Accelerated Biological Aging, Genetic Susceptibility, and Non-Alcoholic Fatty Liver Disease: Two Prospective Cohort Studies
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Outcomes
2.3. Assessment of Biological Aging
2.4. Covariates
2.5. PRS Calculation of NAFLD
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
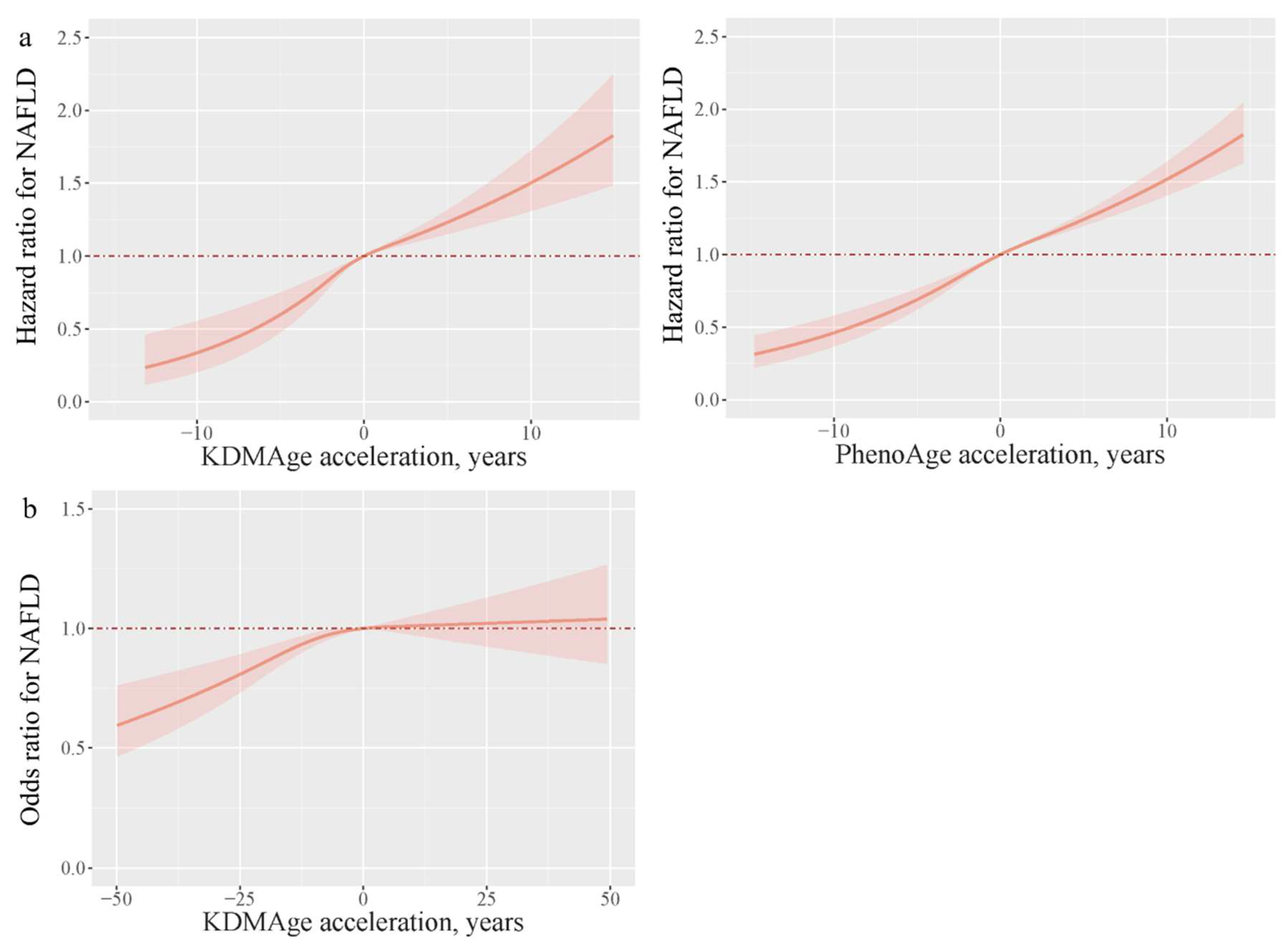
3.2. Association of Biological Aging with NAFLD
3.3. Risk of Incident NAFLD According to PRS and Biological Aging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Clin. Liver Dis. 2018, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Tas, E.; Yakar, S.; Muzumdar, R. Hepatic lipid metabolism and non-alcoholic fatty liver disease in aging. Mol. Cell. Endocrinol. 2017, 455, 115–130. [Google Scholar] [CrossRef]
- Heinze, I.; Bens, M.; Calzia, E.; Holtze, S.; Dakhovnik, O.; Sahm, A.; Kirkpatrick, J.M.; Szafranski, K.; Romanov, N.; Sama, S.N.; et al. Species comparison of liver proteomes reveals links to naked mole-rat longevity and human aging. BMC Biol. 2018, 16, 82. [Google Scholar] [CrossRef]
- Koehler, E.M.; Schouten, J.N.; Hansen, B.E.; van Rooij, F.J.; Hofman, A.; Stricker, B.H.; Janssen, H.L. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: Results from the Rotterdam study. J. Hepatol. 2012, 57, 1305–1311. [Google Scholar] [CrossRef]
- Frith, J.; Day, C.P.; Henderson, E.; Burt, A.D.; Newton, J.L. Non-alcoholic fatty liver disease in older people. Gerontology 2009, 55, 607–613. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Jones, M.A.; Fromal, O.; Powell, F.L.; Khurana, S.; Singh, N.; Martin, P.M. Loss of GPR109A/HCAR2 induces aging-associated hepatic steatosis. Aging 2019, 11, 386–400. [Google Scholar] [CrossRef]
- Huang, Y.L.; Shen, Z.Q.; Huang, C.H.; Lin, C.H.; Tsai, T.F. Cisd2 slows down liver aging and attenuates age-related metabolic dysfunction in male mice. Aging Cell 2021, 20, e13523. [Google Scholar] [CrossRef]
- Lowsky, D.J.; Olshansky, S.J.; Bhattacharya, J.; Goldman, D.P. Heterogeneity in healthy aging. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 640–649. [Google Scholar] [CrossRef]
- Cheng, K.; Cai, N.; Zhu, J.; Yang, X.; Liang, H.; Zhang, W. Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun. 2022, 42, 1112–1140. [Google Scholar] [CrossRef]
- Xia, M.; Li, W.; Lin, H.; Zeng, H.; Ma, S.; Wu, Q.; Ma, H.; Li, X.; Pan, B.; Gao, J.; et al. DNA methylation age acceleration contributes to the development and prediction of non-alcoholic fatty liver disease. Geroscience 2024, 46, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Suo, C.; Zhao, R.; Yuan, H.; Jin, L.; Zhang, T.; Chen, X. Genetic predisposition, lifestyle risk, and obesity associate with the progression of nonalcoholic fatty liver disease. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2021, 53, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Ghodsian, N.; Abner, E.; Emdin, C.A.; Gobeil, É.; Taba, N.; Haas, M.E.; Perrot, N.; Manikpurage, H.D.; Gagnon, É.; Bourgault, J.; et al. Electronic health record-based genome-wide meta-analysis provides insights on the genetic architecture of non-alcoholic fatty liver disease. Cell Rep. Med. 2021, 2, 100437. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Darlay, R.; Cockell, S.; Meroni, M.; Govaere, O.; Tiniakos, D.; Burt, A.D.; Bedossa, P.; Palmer, J.; Liu, Y.L.; et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J. Hepatol. 2020, 73, 505–515. [Google Scholar] [CrossRef]
- Vujkovic, M.; Ramdas, S.; Lorenz, K.M.; Guo, X.; Darlay, R.; Cordell, H.J.; He, J.; Gindin, Y.; Chung, C.; Myers, R.P.; et al. A multiancestry genome-wide association study of unexplained chronic ALT elevation as a proxy for nonalcoholic fatty liver disease with histological and radiological validation. Nat. Genet. 2022, 54, 761–771. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Yao, P.; Li, X.; He, M.; Liu, Y.; Yuan, J.; Chen, W.; Zhou, L.; Min, X.; et al. Cohort Profile: The Dongfeng-Tongji cohort study of retired workers. Int. J. Epidemiol. 2013, 42, 731–740. [Google Scholar] [CrossRef]
- Farrell, G.C.; Chitturi, S.; Lau, G.K.; Sollano, J.D. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: Executive summary. J. Gastroenterol. Hepatol. 2007, 22, 775–777. [Google Scholar] [CrossRef]
- Klemera, P.; Doubal, S. A new approach to the concept and computation of biological age. Mech. Ageing Dev. 2006, 127, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E. Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age? J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 667–674. [Google Scholar] [CrossRef]
- Liu, Z.; Kuo, P.L.; Horvath, S.; Crimmins, E.; Ferrucci, L.; Levine, M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study. PLoS Med. 2018, 15, e1002718. [Google Scholar] [CrossRef]
- Wang, C.; Hong, S.; Guan, X.; Xiao, Y.; Fu, M.; Meng, H.; Feng, Y.; Zhou, Y.; Cao, Q.; Yuan, F.; et al. Associations between multiple metals exposure and biological aging: Evidence from the Dongfeng-Tongji cohort. Sci. Total Environ. 2023, 861, 160596. [Google Scholar] [CrossRef] [PubMed]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Hyppönen, E.; Kuzma, E.; Llewellyn, D.J. Association of Lifestyle and Genetic Risk With Incidence of Dementia. JAMA 2019, 322, 430–437. [Google Scholar] [CrossRef]
- Gao, X.; Geng, T.; Jiang, M.; Huang, N.; Zheng, Y.; Belsky, D.W.; Huang, T. Accelerated biological aging and risk of depression and anxiety: Evidence from 424,299 UK Biobank participants. Nat. Commun. 2023, 14, 2277. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Cao, X.; Li, X.; Zhang, J.; Ma, C.; Zhang, N.; Lu, Q.; Crimmins, E.M.; Gill, T.M.; Chen, X.; et al. Association of Unhealthy Lifestyle and Childhood Adversity With Acceleration of Aging Among UK Biobank Participants. JAMA Netw. Open 2022, 5, e2230690. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, M.L.; Huang, J.J.; Xia, S.S.; Yang, Y.; Dong, K. Association of leukocyte telomere length with non-alcoholic fatty liver disease in patients with type 2 diabetes. Chin. Med. J. 2019, 132, 2927–2933. [Google Scholar] [CrossRef]
- Tang, L.; Li, D.; Ma, Y.; Cui, F.; Wang, J.; Tian, Y. The association between telomere length and non-alcoholic fatty liver disease: A prospective study. BMC Med. 2023, 21, 427. [Google Scholar] [CrossRef]
- Luo, S.; Wong, I.C.K.; Chui, C.S.L.; Zheng, J.; Huang, Y.; Schooling, C.M.; Yeung, S.L.A. Effects of putative metformin targets on phenotypic age and leukocyte telomere length: A mendelian randomisation study using data from the UK Biobank. Lancet Healthy Longev. 2023, 4, e337–e344. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Liu, F.; Chen, H.; Tan, T.; Yao, P.; Tang, Y. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. 2022, 20, 207. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Z.; Fan, H.; Guo, C.; Zhang, X.; Li, Y.; Han, X.; Zhang, T. Association between biological aging and the risk of mortality in individuals with non-alcoholic fatty liver disease: A prospective cohort study. Arch. Gerontol. Geriatr. 2024, 124, 105477. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 2017, 8, 15691. [Google Scholar] [CrossRef] [PubMed]
- Meijnikman, A.S.; Herrema, H.; Scheithauer, T.P.M.; Kroon, J.; Nieuwdorp, M.; Groen, A.K. Evaluating causality of cellular senescence in non-alcoholic fatty liver disease. JHEP Rep. 2021, 3, 100301. [Google Scholar] [CrossRef]
- Sanguino, E.; Bejarano, R.; Alegret, M.; Sánchez, R.M.; Vázquez-Carrera, M.; Laguna, J.C. Sexual dimorphism in lipid metabolic phenotype associated with old age in Sprague-Dawley rats. Exp. Gerontol. 2004, 39, 1295–1306. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a Tool to Target Aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Simons, P.; Wesselius, A.; Stehouwer, C.D.A.; Brouwers, M. Relationship between NAFLD and coronary artery disease: A Mendelian randomization study. Hepatology 2023, 77, 230–238. [Google Scholar] [CrossRef]
- Sveinbjornsson, G.; Ulfarsson, M.O.; Thorolfsdottir, R.B.; Jonsson, B.A.; Einarsson, E.; Gunnlaugsson, G.; Rognvaldsson, S.; Arnar, D.O.; Baldvinsson, M.; Bjarnason, R.G.; et al. Multiomics study of nonalcoholic fatty liver disease. Nat. Genet. 2022, 54, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Speliotes, E.K.; Yerges-Armstrong, L.M.; Wu, J.; Hernaez, R.; Kim, L.J.; Palmer, C.D.; Gudnason, V.; Eiriksdottir, G.; Garcia, M.E.; Launer, L.J.; et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011, 7, e1001324. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, X.; Kuppa, A.; Feitosa, M.F.; Bielak, L.F.; O’Connell, J.R.; Musani, S.K.; Guo, X.; Kahali, B.; Chen, V.L.; et al. Genome-wide association meta-analysis identifies 17 loci associated with nonalcoholic fatty liver disease. Nat. Genet. 2023, 55, 1640–1650. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Borén, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230.e6. [Google Scholar] [CrossRef]

| UK Biobank | Dongfeng–Tongji Cohort | |||||
|---|---|---|---|---|---|---|
| Characteristics | Total (N = 329,040) | Non-NAFLD (N = 325,266) | NAFLD (N = 3774) | Total (N = 6783) | Non-NAFLD (N = 4985) | NAFLD (N = 1798) |
| Age, years | 56.4 ± 8.1 | 56.4 ± 8.1 | 56.8 ± 8.0 | 62.0 ± 7.4 | 62.5 ± 7.4 | 60.6 ± 7.3 |
| Sex (%) | ||||||
| Female | 177,498 (53.9) | 175,556 (54.0) | 1942 (51.5) | 3969 (58.5) | 2710 (54.4) | 1259 (70.1) |
| Male | 151,542 (46.1) | 149,710 (46.0) | 1832 (48.5) | 2810 (41.5) | 2272 (45.6) | 538 (29.9) |
| Race/ethnicity (%) | ||||||
| Non-White | 16,619 (5.1) | 16,372 (5.1) | 247 (6.6) | - | - | - |
| White | 310,927 (94.6) | 307,420 (94.6) | 3507 (93.0) | - | - | - |
| BMI (%) | ||||||
| <25 or 24 | 109,867 (33.5) | 109,504 (33.7) | 363 (9.7) | 4156 (61.3) | 3330 (66.8) | 826 (46.0) |
| 25–30 or 24–28 | 140,526 (42.8) | 139,137 (42.9) | 1389 (37.0) | 2274 (33.5) | 1463 (29.4) | 811 (45.1) |
| ≥30 or 28 | 77,959 (23.7) | 75,955 (23.4) | 2004 (53.3) | 351 (5.2) | 191 (3.8) | 160 (8.9) |
| Smoking status (%) | ||||||
| Never | 180,864 (55.0) | 179,172 (55.1) | 1692(44.9) | 5056 (74.9) | 3598 (72.5) | 1458(81.4) |
| Ever | 113,278 (34.5) | 111,773 (34.4) | 1505 (40.0) | 686 (10.2) | 550 (11.1) | 136 (7.6) |
| Current | 33,351 (10.0) | 32,797 (10.1) | 554 (14.7) | 1012 (15.0) | 815 (16.4) | 197 (11.0) |
| Alcohol intake (%) | ||||||
| Never | 14,071 (4.3) | 13,842 (4.2) | 229 (6.1) | 5621 (77.6) | 3813 (76.5) | 1448 (80.5) |
| Ever | 11,050 (3.4) | 10,796 (3.3) | 254 (6.8) | 358 (5.3) | 296 (6.0) | 62 (3.5) |
| Current | 303,190 (92.2) | 299,917 (92.3) | 3273 (86.8) | 1162 (17.1) | 874 (17.5) | 288(16.0) |
| Education level (%) | ||||||
| Less than high school | 89,372 (33.0) | 88,261 (32.9) | 1111 (39.8) | 4416 (65.1) | 3239 (65.5) | 1177 (65.0) |
| High school or above | 181,870 (67.0) | 180,191 (67.1) | 1679 (60.2) | 2367 (34.9) | 1746 (34.5) | 621 (35.0) |
| Metabolic equivalent task, min/week | 1785.0 (813.0, 3573.0) | 1786.0 (815.0, 3573.0) | 1480.0 (611.0, 3230.0) | 1674.0 (1080.0, 2700.0) | 1680.0 (1050.0, 2744.0) | 1674.0 (1080.0, 2520.0) |
| KDMAge, years | 61.0 (54.0, 67.0) | 61.0 (54.0, 67.0) | 63.0 (56.0, 68.0) | 53.0 (38.0, 71.0) | 53.0 (37.0, 71.0) | 54.0 (39.0, 71.0) |
| PhenoAge, years | 46.0 (38.0, 53.0) | 46.0 (38.0, 53.0) | 49.0 (42.0, 56.0) | - | - | - |
| KDMAge, non-aging (%) | 223,635 (68.0) | 221,375 (68.1) | 2260 (31.9) | 4380 (64.0) | 3267 (64.8) | 1112 (61.7) |
| KDMAge, accelerated aging (%) | 105,405 (32.0) | 103,891 (59.9) | 1514 (40.1) | 2403 (36.0) | 1717 (35.2) | 686 (38.3) |
| PhenoAge, non-aging (%) | 179,531 (54.6) | 178,257 (54.8) | 1274 (33.8) | - | - | - |
| PhenoAge, accelerated aging (%) | 149,509 (45.4) | 147,009 (45.2) | 2500 (66.2) | - | - | - |
| UK Biobank | Dongfeng–Tongji Cohort | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Case/total | 3774/325,266 | 1798/4985 | ||||
| KDMAge acceleration | PhenoAge acceleration | KDMAge acceleration | ||||
| Continuous | 1.06 (1.04, 1.07) | <0.001 | 1.06 (1.05, 1.06) | <0.001 | 1.01 (1.00, 1.01) | 0.001 |
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Q2 | 1.19 (1.04, 1.37) | 0.015 | 1.25 (1.07, 1.45) | 0.005 | 1.34 (1.13, 1.60) | 0.001 |
| Q3 | 1.34 (1.16, 1.55) | <0.001 | 1.44 (1.24, 1.67) | <0.001 | 1.41 (1.18, 1,69) | <0.001 |
| Q4 | 1.76 (1.49, 2.09) | <0.001 | 2.38 (2.06, 2.74) | <0.001 | 1.50 (1.24, 1.83) | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | |||
| Non-aging | 1 (Reference) | 1 (Reference) | 1 (Reference) | 0.019 | ||
| Accelerated aging | 1.35 (1.17, 1.56) | <0.001 | 1.69 (1.54, 1.85) | <0.001 | 1.18 (1.03, 1.36) | |
| Low Genetic Risk | Medium Genetic Risk | High Genetic Risk | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Case/total | 404/50,796 | 1057/101,697 | 623/50,857 | |||
| KDMAge acceleration | ||||||
| Continuous | 1.05 (1.01, 1.10) | 0.008 | 1.09 (1.07, 1.11) | <0.001 | 1.05 (1.02, 1.09) | 0.001 |
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Q2 | 1.06 (0.67, 1.65) | 0.816 | 1.02 (0.78, 1.33) | 0.906 | 1.52 (1.08, 2.14) | 0.016 |
| Q3 | 1.51 (0.96, 2.37) | 0.076 | 1.34 (1.02, 1.77) | 0.036 | 1.51 (1.04, 2.19) | 0.030 |
| Q4 | 1.91 (1.13, 3.25) | 0.017 | 2.55 (1.83, 3.56) | <0.001 | 2.01 (1.30, 3.12) | 0.002 |
| p for trend | 0.011 | <0.001 | 0.003 | |||
| Non-aging | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Accelerated aging | 1.36 (0.86, 2.16) | 0.193 | 1.91 (1.42, 2.57) | <0.001 | 1.53 (1.03, 2.26) | 0.034 |
| PhenoAge acceleration | ||||||
| Continuous | 1.06 (1.04, 1.08) | <0.001 | 1.06 (1.05, 1.07) | <0.001 | 1.07 (1.05, 1.08) | <0.001 |
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Q2 | 1.94 (1.14, 3.33) | 0.015 | 1.27 (0.94, 1.71) | 0.118 | 1.47 (0.98, 2.22) | 0.064 |
| Q3 | 1.93 (1.13, 3.28) | 0.016 | 1.75 (1.32, 2.32) | <0.001 | 1.76 (1.18, 2.61) | 0.005 |
| Q4 | 3.60 (2.17, 5.97) | <0.001 | 2.75 (2.10, 3.61) | <0.001 | 3.26 (2.24, 4.73) | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | |||
| Non-aging | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Accelerated aging | 1.69 (1.27, 2.26) | <0.001 | 1.96 (1.65, 2.32) | <0.001 | 2.08 (1.65, 2.62) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, Y.; Chen, L.; Chen, H.; Tang, Y.; He, Y.; Yao, P. Accelerated Biological Aging, Genetic Susceptibility, and Non-Alcoholic Fatty Liver Disease: Two Prospective Cohort Studies. Nutrients 2025, 17, 1618. https://doi.org/10.3390/nu17101618
Zhao Y, Wang Y, Chen L, Chen H, Tang Y, He Y, Yao P. Accelerated Biological Aging, Genetic Susceptibility, and Non-Alcoholic Fatty Liver Disease: Two Prospective Cohort Studies. Nutrients. 2025; 17(10):1618. https://doi.org/10.3390/nu17101618
Chicago/Turabian StyleZhao, Ying, Yu Wang, Li Chen, Huimin Chen, Yuhan Tang, Yuefeng He, and Ping Yao. 2025. "Accelerated Biological Aging, Genetic Susceptibility, and Non-Alcoholic Fatty Liver Disease: Two Prospective Cohort Studies" Nutrients 17, no. 10: 1618. https://doi.org/10.3390/nu17101618
APA StyleZhao, Y., Wang, Y., Chen, L., Chen, H., Tang, Y., He, Y., & Yao, P. (2025). Accelerated Biological Aging, Genetic Susceptibility, and Non-Alcoholic Fatty Liver Disease: Two Prospective Cohort Studies. Nutrients, 17(10), 1618. https://doi.org/10.3390/nu17101618







