When Timing Matters: Effects of Maternal Separation and Post-Weaning High-Fat Diet on Liver Morphology in a Rodent Model
Highlights
- Early maternal separation enhances the harmful effects of a high-fat diet, worsening hepatic steatosis, inflammation, and hepatocellular ballooning in C57BL/6 mice.
- The combination of neonatal psychosocial stress and an obesogenic diet reduces hepatocytes’ numerical density and increases their surface density, disrupting liver tissue organization.
- These early structural liver alterations indicate a higher risk of chronic damage even before fibrosis onset, reflecting adverse metabolic programming.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Maternal Separation
2.3. Post-Weaning Diet
2.4. Euthanasia
2.5. Histological Processing and Staining
2.6. Histopathological Evaluation
2.7. Collagen Fiber Quantification
2.8. Liver Stereology
2.9. Statistical Analysis
3. Results
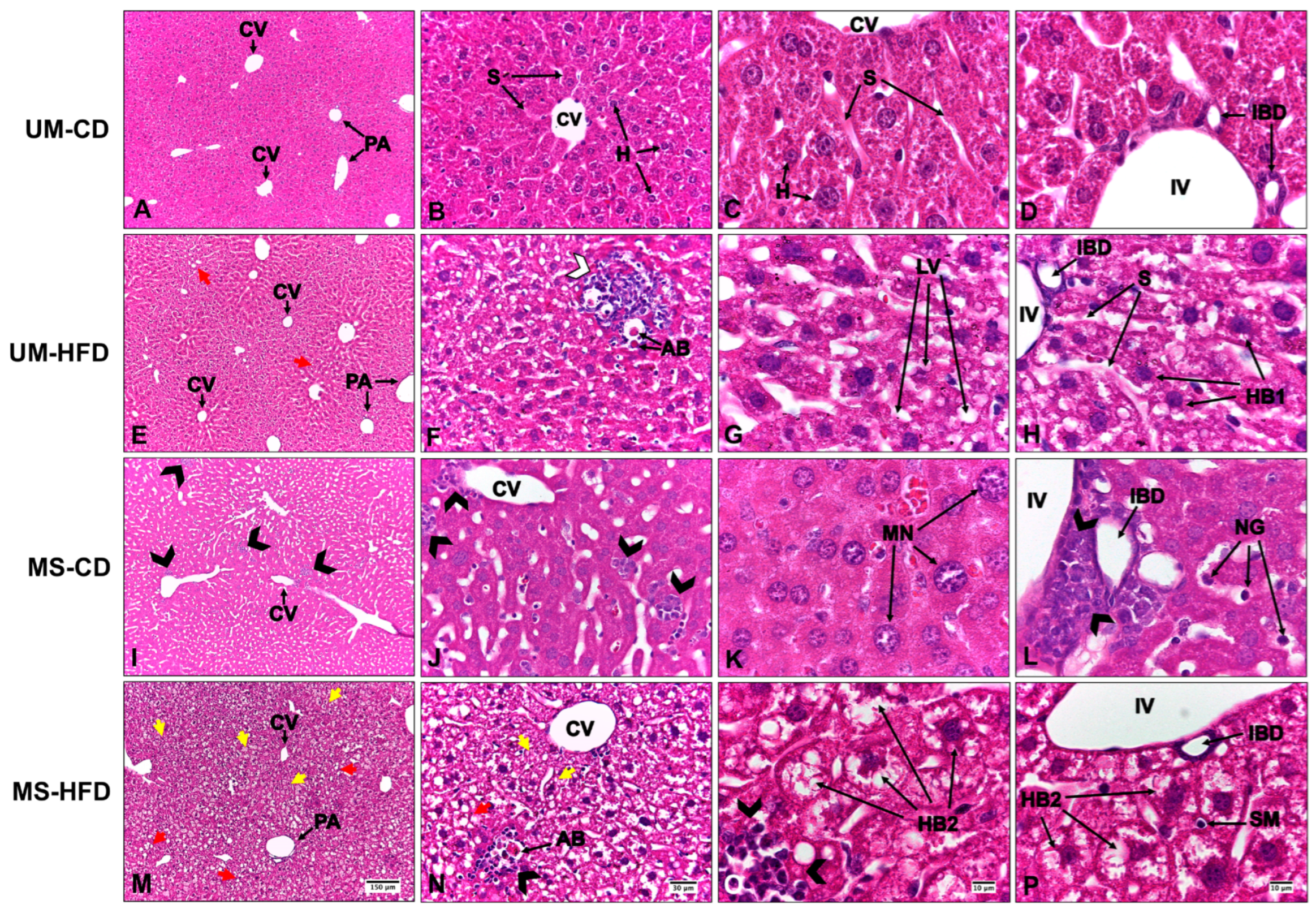
3.1. Maternal Separation and a High-Fat Diet Induce Liver Alterations, Evidenced by Steatosis, Inflammation, and Hepatocellular Ballooning, with Greater Severity Observed in the Group Exposed to Both Conditions
3.2. Maternal Separation and Post-Weaning High-Fat Diet Affect Liver Morphometry and Stereology, Revealing Changes in the Organization and Cellular Composition of Hepatic Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Maternal separation |
| UM | Unmanipulated |
| HFD | High-fat diet |
| CD | Control diet |
| UM-CD | Unmanipulated group with control diet |
| UM-HFD | Unmanipulated group with a high-fat diet |
| MS-CD | Maternal separation group with control diet |
| MS-HFD | Maternal separation group with a high-fat diet |
| Vvhep | Hepatocyte nuclear volume density |
| Svhep | Hepatocyte Nuclear Surface Density |
| Nvhep | Hepatocyte Nuclear Number Density |
References
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Int. J. Epidemiol. 2013, 42, 1215–1222. [Google Scholar] [CrossRef]
- Maniam, J.; Antoniadis, C.; Morris, M.J. Early-life stress, HPA axis adaptation, and mechanisms contributing to later health outcomes. Front. Endocrinol. 2014, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Nishi, M.; Horii-Hayashi, N.; Sasagawa, T. Effects of early life adverse experiences on the brain: Implications from maternal separation models in rodents. Front. Neurosci. 2014, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Tractenberg, S.G.; Levandowski, M.L.; de Azeredo, L.A.; Orso, R.; Roithmann, L.G.; Hoffmann, E.S.; Brenhouse, H.; Grassi-Oliveira, R. An overview of maternal separation effects on behavioural outcomes in mice: Evidence from a four-stage methodological systematic review. Neurosci. Biobehav. Rev. 2016, 68, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.O.; Herald, J.B.; Leachman, J.; Villasante Tezanos, A.; Cohn, D.M.; Loria, A.S. A model of neglect during postnatal life heightens obesity-induced hypertension and is linked to a greater metabolic compromise in female mice. Int. J. Obes. 2018, 42, 1354–1365. [Google Scholar] [CrossRef]
- Leachman, J.R.; Cincinelli, C.; Ahmed, N.; Dalmasso, C.; Xu, M.; Gatineau, E.; Nikolajczyk, B.S.; Yiannikouris, F.; Hinds, T.D., Jr.; Loria, A.S. Early life stress exacerbates obesity in adult female mice via mineralocorticoid receptor-dependent increases in adipocyte triglyceride and glycerol content. Life Sci. 2022, 304, 120718. [Google Scholar] [CrossRef]
- Zhao, J.; Ye, L.; Liu, Z.; Wu, J.; Deng, D.; An, L.; Bai, S.; Yang, L.; Liu, B.; Shi, Y.; et al. The effects of early-life stress on liver transcriptomics and the protective role of EPA in a mouse model of early-life-stress-induced adolescent depression. Int. J. Mol. Sci. 2023, 24, 13131. [Google Scholar] [CrossRef]
- Dalmasso, C.; Ahmed, N.S.; Ghuneim, S.; Cincinelli, C.; Leachman, J.R.; Giani, J.F.; Cassis, L.; Loria, A.S. Obese male mice exposed to early life stress display sympathetic activation and hypertension independent of circulating angiotensin II. J. Am. Heart Assoc. 2024, 13, e029511. [Google Scholar] [CrossRef]
- Laitinen, T.T.; Vahtera, J.; Pahkala, K.; Magnussen, C.G.; Nuotio, J.; Hutri-Kähönen, N.; Kivimäki, M.; Lehtimäki, T.; Jokinen, E.; Laitinen, T.; et al. Childhood socioeconomic disadvantage and risk of fatty liver in adulthood: The cardiovascular risk in young finns study. Hepatology 2020, 71, 67–75. [Google Scholar] [CrossRef]
- Eller, O.C.; Morris, E.M.; Thyfault, J.P.; Christianson, J.A. Early life stress reduces voluntary exercise and its prevention of diet-induced obesity and metabolic dysfunction in mice. Physiol. Behav. 2020, 223, 113000. [Google Scholar] [CrossRef]
- Fu, Q.; North, P.E.; Ke, X.; Huang, Y.W.; Fritz, K.A.; Majnik, A.V.; Lane, R.H. Adverse maternal environment and postweaning western diet alter hepatic CD36 expression and methylation concurrently with nonalcoholic fatty liver disease in mouse offspring. J. Nutr. 2021, 151, 3102–3112. [Google Scholar] [CrossRef]
- Fu, Q.; Frick, J.M.; O’Neil, M.F.; Eller, O.C.; Morris, E.M.; Thyfault, J.P.; Christianson, J.A.; Lane, R.H. Early-life stress perturbs the epigenetics of Cd36 concurrent with adult onset of NAFLD in mice. Pediatr. Res. 2023, 94, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Aljahdali, B.A.; Bajaber, A.S.; Al-Nouri, D.M.; Al-Khalifah, A.S.; Arzoo, S.; Alasmari, A.A. The development of nonalcoholic fatty liver disease and metabolic syndromes in diet-induced rodent models. Life 2023, 13, 1336. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, D.; Łukomska, A.; Dec, K.; Skonieczna-Żydecka, K.; Gutowska, I.; Skórka-Majewicz, M.; Styburski, D.; Misiakiewicz-Has, K.; Pilutin, A.; Palma, J.; et al. Diet-induced rat model of gradual development of non-alcoholic fatty liver disease (NAFLD) with lipopolysaccharides (LPS) secretion. Diagnostics 2019, 9, 205. [Google Scholar] [CrossRef]
- Boicean, A.; Ichim, C.; Sasu, S.-M.; Todor, S.B. Key Insights into Gut Alterations in Metabolic Syndrome. J. Clin. Med. 2025, 14, 2678. [Google Scholar] [CrossRef]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Frick, J.M.; Eller, O.C.; Foright, R.M.; Levasseur, B.M.; Yang, X.; Wang, R.; Winter, M.K.; O’Neil, M.F.; Morris, E.M.; Thyfault, J.P.; et al. High-fat/high-sucrose diet worsens metabolic outcomes and widespread hypersensitivity following early-life stress exposure in female mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 324, R353–R367. [Google Scholar] [CrossRef]
- Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Wolterink-Donselaar, I.G.; Meerding, J.M.; Fernandes, C. A method for gender determination in newborn dark pigmented mice. Lab. Anim. 2009, 38, 35–38. [Google Scholar] [CrossRef]
- George, E.D.; Bordner, K.A.; Elwafi, H.M.; Simen, A.A. Maternal separation with early weaning: A novel mouse model of early life neglect. BMC Neurosci. 2010, 11, 123. [Google Scholar] [CrossRef]
- Morton, D.B.; Griffiths, P.H. Guidelines on the recognition of pain, distress and discomfort in experimental animals and a hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef]
- Aguila, M.B.; Ornellas, F.; Mandarim-de-Lacerda, C. A Nutritional research and fetal programming: Parental nutrition influences the structure and function of the organs. Int. J. Morphol. 2021, 39, 327–334. [Google Scholar] [CrossRef]
- Stephanie, L.R.; Emis, A.; Meir, S.; Alison, S.F. Effects of early deprivation and maternal separation on pup-directed behavior and HPA axis measures in the juvenile female rat. Dev. Psychobiol. 2008, 50, 315–321. [Google Scholar]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Universities Federation for Animal Welfare: Wheathampstead, UK, 1959; reprinted in 1992. [Google Scholar]
- Cruz-Orive, L.M.; Weibel, E.R. Recent stereological methods for cell biology: A brief survey. Am. J. Physiol. 1990, 258, L148–L156. [Google Scholar] [CrossRef]
- Canadian Council on Animal Care. Guide to the Care and Use of Experimental Animals, 2nd ed.; Canadian Council on Animal Care: Ottawa, ON, Canada, 1993; Volume 1, Available online: https://ccac.ca/Documents/Standards/Guidelines/Guide_to_the_Care_and_Use_of_Experimental_Animals_Vol1.pdf (accessed on 27 March 2025).
- Scherle, W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie 1970, 26, 57–60. [Google Scholar]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Mandarim-de-Lacerda, C.A.; del Sol, M. Tips for studies with quantitative morphology (Morphometry and Stereology). Int. J. Morphol. 2017, 35, 1482–1494. [Google Scholar] [CrossRef]
- Sagae, S.C.; Zanardini, B.; Ribeiro-Paz, E.D.; Amaral, A.C.; Bronczek, G.A.; Lubaczeuski, C.; Grassiolli, S.; Koehler-Santos, P.; de Oliveira, J.R.; Donadio, M.V.F.; et al. Metabolic dysfunction in a rat model of early-life scarcity-adversity: Modulatory role of cafeteria diet. Exp. Physiol. 2018, 103, 1481–1493. [Google Scholar] [CrossRef]
- Dong, M.; Dube, S.R.; Felitti, V.J.; Giles, W.H.; Anda, R.F. Adverse childhood experiences and self-reported liver disease: New insights into the causal pathway. Arch Intern. Med. 2003, 163, 1949–1956. [Google Scholar] [CrossRef]
- Molinero, K.C.; Parroquia, A.; Niedzwiecki, D.; Troester, M.A.; Schildkraut, J.M.; Muir, A.J.; Hoyo, C.; Moylan, C.A. S1440 Higher household dysfunction in adverse childhood experiences score & low household income associate with increased risk of cirrhosis in adulthood. Am. J. Gastroenterol. 2023, 118, S1097–S1098. [Google Scholar]
- Sadana, P.; Lin, L.; Aghayev, M.; Ilchenko, S.; Kasumov, T. Early pro-inflammatory remodeling of HDL proteome in a model of diet-induced obesity: 2H2O-metabolic labeling-based kinetic approach. Int. J. Mol. Sci. 2020, 21, 7472. [Google Scholar] [CrossRef]
- Paternain, L.; Martisova, E.; Milagro, F.I.; Ramírez, M.J.; Martínez, J.A.; Campión, J. Postnatal maternal separation modifies the response to an obesogenic diet in adulthood in rats. Dis. Model. Mech. 2012, 5, 691–697. [Google Scholar]
- Tanami, S.; Ben-Moshe, S.; Elkayam, A.; Mayo, A.; Bahar Halpern, K.; Itzkovitz, S. Dynamic zonation of liver polyploidy. Cell Tissue Res. 2017, 368, 405–410. [Google Scholar] [CrossRef]
- Kotulkar, M.; Robarts, D.R.; Apte, U. HNF4α in Hepatocyte Health and Disease. Semin. Liver Dis. 2023, 43, 234–244. [Google Scholar] [CrossRef]
- Li, S.; Hong, M.; Tan, H.Y.; Wang, N.; Feng, Y. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid. Med. Cell Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef]
- Popa, M.L.; Ichim, C.; Anderco, P.; Todor, S.B.; Pop-Lodromanean, D. MicroRNAs in the Diagnosis of Digestive Diseases: A Comprehensive Review. J. Clin. Med. 2025, 14, 2054. [Google Scholar] [CrossRef]
- Nijjar, S.S.; Wallace, L.; Crosby, H.A.; Hubscher, S.G.; Strain, A.J. Altered Notch ligand expression in human liver disease: Further evidence for a role of the Notch signaling pathway in hepatic neovascularization and biliary ductular defects. Am. J. Pathol. 2002, 160, 1695–1703. [Google Scholar] [CrossRef]
- Yang, Y.M.; Noureddin, M.; Liu, C.; Ohashi, K.; Kim, S.Y.; Ramnath, D.; Powell, E.E.; Sweet, M.J.; Roh, Y.S.; Hsin, I.F.; et al. Hyaluronan synthase 2-mediated hyaluronan production mediates Notch1 activation and liver fibrosis. Sci. Transl. Med. 2019, 11, eaat9284. [Google Scholar] [CrossRef]
- Benhamouche, S.; Decaens, T.; Godard, C.; Chambrey, R.; Rickman, D.S.; Moinard, C.; Vasseur-Cognet, M.; Kuo, C.J.; Kahn, A.; Perret, C.; et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev. Cell 2006, 10, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.O.; Monga, S.P. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annu. Rev. Pathol. 2018, 13, 351–378. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | CD | HFD |
|---|---|---|
| Casein (>85% protein) | 200.0 | 230.0 |
| L-cystine (g/kg) | 3.0 | 3.0 |
| Cornstarch (g/kg) | 529.486 | 299.472 |
| Sucrosa (g/kg) | 100.0 | 100.0 |
| Soybean oil (g/kg) | 70.0 | 70.0 |
| Lard (g/kg) | - | 200.0 |
| Fiber (g/kg) | 50.0 | 50.0 |
| Vitamin mixture (g/kg) 1 | 10.0 | 10.0 |
| Mineral mixture (g/kg) | 35.0 | 35.0 |
| Choline bitartrate (g/kg) | 2.5 | 2.5 |
| Antioxidant (g/kg) | 0.014 | 0.028 |
| Total (g) | 1000.0 | 1000.0 |
| Energy (kcal/g) | 3.95 | 4.95 |
| Carbohydrate (% Energy) | 64.0 | 32.0 |
| Protein (% Energy) | 19.0 | 19.0 |
| Lipid (% Energy) | 17.0 | 49.0 |
| Media ± SD | |||||
|---|---|---|---|---|---|
| Collagen | UM-CD | UM-HFD | MS-CD | MS-HFD | p-Value |
| Type I (µm2) | 2.16 ± 0.62 | 2.37 ± 0.63 | 2.75 ± 0.66 | 2.62 ± 0.58 | 0.079 |
| Type III (µm2) | 1.35 ± 0.22 | 1.32 ± 0.18 | 1.18 ± 0.31 | 1.24 ± 0.33 | 0.180 |
| Median ± SD | |||||
|---|---|---|---|---|---|
| Variables | UM-CD | UM-HFD | MS-CD | MS-HFD | p-Value |
| Body mass (g) | 25.28 ± 2.09 | 30.76 ± 1.71 a | 24.39 ± 5.79 b | 30.10 ± 1.55 a,c | 0.013 |
| Liver mass (g) | 1.56 ± 0.21 | 1.31 ± 0.07 | 1.42 ± 0.27 | 1.81 ± 0.36 b | 0.035 |
| Liver volume (mL) | 1.46 ± 0.28 | 1.28 ± 0.10 | 1.36 ± 0.29 | 1.74 ± 0.35 | 0.081 |
| Media ± SD | |||||
|---|---|---|---|---|---|
| Variables | UM-CD | UM-HFD | MS-CD | MS-HFD | p-Value |
| Vvhep (%) | 7.57 ± 1.89 | 7.06 ± 2.84 | 7.88 ± 3.97 | 7.93 ± 4.08 | 0.184 |
| Svhep (mm−1) | 29.19 ± 11.98 | 32.87 ± 14.89 | 41.94 ± 18.97 a,b | 40.15 ± 15.95 a,b | <0.001 |
| Nvhep (mm−3) | 15,217.52 ± 5495.84 | 12,447.95 ± 6305.92 a | 12,323.15 ± 5326.38 a | 7836.26 ± 4732.54 a,b,c | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Sol, M.; Navarrete, J.; García-Orozco, L.; Duque-Colorado, J.; Sócola-Barsallo, Z.; Sandoval, C.; Vásquez, B. When Timing Matters: Effects of Maternal Separation and Post-Weaning High-Fat Diet on Liver Morphology in a Rodent Model. Nutrients 2025, 17, 1619. https://doi.org/10.3390/nu17101619
del Sol M, Navarrete J, García-Orozco L, Duque-Colorado J, Sócola-Barsallo Z, Sandoval C, Vásquez B. When Timing Matters: Effects of Maternal Separation and Post-Weaning High-Fat Diet on Liver Morphology in a Rodent Model. Nutrients. 2025; 17(10):1619. https://doi.org/10.3390/nu17101619
Chicago/Turabian Styledel Sol, Mariano, Javiera Navarrete, Laura García-Orozco, Jhonatan Duque-Colorado, Zaida Sócola-Barsallo, Cristian Sandoval, and Bélgica Vásquez. 2025. "When Timing Matters: Effects of Maternal Separation and Post-Weaning High-Fat Diet on Liver Morphology in a Rodent Model" Nutrients 17, no. 10: 1619. https://doi.org/10.3390/nu17101619
APA Styledel Sol, M., Navarrete, J., García-Orozco, L., Duque-Colorado, J., Sócola-Barsallo, Z., Sandoval, C., & Vásquez, B. (2025). When Timing Matters: Effects of Maternal Separation and Post-Weaning High-Fat Diet on Liver Morphology in a Rodent Model. Nutrients, 17(10), 1619. https://doi.org/10.3390/nu17101619







