Nutrition and Neuroinflammation: Are Middle-Aged Women in the Red Zone?
Abstract
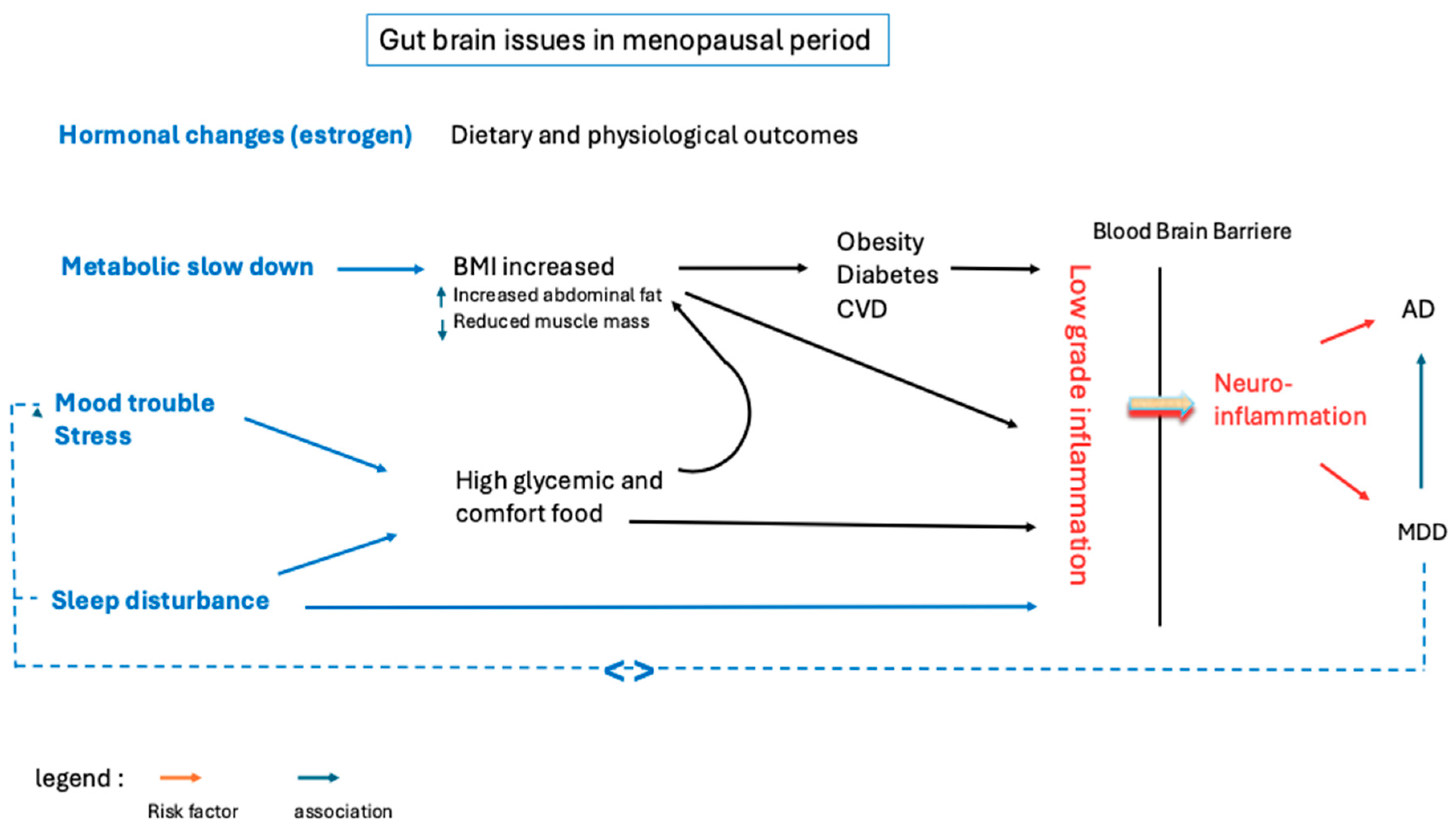
1. Introduction
2. Consequences of Hormonal Changes on Mood and Sleep Disorders, Metabolism, and Nutrition
2.1. Mood and Cognitive Disorders
2.2. Sleep Disturbance
2.3. Mood Disorder, Sleep Disturbance, and Nutrition
2.4. Impact of Decreasing Estrogen Levels on Metabolism and Weight
3. Etiology of a Pro-Inflammatory Diet
3.1. Dietary Intake and the Activation of Pro-Inflammatory Immune Pathways
3.2. Diet and Gut Permeability
4. Mood and Sleep Disturbances and Their Impacts on Inflammation
4.1. Psychological Stress and Gut Permeability
4.2. Sleep and Inflammation
5. Inflammation, a Risk Factor for MDD and AD
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, S.; Zhang, X.; Cai, Y.; Zheng, L.; Pang, H.; Lou, L. Sex Difference in Incidence of Major Depressive Disorder: An Analysis from the Global Burden of Disease Study 2019. Ann. Gen. Psychiatry 2023, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R. Why Is Depression More Prevalent in Women? J. Psychiatry Neurosci. JPN 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population Estimate of People with Clinical Alzheimer’s Disease and Mild Cognitive Impairment in the United States (2020–2060). Alzheimers Dement. J. Alzheimers Assoc. 2021, 17, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Chêne, G.; Beiser, A.; Au, R.; Preis, S.R.; Wolf, P.A.; Dufouil, C.; Seshadri, S. Gender and Incidence of Dementia in the Framingham Heart Study from Mid-Adult Life. Alzheimers Dement. J. Alzheimers Assoc. 2015, 11, 310–320. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.J.; Fulton-Howard, B.; Goate, A. Interpretation of Risk Loci from Genome-Wide Association Studies of Alzheimer’s Disease. Lancet Neurol. 2020, 19, 326–335. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Scheyer, O.; Rahman, A.; Hristov, H.; Berkowitz, C.; Isaacson, R.S.; Brinton, R.D.; Mosconi, L. Female Sex and Alzheimer’s Risk: The Menopause Connection. J. Prev. Alzheimers Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef]
- Better, M.A. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of Low-Grade Inflammation in Depression: A Systematic Review and Meta-Analysis of CRP Levels. Psychol. Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef]
- Qian, X.-H.; Song, X.-X.; Liu, X.-L.; Chen, S.; Tang, H.-D. Inflammatory Pathways in Alzheimer’s Disease Mediated by Gut Microbiota. Ageing Res. Rev. 2021, 68, 101317. [Google Scholar] [CrossRef] [PubMed]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative Meta-Analysis of Interleukins 6 and 1β, Tumour Necrosis Factor α and C-Reactive Protein in Patients with Major Depressive Disorder. Brain. Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Gan, Y.-H.; Yang, L.; Cheng, W.; Yu, J.-T. Depression in Alzheimer’s Disease: Epidemiology, Mechanisms, and Treatment. Biol. Psychiatry 2024, 95, 992–1005. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia Prevention, Intervention, and Care: 2024 Report of the Lancet Standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Gold, E.B. The Timing of the Age at Which Natural Menopause Occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Trévoux, R.; De Brux, J.; Castanier, M.; Nahoul, K.; Soule, J.P.; Scholler, R. Endometrium and Plasma Hormone Profile in the Peri-Menopause and Post-Menopause. Maturitas 1986, 8, 309–326. [Google Scholar] [CrossRef]
- Tandon, V.R.; Sharma, S.; Mahajan, A.; Mahajan, A.; Tandon, A. Menopause and Sleep Disorders. J.-Life Health 2022, 13, 26. [Google Scholar] [CrossRef]
- Greendale, G.A.; Karlamangla, A.S.; Maki, P.M. The Menopause Transition and Cognition. JAMA 2020, 323, 1495–1496. [Google Scholar] [CrossRef]
- Malutan, A.M.; Dan, M.; Nicolae, C.; Carmen, M. Proinflammatory and Anti-Inflammatory Cytokine Changes Related to Menopause. Przegla̜d Menopauzalny Menopause Rev. 2014, 13, 162–168. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet and Lifestyle Drive the Pandemic of Obesity and Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Azzam, A. Is the World Converging to a ‘Western Diet’? Public Health Nutr. 2021, 24, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Alblooshi, S.; Taylor, M.; Gill, N. Does Menopause Elevate the Risk for Developing Depression and Anxiety? Results from a Systematic Review. Australas. Psychiatry Bull. R. Aust. N. Z. Coll. Psychiatr. 2023, 31, 165–173. [Google Scholar] [CrossRef]
- Kundakovic, M.; Rocks, D. Sex Hormone Fluctuation and Increased Female Risk for Depression and Anxiety Disorders: From Clinical Evidence to Molecular Mechanisms. Front. Neuroendocrinol. 2022, 66, 101010. [Google Scholar] [CrossRef]
- Bromberger, J.T.; Epperson, C.N. Depression During and After the Perimenopause: Impact of Hormones, Genetics, and Environmental Determinants of Disease. Obstet. Gynecol. Clin. N. Am. 2018, 45, 663–678. [Google Scholar] [CrossRef]
- Vaziri-Harami, R.; Kazemi, S.N.; Vaziri-Harami, S.; Hazari, V.; Farokh, P.; Valadbeigi, T. The Prevalence of Depression and Anxiety in Premenopausal and Menopausal Women: A Cross-Sectional Study. Health Sci. Rep. 2024, 7, e2267. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, M.K.; Gustafsson, J.A.; Keller, E.; Hurd, Y.L. Estrogen Receptor Beta (ERbeta) Messenger Ribonucleic Acid (mRNA) Expression within the Human Forebrain: Distinct Distribution Pattern to ERalpha mRNA. J. Clin. Endocrinol. Metab. 2000, 85, 3840–3846. [Google Scholar] [CrossRef][Green Version]
- Heller, C.; Güllmar, D.; Koeppel, C.J.; Rojczyk, P.; Stein, H.; Taylor, C.M.; Jacobs, E.G.; Derntl, B.; Kikinis, Z.; Walter, M.; et al. Hippocampal Volume and Affect in Response to Fluctuating Estrogens in Menstrual Cycle Irregularity: A Longitudinal Single-Subject Study. Npj Womens Health 2024, 2, 19. [Google Scholar] [CrossRef]
- Campbell, S.; Macqueen, G. The Role of the Hippocampus in the Pathophysiology of Major Depression. J. Psychiatry Neurosci. JPN 2004, 29, 417–426. [Google Scholar]
- Cha, J.; Greenberg, T.; Song, I.; Blair Simpson, H.; Posner, J.; Mujica-Parodi, L.R. Abnormal Hippocampal Structure and Function in Clinical Anxiety and Comorbid Depression. Hippocampus 2016, 26, 545–553. [Google Scholar] [CrossRef]
- Akbaraly, T.; Sexton, C.; Zsoldos, E.; Mahmood, A.; Filippini, N.; Kerleau, C.; Verdier, J.-M.; Virtanen, M.; Gabelle, A.; Ebmeier, K.P.; et al. Association of Long-Term Diet Quality with Hippocampal Volume: Longitudinal Cohort Study. Am. J. Med. 2018, 131, 1372–1381.e4. [Google Scholar] [CrossRef]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Sachdev, P.; Butterworth, P. Western Diet Is Associated with a Smaller Hippocampus: A Longitudinal Investigation. BMC Med. 2015, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.; Sim, S.C.; Virding, S.; Onishchenko, N.; Schulte, G.; Ingelman-Sundberg, M. Decreased Hippocampal Volume and Increased Anxiety in a Transgenic Mouse Model Expressing the Human CYP2C19 Gene. Mol. Psychiatry 2014, 19, 733–741. [Google Scholar] [CrossRef]
- Baksh, R.A.; Ritchie, C.W.; Terrera, G.M.; Norton, J.; Raymont, V.; Ritchie, K. The Association between Anxiety Disorders and Hippocampal Volume in Older Adults. Psychol. Aging 2021, 36, 288–297. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. A Review and Update of Mechanisms of Estrogen in the Hippocampus and Amygdala for Anxiety and Depression Behavior. Neuropsychopharmacology 2006, 31, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lu, Y.; Pan, B.-X.; Zhang, W.-H. New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder. Int. J. Mol. Sci. 2022, 23, 11076. [Google Scholar] [CrossRef]
- Grogans, S.E.; Fox, A.S.; Shackman, A.J. The Amygdala and Depression: A Sober Reconsideration. Am. J. Psychiatry 2022, 179, 454–457. [Google Scholar] [CrossRef]
- Bendis, P.C.; Zimmerman, S.; Onisiforou, A.; Zanos, P.; Georgiou, P. The Impact of Estradiol on Serotonin, Glutamate, and Dopamine Systems. Front. Neurosci. 2024, 18, 1348551. [Google Scholar] [CrossRef] [PubMed]
- Ethridge, S.B.; Smith, M.A. Estradiol and Mu Opioid-Mediated Reward: The Role of Estrogen Receptors in Opioid Use. Addict. Neurosci. 2023, 9, 100139. [Google Scholar] [CrossRef]
- Aleem, F.A.; McIntosh, T.K. Menopausal Syndrome: Plasma Levels of Beta-Endorphin in Post-Menopausal Women Measured by a Specific Radioimmunoassay. Maturitas 1985, 7, 329–334. [Google Scholar] [CrossRef]
- Pilozzi, A.; Carro, C.; Huang, X. Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism. Int. J. Mol. Sci. 2020, 22, 338. [Google Scholar] [CrossRef]
- Stomati, M.; Bersi, C.; Rubino, S.; Palumbo, M.; Comitini, G.; Genazzani, A.D.; Santuz, M.; Petraglia, F.; Genazzani, A.R. Neuroendocrine Effects of Different Estradiol-Progestin Regimens in Postmenopausal Women. Maturitas 1997, 28, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.R.; Facchinetti, F.; Ricci-Danero, M.G.; Parrini, D.; Petraglia, F.; La Rosa, R.; D’Antona, N. Beta-Lipotropin and Beta-Endorphin in Physiological and Surgical Menopause. J. Endocrinol. Investig. 1981, 4, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Joffe, H.; de Wit, A.; Coborn, J.; Crawford, S.; Freeman, M.; Wiley, A.; Athappilly, G.; Kim, S.; Sullivan, K.A.; Cohen, L.S.; et al. Impact of Estradiol Variability and Progesterone on Mood in Perimenopausal Women with Depressive Symptoms. J. Clin. Endocrinol. Metab. 2020, 105, e642–e650. [Google Scholar] [CrossRef]
- Byers, A.L.; Yaffe, K. Depression and Risk of Developing Dementia. Nat. Rev. Neurol. 2011, 7, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Cheslack-Postava, K.; Keyes, K.M.; Lowe, S.R.; Koenen, K.C. Oral Contraceptive Use and Psychiatric Disorders in a Nationally Representative Sample of Women. Arch. Womens Ment. Health 2015, 18, 103–111. [Google Scholar] [CrossRef]
- Boyle, C.P.; Raji, C.A.; Erickson, K.I.; Lopez, O.L.; Becker, J.T.; Gach, H.M.; Kuller, L.H.; Longstreth, W.; Carmichael, O.T.; Riedel, B.C.; et al. Estrogen, Brain Structure, and Cognition in Postmenopausal Women. Hum. Brain Mapp. 2021, 42, 24–35. [Google Scholar] [CrossRef]
- Bayer, U.; Hausmann, M. Estrogen Treatment Affects Brain Functioning after Menopause. Menopause Int. 2011, 17, 148–152. [Google Scholar] [CrossRef]
- Matyi, J.M.; Rattinger, G.B.; Schwartz, S.; Buhusi, M.; Tschanz, J.T. Lifetime Estrogen Exposure and Cognition in Late Life: The Cache County Study. Menopause 2019, 26, 1366–1374. [Google Scholar] [CrossRef]
- Herson, M.; Kulkarni, J. Hormonal Agents for the Treatment of Depression Associated with the Menopause. Drugs Aging 2022, 39, 607–618. [Google Scholar] [CrossRef]
- Pengo, M.F.; Won, C.H.; Bourjeily, G. Sleep in Women Across the Life Span. Chest 2018, 154, 196–206. [Google Scholar] [CrossRef]
- Toffol, E.; Kalleinen, N.; Haukka, J.; Vakkuri, O.; Partonen, T.; Polo-Kantola, P. Melatonin in Perimenopausal and Postmenopausal Women: Associations with Mood, Sleep, Climacteric Symptoms, and Quality of Life. Menopause 2014, 21, 493–500. [Google Scholar] [CrossRef]
- Kravitz, H.M.; Zhao, X.; Bromberger, J.T.; Gold, E.B.; Hall, M.H.; Matthews, K.A.; Sowers, M.R. Sleep Disturbance during the Menopausal Transition in a Multi-Ethnic Community Sample of Women. Sleep 2008, 31, 979–990. [Google Scholar] [PubMed]
- Naufel, M.F.; Frange, C.; Andersen, M.L.; Girão, M.J.B.C.; Tufik, S.; Beraldi Ribeiro, E.; Hachul, H. Association between Obesity and Sleep Disorders in Postmenopausal Women. Menopause 2018, 25, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Wesstrom, J.; Nilsson, S.; Sundstrom-Poromaa, I.; Ulfberg, J. Restless Legs Syndrome among Women: Prevalence, Co-Morbidity and Possible Relationship to Menopause. Climacteric J. Int. Menopause Soc. 2008, 11, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Bélanger, L.; Ivers, H.; Guay, B.; Zhang, J.; Morin, C.M. Comparison of Subjective and Objective Sleep Quality in Menopausal and Non-Menopausal Women with Insomnia. Sleep Med. 2011, 12, 65–69. [Google Scholar] [CrossRef]
- Dugral, E.; Ordu, G. Differences in Polysomnography Parameters of Women in the Post and Transitional Phases of Menopause. Cureus 2021, 13, e20570. [Google Scholar] [CrossRef]
- Kalleinen, N.; Aittokallio, J.; Lampio, L.; Kaisti, M.; Polo-Kantola, P.; Polo, O.; Heinonen, O.J.; Saaresranta, T. Sleep during Menopausal Transition: A 10-Year Follow-Up. Sleep 2021, 44, zsaa283. [Google Scholar] [CrossRef]
- Bernier, V.; Debarge, M.-H.; Hein, M.; Ammendola, S.; Mungo, A.; Loas, G. Major Depressive Disorder, Inflammation, and Nutrition: A Tricky Pattern? Nutrients 2023, 15, 3438. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, H.; Liu, Z.; Yang, J.; Liu, Y. Association between Dietary Sugar Intake and Depression in US Adults: A Cross-Sectional Study Using Data from the National Health and Nutrition Examination Survey 2011–2018. BMC Psychiatry 2024, 24, 110. [Google Scholar] [CrossRef]
- El Ansari, W.; Adetunji, H.; Oskrochi, R. Food and Mental Health: Relationship between Food and Perceived Stress and Depressive Symptoms among University Students in the United Kingdom. Cent. Eur. J. Public Health 2014, 22, 90–97. [Google Scholar] [CrossRef]
- Yu, B.; He, H.; Zhang, Q.; Wu, H.; Du, H.; Liu, L.; Wang, C.; Shi, H.; Xia, Y.; Guo, X.; et al. Soft Drink Consumption Is Associated with Depressive Symptoms among Adults in China. J. Affect. Disord. 2015, 172, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Westover, A.N.; Marangell, L.B. A Cross-National Relationship between Sugar Consumption and Major Depression? Depress. Anxiety 2002, 16, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Shahdadian, F.; Boozari, B.; Saneei, P. Association between Short Sleep Duration and Intake of Sugar and Sugar-Sweetened Beverages: A Systematic Review and Meta-Analysis of Observational Studies. Sleep Health 2023, 9, 159–176. [Google Scholar] [CrossRef]
- Alahmary, S.A.; Alduhaylib, S.A.; Alkawii, H.A.; Olwani, M.M.; Shablan, R.A.; Ayoub, H.M.; Purayidathil, T.S.; Abuzaid, O.I.; Khattab, R.Y. Relationship Between Added Sugar Intake and Sleep Quality Among University Students: A Cross-Sectional Study. Am. J. Lifestyle Med. 2022, 16, 122–129. [Google Scholar] [CrossRef]
- Benedict, C.; Brooks, S.J.; O’Daly, O.G.; Almèn, M.S.; Morell, A.; Åberg, K.; Gingnell, M.; Schultes, B.; Hallschmid, M.; Broman, J.-E.; et al. Acute Sleep Deprivation Enhances the Brain’s Response to Hedonic Food Stimuli: An fMRI Study. J. Clin. Endocrinol. Metab. 2012, 97, E443–E447. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Wolfe, S.; Sy, M.; Shechter, A.; Hirsch, J. Sleep Restriction Increases the Neuronal Response to Unhealthy Food in Normal-Weight Individuals. Int. J. Obes. 2014, 38, 411–416. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Redondo-Flórez, L.; Tornero-Aguilera, J.F. The Burden of Carbohydrates in Health and Disease. Nutrients 2022, 14, 3809. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Holt, S.H.A.; Pawlak, D.B.; McMillan, J. Glycemic Index and Obesity. Am. J. Clin. Nutr. 2002, 76, 281S–285S. [Google Scholar] [CrossRef] [PubMed]
- Fazzino, T.L.; Dorling, J.L.; Apolzan, J.W.; Martin, C.K. Meal Composition during an Ad Libitum Buffet Meal and Longitudinal Predictions of Weight and Percent Body Fat Change: The Role of Hyper-Palatable, Energy Dense, and Ultra-Processed Foods. Appetite 2021, 167, 105592. [Google Scholar] [CrossRef]
- Kissileff, H.R. Some Suggestions on Dealing with Palatability--Response to Ramirez. Appetite 1990, 14, 162–166; discussion 180. [Google Scholar] [CrossRef]
- Benton, D. Carbohydrate Ingestion, Blood Glucose and Mood. Neurosci. Biobehav. Rev. 2002, 26, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Bohórquez, D.V. The Neural Basis of Sugar Preference. Nat. Rev. Neurosci. 2022, 23, 584–595. [Google Scholar] [CrossRef]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Evidence for Sugar Addiction: Behavioral and Neurochemical Effects of Intermittent, Excessive Sugar Intake. Neurosci. Biobehav. Rev. 2008, 32, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Winterdahl, M.; Noer, O.; Orlowski, D.; Schacht, A.C.; Jakobsen, S.; Alstrup, A.K.O.; Gjedde, A.; Landau, A.M. Sucrose Intake Lowers μ-Opioid and Dopamine D2/3 Receptor Availability in Porcine Brain. Sci. Rep. 2019, 9, 16918. [Google Scholar] [CrossRef] [PubMed]
- Gangwisch, J.E.; Hale, L.; Garcia, L.; Malaspina, D.; Opler, M.G.; Payne, M.E.; Rossom, R.C.; Lane, D. High Glycemic Index Diet as a Risk Factor for Depression: Analyses from the Women’s Health Initiative. Am. J. Clin. Nutr. 2015, 102, 454–463. [Google Scholar] [CrossRef]
- Breymeyer, K.L.; Lampe, J.W.; McGregor, B.A.; Neuhouser, M.L. Subjective Mood and Energy Levels of Healthy Weight and Overweight/Obese Healthy Adults on High-and Low-Glycemic Load Experimental Diets. Appetite 2016, 107, 253–259. [Google Scholar] [CrossRef]
- Kuryłowicz, A. Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines 2023, 11, 690. [Google Scholar] [CrossRef]
- Opoku, A.A.; Abushama, M.; Konje, J.C. Obesity and Menopause. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 88, 102348. [Google Scholar] [CrossRef]
- Davis, S.R.; Castelo-Branco, C.; Chedraui, P.; Lumsden, M.A.; Nappi, R.E.; Shah, D.; Villaseca, P.; Writing Group of the International Menopause Society for World Menopause Day 2012. Understanding Weight Gain at Menopause. Climacteric J. Int. Menopause Soc. 2012, 15, 419–429. [Google Scholar] [CrossRef]
- Hurtado, M.D.; Saadedine, M.; Kapoor, E.; Shufelt, C.L.; Faubion, S.S. Weight Gain in Midlife Women. Curr. Obes. Rep. 2024, 13, 352–363. [Google Scholar] [CrossRef]
- Chedraui, P.; Hidalgo, L.; Chavez, D.; Morocho, N.; Alvarado, M.; Huc, A. Menopausal Symptoms and Associated Risk Factors among Postmenopausal Women Screened for the Metabolic Syndrome. Arch. Gynecol. Obstet. 2007, 275, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Alder, E. The Blatt-Kupperman Menopausal Index: A Critique. Maturitas 1998, 29, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Chatelan, A.; Carrard, I. Diet Quality in Middle-Aged and Older Women with and without Body Weight Dissatisfaction: Results from a Population-Based National Nutrition Survey in Switzerland. J. Nutr. Sci. 2021, 10, e38. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic Stress and Obesity: A New View of “Comfort Food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Guedes Melo, R.; de Souza Medeiros, J.; Queiroz de Medeiros, A.C.; de Araújo Lopes, F.; Federal University of Rio Grande do Norte. Comfort Food Concepts and Contexts in Which They Are Used: A Scoping Review Protocol. PLoS ONE 2024, 19, e0299991. [Google Scholar] [CrossRef]
- Holt, R.R.; Schmitz, H.H.; Mhawish, R.; Komarnytsky, S.; Nguyen, T.; Caveney, P.M.; Munafo, J.P. Comfort Foods in the Twenty-First Century: Friend or Foe? Annu. Rev. Food Sci. Technol. 2024, 16, 433–458. [Google Scholar] [CrossRef]
- Macdonald, H.M.; New, S.A.; Reid, D.M. Longitudinal Changes in Dietary Intake in Scottish Women around the Menopause: Changes in Dietary Pattern Result in Minor Changes in Nutrient Intake. Public Health Nutr. 2005, 8, 409–416. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased Visceral Fat and Decreased Energy Expenditure during the Menopausal Transition. Int. J. Obes. 2005 2008, 32, 949–958. [Google Scholar] [CrossRef]
- Duval, K.; Prud’homme, D.; Rabasa-Lhoret, R.; Strychar, I.; Brochu, M.; Lavoie, J.-M.; Doucet, E. Effects of the Menopausal Transition on Dietary Intake and Appetite: A MONET Group Study. Eur. J. Clin. Nutr. 2014, 68, 271–276. [Google Scholar] [CrossRef]
- Macdonald, H.M.; New, S.A.; Campbell, M.K.; Reid, D.M. Longitudinal Changes in Weight in Perimenopausal and Early Postmenopausal Women: Effects of Dietary Energy Intake, Energy Expenditure, Dietary Calcium Intake and Hormone Replacement Therapy. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2003, 27, 669–676. [Google Scholar] [CrossRef]
- Grisotto, G.; Raguindin, P.F.; Glisic, M.; Bally, L.; Bano, A.; Franco, O.H.; Marques-Vidal, P.; Muka, T. Menopausal Transition Is Not Associated with Dietary Change in Swiss Women. J. Nutr. 2021, 151, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Peters, J.C.; Wyatt, H.R. Using the Energy Gap to Address Obesity: A Commentary. J. Am. Diet. Assoc. 2009, 109, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive Intake of Sugar: An Accomplice of Inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Karmakar, S. Excess Dietary Sugar and Its Impact on Periodontal Inflammation: A Narrative Review. BDJ Open 2024, 10, 78. [Google Scholar] [CrossRef]
- Merino, B.; Fernández-Díaz, C.M.; Cózar-Castellano, I.; Perdomo, G. Intestinal Fructose and Glucose Metabolism in Health and Disease. Nutrients 2019, 12, 94. [Google Scholar] [CrossRef]
- Mayes, P.A. Intermediary Metabolism of Fructose. Am. J. Clin. Nutr. 1993, 58, 754S–765S. [Google Scholar] [CrossRef]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef]
- Douard, V.; Ferraris, R.P. The Role of Fructose Transporters in Diseases Linked to Excessive Fructose Intake. J. Physiol. 2013, 591, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; McCall, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose Consumption as a Risk Factor for Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, J.; Sun, Y.; Zhan, Q.; Zhang, D. High Fructose Diet: A Risk Factor for Immune System Dysregulation. Hum. Immunol. 2022, 83, 538–546. [Google Scholar] [CrossRef]
- Shomali, N.; Mahmoudi, J.; Mahmoodpoor, A.; Zamiri, R.E.; Akbari, M.; Xu, H.; Shotorbani, S.S. Harmful Effects of High Amounts of Glucose on the Immune System: An Updated Review. Biotechnol. Appl. Biochem. 2021, 68, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Semchyshyn, H. Fructose-Mediated AGE-RAGE Axis: Approaches for Mild Modulation. Front. Nutr. 2024, 11, 1500375. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- Porto, M.L.; Lírio, L.M.; Dias, A.T.; Batista, A.T.; Campagnaro, B.P.; Mill, J.G.; Meyrelles, S.S.; Baldo, M.P. Increased Oxidative Stress and Apoptosis in Peripheral Blood Mononuclear Cells of Fructose-Fed Rats. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2015, 29, 1977–1981. [Google Scholar] [CrossRef]
- Jaiswal, N.; Agrawal, S.; Agrawal, A. High Fructose-Induced Metabolic Changes Enhance Inflammation in Human Dendritic Cells. Clin. Exp. Immunol. 2019, 197, 237–249. [Google Scholar] [CrossRef]
- Kaur, H.; Chien, A.; Jialal, I. Hyperglycemia Induces Toll like Receptor 4 Expression and Activity in Mouse Mesangial Cells: Relevance to Diabetic Nephropathy. Am. J. Physiol. Renal Physiol. 2012, 303, F1145–F1150. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Qi, X.; Shao, Y.; Li, Y.; Fu, X.; Feng, S.; Wu, Y. High Glucose Induced-Macrophage Activation through TGF-β-Activated Kinase 1 Signaling Pathway. Inflamm. Res. 2016, 65, 655–664. [Google Scholar] [CrossRef]
- O’Connor, L.; Imamura, F.; Brage, S.; Griffin, S.J.; Wareham, N.J.; Forouhi, N.G. Intakes and Sources of Dietary Sugars and Their Association with Metabolic and Inflammatory Markers. Clin. Nutr. Edinb. Scotl. 2018, 37, 1313–1322. [Google Scholar] [CrossRef]
- Liu, S.; Manson, J.E.; Buring, J.E.; Stampfer, M.J.; Willett, W.C.; Ridker, P.M. Relation between a Diet with a High Glycemic Load and Plasma Concentrations of High-Sensitivity C-Reactive Protein in Middle-Aged Women. Am. J. Clin. Nutr. 2002, 75, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, S.; Hancock, D.P.; Petocz, P.; Ceriello, A.; Brand-Miller, J. High-Glycemic Index Carbohydrate Increases Nuclear Factor-kappaB Activation in Mononuclear Cells of Young, Lean Healthy Subjects. Am. J. Clin. Nutr. 2008, 87, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Schwarz, Y.; Wang, C.; Breymeyer, K.; Coronado, G.; Wang, C.-Y.; Noar, K.; Song, X.; Lampe, J.W. A Low-Glycemic Load Diet Reduces Serum C-Reactive Protein and Modestly Increases Adiponectin in Overweight and Obese Adults. J. Nutr. 2012, 142, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Núñez, B.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. The Relation of Saturated Fatty Acids with Low-Grade Inflammation and Cardiovascular Disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio, M.d.C.G.; Alfenas, R.d.C.G. Influence of a High-Fat Diet on Gut Microbiota, Intestinal Permeability and Metabolic Endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Langley, K.G.; Berglund, N.A.; Kammoun, H.L.; Reibe, S.; Estevez, E.; Weir, J.; Mellett, N.A.; Pernes, G.; Conway, J.R.W.; et al. Evidence That TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab. 2018, 27, 1096–1110.e5. [Google Scholar] [CrossRef]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.-H.; Brickey, W.J.; Ting, J.P.-Y. Fatty Acid-Induced NLRP3-ASC Inflammasome Activation Interferes with Insulin Signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef]
- Nishida, C.; Uauy, R. WHO Scientific Update on Health Consequences of Trans Fatty Acids: Introduction. Eur. J. Clin. Nutr. 2009, 63 (Suppl. S2), S1–S4. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health Effects of Trans-Fatty Acids: Experimental and Observational Evidence. Eur. J. Clin. Nutr. 2009, 63 (Suppl. S2), S5–S21. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Very Long-Chain n-3 Fatty Acids and Human Health: Fact, Fiction and the Future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- La Torre, D.; Van Oudenhove, L.; Vanuytsel, T.; Verbeke, K. Psychosocial Stress-Induced Intestinal Permeability in Healthy Humans: What Is the Evidence? Neurobiol. Stress 2023, 27, 100579. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef]
- Nativel, B. Pathologie Inflammatoire: Étude de la Contribution des PAMP et DAMP. Ph.D. Thesis, Université de la Réunion, Saint-Denis, Réunion, 2017. [Google Scholar]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s That Spur Autophagy and Immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Staltner, R.; Burger, K.; Baumann, A.; Bergheim, I. Fructose: A Modulator of Intestinal Barrier Function and Hepatic Health? Eur. J. Nutr. 2023, 62, 3113–3124. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Bosco, N.; Noti, M. The Aging Gut Microbiome and Its Impact on Host Immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; Aidy, S.E.; Hugenholtz, F.; van der Gaast-de Jongh, C.; Savelkoul, H.F.J.; De Jonge, M.I.; Boekschoten, M.V.; Smidt, H.; et al. Aged Gut Microbiota Contributes to Systemical Inflammaging after Transfer to Germ-Free Mice. Front. Immunol. 2017, 8, 1385. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and Anti-Inflammaging: A Systemic Perspective on Aging and Longevity Emerged from Studies in Humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Cho, Y.-E.; Kim, D.-K.; Seo, W.; Gao, B.; Yoo, S.-H.; Song, B.-J. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1-Mediated Oxidative and Nitrative Stress. Hepatology 2021, 73, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Guney, C.; Bal, N.B.; Akar, F. The Impact of Dietary Fructose on Gut Permeability, Microbiota, Abdominal Adiposity, Insulin Signaling and Reproductive Function. Heliyon 2023, 9, e18896. [Google Scholar] [CrossRef]
- Jung, S.; Bae, H.; Song, W.-S.; Jang, C. Dietary Fructose and Fructose-Induced Pathologies. Annu. Rev. Nutr. 2022, 42, 45–66. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef]
- Garcia, K.; Ferreira, G.; Reis, F.; Viana, S. Impact of Dietary Sugars on Gut Microbiota and Metabolic Health. Diabetology 2022, 3, 549–560. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Bindels, L.B.; Neyrinck, A.M.; Walter, J. The Gut Microbiome and Dietary Fibres: Implications in Obesity, Cardiometabolic Diseases and Cancer. Nat. Rev. Microbiol. 2025, 23, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Chen, S.-Y.; Sheng, L.; Jena, P.K.; Kalanetra, K.M.; Mills, D.A.; Wan, Y.-J.Y.; Slupsky, C.M. Long-Term Effects of Western Diet Consumption in Male and Female Mice. Sci. Rep. 2020, 10, 14686. [Google Scholar] [CrossRef]
- Firth, J.; Gangwisch, J.E.; Borisini, A.; Wootton, R.E.; Mayer, E.A. Food and Mood: How Do Diet and Nutrition Affect Mental Wellbeing? BMJ 2020, 369, m2382. [Google Scholar] [CrossRef]
- Cao, J.; Papadopoulou, N.; Kempuraj, D.; Boucher, W.S.; Sugimoto, K.; Cetrulo, C.L.; Theoharides, T.C. Human Mast Cells Express Corticotropin-Releasing Hormone (CRH) Receptors and CRH Leads to Selective Secretion of Vascular Endothelial Growth Factor. J. Immunol. 2005, 174, 7665–7675. [Google Scholar] [CrossRef]
- Vicario, M.; Guilarte, M.; Alonso, C.; Yang, P.; Martínez, C.; Ramos, L.; Lobo, B.; González, A.; Guilà, M.; Pigrau, M.; et al. Chronological Assessment of Mast Cell-Mediated Gut Dysfunction and Mucosal Inflammation in a Rat Model of Chronic Psychosocial Stress. Brain. Behav. Immun. 2010, 24, 1166–1175. [Google Scholar] [CrossRef]
- Saunders, P.R.; Santos, J.; Hanssen, N.P.M.; Yates, D.; Groot, J.A.; Perdue, M.H. Physical and Psychological Stress in Rats Enhances Colonic Epithelial Permeability via Peripheral CRH. Dig. Dis. Sci. 2002, 47, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Vanuytsel, T.; van Wanrooy, S.; Vanheel, H.; Vanormelingen, C.; Verschueren, S.; Houben, E.; Salim Rasoel, S.; Tόth, J.; Holvoet, L.; Farré, R.; et al. Psychological Stress and Corticotropin-Releasing Hormone Increase Intestinal Permeability in Humans by a Mast Cell-Dependent Mechanism. Gut 2014, 63, 1293–1299. [Google Scholar] [CrossRef]
- Albert-Bayo, M.; Paracuellos, I.; González-Castro, A.M.; Rodríguez-Urrutia, A.; Rodríguez-Lagunas, M.J.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Intestinal Mucosal Mast Cells: Key Modulators of Barrier Function and Homeostasis. Cells 2019, 8, 135. [Google Scholar] [CrossRef]
- Gerdin, L.; González-Castro, A.M.; Ericson, A.-C.; Persborn, M.; Santos, J.; Walter, S.A.; Keita, Å.V.; Vicario, M.; Söderholm, J.D. Acute Psychological Stress Increases Paracellular Permeability and Modulates Immune Activity in Rectal Mucosa of Healthy Volunteers. United Eur. Gastroenterol. J. 2023, 11, 31–41. [Google Scholar] [CrossRef] [PubMed]
- de Punder, K.; Pruimboom, L. Stress Induces Endotoxemia and Low-Grade Inflammation by Increasing Barrier Permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef]
- Schäper, J.; Wagner, A.; Enigk, F.; Brell, B.; Mousa, S.A.; Habazettl, H.; Schäfer, M. Regional Sympathetic Blockade Attenuates Activation of Intestinal Macrophages and Reduces Gut Barrier Failure. Anesthesiology 2013, 118, 134–142. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and Inflammation: Partners in Sickness and in Health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- Irwin, M.R.; Wang, M.; Campomayor, C.O.; Collado-Hidalgo, A.; Cole, S. Sleep Deprivation and Activation of Morning Levels of Cellular and Genomic Markers of Inflammation. Arch. Intern. Med. 2006, 166, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Dumaine, J.E.; Ashley, N.T. Acute Sleep Fragmentation Induces Tissue-Specific Changes in Cytokine Gene Expression and Increases Serum Corticosterone Concentration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R1062–R1069. [Google Scholar] [CrossRef]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef]
- Summa, K.C.; Voigt, R.M.; Forsyth, C.B.; Shaikh, M.; Cavanaugh, K.; Tang, Y.; Vitaterna, M.H.; Song, S.; Turek, F.W.; Keshavarzian, A. Disruption of the Circadian Clock in Mice Increases Intestinal Permeability and Promotes Alcohol-Induced Hepatic Pathology and Inflammation. PLoS ONE 2013, 8, e67102. [Google Scholar] [CrossRef]
- Irwin, M.R.; Carrillo, C.; Olmstead, R. Sleep Loss Activates Cellular Markers of Inflammation: Sex Differences. Brain. Behav. Immun. 2010, 24, 54–57. [Google Scholar] [CrossRef]
- Prather, A.A.; Epel, E.S.; Cohen, B.E.; Neylan, T.C.; Whooley, M.A. Gender Differences in the Prospective Associations of Self-Reported Sleep Quality with Biomarkers of Systemic Inflammation and Coagulation: Findings from the Heart and Soul Study. J. Psychiatr. Res. 2013, 47, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine, Sickness Behavior, and Depression. Immunol. Allergy Clin. N. Am. 2009, 29, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines Sing the Blues: Inflammation and the Pathogenesis of Depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Gumnick, J.F.; Musselman, D.L.; Lawson, D.H.; Reemsnyder, A.; Nemeroff, C.B.; Miller, A.H. Neurobehavioral Effects of Interferon-Alpha in Cancer Patients: Phenomenology and Paroxetine Responsiveness of Symptom Dimensions. Neuropsychopharmacology 2002, 26, 643–652. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s Metabolites in Exercise, Inflammation, and Mental Health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Jha, M.K.; Minhajuddin, A.; Gadad, B.S.; Greer, T.; Grannemann, B.; Soyombo, A.; Mayes, T.L.; Rush, A.J.; Trivedi, M.H. Can C-Reactive Protein Inform Antidepressant Medication Selection in Depressed Outpatients? Findings from the CO-MED Trial. Psychoneuroendocrinology 2017, 78, 105–113. [Google Scholar] [CrossRef]
- Uher, R.; Tansey, K.E.; Dew, T.; Maier, W.; Mors, O.; Hauser, J.; Dernovsek, M.Z.; Henigsberg, N.; Souery, D.; Farmer, A.; et al. An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment with Escitalopram and Nortriptyline. Am. J. Psychiatry 2014, 171, 1278–1286. [Google Scholar] [CrossRef]
- Huffman, J.C.; Celano, C.M.; Beach, S.R.; Motiwala, S.R.; Januzzi, J.L. Depression and Cardiac Disease: Epidemiology, Mechanisms, and Diagnosis. Cardiovasc. Psychiatry Neurol. 2013, 2013, 695925. [Google Scholar] [CrossRef]
- Haroon, E.; Daguanno, A.W.; Woolwine, B.J.; Goldsmith, D.R.; Baer, W.M.; Wommack, E.C.; Felger, J.C.; Miller, A.H. Antidepressant Treatment Resistance Is Associated with Increased Inflammatory Markers in Patients with Major Depressive Disorder. Psychoneuroendocrinology 2018, 95, 43–49. [Google Scholar] [CrossRef]
- Freeman, M.P.; Lee, H.; Savella, G.M.; Sosinsky, A.Z.; Marfurt, S.P.; Murphy, S.K.; Cohen, L.S. Predictors of Depressive Relapse in Women Undergoing Infertility Treatment. J. Womens Health 2018, 27, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.S.P.; Liu, C.S.; Rau, A.; Lanctôt, K.L.; Köhler, C.A.; Pakosh, M.; Carvalho, A.F.; Herrmann, N. Peripheral Inflammatory Markers in Alzheimer’s Disease: A Systematic Review and Meta-Analysis of 175 Studies. J. Neurol. Neurosurg. Psychiatry 2017, 88, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Galea, I. The Blood-Brain Barrier in Systemic Inflammation. Brain. Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Le Thuc, O.; García-Cáceres, C. Obesity-Induced Inflammation: Connecting the Periphery to the Brain. Nat. Metab. 2024, 6, 1237–1252. [Google Scholar] [CrossRef]
- Holmes, C. Review: Systemic Inflammation and A Lzheimer’s Disease. Neuropathol. Appl. Neurobiol. 2013, 39, 51–68. [Google Scholar] [CrossRef]
- Lim, S.L.; Rodriguez-Ortiz, C.J.; Kitazawa, M. Infection, Systemic Inflammation, and Alzheimer’s Disease. Microbes Infect. 2015, 17, 549–556. [Google Scholar] [CrossRef]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western Diet as a Trigger of Alzheimer’s Disease: From Metabolic Syndrome and Systemic Inflammation to Neuroinflammation and Neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernier, V.; Chatelan, A.; Point, C.; Strauss, M. Nutrition and Neuroinflammation: Are Middle-Aged Women in the Red Zone? Nutrients 2025, 17, 1607. https://doi.org/10.3390/nu17101607
Bernier V, Chatelan A, Point C, Strauss M. Nutrition and Neuroinflammation: Are Middle-Aged Women in the Red Zone? Nutrients. 2025; 17(10):1607. https://doi.org/10.3390/nu17101607
Chicago/Turabian StyleBernier, Veronique, Angeline Chatelan, Camille Point, and Mélanie Strauss. 2025. "Nutrition and Neuroinflammation: Are Middle-Aged Women in the Red Zone?" Nutrients 17, no. 10: 1607. https://doi.org/10.3390/nu17101607
APA StyleBernier, V., Chatelan, A., Point, C., & Strauss, M. (2025). Nutrition and Neuroinflammation: Are Middle-Aged Women in the Red Zone? Nutrients, 17(10), 1607. https://doi.org/10.3390/nu17101607






