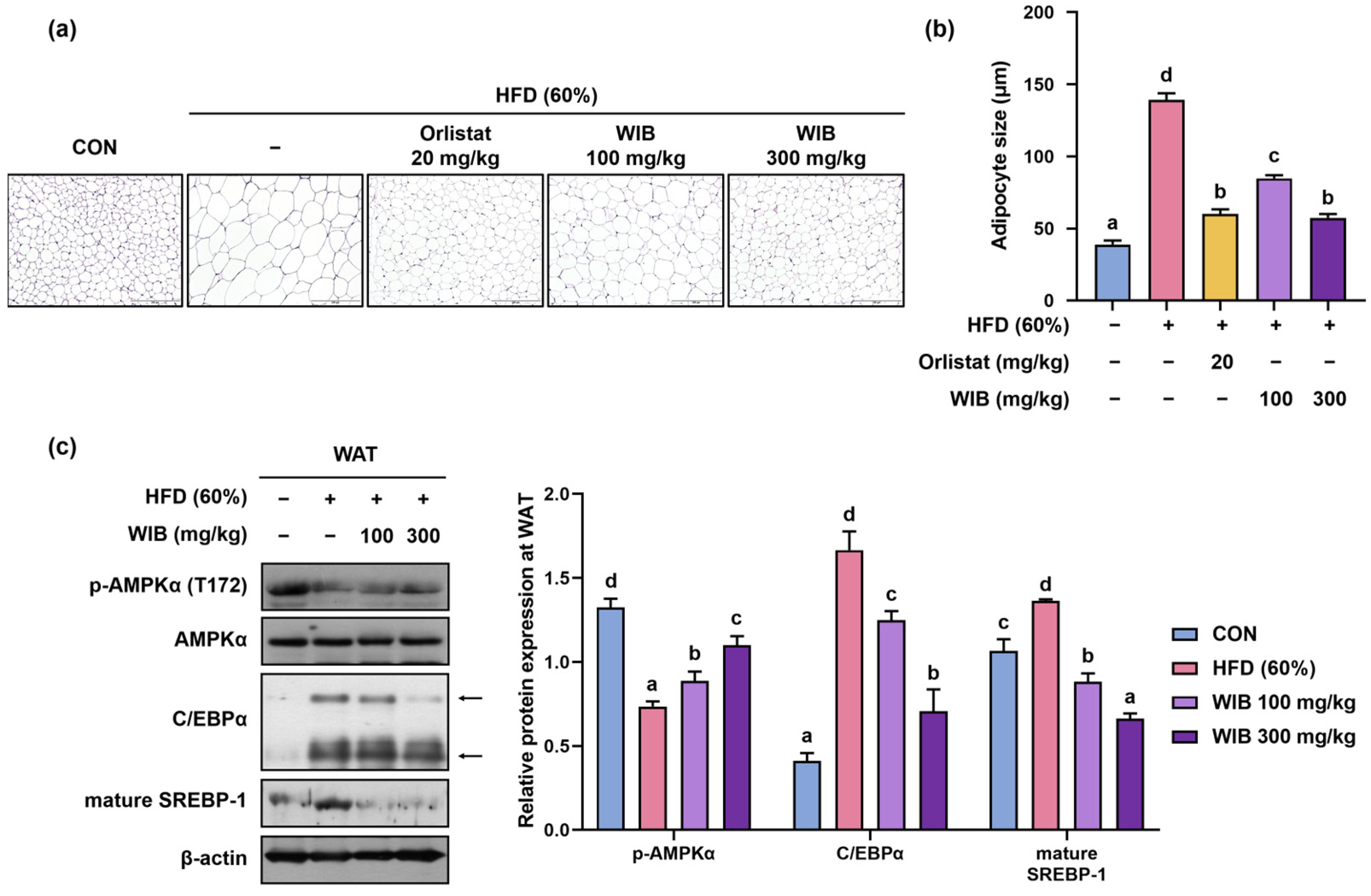
3.6. WIB Reduced Hypertrophy of Lipid Droplets and Lipogenesis in Subcutaneous Tissue of HFD-Induced Mice
3.7. WIB Prevents Lipid Accumulation in Liver Tissue of HFD-Induced Mice
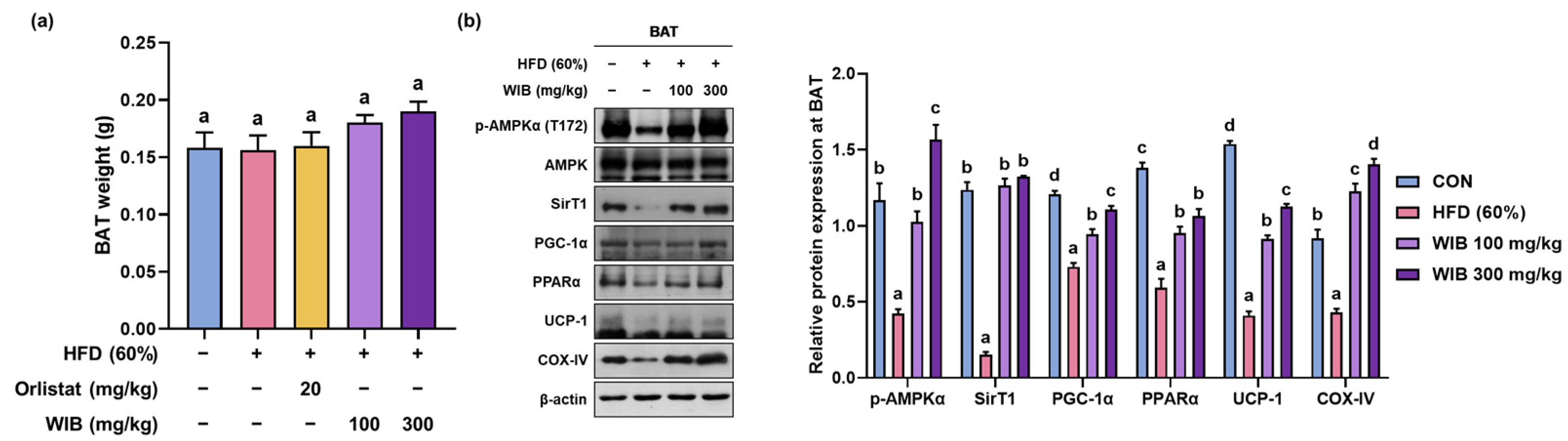
3.8. WIB Upregulates the Levels of Thermogenic-Related Proteins in BAT of HFD-Induced Mice
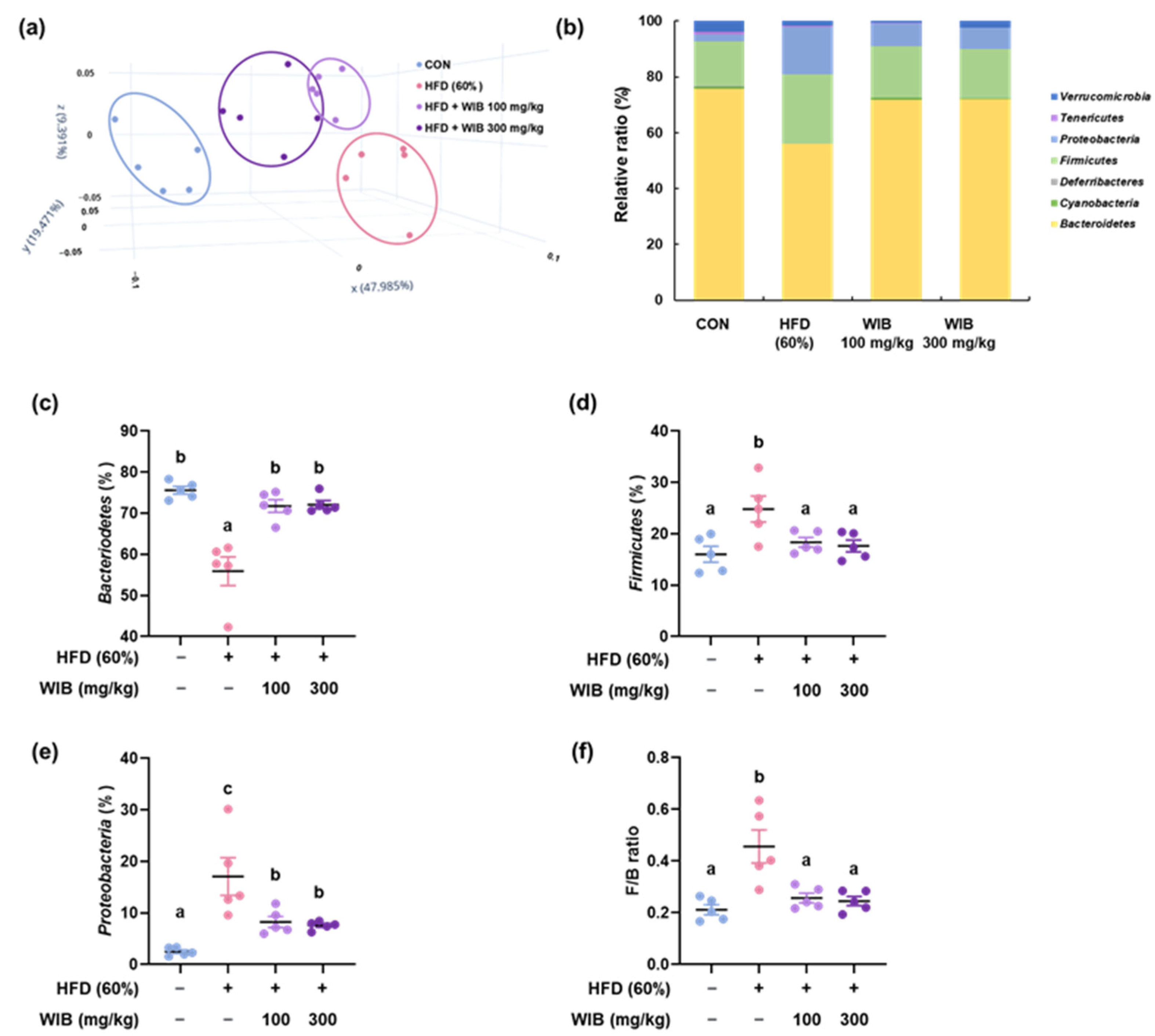
3.9. WIB Ameliorates the Dysbiosis by Regulating the Gut Microbiota Composition in HFD-Induced Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prattichizzo, F.; Ceriello, A.; Shah, V.N. Obesity, NAFLD/NASH, and Diabetes. Diabetes Technol. Ther. 2024, 26, S231–S240. [Google Scholar] [CrossRef]
- Blackstone, R.P.; Blackstone, R.P. Obesity-related diseases and syndromes: Insulin resistance, type 2 diabetes mellitus, non-alcoholic fatty liver disease, cardiovascular disease, and metabolic syndrome. Obes. Med. Pract. Essent. Guide 2016, 83–108. [Google Scholar]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Petito, G.; Cioffi, F.; Magnacca, N.; de Lange, P.; Senese, R.; Lanni, A. Adipose Tissue Remodeling in Obesity: An Overview of the Actions of Thyroid Hormones and Their Derivatives. Pharmaceuticals 2023, 16, 572. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Lee, H.-H.; Gil, H.-S.; Chung, K.-S.; Kim, J.-K.; Kim, D.-H.; Yoon, J.; Chung, E.K.; Lee, J.K.; Yang, W.M. Standardized hot water extract from the leaves of Hydrangea serrata (Thunb.) Ser. alleviates obesity via the AMPK pathway and modulation of the gut microbiota composition in high fat diet-induced obese mice. Food Funct. 2021, 12, 2672–2685. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Chung, K.S.; Lee, S.Y.; Heo, S.W.; Kim, Y.R.; Lee, J.K.; Kim, H.; Park, S.; Shin, Y.K.; Lee, K.T. Anti-obesity effects of a standardized ethanol extract of Eisenia bicyclis by regulating the AMPK signaling pathway in 3T3-L1 cells and HFD-induced mice. Food Funct. 2024, 15, 6424–6437. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1alpha Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Lehman, J.J.; Barger, P.M.; Kovacs, A.; Saffitz, J.E.; Medeiros, D.M.; Kelly, D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Investig. 2000, 106, 847–856. [Google Scholar] [CrossRef]
- Meng, D.; Yin, G.; Chen, S.; Zhang, X.; Yu, W.; Wang, L.; Liu, H.; Jiang, W.; Sun, Y.; Zhang, F. Diosgenin attenuates nonalcoholic hepatic steatosis through the hepatic SIRT1/PGC-1alpha pathway. Eur. J. Pharmacol. 2024, 977, 176737. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.F.; Ku, H.C.; Lin, H. PGC-1alpha as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef]
- Sankararaman, S.; Noriega, K.; Velayuthan, S.; Sferra, T.; Martindale, R. Gut Microbiome and Its Impact on Obesity and Obesity-Related Disorders. Curr. Gastroenterol. Rep. 2023, 25, 31–44. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martinez-Florez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Zsálig, D.; Berta, A.; Tóth, V.; Szabó, Z.; Simon, K.; Figler, M.; Pusztafalvi, H.; Polyák, É. A review of the relationship between gut microbiome and obesity. Appl. Sci. 2023, 13, 610. [Google Scholar] [CrossRef]
- Walker, A.W.; Parkhill, J. Microbiology. Fighting obesity with bacteria. Science 2013, 341, 1069–1070. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, A.; Afkhami, H.; Javadi, A.; Kashfi, M. Understanding the complex function of gut microbiota: Its impact on the pathogenesis of obesity and beyond: A comprehensive review. Diabetol. Metab. Syndr. 2024, 16, 308. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Hirano, K.; Tsujimoto, G. The SCFA Receptor GPR43 and Energy Metabolism. Front. Endocrinol. 2014, 5, 85. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef]
- Halpern, B.; Halpern, A. Safety assessment of FDA-approved (orlistat and lorcaserin) anti-obesity medications. Expert Opin. Drug Saf. 2015, 14, 305–315. [Google Scholar] [CrossRef]
- Kim, G.N.; Shin, M.R.; Shin, S.H.; Lee, A.R.; Lee, J.Y.; Seo, B.I.; Kim, M.Y.; Kim, T.H.; Noh, J.S.; Rhee, M.H.; et al. Study of Antiobesity Effect Through Inhibition of Pancreatic Lipase Activity of Diospyros Kaki Fruit and Citrus Unshiu Peel. BioMed Res. Int. 2016, 2016, 1723042. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review About Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, C.T.; Kim, Y. Green tea (-)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann. Nutr. Metab. 2009, 54, 151–157. [Google Scholar] [CrossRef]
- Barrios-Nolasco, A.; Dominguez-Lopez, A.; Miliar-Garcia, A.; Cornejo-Garrido, J.; Jaramillo-Flores, M.E. Anti-Inflammatory Effect of Ethanolic Extract from Tabebuia rosea (Bertol.) DC., Quercetin, and Anti-Obesity Drugs in Adipose Tissue in Wistar Rats with Diet-Induced Obesity. Molecules 2023, 28, 3801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tu, Z.C.; Yuan, T.; Wang, H.; Xie, X.; Fu, Z.F. Antioxidants and alpha-glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships. Food Chem. 2016, 208, 61–67. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.; Sun, H. Sweet potato (Ipomoea batatas L.) leaf polyphenols ameliorate hyperglycemia in type 2 diabetes mellitus mice. Food Funct. 2021, 12, 4117–4131. [Google Scholar] [CrossRef]
- Shih, C.K.; Chen, C.M.; Varga, V.; Shih, L.C.; Chen, P.R.; Lo, S.F.; Shyur, L.F.; Li, S.C. White sweet potato ameliorates hyperglycemia and regenerates pancreatic islets in diabetic mice. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Lee, S.L.; Chin, T.Y.; Tu, S.C.; Wang, Y.J.; Hsu, Y.T.; Kao, M.C.; Wu, Y.C. Purple Sweet Potato Leaf Extract Induces Apoptosis and Reduces Inflammatory Adipokine Expression in 3T3-L1 Differentiated Adipocytes. Evid. Based Complement. Altern. Med. 2015, 2015, 126302. [Google Scholar] [CrossRef]
- Lee, C.L.; Lee, S.L.; Chen, C.J.; Chen, H.C.; Kao, M.C.; Liu, C.H.; Chen, J.Y.; Lai, Y.T.; Wu, Y.C. Characterization of Secondary Metabolites from Purple Ipomoea batatas Leaves and Their Effects on Glucose Uptake. Molecules 2016, 21, 745. [Google Scholar] [CrossRef]
- Pestel, J.; Blangero, F.; Watson, J.; Pirola, L.; Eljaafari, A. Adipokines in obesity and metabolic-related-diseases. Biochimie 2023, 212, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Patel, S.; Rajbhandari, P. Adipose tissue lipid metabolism: Lipolysis. Curr. Opin. Genet. Dev. 2023, 83, 102114. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Yu, B.; Shi, S.; Liu, J.; Zhang, R.; Ayada, I.; Verstegen, M.M.A.; van der Laan, L.J.W.; Peppelenbosch, M.P.; et al. Recapitulating lipid accumulation and related metabolic dysregulation in human liver-derived organoids. J. Mol. Med. 2022, 100, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—A systematic review. Genes. Nutr. 2022, 17, 2. [Google Scholar] [CrossRef]
- Obesity: Causes, consequences, treatments, and challenges. J. Mol. Cell Biol. 2021, 13, 463–465. [CrossRef]
- Ghosh, S.; Dhar, S.; Bhattacharjee, S.; Bhattacharjee, P. Contribution of environmental, genetic and epigenetic factors to obesity-related metabolic syndrome. Nucleus 2023, 66, 215–237. [Google Scholar] [CrossRef]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar] [CrossRef]
- Shaik Mohamed Sayed, U.F.; Moshawih, S.; Goh, H.P.; Kifli, N.; Gupta, G.; Singh, S.K.; Chellappan, D.K.; Dua, K.; Hermansyah, A.; Ser, H.L.; et al. Natural products as novel anti-obesity agents: Insights into mechanisms of action and potential for therapeutic management. Front. Pharmacol. 2023, 14, 1182937. [Google Scholar] [CrossRef]
- Bodjrenou, D.M.; Li, X.; Lu, X.; Lei, S.; Zheng, B.; Zeng, H. Resistant starch from sweet potatoes: Recent advancements and applications in the food sector. Int. J. Biol. Macromol. 2023, 225, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.S.; Cavaco, T.; Carvalho, L.M.; Duque, P. Effect of photoperiod on flavonoid pathway activity in sweet potato (Ipomoea batatas (L.) Lam.) leaves. Food Chem. 2010, 118, 384–390. [Google Scholar] [CrossRef]
- Pochapski, M.T.; Fosquiera, E.C.; Esmerino, L.A.; Dos Santos, E.B.; Farago, P.V.; Santos, F.A.; Groppo, F.C. Phytochemical screening, antioxidant, and antimicrobial activities of the crude leaves’ extract from Ipomoea batatas (L.) Lam. Pharmacogn. Mag. 2011, 7, 165–170. [Google Scholar] [CrossRef]
- Chagas, M.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Goncalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef]
- Sato, S.; Mukai, Y. Modulation of Chronic Inflammation by Quercetin: The Beneficial Effects on Obesity. J. Inflamm. Res. 2020, 13, 421–431. [Google Scholar] [CrossRef]
- White, U. Adipose tissue expansion in obesity, health, and disease. Front. Cell Dev. Biol. 2023, 11, 1188844. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Stefanovic, A.; Zeljkovic, A. Obesity and Dyslipidemia: A Review of Current Evidence. Curr. Obes. Rep. 2023, 12, 207–222. [Google Scholar] [CrossRef]
- Huang, J.K.; Lee, H.C. Emerging Evidence of Pathological Roles of Very-Low-Density Lipoprotein (VLDL). Int. J. Mol. Sci. 2022, 23, 4300. [Google Scholar] [CrossRef]
- Carpentier, A.C. 100(th) anniversary of the discovery of insulin perspective: Insulin and adipose tissue fatty acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E653–E670. [Google Scholar] [CrossRef]
- Kumar, A.; Sundaram, K.; Mu, J.; Dryden, G.W.; Sriwastva, M.K.; Lei, C.; Zhang, L.; Qiu, X.; Xu, F.; Yan, J.; et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat. Commun. 2021, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef]
- Guerre-Millo, M. Adipose tissue hormones. J. Endocrinol. Investig. 2002, 25, 855–861. [Google Scholar] [CrossRef]
- Angin, Y.; Beauloye, C.; Horman, S.; Bertrand, L. Regulation of Carbohydrate Metabolism, Lipid Metabolism, and Protein Metabolism by AMPK. Exp. Suppl. 2016, 107, 23–43. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Kim, S.M. Vanadium-protein complex inhibits human adipocyte differentiation through the activation of beta-catenin and LKB1/AMPK signaling pathway. PLoS ONE 2020, 15, e0239547. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.T.; Arshad, A.; Arockiaraj, J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef] [PubMed]
- Reverte-Salisa, L.; Siddig, S.; Hildebrand, S.; Yao, X.; Zurkovic, J.; Jaeckstein, M.Y.; Heeren, J.; Lezoualc’h, F.; Krahmer, N.; Pfeifer, A. EPAC1 enhances brown fat growth and beige adipogenesis. Nat. Cell Biol. 2024, 26, 113–123. [Google Scholar] [CrossRef]
- Gaspar, R.C.; Pauli, J.R.; Shulman, G.I.; Munoz, V.R. An update on brown adipose tissue biology: A discussion of recent findings. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E488–E495. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhaes, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Haman, F.; Richard, D. Brown Adipose Tissue—A Translational Perspective. Endocr. Rev. 2023, 44, 143–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism Through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Blaut, M. Gut microbiota and energy balance: Role in obesity. Proc. Nutr. Soc. 2015, 74, 227–234. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Zhang, W.; Zhou, J.; Cong, H.; Yang, G.; Liu, G. Rumen microbiota helps Tibetan sheep obtain energy more efficiently to survive in the extreme environment of the Qinghai-Tibet Plateau. Front. Microbiol. 2024, 15, 1431063. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Strukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, L.; Liu, Y. Targeting AMPK Signaling in the Liver: Implications for Obesity and Type 2 Diabetes Mellitus. Curr. Drug Targets 2022, 23, 1057–1071. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chung, K.S.; Son, S.R.; Lee, S.Y.; Jang, D.S.; Lee, J.K.; Kim, H.J.; Na, C.S.; Lee, S.H.; Lee, K.T. A Botanical Mixture Consisting of Inula japonica and Potentilla chinensis Relieves Obesity via the AMPK Signaling Pathway in 3T3-L1 Adipocytes and HFD-Fed Obese Mice. Nutrients 2022, 14, 3685. [Google Scholar] [CrossRef]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef]
- Zhang, E.; Jin, L.; Wang, Y.; Tu, J.; Zheng, R.; Ding, L.; Fang, Z.; Fan, M.; Al-Abdullah, I.; Natarajan, R.; et al. Intestinal AMPK modulation of microbiota mediates crosstalk with brown fat to control thermogenesis. Nat. Commun. 2022, 13, 1135. [Google Scholar] [CrossRef]


| Comparative Profiles | Normal Diet | High-Fat Diet | ||
|---|---|---|---|---|
| g% | kcal% | g% | kcal% | |
| Protein | 19.2 | 20 | 26 | 20 |
| Carbohydrate | 67.3 | 70 | 26 | 20 |
| Fat | 4.3 | 10 | 35 | 60 |
| Total | 100 | 100 | ||
| kcal/g | 3.85 | 5.24 | ||
| Peak No. | Rt (min) | Precursor Ion (m/z) | Elemental Composition (or Molecular Formula) | Mass Difference (mmu) | Identification |
|---|---|---|---|---|---|
| 1 | 6.6 | 205.09655 [M+H]+ 203.07701 [M−H]− | C11H12N2O2 | −1.16 −5.04 | L-tryptophan 1 |
| 2 | 10.8 | 355.10230 [M+H]+ 377.08625 [M+Na]+ 353.08623 [M−H]− | C16H18O9 | −0.61 1.39 −1.03 | Neochlorogenic acid 1 |
| 3 | 14. | 181.04995 [M+H]+ 179.03368 [M−H]− | C9H8O4 | −0.13 −0.75 | Caffeic acid 1 |
| 4 | 14.9 | 355.10254 [M+H]+ 377.08614 [M+Na]+ 353.08607 [M−H]− | C16H18O9 | −0.36 1.29 −1.18 | Chlorogenic acid 1 |
| 5 | 15. 3 | 355.10254 [M+H]+ 377.08647 [M+Na]+ 353.08644 [M−H]− | C16H18O9 | −0.36 1.62 −0.82 | Cryptochlorogenic acid 1 |
| 6 | 22.3 | 627.16048 [M+H]+ 625.14879 [M−H]− | C27H30O17 | 4.35 8.31 | Quercetin 3-O-β-D-sophoroside |
| 7 | 25.1 | 465.10362 [M+H]+ 463.08995 [M−H]− | C21H20O12 | 0.32 2.30 | Hyperoside 1 |
| 8 | 25.6 | 465.10496 [M+H]+ 463.08931 [M−H]− | C21H20O12 | 1.66 1.66 | Isoquercetin 1 |
| 9 | 27.4 | 517.13593 [M+H]+ 539.11944 [M+Na]+ 515.12501 [M−H]− | C25H24O12 | 1.33 2.89 6.06 | Isochlorogenic acid B 1 |
| 10 | 28.1 | 517.13653 [M+H]+ 539.11977 [M+Na]+ 515.12431 [M−H]− | C25H24O12 | 1.93 3.23 5.36 | Isochlorogenic acid A 1 |
| 11 | 30.0 | 517.13486 [M+H]+ 515.12623 [M−H]− | C25H24O12 | 0.26 7.28 | Isochlorogenic acid C 1 |
| Variable (mg/dL) | CON | HFD | Orlistat (20 mg/kg) | WIB (100 mg/kg) | WIB (300 mg/kg) |
|---|---|---|---|---|---|
| TC | 103.43 ± 14.88 a | 158.71 ± 8.04 c | 125.71 ± 37.84 ab | 148.57 ± 23.14 bc | 147.29 ± 10.73 bc |
| TG | 52.86 ± 9.91 a | 115.14 ± 15.60 c | 66.57 ± 25.25 ab | 86.43 ± 31.72 bc | 68.00 ± 42.49 ab |
| LDL | 9.71 ± 2.14 a | 15.29 ± 1.25 c | 11.57 ± 2.64 ab | 12.86 ± 2.04 b | 11.57 ± 2.23 ab |
| HDL | 86.57 ± 5.91 a | 91.14 ± 14.24 a | 97.71 ± 13.83 b | 96.00 ± 9.07 ab | 99.57 ± 8.79 ab |
| VLDL | 8.86 ± 1.65 a | 18.29 ± 2.70 c | 10.12 ± 2.29 ab | 14.40 ± 5.29 bc | 11.21 ± 6.93 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-W.; Yoon, Y.S.; Yoon, Y.-S.; Chung, K.-S.; Kim, M.-j.; Park, G.; Choi, M.; Jang, Y.-P.; Lee, K.-T. Protective Effects of a Standardized Water Extract from the Stem of Ipomoea batatas L. Against High-Fat Diet-Induced Obesity. Nutrients 2025, 17, 1643. https://doi.org/10.3390/nu17101643
Lee C-W, Yoon YS, Yoon Y-S, Chung K-S, Kim M-j, Park G, Choi M, Jang Y-P, Lee K-T. Protective Effects of a Standardized Water Extract from the Stem of Ipomoea batatas L. Against High-Fat Diet-Induced Obesity. Nutrients. 2025; 17(10):1643. https://doi.org/10.3390/nu17101643
Chicago/Turabian StyleLee, Chae-Won, Ye Seul Yoon, Young-Seo Yoon, Kyung-Sook Chung, Mi-ju Kim, Geonha Park, Minsik Choi, Young-Pyo Jang, and Kyung-Tae Lee. 2025. "Protective Effects of a Standardized Water Extract from the Stem of Ipomoea batatas L. Against High-Fat Diet-Induced Obesity" Nutrients 17, no. 10: 1643. https://doi.org/10.3390/nu17101643
APA StyleLee, C.-W., Yoon, Y. S., Yoon, Y.-S., Chung, K.-S., Kim, M.-j., Park, G., Choi, M., Jang, Y.-P., & Lee, K.-T. (2025). Protective Effects of a Standardized Water Extract from the Stem of Ipomoea batatas L. Against High-Fat Diet-Induced Obesity. Nutrients, 17(10), 1643. https://doi.org/10.3390/nu17101643






