Beneficial Effects of Traditional Fermented Soybean Sauce (Kanjang) on Memory Function, Body Water, and Glucose Metabolism: Roles of Gut Microbiota and Neuroinflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Kanjang Production and Sample Collection
2.2. Sodium, Aflatoxin, and Biogenic Amine Analyses of Kanjang Samples
2.3. Animal Care
2.4. Induction of Memory Deficit in an Animal Model
2.5. Experimental Design and Diet Preparation
2.6. Oral Glucose Tolerance Test (OGTT) and Intraperitoneal Insulin Tolerance Test (IPITT)
2.7. Assessments of Memory Deficit
2.8. Sample Collection and Biochemical Assays
2.9. Quantitative Real-Time PCR to Evaluate mRNA Expression in the Hippocampal Tissue
2.10. Histological Analysis of the Brain and Large Intestinal Tissues
2.11. Assessment of Serum Short-Chain Fatty Acid (SCFA) Concentrations
2.12. Gut Microbiome Composition and Metagenome Analysis
2.13. Statistical Analyses
3. Results
3.1. Characteristics of the Kanjang Samples
3.2. Effects of Kanjang Treatments on Weight Gain
3.3. Effects of Kanjang Treatments on Water Homeostasis
3.4. Effects of Kanjang Treatments on Glucose Metabolism
3.5. Effects of Kanjang Treatments on Serum Markers of Inflammation
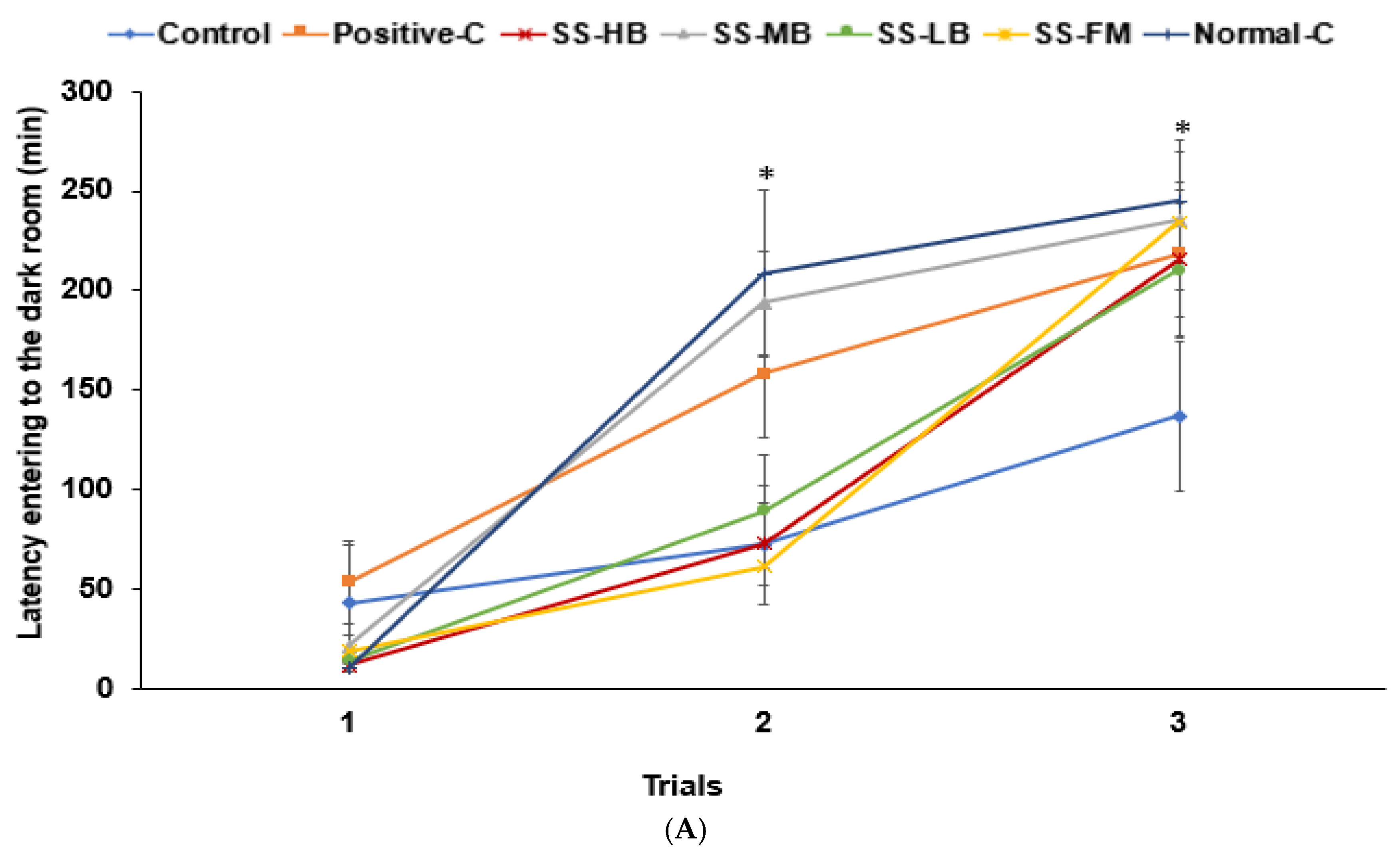
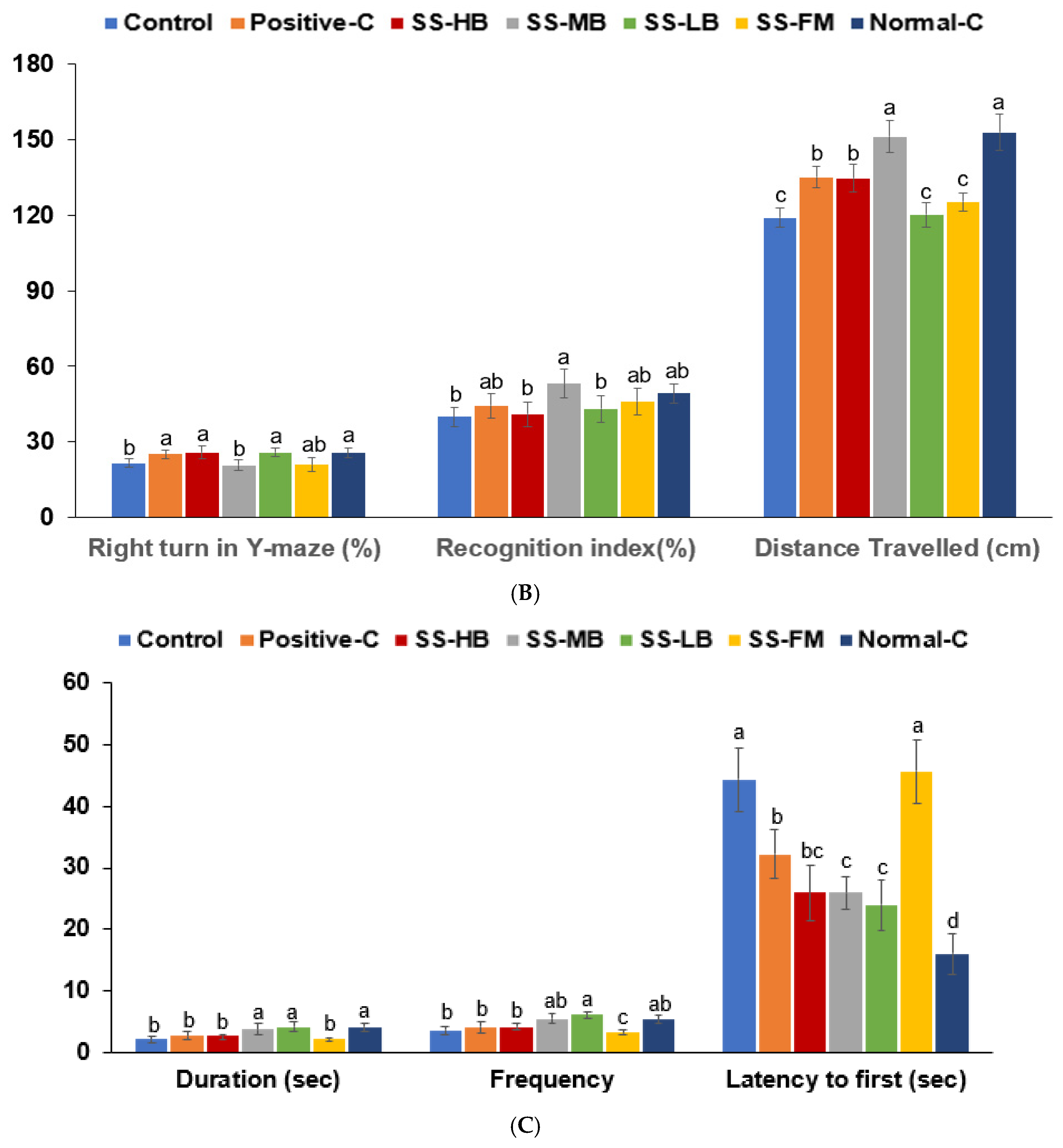
3.6. Effects of Kanjang Treatments on Memory Impairment
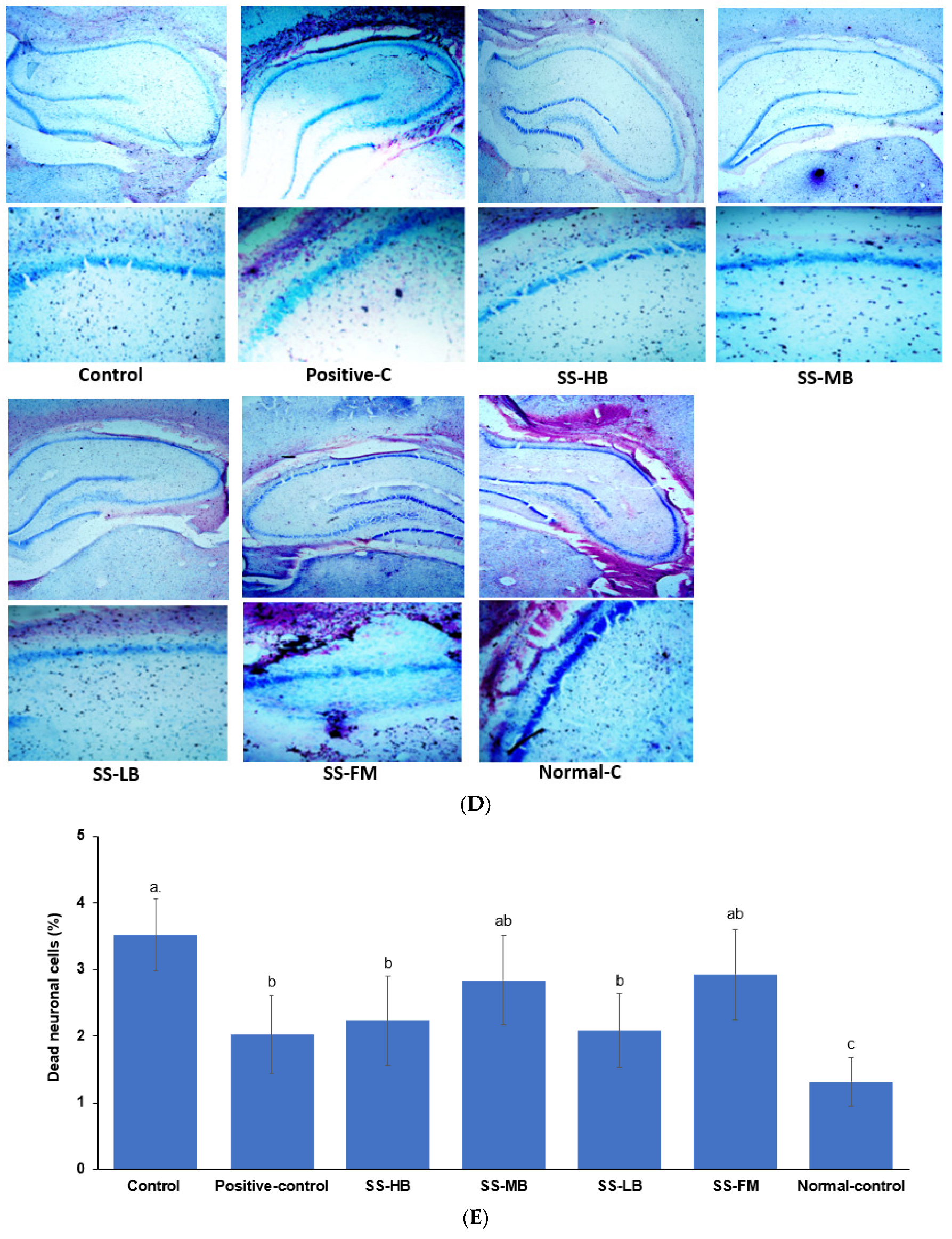
3.7. Effects of Kanjang Treatments on Brain Neuronal Cell Death
3.8. Effects of Kanjang Treatments on Goblet Cells and Serum SCFA Concentrations
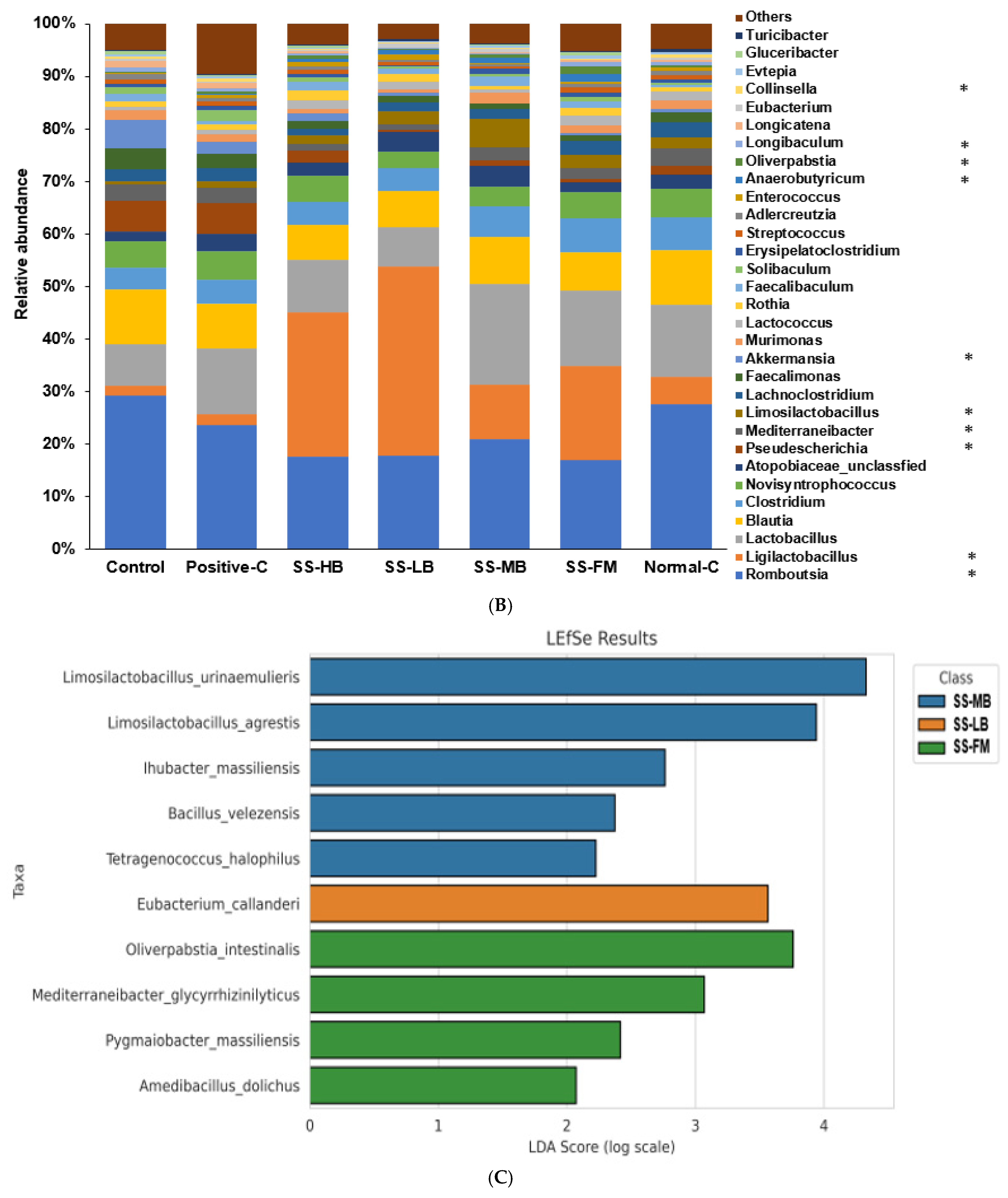
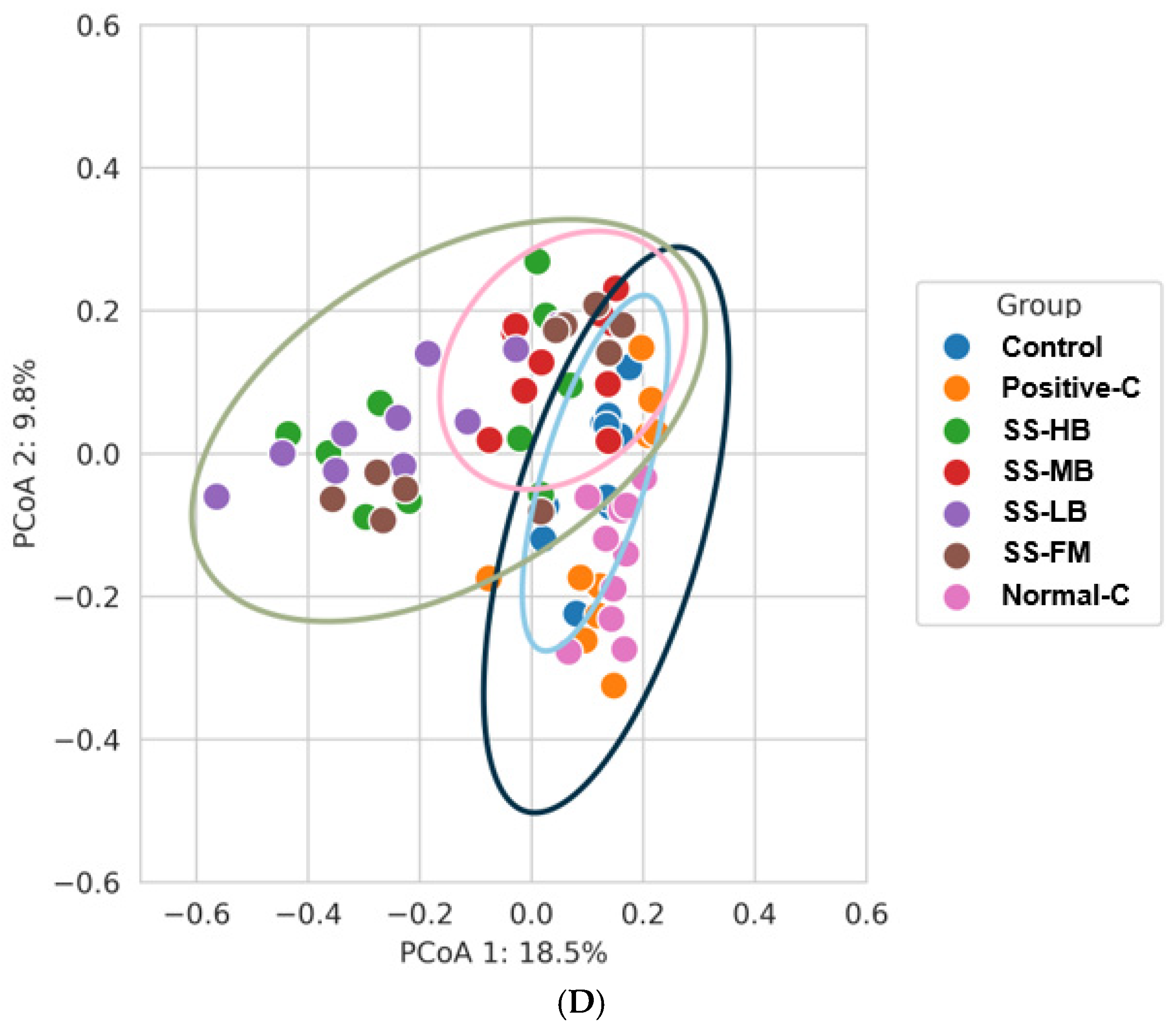
3.9. Effects of Kanjang Treatments on Gut Microbiota and Metagenome Function
4. Discussion
Clinical Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.-H.; Ko, J.; Kwon, D.Y. Jang, Korean fermented soybean product, the result of endeavors of ancients for the best taste of Korean diet. J. Ethn. Foods 2023, 10, 33. [Google Scholar] [CrossRef]
- Faraco, G.; Brea, D.; Garcia-Bonilla, L.; Wang, G.; Racchumi, G.; Chang, H.; Buendia, I.; Santisteban, M.M.; Segarra, S.G.; Koizumi, K.; et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 2018, 21, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Yang, H.J.; Yang, H.G.; Ryu, M.S.; Ha, G.; Jeong, D.Y.; Park, S. Inverse association of daily fermented soybean paste (“Jang”) intake with metabolic syndrome risk, especially body fat and hypertension, in men of a large hospital-based cohort. Front. Nutr. 2023, 10, 1122945. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhang, T.; Yue, Y.; Jeong, S.J.; Ryu, M.S.; Wu, X.; Yang, H.J.; Jeong, D.Y. Alleviation of metabolic disturbance by substituting kanjang high in Bacillus for salt through modulation of gut microbiota in estrogen-deficient Rats. Foods 2022, 11, 1951. [Google Scholar] [CrossRef]
- Zheng, Y.; Bonfili, L.; Wei, T.; Eleuteri, A.M. Understanding the gut-brain axis and Its therapeutic implications for neurodegenerative disorders. Nutrients 2023, 15, 4631. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, Q.; Guan, Y.; Sun, Z.; Li, W.; Guo, S.; Zhang, A. The communication mechanism of the gut-brain axis and its effect on central nervous system diseases: A systematic review. Biomed. Pharmacother. 2024, 178, 117207. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Gritsenko, V.A.; Martins, A.C.; Tizabi, Y.; Korobeinikova, T.V.; Paoliello, M.M.B.; Tinkov, A.A. Modulation of gut microbiota with probiotics as a strategy to counteract endogenous and exogenous neurotoxicity. Adv. Neurotoxicol. 2024, 11, 133–176. [Google Scholar] [CrossRef]
- Martin, M.; Boulaire, M.; Lucas, C.; Peltier, A.; Pourtau, L.; Gaudout, D.; Layé, S.; Pallet, V.; Joffre, C.; Dinel, A.-L. Plant extracts and ω-3 improve short-term memory and modulate the microbiota–gut–brain axis in D-galactose model mice. J. Nutr. 2024, 154, 3704–3717. [Google Scholar] [CrossRef]
- Poceviciute, I.; Brazaityte, A.; Buisas, R.; Vengeliene, V. Scopolamine animal model of memory impairment. Behav. Brain Res. 2025, 479, 115344. [Google Scholar] [CrossRef]
- Mun, E.G.; Sohn, H.S.; Kim, M.S.; Cha, Y.S. Antihypertensive effect of Ganjang (traditional Korean soy sauce) on Sprague-Dawley rats. Nutr. Res. Pract. 2017, 11, 388–395. [Google Scholar] [CrossRef]
- Lee, E.J.; Song, J.; Park, C.H.; Mun, E.G.; Wang, J.; Han, A.; Park, J.E.; Cha, Y.S. Soy sauce lowers body weight and fat mass in high-fat diet-induced obese rats. J. Med. Food 2023, 26, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.E.; Clarke, S.L.; Wu, K.-H.H.; Kanoni, S.; Zajac, G.J.M.; Ramdas, S.; Surakka, I.; Ntalla, I.; Vedantam, S.; Winkler, T.W.; et al. The power of genetic diversity in genome-wide association studies of lipids. Nature 2021, 600, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Zhang, T.; An, T.; Lu, R. Soy isoflavones ameliorate the cognitive dysfunction of Goto-Kakizaki rats by activating the Nrf2-HO-1 signaling pathway. Aging 2020, 12, 21344–21354. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishna, T.; Suresh Kumar, H.K.; Perumal, K.; Elangovan, E. Impact of Bacillus in fermented soybean foods on human health. Ann. Microbiol. 2021, 71, 30. [Google Scholar] [CrossRef]
- Kim, B.V.; Yoon, S.; Kim, J.E. A review of fermented foods with beneficial effects on brain and cognitive function. Prev. Nutr. Food Sci. 2016, 21, 297–305. [Google Scholar] [CrossRef]
- do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented soy products and their potential health benefits: A review. Microorganisms 2022, 10, 1606. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, K.; Zhang, Z.; Zhang, C.; Sun, Y.; Feng, Z. Fermented soybean foods: A review of their functional components, mechanism of action and factors influencing their health benefits. Food Res. Int. 2022, 158, 111575. [Google Scholar] [CrossRef]
- Ryu, M.S.; Yue, Y.; Li, C.; Yang, H.-J.; Zhang, T.; Wu, X.; Jeong, D.Y.; Park, S. Moderate capsaicin-containing kochujang alleviates memory impairment through the gut-brain axis in rats with scopolamine-induced amnesia. Biomed. Pharmacother. 2024, 178, 117091. [Google Scholar] [CrossRef]
- Zhong, B.; Mun, E.-G.; Wang, J.-X.; Cha, Y.-S. Chinese traditional fermented soy sauce exerts protective effects against high-fat and high-salt diet-induced hypertension in Sprague-Dawley rats by improving adipogenesis and renin-angiotensin-aldosterone system activity. Fermentation 2021, 7, 52. [Google Scholar] [CrossRef]
- Zaprovalna, O.E.; Kolesnikova, O.V.; Potapenko, A.V.; Radchenko, A.O. Association between metabolic parameters and cognitive dysfunction in different age groups. Atherosclerosis 2022, 355, 156–157. [Google Scholar] [CrossRef]
- Yulug, B.; Altay, O.; Li, X.; Hanoglu, L.; Cankaya, S.; Lam, S.; Velioglu, H.A.; Yang, H.; Coskun, E.; Idil, E.; et al. Combined metabolic activators improve cognitive functions in Alzheimer’s disease patients: A randomized, double-blinded, placebo-controlled phase-II trial. Transl. Neurodegener. 2023, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Shalal, O.S. Anti-inflammatory activity of fermented soybean (Glycine max) extract on macrophages by inhibiting cytokines expression. Gene Rep. 2023, 30, 101729. [Google Scholar] [CrossRef]
- Das, D.; Sarkar, S.; Borsingh Wann, S.; Kalita, J.; Manna, P. Current perspectives on the anti-inflammatory potential of fermented soy foods. Food Res. Int. 2022, 152, 110922. [Google Scholar] [CrossRef] [PubMed]
- Arcusa, R.; Villaño, D.; Marhuenda, J.; Cano, M.; Cerdà, B.; Zafrilla, P. Potential role of ginger (Zingiber officinale Roscoe) in the prevention of neurodegenerative diseases. Front. Nutr. 2022, 9, 809621. [Google Scholar] [CrossRef]
- Muniyappan, M.; Shanmugam, S.; Park, J.H.; Han, K.; Kim, I.H. Effects of fermented soybean meal supplementation on the growth performance and apparent total tract digestibility by modulating the gut microbiome of weaned piglets. Sci. Rep. 2023, 13, 3691. [Google Scholar] [CrossRef]
- Zhang, L.; Su, H.; Wang, S.; Fu, Y.; Wang, M. Gut microbiota and neurotransmitter regulation: Functional effects of four traditional Chinese fermented soybeans (Sojae Semen praeparatum). Foods 2025, 14, 671. [Google Scholar] [CrossRef]
- Song, E.-J.; Kim, M.J.; Jung, C.H.; Chung, W.-H.; Nam, Y.-D.; Lim, M.Y. Early response of the gut microbiome and serum metabolites to Cheonggukjang intake in healthy Korean subjects. J. Funct. Foods 2023, 101, 105420. [Google Scholar] [CrossRef]
- Karbownik, M.S.; Mokros, Ł.; Dobielska, M.; Kowalczyk, M.; Kowalczyk, E. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: A prospective cohort study. Front. Nutr. 2022, 9, 850249. [Google Scholar] [CrossRef]
- Zhu, J.-j.; Gao, M.-x.; Song, X.-j.; Zhao, L.; Li, Y.-w.; Hao, Z.-h. Changes in bacterial diversity and composition in the feces and colon of weaned piglets after feeding fermented soybean meal. J. Med. Microbiol. 2018, 67, 1181–1190. [Google Scholar] [CrossRef]
- Jang, S.-E.; Kim, K.-A.; Han, M.J.; Kim, D.-H. Doenjang, a fermented Korean soybean paste, inhibits lipopolysaccharide production of gut microbiota in mice. J. Med. Food 2014, 17, 67–75. [Google Scholar] [CrossRef]
- Lim, H.J.; Park, I.S.; Seo, J.W.; Ha, G.; Yang, H.J.; Jeong, D.Y.; Kim, S.Y.; Jung, C.H. Anti-inflammatory effect of Korean soybean sauce (Ganjang) on mice with induced colitis. J. Microbiol. Biotechnol. 2024, 34, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Uchida, R.; Horiba, T.; Kawaguchi, T.; Gomi, K.; Goto, Y. Soy sauce-like seasoning enhances the growth of Agathobacter rectalis and the production of butyrate, propionate, and lactate. Biosci. Microbiota Food Health 2024, 43, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.M.A.D.B.; Martins, I.J.; Morici, M.; Bharadwaj, P.; Rainey-Smith, S.R.; Lim, W.L.F.; Martins, R.N. Sodium butyrate reduces brain amyloid-β levels and improves cognitive memory performance in an Alzheimer’s disease transgenic mouse model at an early disease stage. J. Alzheimers Dis. 2020, 74, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ziemka-Nalecz, M.; Jaworska, J.; Sypecka, J.; Polowy, R.; Filipkowski, R.K.; Zalewska, T. Sodium butyrate, a histone deacetylase inhibitor, exhibits neuroprotective/neurogenic effects in a rat model of neonatal hypoxia-ischemia. Mol. Neurobiol. 2017, 54, 5300–5318. [Google Scholar] [CrossRef]
- Ge, X.; Zheng, M.; Hu, M.; Fang, X.; Geng, D.; Liu, S.; Wang, L.; Zhang, J.; Guan, L.; Zheng, P.; et al. Butyrate ameliorates quinolinic acid-induced cognitive decline in obesity models. J. Clin. Investig. 2023, 133, e154612. [Google Scholar] [CrossRef]
- Basson, A.R.; Ahmed, S.; Almutairi, R.; Seo, B.; Cominelli, F. Regulation of intestinal inflammation by soybean and soy-derived compounds. Foods 2021, 10, 774. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Zhang, T.; Park, S. The impact of gut microbiome enterotypes on ulcerative colitis: Identifying key bacterial species and revealing species co-occurrence networks using machine learning. Gut Microbes 2024, 16, 2292254. [Google Scholar] [CrossRef]
- Tooley, K.L. Effects of the Human gut microbiota on cognitive performance, brain structure, and function: A narrative review. Nutrients 2020, 12, 3009. [Google Scholar] [CrossRef]
- Park, S.; Wu, X. Modulation of the gut microbiota in memory impairment and Alzheimer’s disease via the inhibition of the parasympathetic nervous system. Int. J. Mol. Sci. 2022, 23, 13574. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus nerve and underlying impact on the gut microbiota-brain axis in behavior and neurodegenerative diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Schneider, E.; Gunnigle, E.; Cotter, P.D.; Cryan, J.F. Fermented foods: Harnessing their potential to modulate the microbiota-gut-brain axis for mental health. Neurosci. Biobehav. Rev. 2024, 158, 105562. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.C.; Pathak, S.; Kumar, V.; Panda, B.P. Attenuation of neurobehavioral and neurochemical abnormalities in an animal model of cognitive deficits of Alzheimer’s disease by fermented soybean nano nutraceutical. Inflammopharmacology 2018, 26, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hwang, Y.H.; Kim, D.H. Lactobacillus plantarum C29-fermented soybean (DW2009) alleviates memory impairment in 5XFAD transgenic mice by regulating microglia activation and gut microbiota composition. Mol. Nutr. Food Res. 2018, 62, e1800359. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.Y.; Ryu, M.S.; Yang, H.J.; Park, S. γ-PGA-rich chungkookjang, short-term fermented soybeans: Prevents memory impairment by modulating brain insulin sensitivity, neuro-inflammation, and the gut-microbiome-brain axis. Foods 2021, 10, 221. [Google Scholar] [CrossRef]
- Nithya, A.; Misra, S.; Panigrahi, C.; Dalbhagat, C.G.; Mishra, H.N. Probiotic potential of fermented foods and their role in non-communicable diseases management: An understanding through recent clinical evidence. Food Chem. Adv. 2023, 3, 100381. [Google Scholar] [CrossRef]
- Valentino, V.; Magliulo, R.; Farsi, D.; Cotter, P.D.; O’Sullivan, O.; Ercolini, D.; De Filippis, F. Fermented foods, their microbiome and its potential in boosting human health. Microb. Biotechnol. 2024, 17, e14428. [Google Scholar] [CrossRef]





| Control | Positive-C | SS-HB | SS-MB | SS-LB | SS-FM | Normal-C | |
|---|---|---|---|---|---|---|---|
| Final weight (g) | 356 ± 5.44 a | 362 ± 6.2 a | 280 ± 13.3 d | 287 ± 7.22 cd | 281 ± 13.9 d | 299 ± 8.41 c | 347 ± 5.61 b |
| Body weight gain (g) | 182 ± 5.44 a | 189 ± 6.2 a | 96.6 ± 13.3 d | 116 ± 7.22 c | 110 ± 13.9 c | 116 ± 8.41 c | 175 ± 5.61 b |
| Retroperitoneal mass (g) | 6.45 ± 0.63 a | 6.99 ± 0.64 a | 3.08 ± 0.45 c | 4.01 ± 0.42 b | 2.84 ± 0.32 c | 3.9 ± 0.19 b | 6.5 ± 0.39 a |
| Epididymal fat mass | 5.14 ± 0.73 a | 5.89 ± 0.41 a | 1.91 ± 0.43 d | 3.22 ± 0.5 c | 1.36 ± 0.3 e | 2.91 ± 0.33 c | 5.43 ± 0.58 a |
| Visceral fat (% of bw) | 3.18 ± 0.32 a | 3.53 ± 0.18 a | 1.7 ± 0.24 c | 2.5 ± 0.19 b | 1.47 ± 0.19 d | 2.28 ± 0.1 b | 2.93 ± 0.24 a |
| Skeletal muscle (% of bw) | 4.39 ± 0.2 b | 4.46 ± 0.07 b | 4.79 ± 0.1 a | 4.73 ± 0.2 a | 4.55 ± 0.3 b | 4.91 ± 0.15 a | 4.76 ± 0.14 a |
| Food efficiency (%) | 0.66 ± 0.03 b | 0.74 ± 0.04 a | 0.34 ± 0.05 d | 0.46 ± 0.03 c | 0.38 ± 0.05 d | 0.45 ± 0.04 c | 0.68 ± 0.04 b |
| Water intake (mL/day) | 17.2 ± 1.27 c | 18.6 ± 2 c | 27.2 ± 2.27 a | 29.7 ± 1.34 a | 21.3 ± 1.91 b | 29.4 ± 1.34 a | 28.3 ± 1.88 a |
| Serum aldosterone level (ng/mL) | 1.24 ± 0.18 a | 1.18 ± 0.17 a | 0.88 ± 0.11 c | 0.73 ± 0.09 d | 1.01 ± 0.15 b | 0.76 ± 0.09 cd | 0.79 ± 0.10 d |
| Serum renin (pg/mL) | 29.4 ± 3.24 a | 28.9 ± 3.05 a | 22.9 ± 2.23 c | 19.4 ± 2.27 d | 25.3 ± 2.31 b | 19.8 ± 2.32 d | 19.1 ± 2.43 d |
| Serum Angiotensin II (ng/mL) | 3.14 ± 0.32 a | 3.05 ± 0.21 a | 2.49 ± 0.23 bc | 2.42 ± 0.32 c | 2.75 ± 0.23 b | 2.35 ± 0.21 c | 2.32 ± 0.21 c |
| Angiotensin-converting-enzyme activity (U/mL) | 2.07 ± 0.27 a | 1.98 ± 0.24 a | 1.46 ± 0.18 c | 1.21 ± 0.17 d | 1.67 ± 0.16 b | 1.25 ± 0.16 d | 1.18 ± 0.17 d |
| Control | Positive-C | SS-HB | SS-MB | SS-LB | SS-FM | Normal-C | |
|---|---|---|---|---|---|---|---|
| Serum glucose (mg/dL) | 120 ± 6.53 ab | 125 ± 8.42 a | 117 ± 1.69 b | 94 ± 4.78 c | 116 ± 4.85 b | 120 ± 3.4 ab | 120 ± 3.86 ab |
| Serum insulin (mg/dL) | 0.25 ± 0.03 a | 0.22 ± 0.01 b | 0.15 ± 0.02 b | 0.23 ± 0.02 a | 0.18 ± 0.01 b | 0.2 ± 0.02 ab | 0.16 ± 0.02 b |
| HOMA-IR | 6.61 ± 0.63 a | 6.43 ± 0.52 a | 3.95 ± 0.34 c | 4.95 ± 0.33 b | 4.59 ± 0.28 b | 5.3 ± 0.31 b | 4.35 ± 0.3 c |
| Serum TNF-α (pg/mL) | 0.79 ± 0.08 a | 0.73 ± 0.08 a | 0.68 ± 0.09 b | 0.65 ± 0.08 b | 0.68 ± 0.08 b | 0.73 ± 0.06 a | 0.46 ± 0.05 c |
| Serum IL-1β (pg/mL) | 19.5 ± 1.87 a | 18.6 ± 1.63 a | 15.3 ± 1.71 b | 14.7 ± 1.54 b | 17.5 ± 1.58 a | 17.2 ± 1.62 a | 11.2 ± 1.43 c |
| Hippocampus | |||||||
| Glycogen (mg/g) | 29.7 ± 1.4 b | 30.8 ± 2.1 b | 33.4 ± 2.7 a | 34.2 ± 2.4 a | 33.5 ± 2.5 a | 34.8 ± 2.1 a | 33.7 ± 2.8 a |
| Cholesterol (mg/g) | 85.8 ± 8.3 a | 62.6 ± 8.66 c | 69.1 ± 10.1 bc | 76.7 ± 9.26 b | 71.6 ± 6.16 b | 74.9 ± 2.63 b | 76.1 ± 8.12 b |
| Triglyceride (mg/g) | 68.1 ± 3.85 a | 71.3 ± 3.5 a | 61.7 ± 2.62 b | 72.5 ± 3.62 a | 63.5 ± 4.51 b | 69.1 ± 1.93 a | 61.1 ± 2.87 b |
| AChE activity (U/mg protein) | 0.85 ± 0.10 a | 0.36 ± 0.05 d | 0.59 ± 0.07 b | 0.43 ± 0.06 c | 0.48 ± 0.23 c | 0.67 ± 0.08 b | 0.22 ± 0.04 e |
| Relative mRNA of TNF-α (AU) | 1 a | 0.94 ± 0.08 a | 0.95 ± 0.09 a | 0.62 ± 0.09 c | 0.75 ± 0.08 b | 0.88 ± 0.10 b | 0.68 ± 0.09 c |
| Relative mRNA of IL-1β (AU) | 1 a | 0.78 ± 0.13 b | 0.53 ± 0.15 c | 0.31 ± 0.09 e | 0.64 ± 0.12 c | 0.51 ± 0.09 d | 0.48 ± 0.04 d |
| Relative mRNA of BDNF (AU) | 1 e | 1.53 ± 0.38 c | 1.90 ± 0.39 bc | 1.47 ± 0.27 c | 2.36 ± 0.24 b | 1.78 ± 0.33 c | 2.94 ± 0.16 a |
| Control | Positive-C | SS-HB | SS-MB | SS-LB | SS-FM | Normal-C | |
|---|---|---|---|---|---|---|---|
| map00010 glycolysis/gluconeogenesis (86) | 2.28 ± 0.03 c | 2.31 ± 0.03 bc | 2.86 ± 0.09 a | 2.66 ± 0.03 ab | 3 ± 0.05 a | 2.75 ± 0.07 a | 2.44 ± 0.02 b |
| map04973 carbohydrate digestion and absorption (6) | 0.02 ± 0.002 c | 0.021 ± 0.002 c | 0.044 ± 0.005 a | 0.025 ± 0.002 c | 0.047 ± 0.005 a | 0.033 ± 0.005 b | 0.022 ± 0.001 c |
| map03320 PPAR signaling pathway (25) | 0.143 ± 0.003 c | 0.148 ± 0.006 bc | 0.177 ± 0.004 a | 0.168 ± 0.006 ab | 0.182 ± 0.004 a | 0.172 ± 0.003 a | 0.151 ± 0.002 b |
| map04066 HIF-1 signaling pathway (17) | 0.528 ± 0.007 c | 0.533 ± 0.013 bc | 0.616 ± 0.013 a | 0.616 ± 0.005 a | 0.635 ± 0.006 a | 0.612 ± 0.007 a | 0.577 ± 0.007 b |
| map00480 glutathione metabolism (30) | 0.327 ± 0.004 c | 0.345 ± 0.003 c | 0.471 ± 0.007 a | 0.395 ± 0.006 b | 0.497 ± 0.004 a | 0.417 ± 0.008 ab | 0.346 ± 0.004 c |
| map00540 lipopolysaccharide biosynthesis (53) | 0.191 ± 0.026 a | 0.204 ± 0.025 a | 0.115 ± 0.017 b | 0.083 ± 0.004 c | 0.088 ± 0.006 c | 0.089 ± 0.007 c | 0.109 ± 0.011 b |
| map04657 IL-17 signaling pathway (11) | 0.057 ± 0.002 a | 0.055 ± 0.002 a | 0.032 ± 0.003 c | 0.041 ± 0.002 c | 0.027 ± 0.002 d | 0.038 ± 0.003 c | 0.052 ± 0.001 b |
| map04137 mitophagy–animal (15) | 0.009 ± 0.001 a | 0.009 ± 0.001 a | 0.004 ± 0.001 c | 0.004 ± 0 c | 0.003 ± 0 c | 0.003 ± 0.001 c | 0.006 ± 0 b |
| map00650 butanoate metabolism (96) | 1.01 ± 0.01 c | 1.03 ± 0.01 bc | 1.1 ± 0.02 a | 1.08 ± 0.01 b | 1.13 ± 0.02 a | 1.07 ± 0.02 b | 1.03 ± 0.01 bc |
| map00640 propanoate metabolism (84) | 1.07 ± 0.02 a | 1.11 ± 0.01 a | 1.01 ± 0.02 b | 1.04 ± 0.01 ab | 0.97 ± 0.01 b | 1.03 ± 0.02 ab | 1.06 ± 0.01 b |
| Kanjang Variety | Improved Memory Function | Neuroinflammation by the Hippocampal mRNA Expression | Neuroplasticity (BDNF) | Cholinergic Function (AChE) | Overall Brain Health Effects |
|---|---|---|---|---|---|
| SS-HB | Y-maze, locomotive activity, and passive avoidance | ↓ TNF-α, ↓↓ IL-1β | ↑ BDNF | ↓ AChE | Strong multi-dimensional effect |
| SS-MB | Locomotive activity, novel object recognition, water maze, and passive avoidance | ↓↓ TNF-α, ↓↓↓ IL-1β | ↑ BDNF | ↓↓ AChE | Best behavioral outcome; good mechanistic support |
| SS-LB | Y-maze, water maze, and passive avoidance | ↓↓ TNF-α, ↓↓ IL-1β | ↑↑ BDNF | ↓↓ AChE | Strong anti-inflammatory and plasticity-related effects |
| SS-FM | Passive avoidance | Mild anti-inflammatory | ↑ BDNF | ↓ AChE | Weaker behavioral and molecular effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Y.; Yang, H.-J.; Li, C.; Ryu, M.-S.; Seo, J.-W.; Jeong, D.Y.; Park, S. Beneficial Effects of Traditional Fermented Soybean Sauce (Kanjang) on Memory Function, Body Water, and Glucose Metabolism: Roles of Gut Microbiota and Neuroinflammation. Nutrients 2025, 17, 1617. https://doi.org/10.3390/nu17101617
Yue Y, Yang H-J, Li C, Ryu M-S, Seo J-W, Jeong DY, Park S. Beneficial Effects of Traditional Fermented Soybean Sauce (Kanjang) on Memory Function, Body Water, and Glucose Metabolism: Roles of Gut Microbiota and Neuroinflammation. Nutrients. 2025; 17(10):1617. https://doi.org/10.3390/nu17101617
Chicago/Turabian StyleYue, Yu, Hee-Jong Yang, Chen Li, Myeong-Seon Ryu, Ji-Won Seo, Do Youn Jeong, and Sunmin Park. 2025. "Beneficial Effects of Traditional Fermented Soybean Sauce (Kanjang) on Memory Function, Body Water, and Glucose Metabolism: Roles of Gut Microbiota and Neuroinflammation" Nutrients 17, no. 10: 1617. https://doi.org/10.3390/nu17101617
APA StyleYue, Y., Yang, H.-J., Li, C., Ryu, M.-S., Seo, J.-W., Jeong, D. Y., & Park, S. (2025). Beneficial Effects of Traditional Fermented Soybean Sauce (Kanjang) on Memory Function, Body Water, and Glucose Metabolism: Roles of Gut Microbiota and Neuroinflammation. Nutrients, 17(10), 1617. https://doi.org/10.3390/nu17101617






